Abstract
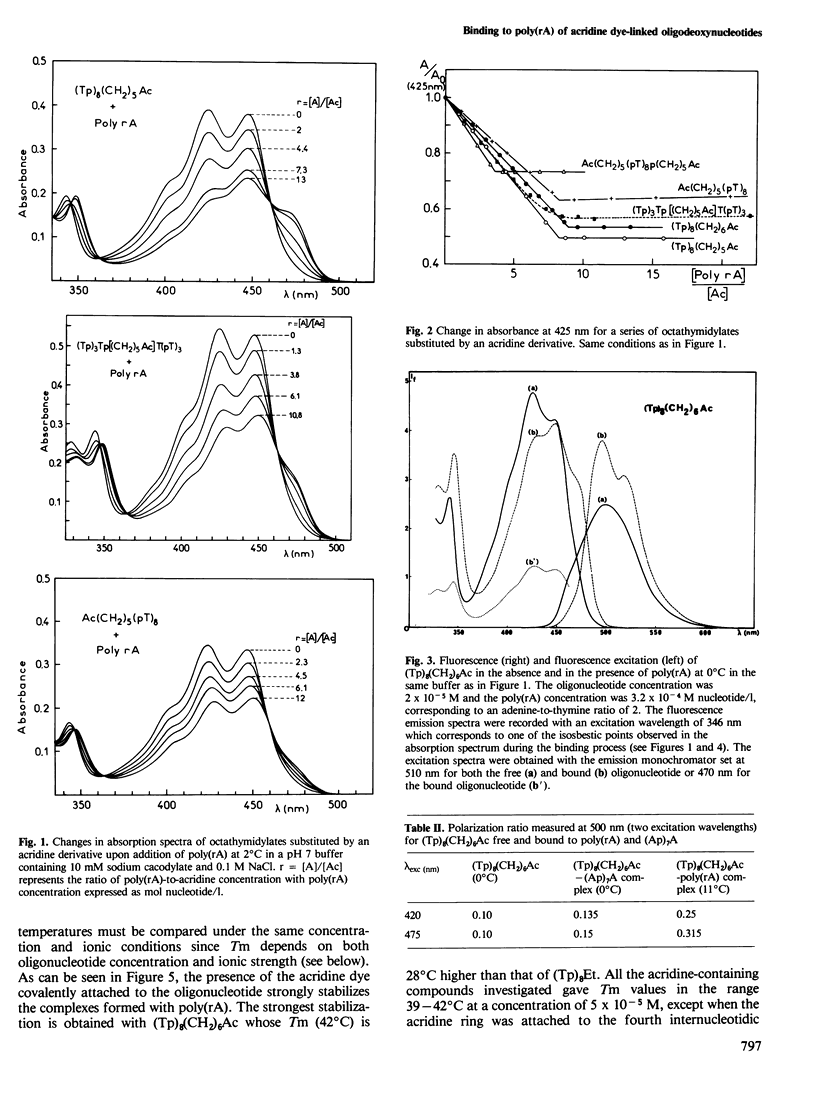
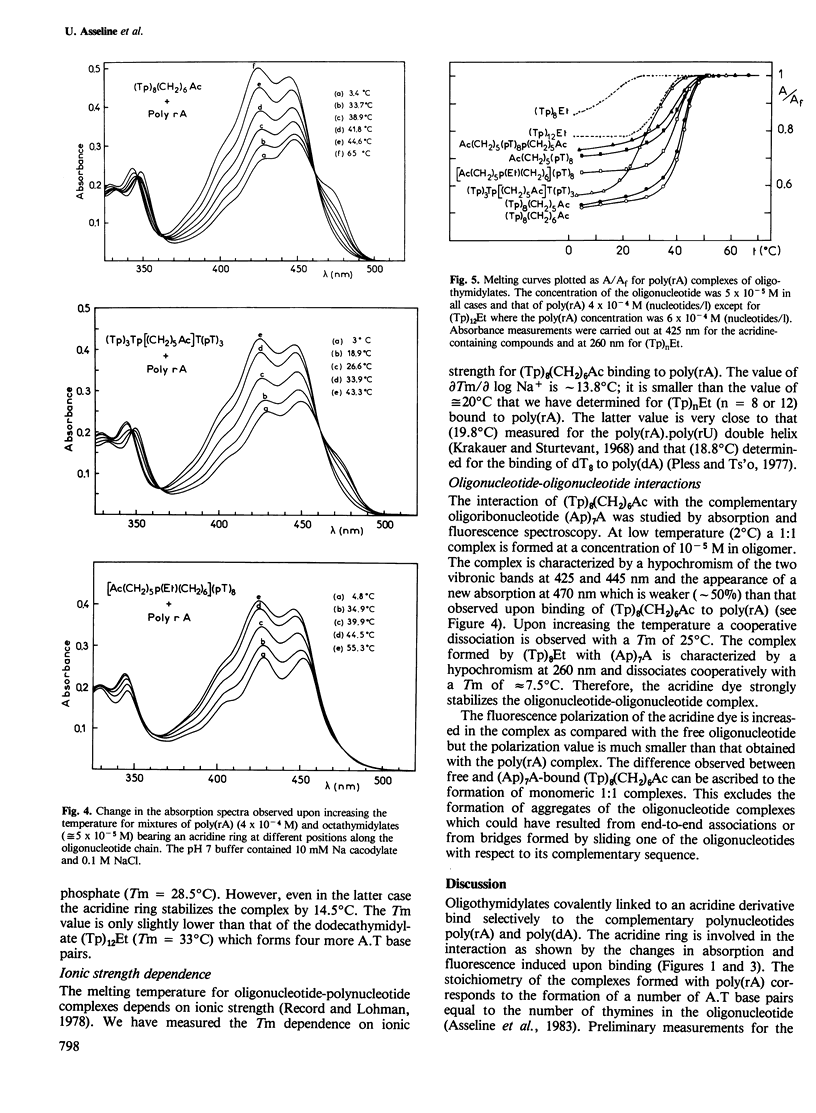
New molecules with high and specific affinity for nucleic acid base sequences have been synthesized. They involve an oligodeoxynucleotide covalently attached to an intercalating dye. Visible absorption spectroscopy and fluorescence have been used to investigate the binding of poly(rA) to octadeoxythymidylates substituted by a 9-aminoacridine derivative in different positions along the oligonucleotide chain. The 9-amino group of the acridine dye was linked through a polymethylene bridge to the 3'-phosphate, the 5'-phosphate, the fourth internucleotidic phosphate or to both the 3'- and 5'-phosphates. Different interactions of the acridine dye were exhibited by these different substituted oligodeoxynucleotides when they bind to poly(rA). The interaction was shown to be specific for adenine-containing polynucleotides. The stability of these complexes was compared with that of oligodeoxynucleotides substituted by an alkyl group on the 3'-phosphate. The increase in stability due to the presence of the intercalating dye has been determined from the comparison of melting temperatures. These results are discussed with respect to the strategy of synthesis of a new class of molecules with high affinity and high specificity for nucleic acid base sequences.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Nguyen T. T., Hélène C. Nouvelles substances à forte affinité spécifique pour des séquences d'acides nucléiques: oligodésoxynucléotides liés de façon covalente à un agent intercalant. C R Seances Acad Sci III. 1983;297(7):369–372. [PubMed] [Google Scholar]
- Baldini G., Doglia S., Dolci S., Sassi G. Fluorescence-determined preferential binding of quinacrine to DNA. Biophys J. 1981 Dec;36(3):465–477. doi: 10.1016/S0006-3495(81)84746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps J., Fredericq E. Comparative binding study of the interaction of quinacrine and ethidium bromide with DNA and nucleohistone. Biophys Chem. 1974 Jun;2(1):1–22. doi: 10.1016/0301-4622(74)80019-0. [DOI] [PubMed] [Google Scholar]
- Hélène C., Lancelot G. Interactions between functional groups in protein-nucleic acid associations. Prog Biophys Mol Biol. 1982;39(1):1–68. doi: 10.1016/0079-6107(83)90013-5. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Lanier A. C., Keel R. A., Wilson W. D. The effect of ionic strength on DNA-ligand unwinding angles for acridine and quinoline derivatives. Nucleic Acids Res. 1980 Apr 11;8(7):1613–1624. doi: 10.1093/nar/8.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer H., Sturtevant J. M. Heats of the helix-coil transitions of the poly A-poly U complexes. Biopolymers. 1968 Apr;6(4):491–512. doi: 10.1002/bip.1968.360060406. [DOI] [PubMed] [Google Scholar]
- Pless R. C., Ts'o P. O. Duplex formation of a nonionic oligo(deoxythymidylate) analogue (heptadeoxythymidylyl-(3'-5')-deoxythymidine heptaethyl ester (d-(Tp(Et))7T)) with poly(deoxyadenylate). Evaluation of the electrostatic interaction. Biochemistry. 1977 Mar 22;16(6):1239–1250. doi: 10.1021/bi00625a033. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T. Plasmid ColE1 incompatibility determined by interaction of RNA I with primer transcript. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6096–6100. doi: 10.1073/pnas.78.10.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]



