Abstract
The major mRNA degradation pathway involves deadenylation of the target molecule followed by decapping and, finally, 5′→3′ exonuclease digestion of the mRNA body. While yeast factors involved in the decapping and exonuclease degradation steps have been identified, the nature of the factor(s) involved in the deadenylation step remained elusive. Database searches for yeast proteins related to the mammalian deadenylase PARN identified the Pop2 protein (Pop2p) as a potential deadenylase. While Pop2p was previously identified as a factor affecting transcription, we identified a non-canonical RNase D sequence signature in its sequence. Analysis of the fate of a reporter mRNA in a pop2 mutant demonstrates that Pop2p is required for efficient mRNA degradation in vivo. Characterisation of mRNA degradation intermediates accumulating in this mutant supports the involvement of Pop2p in mRNA deadenylation in vivo. Similar phenotypes are observed in yeast strains lacking the Ccr4 protein, which is known to be associated with Pop2p. A recombinant Pop2p fragment encompassing the putative catalytic domain degrades poly(A) in vitro demonstrating that Pop2p is a nuclease. We also demonstrate that poly(A) is a better competitor than poly(G) or poly(C) of the Pop2p nuclease activity. Altogether, our study indicates that Pop2p is a nuclease subunit of the yeast deadenylase and suggests that Pop2p homologues in other species may have similar functions.
INTRODUCTION
Protein synthesis is in part regulated by the availability of mRNA templates. The levels of the various cellular mRNAs are controlled both by their rates of synthesis and degradation. While much is known about transcriptional control, the mechanisms involved in mRNA degradation have only been identified recently, mostly following analysis in the yeast Saccharomyces cerevisiae (1, reviewed in 2). The major mRNA degradation pathway involves first degradation of the poly(A) tail. This deadenylation appears to be the rate-limiting step of the degradation pathway. By an unknown mechanism, deadenylation induces decapping of the target mRNAs. The generation of a monophosphate at the 5′ mRNA extremity allows the 5′→3′ exonucleolytic digestion of the mRNA body. The structure of mRNA degradation intermediates characterised in mammalian cells indicate that the major mRNA degradation pathway is conserved in eukaryotes (3). In addition to the major mRNA degradation pathway, mRNAs may be degraded by a minor pathway involving 3′→5′ exonucleolytic trimming (4). Interestingly, this process constitutes the major route of regulated pre-mRNA degradation in the yeast cell nucleus (5). Additionally, mRNAs containing premature non-sense codons are degraded by a specific pathway (NMD, for Non-sense Mediated Decay) involving mRNA decapping and 5′→3′ exonuclease trimming of the mRNA body (6). The factors involved in these two steps are identical to those implicated in the similar steps of the major mRNA degradation pathway. It is noteworthy, however, that the signals leading to mRNA decapping are different in the two pathways with deadenylation inducing decapping in the major mRNA degradation pathway (7,8) and recognition of a premature stop codon and associated downstream sequence by the ribosome and specific factors inducing decapping in the NMD pathway (6,9).
Purification of the major yeast exoribonuclease led to the identification of the XRN1 gene (10). The role of Xrn1p in the degradation of mRNAs was demonstrated in vivo. Chase experiments showed that the half-life of reporter mRNAs was greatly extended in an xrn1 mutant while characterisation of mRNA degradation intermediates accumulating in wild-type and mutant cells indicated that Xrn1p was responsible for the 5′→3′ exonucleolytic trimming occurring after decapping (7,11). Similarly, purification of the major yeast decapping enzyme led to the identification of the DCP1 gene (12,13). In vivo analyses revealed that the Dcp1 protein is required for mRNA degradation in both the major and NMD pathways (8). Furthermore, Dcp1p was reported to exert decapping activity on its own (14). Dcp2p, identified as a multicopy suppressor of a dcp1 mutant, is also required for decapping, probably by controlling Dcp1p activity (15). Recently, Edc1p and Edc2p have also been proposed to modulate decapping (16). A group of seven Sm-like proteins (Lsm1p–Lsm7p) associated with the Pat1 factor are required to activate decapping in the major mRNA degradation pathway (17–20). Consistent with their role in mRNA degradation, these proteins copurified biochemically with the Xrn1p exonuclease (17). Two-hybrid screens have revealed significant interactions between various Lsm proteins (Lsm1p–Lsm8p), Pat1p and Dcp2p (21). However, the results of biochemical purifications (17) suggest that Lsm8p is not involved in mRNA degradation. Co-immunoprecipitation studies revealed an RNA-dependent interaction between several Lsm (Lsm1, 2, 3, 5, 6 and 7) proteins and Dcp1p (18). However, as co-immunoprecipitations were inefficient it remains possible that they resulted from the fortuitous association of RNA binding proteins on a bridging RNA molecule. In addition to these factors, several RNA binding proteins have been shown to affect the rate of degradation of specific mRNAs (22,23). In contrast, the nature of the factor(s) mediating the mRNA deadenylation remained unclear. A heterodimeric protein with the corresponding activity was purified from yeast and its constituents identified. Surprisingly, however, disruption of the corresponding genes, PAN2 and PAN3, did not generate a strong growth phenotype nor a general reduction in the deadenylation rate of mRNAs in vivo (24–26). This suggested that an additional mRNA deadenylase was present in yeast cells.
We report here the identification of Pop2p, a protein previously identified as a transcription factor, as a nuclease belonging to the RNase D family. Characterisation of a pop2 mutant demonstrates that Pop2p is a subunit of the major yeast deadenylase involved in mRNA turn-over. Similar results were reported independently by another group during the course of this study (27).
MATERIALS AND METHODS
Biocomputing
Database searches were performed using the BLAST (28) and PSI-BLAST (29) software. Multiple alignments were created with the CLUSTALX (30) program and manually edited. Sequences with the following accession numbers were used for the multiple alignment: SWISS-PROT: P39008, Q17345, CAC27008, P53010; SPTrEMBL: O95709, Q9VTS4, O74856, Q9SHJ0, O95453, Q9SHJ0; and the NCBI protein database: XP_005074.
Strains and media
Escherichia coli strain MH1 was used for standard cloning procedures while strain BL21CodonPlus (Stratagene) was used for protein expression. Yeast mutants were constructed by PCR-mediated gene disruption in the W303 background (31). The POP2 and CCR4 genes were disrupted in isogenic diploids with the help of the HISMX6 cassette (32). Following sporulation, haploid segregants were recovered and named BSY1091 (MATa, ade2-1, can1-100, his3-11,15, leu2-3,112, trp1-1, ura3-1, ccr4::HISMX6) and BSY1093 (MATa, ade2-1, can1-100, his3-11,15, leu2-3,112, trp1-1, ura3-1, pop2::HISMX6). Standard complete and synthetic media were used for yeast and E.coli growth (33,34).
In vivo assay for mRNA degradation
The plasmid pRP485 encoding the MFA2pG reporter (1) was introduced into wild-type and mutant strains by transformation. RNA chase experiments were performed as described previously (1). Following northern blotting and detection with an oligonucleotide probe complementary to the oligo(G) insertion of pRP485, signals were detected using a PhosphorImager (Molecular Dynamics). mRNA half-lives were compared by plotting the level of reporter MFA2 mRNA per unit of total RNA over time, using the best fit for an exponential decay. RNase H-mediated degradation of the poly(A) tail in the presence of oligo(dT) was performed essentially as described (19) except that oligo(dT) and total yeast RNA were denatured by heating to 65°C for 10 min and annealed by slowly cooling to room temperature.
Overexpression of the RNase D domain of yeast Pop2p
The sequence coding for amino acids 147–433 of the yeast POP2 gene was amplified from yeast genomic DNA by PCR and inserted in a modified pET24d vector carrying 6× HIS and GST tags (a kind gift from Günther Stier, EMBL). Absence of mutation in the coding sequence was checked by DNA sequencing. Protein expression and purification on Ni-agarose (Qiagen) were performed essentially as suggested by the manufacturer. As a negative control, we used a clone expressing a similarly sized fragment of human α2-actinin inserted in the same vector and purified under identical conditions.
In vitro RNase assays
Radiolabelled poly(A) was prepared by incubating poly(A) (Pharmacia) in the presence of [α-32P]ATP (3000 Ci/mmol; Amersham) and yeast poly(A) polymerase (USB) as described previously (25). The probe was purified by gel filtration and ethanol precipitation. For nuclease activity tests, ∼50 000 c.p.m. of probe was incubated with a given amount of recombinant Pop2p or human α2-actinin at 30°C in a 50 µl volume reaction containing 10 mM HEPES pH 7.5, 1 mM magnesium acetate, 2 mM spermidine, 2 mM DTT, 0.02% NP-40, 1 U/µl RNasin (Promega). At various time points aliquots were precipitated with TCA and the level of soluble radioactivity released was measured by scintillation or Cerenkov counting, essentially as described previously (35). Radioactivity released was converted into picomoles, taking into account the specific activity of the ATP incorporated and assuming that AMP is released. Activity is therefore underestimated as degradation of the starting unlabelled poly(A) is not taken into account. Experiments using identical quantities of proteins or identical volumes from the eluted fractions (to rule out differential activity due to presence of E.coli contaminants present at different relative ratio compared to the recombinant protein) led to the same conclusion (data not shown). Poly(A), poly(C) and poly(G) for competition assays were obtained from Pharmacia.
RESULTS
Yeast Pop2p contains an RNase D sequence signature
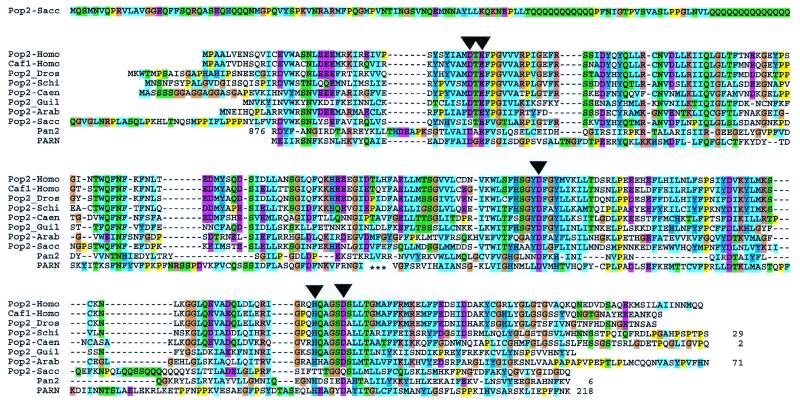
In order to identify a putative yeast deadenylase, we searched proteins derived from the yeast genome sequence for similarity with the peptidic sequence of human PARN, a human poly(A)-specific nuclease (36). One of the best matching sequences turned out to be yeast Pop2p. (Pop2p is also called Caf1p; as POP2 is the official name in the yeast gene database, we will use this nomenclature throughout this paper.) While this protein has been previously extensively characterised as a transcription factor negatively regulating transcription (37,38), we noticed that Pop2p was similar to PARN over its RNase D domain (Fig. 1) (36,39,40). Interestingly, a general analysis of RNase D-related proteins failed to identify Pop2p as a bona fide member of this family, partly because it contains non-canonical residues at three of the five residues involved in the formation of the catalytic site (Fig. 1, arrowheads) (40,41). However, putative Pop2p homologues from plants, animals or fungi contain consensus residues at these conserved positions suggesting that the lack of conservation observed in S.cerevisiae represents an exceptional situation rather than the selection for a non-functional protein (Fig. 1). The S.cerevisiae protein is also unique because it contains a long N-terminal extension that is not present in other putative homologues. Interestingly, the N-terminal extension of yeast Pop2p is rich in glutamine residues but dispensable for function (Fig. 1) (42). Overall, these observations suggested that yeast Pop2p might be endowed with a 3′→5′ exonuclease activity similar to that of RNase D and other members of this family. Previous analyses had revealed the presence of 11 additional proteins containing the characteristic RNase D motif encoded by the yeast genome (41). Among them figured the Pan2 protein that had been characterised as a deadenylase (24). The other yeast proteins from this family have been ascribed other functions involving exonucleolytic activities, such as proofreading associated with DNA polymerases or rRNA maturation (41,43). Except for Pan2p, previous analyses have failed to reveal an involvement of the other yeast proteins from this family in mRNA degradation. Taken together with the similarity to PARN and PAN2, the fact that Pop2p was the only remaining member from this family without a direct function in RNA degradation made it the best candidate for the elusive yeast deadenylase. This hypothesis was strengthened by the observation that the Pop2p-associated protein Ccr4 was itself recently shown to contain an endonuclease motif (44).
Figure 1.
Alignment of the Pop2p (Pop2-Sacc) sequence with the sequences of two putative human orthologues of Pop2p, human Pop2 (Pop2-Homo) and human Caf1p (Caf1-Homo); putative Pop2p orthologues from plants, Arabidopsis thaliana (Pop2-Arab, note that several isoforms are present in the database) and Guillardia theta (Pop2-Guil); animals, Caenorhabditis elegans (Pop2-Caen), Drosophila melanogaster (Pop2-Dros); and fungi, Schizosaccharomyces pombe (Pop2-Schi), and with the RNase D domain of human PARN and of the yeast Pan2 protein, two previously characterised deadenylases. The complete sequence of Pop2p homologues is shown except for those having extension at the C-terminus, in which case the length of this extension is given. The number of residues preceding and following the RNase D domain of Pan2 is indicated. The RNase D domain of PARN is located at its N-terminus. The number of residues not shown at its C-terminus is indicated. We noticed that the RNase D domain of PARN is interrupted by a R3H single-stranded nucleic acid binding domain at the location indicated with asterisks (136 residues not shown). Note the long N-terminal extension of Pop2p compared to putative orthologues of other species; this sequence is not essential in vivo or in vitro (42). The Pop2p RNase D domain covers therefore amino acids 147–433 of the protein while putative orthologues consist mainly of the RNase D domain. In addition, Pop2p carries the substitution of three characteristic residues out of the five amino acids located close to the catalytic centre in the RNase D domain of Pop2p (arrowheads). These substitutions are, however, not present in putative orthologues. The special features of the yeast protein appear to be conserved in other ascomycetes (data not shown).
Pop2p and Ccr4p are both required for efficient in vivo mRNA degradation
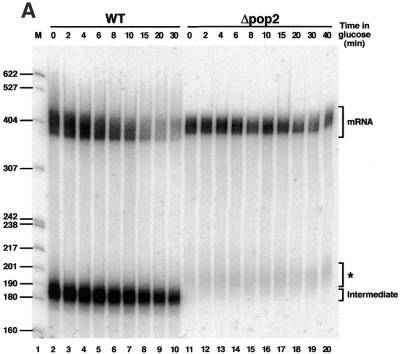
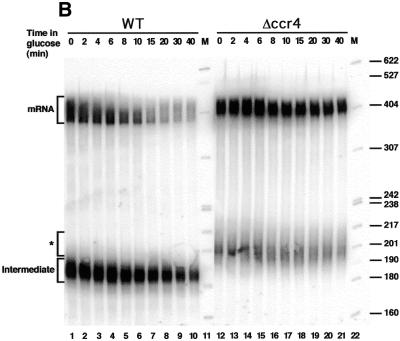
Previous studies have shown that both Pop2p and Ccr4p were not required for vegetative growth of yeast, even though disruption of the corresponding genes did produce a complex slow growth phenotype (45,46). Therefore, to test the possibility that Pop2p and/or Ccr4p were involved in mRNA degradation we constructed haploid yeast stains lacking these genes (see Materials and Methods). Strains disrupted for either of these two genes grew slowly in agreement with published results. The pop2 mutant strain was slightly more affected than the ccr4 mutant, suggesting a more important role for cell growth for Pop2p (data not shown). The pRP485 reporter plasmid encoding a MFA2 mRNA marked with an oligo(G) tract under the control of a GAL regulated promoter (1) was then introduced into wild-type and disrupted cells. Transformants were recovered and grown in selective media containing galactose before being switched to glucose-containing medium at time 0. This procedure turns off the GAL promoter, allowing the analysis of the decay rate of the reporter RNA. At various time points following the shut-off of the reporter, total RNA was extracted from cells, fractionated on denaturing gels and transferred to a membrane that was hybridised with a probe complementary to the oligo(G) track to reveal specifically the reporter RNA (Fig. 2). The RNA profile observed in wild-type cells was similar to that reported previously: a high level of mRNA and a characteristic degradation intermediate fragment starting at the oligo(G) track and ending with a few A residues remaining from the poly(A) tail were present at time 0 (glucose addition) (1). Following transcriptional shut-off, the average size of the full-length mRNA population decreased rapidly due to deadenylation with concomitant disappearance of the MFA2 reporter mRNA. In contrast, the size of the degradation intermediate was only marginally affected while its level decreased with slower kinetics (Fig. 2A, lanes 2–10). Interestingly, in the mutant pop2 strain, the size of the full-length reported MFA2 mRNA was slightly larger than in the wild-type strain. Furthermore, no apparent shortening of this species was visible following transcriptional shut-off. In addition, the mRNA level diminished only slowly over time with a half-life doubled in the pop2 disruption mutant compared to the wild-type strain (data not shown). It is also noteworthy that the major degradation intermediate was not detected at any time point and that a low level of a new heterogeneous species slightly larger than the degradation intermediate was detectable in the mutant strain (Fig. 2A, lanes 11–20). A similar experiment was performed with the ccr4 mutant strain (Fig. 2B). Strikingly similar observations were made including the larger size and increased half-life of the MFA2 mRNA, the absence of normal degradation intermediate and the appearance of a new diffuse species in the mutant strain (compare Fig. 2A and B).
Figure 2.
Decay of the MFA2 mRNA in pop2 and ccr4 mutants. (A) Analysis of MFA2 mRNA decay by transcriptional shut-off in isogenic wild-type (lanes 2–10) and pop2 deletion (lanes 11–20) strains. RNA extracted at various time points following glucose-mediated repression of the GAL promoter was fractionated by denaturing gel electrophoresis, blotted onto a membrane and revealed by probing the membrane with an oligonucleotide complementary to the oligo(G) insertion present in the reporter RNA. The positions of migration of complete mRNA (mRNA), a previously characterised degradation intermediate (Intermediate) and a new species present in the pop2 mutant (*) are indicated. A DNA molecular weight standard was loaded in lane 1. Sizes of the corresponding fragments are indicated on the left. (B) Analysis of MFA2 mRNA decay by transcriptional shut-off in isogenic wild-type (lanes 1–10) and ccr4 deletion (lanes 12–21) strains. See (A) for other details.
MFA2 mRNA and degradation intermediates have extended poly(A) tails in the pop2 and ccr4 mutants
These data revealed that deletion of pop2 and ccr4 had a dramatic effect on the fate of the MFA2 mRNA. In particular, the absence of the normal degradation intermediate was highly suggestive of defective mRNA turnover, possibly through reduced deadenylation. This possibility was consistent with the presence of the longer mRNA and degradation intermediate species potentially containing extended poly(A) tails. However, because Pop2p and Ccr4p have been implicated in transcription, the presence of larger transcripts might also have resulted from choice of new aberrant transcription initiation and/or polyadenylation sites. To discriminate between these possibilities, we treated representative RNA samples from the promoter shut-off experiment with RNase H in the presence of oligo(dT) to degrade poly(A) tails attached to the transcripts. The products of this reaction were compared to the starting material by northern blotting (Fig. 3). This experiment revealed that the size of the deadenylated mRNA was identical in the wild-type and mutant strains. We conclude therefore that differences in the length of the poly(A) tail rather than changes in the structure of the mRNA body account for the larger size of the MFA2 mRNA in mutant versus wild-type cells. Consistent with previous analysis, the degradation intermediate species detected in the wild-type cell was only marginally affected by RNase H treatment indicating the presence of only an oligo(A) tail at its 3′ end. In contrast, the new heterogeneous RNA species detected in the mutant strains was dramatically shortened following RNase H treatment to produce an homogenous population of RNA of size identical to the degradation intermediate detected in wild-type cells (Fig. 3, lanes 4–7). Taken together with the presence of the oligo(G) sequence in this species and its size, these data revealed that the new species accumulating in the mutant strains represented a 5′ truncated fragment of the MFA2 mRNA extending from the oligo(G) track and including an heterogeneous collection of extended 3′ poly(A) tails.
Figure 3.
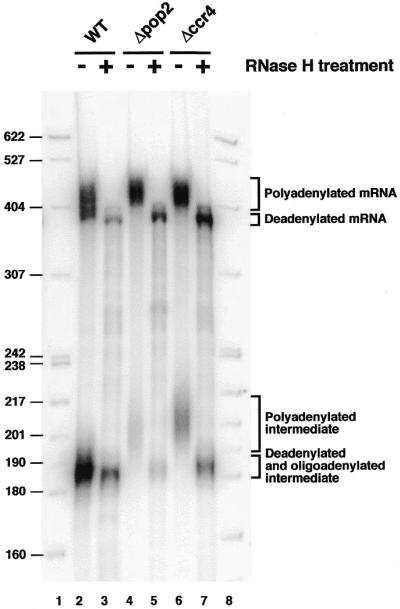
Deduction of the structure of the new species present in pop2 and ccr4 strains following RNase H-mediated degradation of poly(A) tails in the presence of oligo(dT). RNA samples corresponding to the 2 min time point presented in Figure 2A (wild-type and pop2 mutant) or 2B (ccr4 mutant) were treated with RNase H and oligo(dT) (lanes 3, 5 and 7). Untreated material is shown for comparison (lanes 2, 4 and 6). RNA samples were fractionated by gel electrophoresis and revealed following northern blotting (see Fig. 2 for details). A DNA molecular weight standard was loaded in lanes 1 and 8. Sizes of the corresponding fragments are indicated on the left. The structures of the various RNA species are given on the right.
Taken together, our results indicate that Pop2p and Ccr4p are required for efficient degradation of the MFA2 mRNA in vivo. Furthermore, accumulation of species harbouring extended poly(A) tails was consistent with the involvement of these factors in deadenylation, as suggested by the finding of RNase D and endonuclease motifs in their amino acid sequences.
A recombinant Pop2p fragment exhibits nuclease activity in vitro
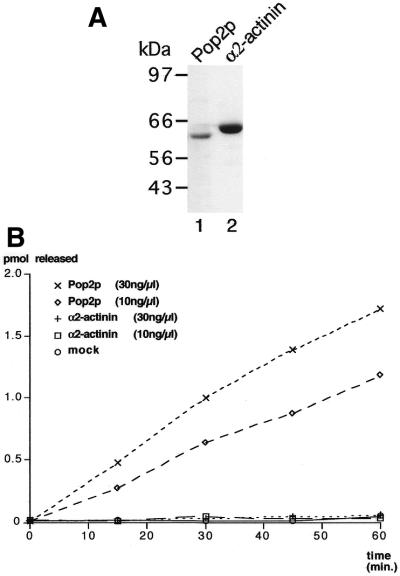
To test for a direct role of Pop2p in deadenylation, we produced a recombinant Pop2p fragment covering the RNase D motif by overexpression in E.coli. [This domain was selected because (i) the N-terminal yeast specific extension is not known to be expressed in vivo, (ii) this region is not conserved in other species and (iii) it contains long stretches of glutamine residues that may prevent expression or induce insolubility in E.coli.] The recombinant fusion protein was purified to near homogeneity on Ni-agarose beads using a 6× HIS tag (Fig. 4A, lane 1). As a control, we expressed and purified a fragment of human α2-actinin (Fig. 4A, lane 2) under the same conditions. To test the activity of these proteins in poly(A) degradation, we incubated in vitro-synthesised radioactively labelled poly(A) with different amounts of these recombinant factors. Poly(A) degradation was assayed by the release of TCA soluble radioactivity from the poly(A) substrate at various time points after the addition of the protein sample (35). This revealed a time and concentration dependent degradation of poly(A) mediated by the recombinant Pop2p fragment (Fig. 4B). In contrast, incubation of the recombinant α2-actinin fragment did not lead to the production of TCA soluble radioactivity compared to the incubation performed in the absence of protein (Fig. 4B, mock). These controls demonstrate that poly(A) degradation is mediated directly by Pop2p rather than by trace amount of a contaminating factor from E.coli. Therefore, we conclude that Pop2p is a nuclease able to degrade poly(A).
Figure 4.
Poly(A) degradation by recombinant Pop2p in vitro. (A) Protein fractions used for the assay. Equivalent volumes of eluate from the Ni-agarose purification column were fractionated by gel electrophoresis and stained with Coomassie brilliant blue. The product harbouring the RNase D domain of Pop2p fused to the GST and His tags is shown in lane 1, while the control product containing a fragment of human α2-actinin is shown in lane 2. The position of migration of molecular weight marker is indicated on the left. (B) Pop2p is a nuclease. Release of TCA soluble radioactivity from a radiolabelled poly(A) substrate was measured in a time course experiment at various concentrations of recombinant Pop2p, control protein (human α2-actinin) or in a mock-treated sample and plotted. In the reaction incubated for 60 min with 30 ng/µl of recombinant Pop2p, ∼60% of the input substrate was degraded. Similar results were obtained using independent protein preparation. Control reactions using an equivalent volume of eluate rather than an equivalent concentration of protein give similar results, confirming that the nuclease activity does not originate from E.coli contaminant(s) (data not shown).
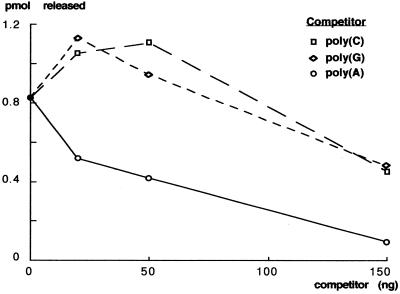
Competition experiments reveal the Pop2p specificity
To determine whether the recombinant Pop2p nuclease display some specificity toward specific substrates, we performed competition experiments. Briefly, increasing amounts of poly(A), poly(G) or poly(C) were added to a standard degradation reaction containing radiolabelled poly(A) and a fixed amount of recombinant Pop2p. After a 30 min incubation, TCA soluble radioactivity released in the assay was measured. The results, depicted in Figure 5, demonstrate that, as expected, excess poly(A) was able to prevent degradation of the radiolabelled poly(A) substrate. In contrast, low concentrations of poly(G) or poly(C) did not inhibit Pop2p mediated poly(A) degradation but rather led to a slight activation of the nuclease activity. At higher concentrations, poly(C) or poly(G) led to inhibition of the Pop2p nuclease activity possibly through non-specific interaction with the enzyme. The effect of poly(U) could not be tested in this assay because it would non-specifically prevent access to poly(A) by formation of double-stranded RNA. We conclude that Pop2p is a nuclease degrading poly(A) preferentially.
Figure 5.
Specificity of the degradation reaction. Competition experiments with poly(A), poly(G) and poly(C) demonstrate that poly(A) is a stronger competitor for the nuclease activity of Pop2p.
DISCUSSION
Our results demonstrate that yeast Pop2p is a nuclease involved in poly(A) degradation. A yeast strain lacking Pop2p is defective in mRNA degradation and accumulates mRNA and degradation products containing extended poly(A) tail, consistent with a defect in the deadenylation of mRNAs. While this work was in progress, a similar observation was independently reported by another group (27). It was unexpected to find some polyadenylated degradation intermediate fragments starting at the oligo(G) insertion and ending with a poly(A) tail in a deadenylase mutant. Indeed, previous studies had suggested that poly(A) tail shortening was required to activate decapping (8). While the pathway of formation of this new degradation fragment remains to be determined, it is likely to involve Dcp1p-mediated decapping and Xrn1p-mediated 5′→3′ exonuclease trimming of fully polyadenylated mRNA. The finding of significant levels of polyadenylated degradation intermediate in the pop2 and ccr4 mutant strains indicates that decapping is not absolutely coupled to the absence (or shortening) of the poly(A) tail. It will be of interest to test whether this intermediate is generated through alternative pathways (e.g., decapping mediated by factors involved in the NMD pathway) and if this alternative route contributes to mRNA decay in wild-type cells. Similarly, the subcellular location of this degradation process and the relative role of Pop2p in nuclear versus cytoplasmic mRNA degradation remain to be determined.
The Pan2–Pan3 complex had been previously described as a deadenylase in yeast. Surprisingly, however, pan2 or pan3 mutant displayed no growth phenotype and only weak reduction in the rate of mRNA deadenylation (24–26). The identification of Pop2p as a second deadenylase in yeast offers an explanation for this situation. Furthermore, because a pop2 disruption mutant displays a severe growth phenotype and a strong effect on mRNA deadenylation in vivo, one can conclude that it is required for activity of the major yeast deadenylase. Interestingly, the protein Ccr4p had been described previously as a Pop2p-associated factor (46). Our characterisation of a ccr4 mutant demonstrates that it is also involved in mRNA degradation. In this context, it is noteworthy that Ccr4p contains a putative endonuclease domain (44). Further study will be required to test whether this endonuclease domain contributes catalytically to poly(A) tail shortening. However, the analysis of pop2 and ccr4 mutant clearly demonstrates that both subunits are required for efficient deadenylation in vivo. It is likely that yeast Pop2p contributes catalytically to poly(A) degradation. Indeed, database searches revealed that Pop2p contains an RNase D domain (39–41) that is highly similar to the RNase D domain found in the mammalian deadenylase PARN (36). The founding member of this family is E.coli RNase D that is involved in tRNA 3′ end processing through its 3′→5′ exonuclease activity (47). Other enzymes harbouring this motif also exhibit 3′→5′ exonuclease activity, including PARN and Pan2p. The presence of this motif in Pop2p is therefore consistent with its involvement in deadenylation that occurs in a 3′→5′ direction even if several of the highly conserved residues typical of the RNase D family have been substituted in Pop2p during evolution. A direct catalytic role for Pop2p in deadenylation is further supported by our demonstration that a recombinant Pop2p RNase D domain purified from E.coli is endowed with nuclease activity with a preferred specificity for poly(A). These results demonstrate that the amino acid substitutions present in Pop2p do not prevent catalytic activity. We do not know, however, whether this activity is sufficient for deadenylation or if additional enzymatic activities are required (e.g., endonuclease activity of Ccr4p). Indeed, at this stage, it remains possible that both proteins are catalytically active or that Ccr4p is required to stabilise and/or activate Pop2p in vivo. Further analyses will be required to discriminate between these possibilities. Interestingly, Pop2p and Ccr4p have been previously described as factors affecting transcription. While our study does not address the role of this protein in mRNA synthesis, we clearly establish that these factors are involved in mRNA turnover. It will therefore be important in the future to discriminate between phenotypes resulting from an involvement of Pop2p in transcription and those resulting from its role in mRNA turnover.
While Pop2p displays significant similarity to human PARN, it does not appear to represent a PARN homologue in yeast. Indeed, at least two proteins highly related to Pop2p are encoded in the human genome (Fig. 1) and represent putative orthologues. These proteins are also likely to have nuclease activity and to be involved in mRNA deadenylation (data not shown). Because deadenylation appears to be the rate-limiting step of mRNA degradation, it is the potential target for mRNA turnover control. The identification of yeast Pop2p as a nuclease involved in mRNA deadenylation and of human homologues will allow us to better understand the control of mRNA degradation.
Acknowledgments
ACKNOWLEDGEMENTS
We thank F.Lacroute, M.Minet and F.Wyers for discussion and support. We are indebted to G.Stier for the gift of the modified pET24d plasmid expressing human α2-actinin. This work was funded by La Ligue contre le Cancer (Equipe Labellisée), le Ministère de la Recherche Scientifique and the CNRS.
References
- 1.Decker C.J. and Parker,R. (1993) A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev., 7, 1632–1643. [DOI] [PubMed] [Google Scholar]
- 2.Caponigro G. and Parker,R. (1996) Mechanisms and control of mRNA turnover in Saccharomyces cerevisiae. Microbiol. Rev., 60, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couttet P., Fromont-Racine,M., Steel,D., Pictet,R. and Grange,T. (1997) Messenger RNA deadenylation precedes decapping in mammalian cells. Proc. Natl Acad. Sci. USA, 94, 5628–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hoof A. and Parker,R. (1999) The exosome: a proteasome for RNA? Cell, 99, 347–350. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet-Antonelli C., Presutti,C. and Tollervey,D. (2000) Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell, 102, 765–775. [DOI] [PubMed] [Google Scholar]
- 6.Czaplinski K., Ruiz-Echevarria,M.J., Gonzalez,C.I. and Peltz,S.W. (1999) Should we kill the messenger? The role of the surveillance complex in translation termination and mRNA turnover. Bioessays, 21, 685–696. [DOI] [PubMed] [Google Scholar]
- 7.Muhlrad D., Decker,C.J. and Parker,R. (1994) Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′→3′ digestion of the transcript. Genes Dev., 8, 855–866. [DOI] [PubMed] [Google Scholar]
- 8.Tucker M. and Parker,R. (2000) Mechanisms and control of mRNA decapping in Saccharomyces cerevisiae. Annu. Rev. Biochem., 69, 571–595. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez C.I., Ruiz-Echevarria,M.J., Vasudevan,S., Henry,M.F. and Peltz,S.W. (2000) The yeast hnRNP-like protein Hrp1/Nab4 marks a transcript for nonsense-mediated mRNA decay. Mol. Cell, 5, 489–499. [DOI] [PubMed] [Google Scholar]
- 10.Larimer F.W., Hsu,C.L., Maupin,M.K. and Stevens,A. (1992) Characterization of the XRN1 gene encoding a 5′→3′ exoribonuclease: sequence data and analysis of disparate protein and mRNA levels of gene-disrupted yeast cells. Gene, 120, 51–57. [DOI] [PubMed] [Google Scholar]
- 11.Hsu C.L. and Stevens,A. (1993) Yeast cells lacking 5′→3′ exoribonuclease 1 contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol. Cell. Biol., 13, 4826–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens A. (1988) mRNA-decapping enzyme from Saccharomyces cerevisiae: purification and unique specificity for long RNA chains. Mol. Cell. Biol., 8, 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beelman C.A., Stevens,A., Caponigro,G., LaGrandeur,T.E., Hatfield,L., Fortner,D.M. and Parker,R. (1996) An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature, 382, 642–646. [DOI] [PubMed] [Google Scholar]
- 14.Vilela C., Velasco,C., Ptushkina,M. and McCarthy,J.E. (2000) The eukaryotic mRNA decapping protein Dcp1 interacts physically and functionally with the eIF4F translation initiation complex. EMBO J., 19, 4372–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunckley T. and Parker,R. (1999) The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J., 18, 5411–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunckley T., Tucker,M. and Parker,R. (2001) Two related proteins, Edc1p and Edc2p, stimulate mRNA decapping in Saccharomyces cerevisiae. Genetics, 157, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouveret E., Rigaut,G., Shevchenko,A., Wilm,M. and Séraphin,B. (2000) A Sm-like protein complex that participates in mRNA degradation. EMBO J., 19, 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tharun S., He,W., Mayes,A.E., Lennertz,P., Beggs,J.D. and Parker,R. (2000) Yeast Sm-like proteins function in mRNA decapping and decay. Nature, 404, 515–518. [DOI] [PubMed] [Google Scholar]
- 19.Boeck R., Lapeyre,B., Brown,C.E. and Sachs,A.B. (1998) Capped mRNA degradation intermediates accumulate in the yeast spb8-2 mutant. Mol. Cell. Biol., 18, 5062–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnerot C., Boeck,R. and Lapeyre,B. (2000) The two proteins pat1p (Mrt1p) and spb8p interact in vivo, are required for mRNA decay and are functionally linked to pab1p. Mol. Cell. Biol., 20, 5939–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fromont-Racine M., Mayes,A.E., Brunet-Simon,A., Rain,J.C., Colley,A., Dix,I., Decourty,L., Joly,N., Ricard,F., Beggs,J.D. and Legrain,P. (2000) Genome-wide protein interaction screens reveal functional networks involving Sm-like proteins. Yeast, 17, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tadauchi T., Matsumoto,K., Herskowitz,I. and Irie,K. (2001) Post-transcriptional regulation through the HO 3′-UTR by Mpt5, a yeast homolog of Pumilio and FBF. EMBO J., 20, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivas W. and Parker,R. (2000) The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J., 19, 6602–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeck R., Tarun,S., Rieger,M., Deardorff,J.A., Muller-Auer,S. and Sachs,A.B. (1996) The yeast Pan2 protein is required for poly(A)-binding protein-stimulated poly(A)-nuclease activity. J. Biol. Chem., 271, 432–438. [DOI] [PubMed] [Google Scholar]
- 25.Brown C.E., Tarun,S.Z., Boeck,R. and Sachs,A.B. (1996) PAN3 encodes a subunit of the Pab1p-dependent poly(A) nuclease in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 5744–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown C.E. and Sachs,A.B. (1998) Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation. Mol. Cell. Biol., 18, 6548–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker M., Valencia-Sanchez,M.A., Staples,R.R., Chen,J., Denis,C.L. and Parker,R. (2001) The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell, 104, 377–386. [DOI] [PubMed] [Google Scholar]
- 28.Altschul S.F., Gish,W., Miller,W., Myers,E.W. and Lipman,D.J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. [DOI] [PubMed] [Google Scholar]
- 29.Altschul S.F., Madden,T.L., Schaffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson J.D., Gibson,T.J., Plewniak,F., Jeanmougin,F. and Higgins,D.G. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res., 25, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas B.J. and Rothstein,R. (1989) Elevated recombination rates in transcriptionally active DNA. Cell, 56, 619–630. [DOI] [PubMed] [Google Scholar]
- 32.Wach A., Brachat,A., Alberti-Segui,C., Rebischung,C. and Philippsen,P. (1997) Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast, 13, 1065–1075. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J., Fritsch,E.J. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, NY.
- 34.Sherman F. (1991) Getting started with yeast. In Guthrie,C. and Fink,G. (eds), Guide to Yeast Genetics and Molecular Biology—Methods in Enzymology. Academic Press Inc., San Diego, CA, Vol.194, pp.3––21.. [DOI] [PubMed]
- 35.Körner C.G. and Wahle,E. (1997) Poly(A) tail shortening by a mammalian poly(A)-specific 3′-exoribonuclease. J. Biol. Chem., 272, 10448–10456. [DOI] [PubMed] [Google Scholar]
- 36.Körner C.G., Wormington,M., Muckenthaler,M., Schneider,S., Dehlin,E. and Wahle,E. (1998) The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. EMBO J., 17, 5427–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maillet L., Tu,C., Hong,Y.K., Shuster,E.O. and Collart,M.A. (2000) The essential function of Not1 lies within the Ccr4–Not complex. J. Mol. Biol., 303, 131–143. [DOI] [PubMed] [Google Scholar]
- 38.Liu H.Y., Badarinarayana,V., Audino,D.C., Rappsilber,J., Mann,M. and Denis,C.L. (1998) The NOT proteins are part of the CCR4 transcriptional complex and affect gene expression both positively and negatively. EMBO J., 17, 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mian I.S. (1997) Comparative sequence analysis of ribonucleases HII, III, II PH and D. Nucleic Acids Res., 25, 3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo Y. and Deutscher,M.P. (2001) Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res., 29, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moser M.J., Holley,W.R., Chatterjee,A. and Mian,I.S. (1997) The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res., 25, 5110–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu-Yoshida Y., Sasamoto,M., Yoshida,A., Yoshioka,T., Matsumoto,A. and Sakai,A. (1999) Mouse CAF1, a mouse homologue of the yeast POP2 gene, complements the yeast pop2 null mutation. Yeast, 15, 1357–1364. [DOI] [PubMed] [Google Scholar]
- 43.van Hoof A., Lennertz,P. and Parker,R. (2000) Three conserved members of the RNase D family have unique and overlapping functions in the processing of 5S, 5.8S, U4, U5, RNase MRP and RNase P RNAs in yeast. EMBO J., 19, 1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dlakic M. (2000) Functionally unrelated signalling proteins contain a fold similar to Mg2+-dependent endonucleases. Trends Biochem. Sci., 25, 272–273. [DOI] [PubMed] [Google Scholar]
- 45.Malvar T., Biron,R.W., Kaback,D.B. and Denis,C.L. (1992) The CCR4 protein from Saccharomyces cerevisiae contains a leucine-rich repeat region which is required for its control of ADH2 gene expression. Genetics, 132, 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Draper M.P., Salvadore,C., Denis,C.L., Malvar,T., Biron,R.W. and Kaback,D.B. (1995) Identification of a mouse protein whose homolog in Saccharomyces cerevisiae is a component of the CCR4 transcriptional regulatory complex. Mol. Cell. Biol., 15, 3487–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J.R. and Deutscher,M.P. (1988) Transfer RNA is a substrate for RNase D in vivo. J. Biol. Chem., 263, 17909–17912. [PubMed] [Google Scholar]