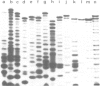
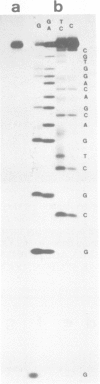
Abstract
An inexpensive, extremely rapid manual method for simultaneous synthesis of large numbers of oligodeoxyribonucleotides on 50 or 150 nanomole scale is described. The oligonucleotides are assembled in parallel by the phosphotriester method on small cellulose paper disks in a simple gas pressure-controlled continuous-flow system. For each addition of a nucleotide the disks are sorted into four sets which are placed in four columns for addition of A, C, G and T, respectively. During one 2-week period, three rounds of synthesis by this method yielded 254 oligomers (8- to 22-mers), many of which were also purified during this time. Using 50 nanomole scale reactions the yields for 17-mers, for example, were in the range of 0.5 O.D.260 units (˜5 nmol, i.e., ˜10% yield), an amount sufficient for most purposes. The equipment required is relatively inexpensive and for the most part usually already available in molecular biology laboratories. All chemicals are commercially available and the current reagent cost per oligonucleotide (25 μg, average length 17-mer) is ˜3 US dollars.
Keywords: gene synthesis, oligonucleotide purification, oligonucleotide synthesis, phosphotriester method, solidphase DNA synthesis
Full text
PDF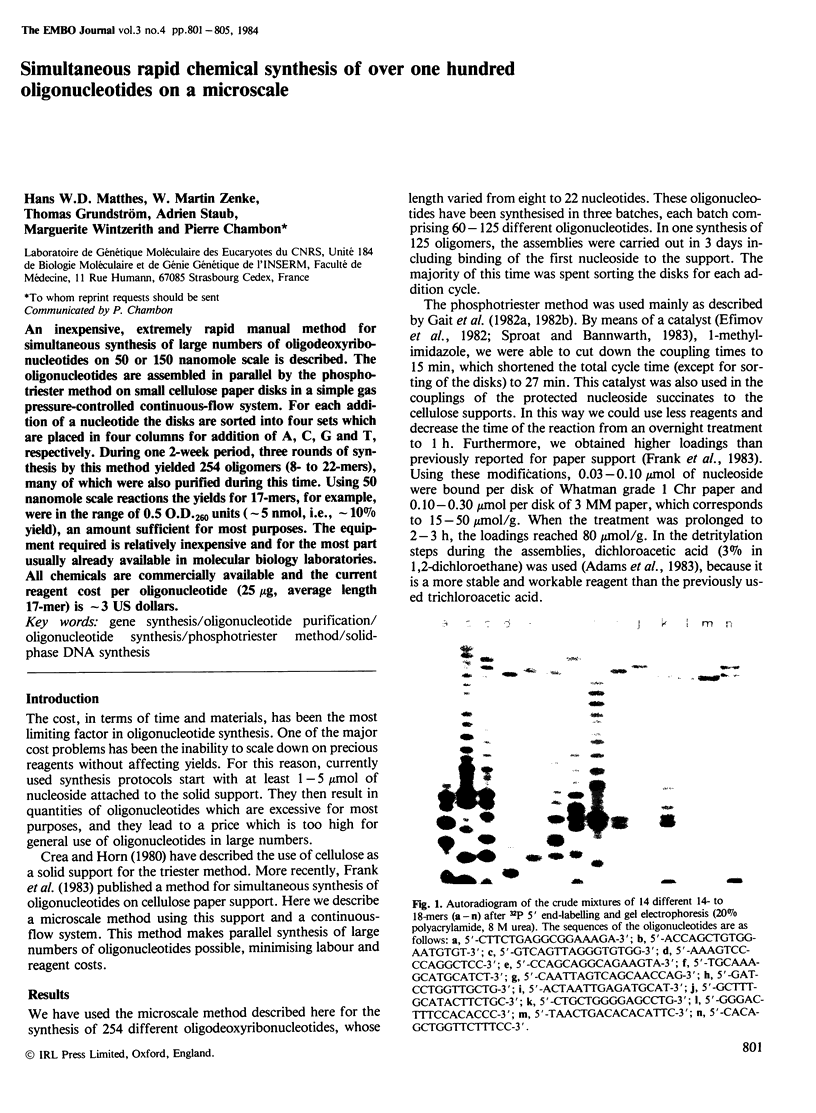



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Kingston I. B. Isolation of a genomic clone for bovine pancreatic trypsin inhibitor by using a unique-sequence synthetic DNA probe. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6838–6842. doi: 10.1073/pnas.80.22.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crea R., Horn T. Synthesis of oligonucleotides on cellulose by a phosphotriester method. Nucleic Acids Res. 1980 May 24;8(10):2331–2348. doi: 10.1093/nar/8.10.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov V. A., Reverdatto S. V., Chakhmakhcheva O. G. New effective method for the synthesis of oligonucleotides via phosphotriester intermediates. Nucleic Acids Res. 1982 Nov 11;10(21):6675–6694. doi: 10.1093/nar/10.21.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R., Heikens W., Heisterberg-Moutsis G., Blöcker H. A new general approach for the simultaneous chemical synthesis of large numbers of oligonucleotides: segmental solid supports. Nucleic Acids Res. 1983 Jul 11;11(13):4365–4377. doi: 10.1093/nar/11.13.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gait M. J., Matthes H. W., Singh M., Sproat B. S., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides. VII. Solid phase synthesis of oligodeoxyribonucleotides by a continuous flow phosphotriester method on a kieselguhr-polyamide support. Nucleic Acids Res. 1982 Oct 25;10(20):6243–6254. doi: 10.1093/nar/10.20.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Seth A. K., Rommens J., Sood A., Jay G. Gene expression: chemical synthesis of E. coli ribosome binding sites and their use in directing the expression of mammalian proteins in bacteria. Nucleic Acids Res. 1982 Oct 25;10(20):6319–6329. doi: 10.1093/nar/10.20.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli V., Balland A., Wintzerith M., Sauerwald R., Staub A., Lecocq J. P. Silica gel: an improved support for the solid-phase phosphotriester synthesis of oligonucleotides. Nucleic Acids Res. 1982 Nov 25;10(22):7439–7448. doi: 10.1093/nar/10.22.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]