Abstract
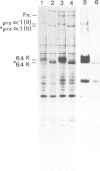
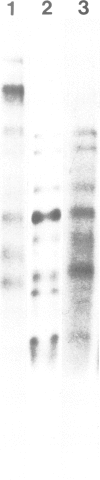
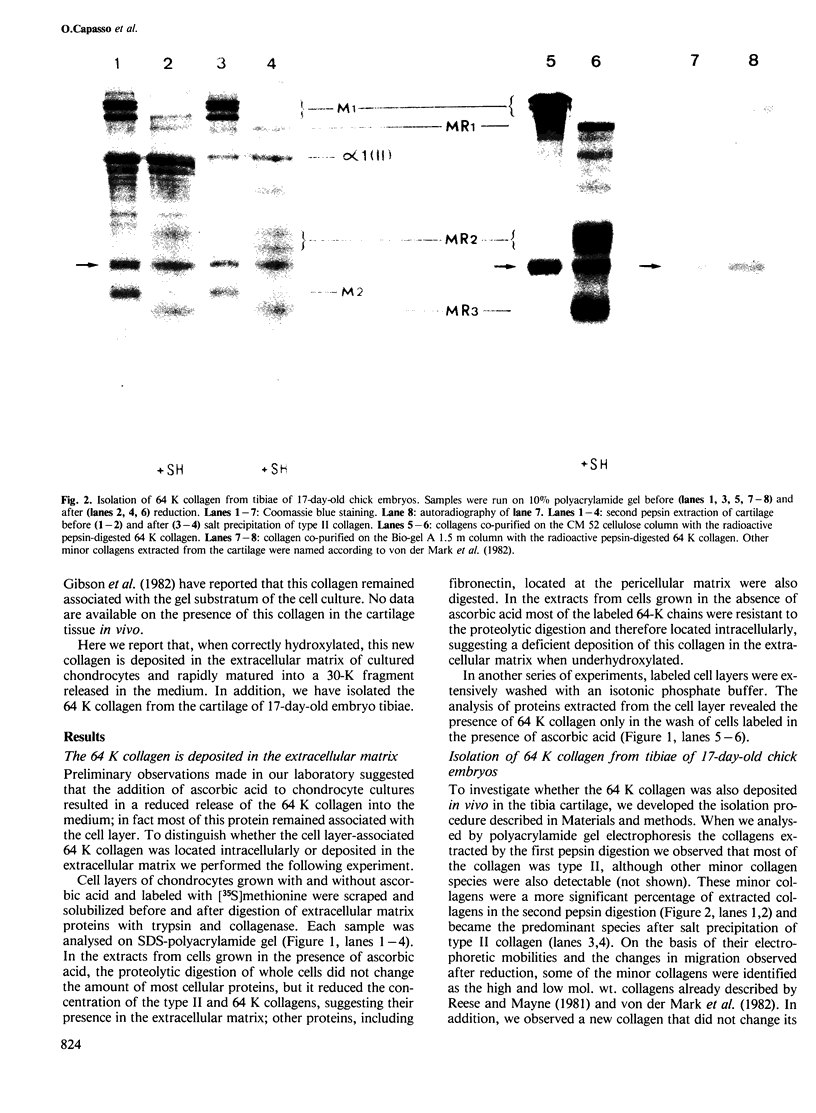
The low mol. wt. collagen (64 K) synthesized by chick embryo chondrocytes in culture is deposited in the extracellular matrix; its deposition is strictly dependent upon a correct hydroxylation. In vivo the 64 K collagen has been isolated from the cartilage of tibiae obtained from 17-day-old chick embryos. The turnover of this collagen in the extracellular matrix is very rapid: within a few hours it is matured into a 30-K fragment released in the medium. Also this maturation is dependent upon a correct hydroxylation of the molecule. The underhydroxylated form, synthesized in the absence of ascorbic acid or in the presence of alpha-alpha' dipyridyl, is not deposited in the extracellular matrix and is directly secreted as 64 K collagen in the culture medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S., Abedin M. Z., Grundy S. M., Weiss J. B. Isolation and characterisation of an unusual collagen from hyaline cartilage and intervertebral disc. FEBS Lett. 1981 Jan 26;123(2):195–199. doi: 10.1016/0014-5793(81)80286-4. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Cancedda F. D. Posttranslational modifications of Sindbis virus glycoproteins: electrophoretic analysis of pulse-chase-labeled infected cells. J Virol. 1982 Apr;42(1):64–70. doi: 10.1128/jvi.42.1.64-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Capasso O., Gionti E., Pontarelli G., Ambesi-Impiombato F. S., Nitsch L., Tajana G., Cancedda R. The culture of chick embryo chondrocytes and the control of their differentiated functions in vitro. I. Characterization of the chondrocyte-specific phenotypes. Exp Cell Res. 1982 Nov;142(1):197–206. doi: 10.1016/0014-4827(82)90423-2. [DOI] [PubMed] [Google Scholar]
- Dessau W., Vertel B. M., von der Mark H., von der Mark K. Extracellular matrix formation by chondrocytes in monolayer culture. J Cell Biol. 1981 Jul;90(1):78–83. doi: 10.1083/jcb.90.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Kielty C. M., Garner C., Schor S. L., Grant M. E. Identification and partial characterization of three low-molecular-weight collagenous polypeptides synthesized by chondrocytes cultured within collagen gels in the absence and in the presence of fibronectin. Biochem J. 1983 May 1;211(2):417–426. doi: 10.1042/bj2110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Schor S. L., Grant M. E. Effects of matrix macromolecules on chondrocyte gene expression: synthesis of a low molecular weight collagen species by cells cultured within collagen gels. J Cell Biol. 1982 Jun;93(3):767–774. doi: 10.1083/jcb.93.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionti E., Capasso O., Cancedda R. The culture of chick embryo chondrocytes and the control of their differentiated functions in vitro. Transformation by rous sarcoma virus induces a switch in the collagen type synthesis and enhances fibronectin expression. J Biol Chem. 1983 Jun 10;258(11):7190–7194. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Müller P. K., Lemmen C., Gay S., Gauss V., Kühn K. Immunochemical and biochemical study of collagen synthesis by chondrocytes in culture. Exp Cell Res. 1977 Aug;108(1):47–55. [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982 Oct 25;257(20):12444–12450. [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. Metabolism of low molecular weight collagen by chondrocytes obtained from histologically distinct zones of the chick embryo tibiotarsus. J Biol Chem. 1982 Oct 25;257(20):12451–12457. [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. A short chain (pro)collagen from aged endochondral chondrocytes. Biochemical characterization. J Biol Chem. 1983 Aug 10;258(15):9504–9509. [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of a new disulphide bonded collagen from cartilage. FEBS Lett. 1980 Nov 17;121(1):51–54. doi: 10.1016/0014-5793(80)81265-8. [DOI] [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of new collagens from chick cartilage. Eur J Biochem. 1982 May;124(1):57–62. doi: 10.1111/j.1432-1033.1982.tb05905.x. [DOI] [PubMed] [Google Scholar]