Abstract
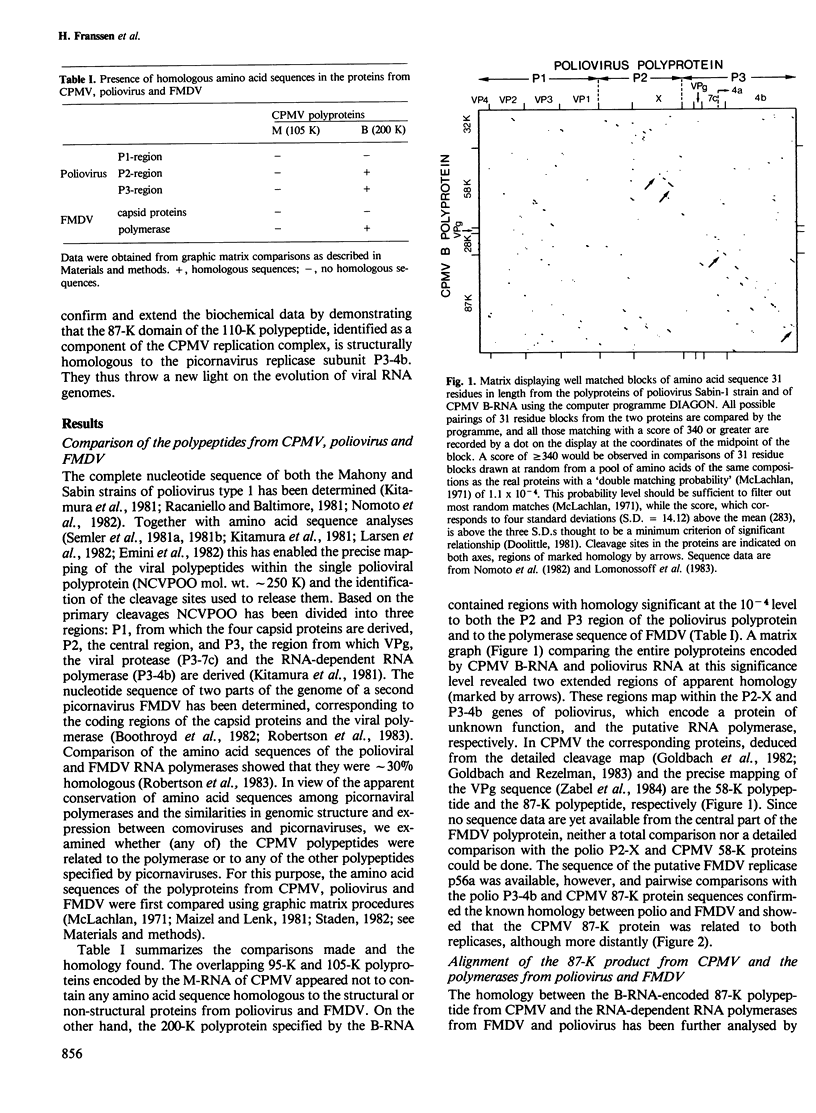
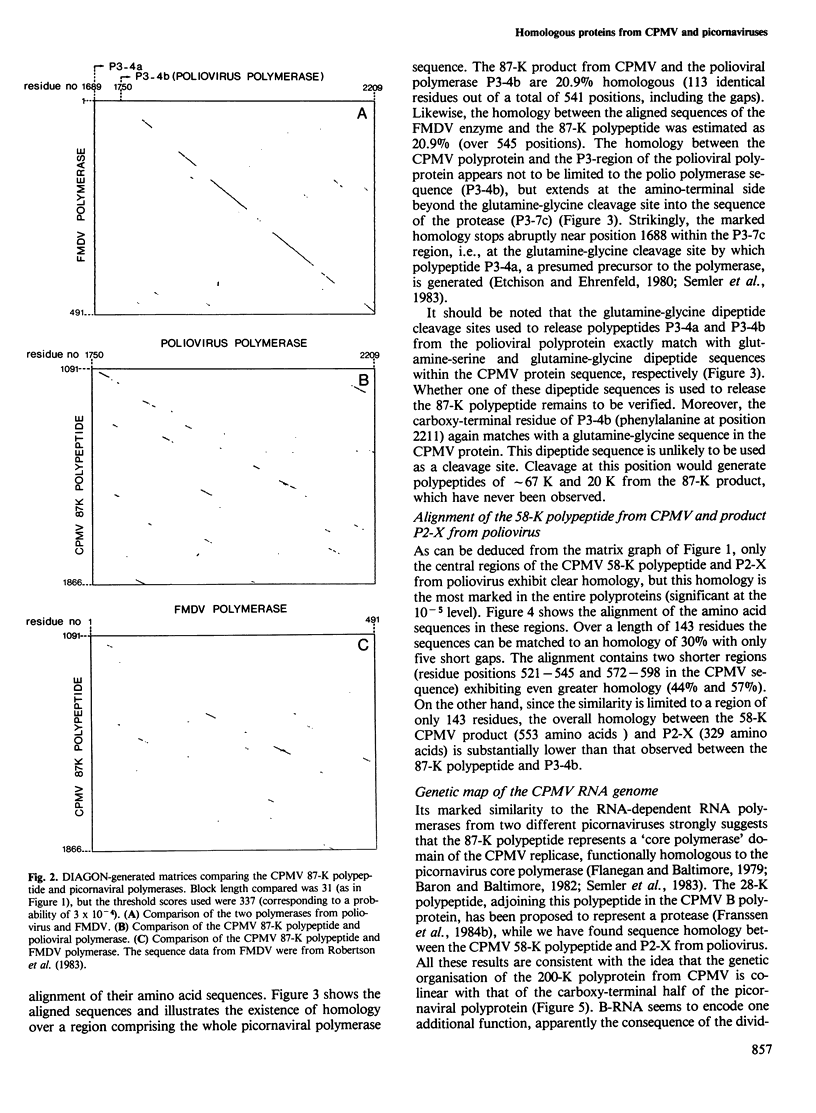
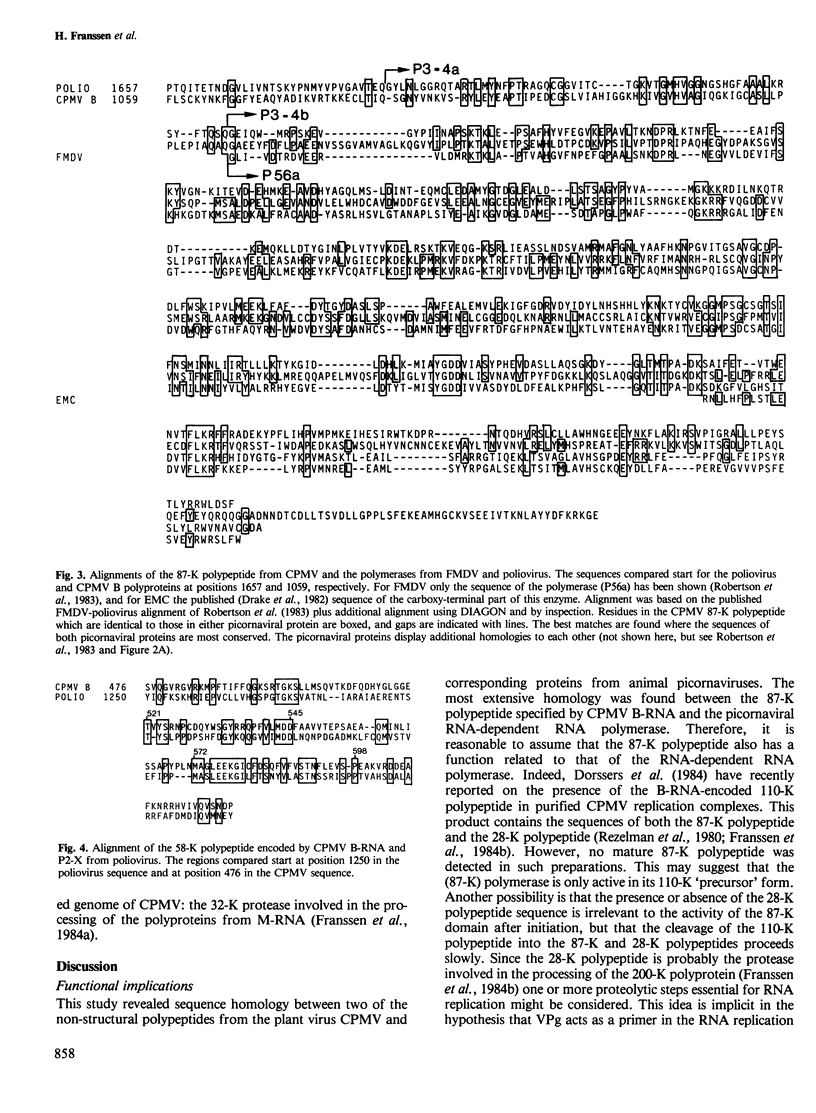
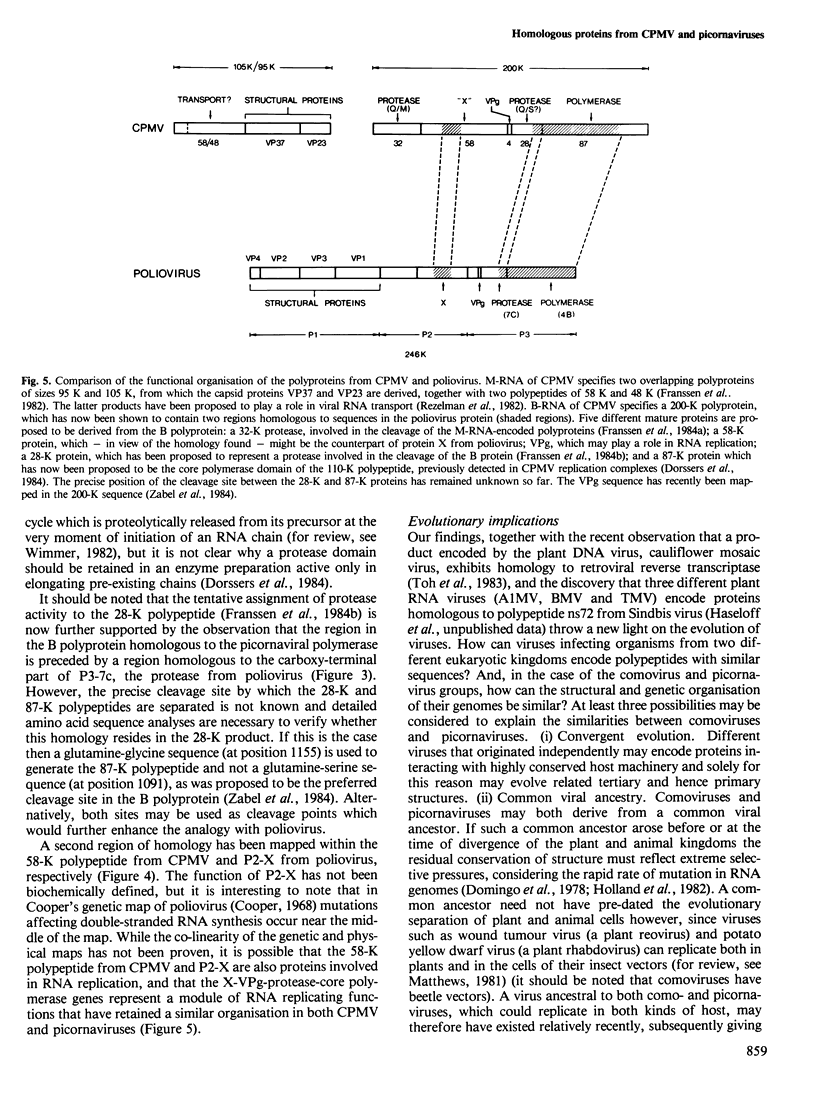
Computer analyses have revealed sequence homology between two non-structural proteins encoded by cowpea mosaic virus (CPMV), and corresponding proteins encoded by two picornaviruses, poliovirus and foot-and-mouth disease virus. A region of 535 amino acids in the 87-K polypeptide from CPMV was found to be homologous to the RNA-dependent RNA polymerases from both picornaviruses, the best matches being found where the picornaviral proteins most resemble each other. Additionally, the 58-K polypeptide from CPMV and polypeptide P2-X from poliovirus contain a conserved region of 143 amino acids. Based on the homology observed, a genetic map of the CPMV genome has been constructed in which the 87-K polypeptide represents the core polymerase domain of the CPMV replicase. These results have implications for the evolution of RNA viruses, and mechanisms are discussed which may explain the existence of homology between picornaviruses (animal viruses with single genomic RNAs) and comoviruses (plant viruses with two genomic RNAs).
Keywords: cowpea mosaic virus, picornavirus, RNA-dependent RNA polymerase, protein sequence homology, evolution
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. H., Baltimore D. Antibodies against a synthetic peptide of the poliovirus replicase protein: reaction with native, virus-encoded proteins and inhibition of virus-specific polymerase activities in vitro. J Virol. 1982 Sep;43(3):969–978. doi: 10.1128/jvi.43.3.969-978.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C., Harris T. J., Rowlands D. J., Lowe P. A. The nucleotide sequence of cDNA coding for the structural proteins of foot-and-mouth disease virus. Gene. 1982 Feb;17(2):153–161. doi: 10.1016/0378-1119(82)90068-3. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Cooper P. D. A genetic map of poliovirus temperature-sensitive mutants. Virology. 1968 Aug;35(4):584–596. doi: 10.1016/0042-6822(68)90287-0. [DOI] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Drake N. L., Palmenberg A. C., Ghosh A., Omilianowski D. R., Kaesberg P. Identification of the polyprotein termination site on encephalomyocarditis viral RNA. J Virol. 1982 Feb;41(2):726–729. doi: 10.1128/jvi.41.2.726-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Elzinga M., Wimmer E. Carboxy-terminal analysis of poliovirus proteins: termination of poliovirus RNA translation and location of unique poliovirus polyprotein cleavage sites. J Virol. 1982 Apr;42(1):194–199. doi: 10.1128/jvi.42.1.194-199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Ehrenfeld E. Viral polypeptides associated with the RNA replication complex in poliovirus-infected cells. Virology. 1980 Nov;107(1):135–142. doi: 10.1016/0042-6822(80)90279-2. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus polyuridylic acid polymerase and RNA replicase have the same viral polypeptide. J Virol. 1979 Jan;29(1):352–360. doi: 10.1128/jvi.29.1.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Goldbach R., Broekhuijsen M., Moerman M., van Kammen A. Expression of Middle-Component RNA of Cowpea Mosaic Virus: In Vitro Generation of a Precursor to Both Capsid Proteins by a Bottom-Component RNA-Encoded Protease from Infected Cells. J Virol. 1982 Jan;41(1):8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs A. J., McIntyre G. A. The diagram, a method for comparing sequences. Its use with amino acid and nucleotide sequences. Eur J Biochem. 1970 Sep;16(1):1–11. doi: 10.1111/j.1432-1033.1970.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Goldbach R., Rezelman G. Orientation of the cleavage map of the 200-kilodalton polypeptide encoded by the bottom-component RNA of cowpea mosaic virus. J Virol. 1983 May;46(2):614–619. doi: 10.1128/jvi.46.2.614-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R., Rezelman G., Zabel P., van Kammen A. Expression of the Bottom-Component RNA of Cowpea Mosaic Virus: Evidence that the 60-Kilodalton VPg Precursor Is Cleaved into Single VPg and a 58-Kilodalton Polypeptide. J Virol. 1982 May;42(2):630–635. doi: 10.1128/jvi.42.2.630-635.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Biochemical evidence of recombination within the unsegmented RNA genome of aphthovirus. J Virol. 1982 Jan;41(1):66–77. doi: 10.1128/jvi.41.1.66-77.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Recombination in RNA. Cell. 1982 Jul;29(3):921–928. doi: 10.1016/0092-8674(82)90454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Anderson C. W., Dorner A. J., Semler B. L., Wimmer E. Cleavage sites within the poliovirus capsid protein precursors. J Virol. 1982 Jan;41(1):340–344. doi: 10.1128/jvi.41.1.340-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezelman G., Goldbach R., Van Kammen A. Expression of bottom component RNA of cowpea mosaic virus in cowpea protoplasts. J Virol. 1980 Nov;36(2):366–373. doi: 10.1128/jvi.36.2.366-373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Morgan D. O., Moore D. M., Grubman M. J., Card J., Fischer T., Weddell G., Dowbenko D., Yansura D. Identification of amino acid and nucleotide sequence of the foot-and-mouth disease virus RNA polymerase. Virology. 1983 Apr 30;126(2):614–623. doi: 10.1016/s0042-6822(83)80017-8. [DOI] [PubMed] [Google Scholar]
- Romanova L. I., Tolskaya E. A., Kolesnikova M. S., Agol V. I. Biochemical evidence for intertypic genetic recombination of polioviruses. FEBS Lett. 1980 Aug 25;118(1):109–112. doi: 10.1016/0014-5793(80)81229-4. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Anderson C. W., Wimmer E. Cleavage sites in the polypeptide precursors of poliovirus protein P2-X. Virology. 1981 Oct 30;114(2):589–594. doi: 10.1016/0042-6822(81)90242-7. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Dorner L. F., Anderson C. W., Wimmer E. Poliovirus RNA synthesis in vitro: structural elements and antibody inhibition. Virology. 1983 Apr 30;126(2):624–635. doi: 10.1016/s0042-6822(83)80018-x. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Tolskaya E. A., Romanova L. A., Kolesnikova M. S., Agol V. I. Intertypic recombination in poliovirus: genetic and biochemical studies. Virology. 1983 Jan 15;124(1):121–132. doi: 10.1016/0042-6822(83)90295-7. [DOI] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J., Dorssers L., Zabel P. Antibody-linked polymerase assay on protein blots: a novel method for identifying polymerases following SDS-polyacrylamide gel electrophoresis. EMBO J. 1983;2(2):233–237. doi: 10.1002/j.1460-2075.1983.tb01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



