Abstract
The results of immunocytochemical studies using two different monoclonal antibodies against the M1 subunit of ribonucleotide reductase show an exclusively cytoplasmic localization of this subunit both in cultured MDBK and mouse 3T6 cells, and in cells from various rat tissues. By fluorescent light microscopy, there is a diffuse staining of the cytoplasm, while by electron microscopy the immunoreactive material appears to be associated with ribosomes. In the rat tissues, only actively dividing cells show M1-specific immunofluorescence revealing a strong correlation between the presence of protein M1 and DNA synthesis. Therefore M1 immunofluorescence could be used to study cell proliferation in normal, inflammatory or neoplastic tissue. A lesser variation in M1 staining is observed between individual cells in tissue culture, where most cells are positive, but neither here nor in the tissues examined are any cells with nuclear staining detected. We interpret our results to mean that in mammalian cells ribonucleotide reduction takes place in the cytoplasm and from there the deoxyribonucleotides are transported into the nucleus to serve in DNA synthesis.
Full text
PDF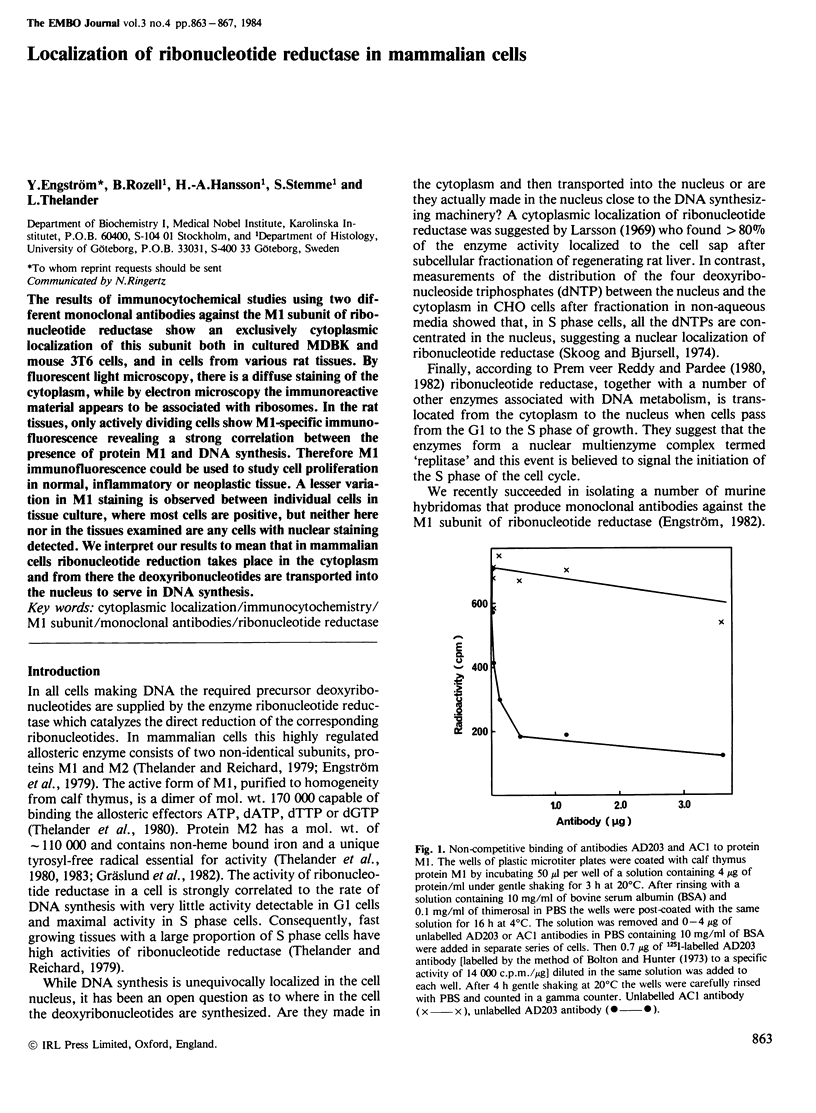



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensch K. G., Tanaka S., Hu S. Z., Wang T. S., Korn D. Intracellular localization of human DNA polymerase alpha with monoclonal antibodies. J Biol Chem. 1982 Jul 25;257(14):8391–8396. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
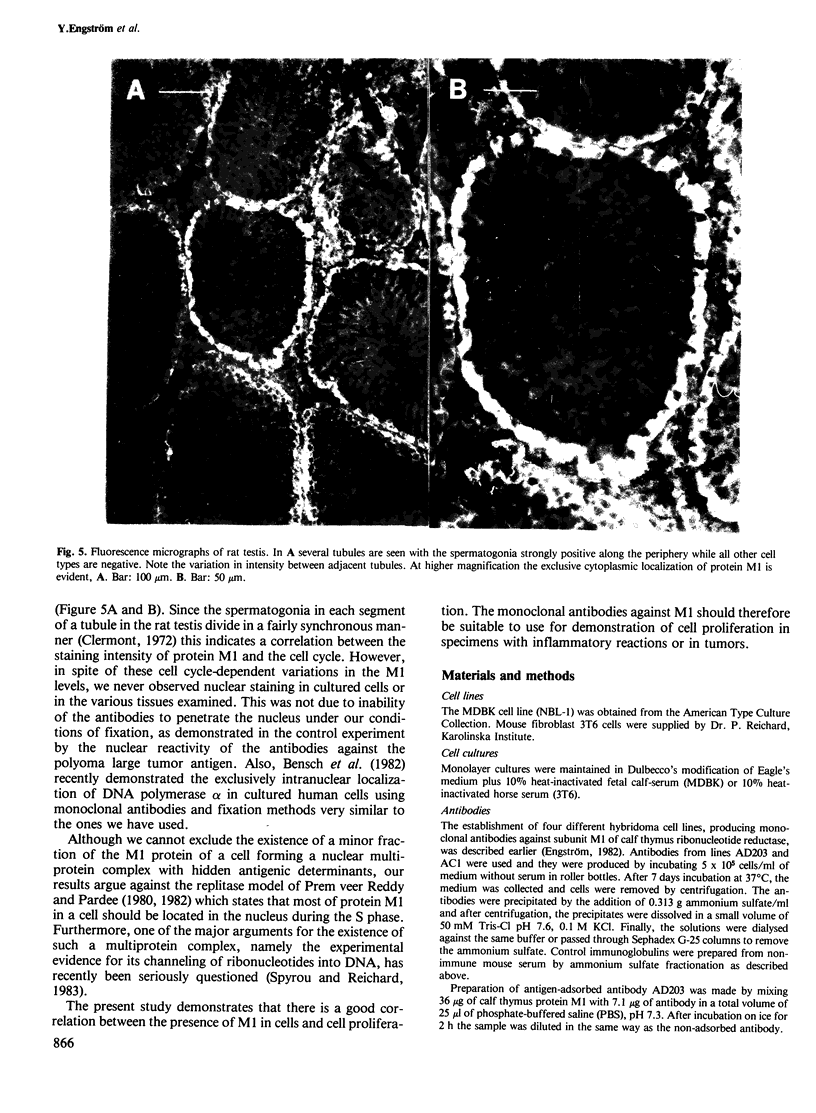
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972 Jan;52(1):198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Dilworth S. M., Griffin B. E. Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Thelander L., Akerman M. Ribonucleotide reductase from calf thymus. Purification and properties. Biochemistry. 1979 Jul 10;18(14):2941–2948. doi: 10.1021/bi00581a004. [DOI] [PubMed] [Google Scholar]
- Engström Y. Monoclonal antibodies against mammalian ribonucleotide reductase. Acta Chem Scand B. 1982;36(5):343–344. doi: 10.3891/acta.chem.scand.36b-0343. [DOI] [PubMed] [Google Scholar]
- Gräslund A., Ehrenberg A., Thelander L. Characterization of the free radical of mammalian ribonucleotide reductase. J Biol Chem. 1982 May 25;257(10):5711–5715. [PubMed] [Google Scholar]
- Larsson A. Ribonucleotide reductase rom regenerating rat liver. Eur J Biochem. 1969 Nov;11(1):113–121. doi: 10.1111/j.1432-1033.1969.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog L., Bjursell G. Nuclear and cytoplasmic pools of deoxyribonucleoside triphosphates in Chinese hamster ovary cells. J Biol Chem. 1974 Oct 25;249(20):6434–6438. [PubMed] [Google Scholar]
- Spyrou G., Reichard P. Ribonucleotides are not channeled into DNA in permeabilized mammalian cells. Biochem Biophys Res Commun. 1983 Sep 30;115(3):1022–1026. doi: 10.1016/s0006-291x(83)80037-0. [DOI] [PubMed] [Google Scholar]
- Thelander L., Eriksson S., Akerman M. Ribonucleotide reductase from calf thymus. Separation of the enzyme into two nonidentical subunits, proteins M1 and M2. J Biol Chem. 1980 Aug 10;255(15):7426–7432. [PubMed] [Google Scholar]
- Thelander L., Gräslund A., Thelander M. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: possible regulation mechanism. Biochem Biophys Res Commun. 1983 Feb 10;110(3):859–865. doi: 10.1016/0006-291x(83)91040-9. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- veer Reddy G. P., Pardee A. B. Coupled ribonucleoside diphosphate reduction, channeling, and incorporation into DNA of mammalian cells. J Biol Chem. 1982 Nov 10;257(21):12526–12531. [PubMed] [Google Scholar]