Abstract
Liver abscesses constitute a prominent concern regarding animal health and profitability of the beef industry. Our objective was to evaluate potential biliary and blood indicators of liver abscesses. Twenty-nine beef bulls (initially averaging 356±70.5 kg and 253±30 days of age) were fed a high-concentrate diet during a performance test of 112 days, during which blood was collected at nine time points spaced 0.5–13 days apart within 56 days before slaughter. At the abattoir, blood and bile were collected and livers were inspected for liver abscesses. Results indicated that liver abscesses are associated with elevated levels of plasma cortisol and aspartate aminotransferase, and decreased levels of albumin, cholesterol and testosterone over the period before slaughter. Based on the blood samples collected during exsanguination, the presence of liver abscesses was associated with lower concentrations of thyroxine, albumin, cholesterol and alkaline phosphatase, and is suggested to be associated with lower blood carbon dioxide (P=0.08) and lower biliary cortisol metabolites (P=0.07). Albumin and cholesterol are established indicators of hepatic function and are consistently related to the presence of liver abscesses. Identifying blood parameters that predict liver abscesses has practical implications for cattle husbandry and for ensuring food safety.
Keywords: albumin, cholesterol, thyroxine, testosterone, feedlot, high-grain diet
Introduction
Livestock farming practices have evolved over the past several decades in a general attempt to maximise gain and profit. However, intensification of farming practices such as those incorporated into beef production systems is associated with heightened incidence of metabolic disease and liver abscesses within a herd (Lister 1971, Dixon 1973, Nagaraja and Lechtenberg 2007). Liver abscesses are a threat to many beef cattle industries globally; for example, they account for losses approximating to nearly $30 million annually in Canada (Beef Cattle Research Council 2011).
Financial damages stem from several factors, including loss of liver due to condemnation, loss of contaminated carcases and increased carcase trimming (Fox and others 2009). Liver abscesses present challenges to animal health and performance (Nagaraja and others 1996), as well as to food safety, where failure of detection may occur during regular inspection practices. Severe liver abscesses are associated with decreased average daily gain (ADG) and are often concomitant with lung and rumen lesions (Rezac and others 2014). Similarly, abscesses are associated with carcase devaluation due to decreased dressing yield, lower carcase weight and diminished marbling (Brown and Lawrence 2010). Together, these amount to a decrease in profitability for the producer and increased risk that a diseased organ might enter the food chain (Mellau and others 2010).
Liver abscesses display an average incidence of 12 to 32 per cent (Nagaraja and others 1996, Radostits 2000) and are particularly common in feedlot cattle fed high-concentrate finishing rations with lower inclusion of roughage (Plaizier and others 2008, Hernández and others 2014). This type of diet is high in non-structural carbohydrates which are rapidly fermented by ruminal microbes, resulting in an excessive production rate of lactate and drop in rumen pH, which predisposes the rumen wall to invasion by opportunistic pathogens (Russell 1999, Nagaraja and others 2005). Fusobacterium necrophorum is the most common cause of liver abscesses (Calkins and Dewey 1968, Narayanan and others 1997). This proteolytic bacterium uses lactate as an energy source and possesses several virulence factors such as leucotoxin and endotoxin, which mediate its invasion into tissues (Tadepalli and others 2009, Kumar and others 2015). Once bacterial colonies are established in the rumen wall, they may shed emboli through the portal circulation to reach the liver, where they form lesions that progress to abscesses (Herenda and others 2000, Kersting and others 2009).
The detection of liver abscesses before slaughter is laborious and often produces inconsistent results (Braun 2009). Ultrasound of the bovine liver has been used with some success to identify abscesses (Lechtenberg and Nagaraja 1991, Liberg and Jönsson 1993). However, current diagnostic techniques overlook deeper regions of the liver and ultrasonograms may not definitively identify abscesses (Doré and others 2007, Braun 2009). Thus, there is a need for a safe, affordable and accurate method of detecting liver abscesses before slaughter. Such a technique would allow for early detection of this condition and aid in effective management decisions, potentially decreasing the impact of liver abscesses on animal health and production. Additionally, a readily available rapid test for liver abscesses used at slaughter could alert abattoir inspectors to potentially infected animals before livers are inspected by palpation and incision.
Previous studies have noted that complete blood cell counts and serum biochemistry in cattle with liver abscesses are consistent with chronic active inflammation (Branum and others 1990, Doré and others 2007). A decrease in serum albumin concentration indicative of liver damage has been correlated with the onset of liver abscesses (Lechtenberg and Nagaraja 1991). Blood testosterone is also reduced when liver function is compromised by inflammatory disease (Kent and others 1973). Similarly, the constituents of the gall bladder bile might reflect inflammation of the liver (Wagner and others 2007). The liver is an energetically demanding organ, and its physiological state relates to variation in metabolic output of an animal (Johnson and others 1990, Müller 1998); thus, markers of hepatic function may reflect the presence of abscesses and may also correlate with associated changes in performance of the animal. We hypothesised that components of blood and bile will differ between cattle with abscessed and normal livers. Therefore, the objective of this study was to compare the bile and blood of cattle with demonstrated presence or absence of liver abscesses in order to identify the following: (1) measurements that may indicate the presence of abscess towards the end of the finishing period and (2) measurements that may provide evidence of liver abscesses at the abattoir.
Materials and methods
Animal care, husbandry and productive performance
Experimental procedures were in accordance with the Canadian Council on Animal Care (2009). A group of 29 cross-bred beef bulls (initially averaging 356±70.5 kg and 253±30.0 days of age) were housed at the Elora Beef Research Centre of the University of Guelph, Ontario, Canada, in an indoor pen bedded with wood shavings. Breed composition consisted of 54 per cent Angus, 31 per cent Simmental and 15 per cent other European breeds and crosses. Bulls were fed a high-moisture, corn-based diet ad libitum, and individual daily feed intake was measured using an automated feeding system (Insentec , Marknesse, The Netherlands). Diet composition (dry matter basis) was as follows: 80 per cent high-moisture corn, 10 per cent alfalfa silage, 4.7 per cent soybean meal, 1.8 per cent corn gluten meal, 1.7 per cent limestone and 1.8 per cent mineral and vitamin premix, as detailed by Montanholi and others (2009). Bulls underwent a 112-day performance test; assessments included daily feed intake, ADG and feed efficiency (Montanholi and others 2009). Following the performance test, bulls were slaughtered at an average of 403±31.7 days of age and 606±85.6 kg body weight in groups of four to eight bulls weekly, with heavier bulls processed first. Productive performance measures such as dry matter intake, ADG, feed to gain ratio (FG) and residual feed intake (RFI; kg/day) were calculated following the methodology of Montanholi and others (2010).
Blood sampling and liver abscess evaluation
A total of nine blood samples were obtained over a 56-day sampling period on days 0.5, 1, 5, 18, 22, 26, 28 and 33 before the start of the slaughter, with a final blood sample taken the day before slaughter, which occurred at approximately 44±8 days of the first sampling. During each sampling occasion, bulls were restrained in a squeeze chute (Silencer, Hydraulic Squeeze Chute, Moly Manufacturing, Lorraine, Kansas, USA) and blood was collected via jugular venepuncture using a 10-ml tube with sodium heparin (plasma) or plain glass (serum) (Vacutainer; Becton Dickinson, Franklin Lakes, New Jersey, USA) connected to a 2.5 cm 20-G needle. Samples were also collected at slaughter immediately after stunning as each animal was bled. Serum samples were left at room temperature for approximately 30 minutes before centrifugation (3000 g at 4°C for 25 minutes). Plasma samples were stored on ice until centrifugation, then plasma was collected and stored at −80°C until further use. During slaughter, livers were evaluated for the presence of abscesses by a certified meat inspector from the Canadian Food Inspection Agency (Herenda and others 2000, Canadian Food Inspection Agency 2015). Bulls were recorded as either abscessed or normal.
Plasma metabolic profile
Concentrations of plasma albumin (g/l), aspartate aminotransferase (AST; U/l), glucose (mmol/l), sodium (mmol/l), potassium (mmol/l), chloride (mmol/l), urea (mmol/l), cholesterol (mmol/l) and alkaline phosphatase (ALP; U/l) were obtained using an automated blood analyser (Cobas c311/501 analyzer, Roche Diagnostics, Indianapolis, Indiana, USA). Plasma carbon dioxide (CO2; mmol/l) was measured using a similar automated analyser (Cobas 400 c311; Roche Diagnostics, Mannheim, Germany). Plasma cortisol (ng/ml) was obtained using a commercially available kit (Coat-A-Count; Diagnostic Products, Los Angeles, California, USA). Plasma acetate (g/l) was measured using a colorimetric kit (K-ACETRM; Megazyme International, Wicklow, Ireland). Osmolality (mmol/l) was calculated as the sum of individual solute concentrations (Bhagat and others 1984). Plasma levels of insulin-like growth factor-1 (ng/ml), triiodothyronine (nmol/l) and thyroxine (T4; nmol/l) were quantified using ELISA tests (IMMULITE 1000; Siemens Healthcare Diagnostic Products, Malvern, Pennsylvania, USA). Plasma testosterone (ng/mL) was analysed by radioimmunoassay (Coat-A-Count, Siemens Healthcare Diagnostic Products) using charcoal-stripped serum to prepare the standards. Luteinising hormone (ng/ml) and follicle stimulating hormone (ng/ml) in plasma were both analysed by radioimmunoassay (Rawlings and Evans 1995). Plasma prolactin (ng/ml) was also analysed by radioimmunoassay as per methodology optimised for bovine assessment (Prairie Diagnostic Services, Saskatoon, Canada), as was plasma leptin (ng/ml) (Multispecies Leptin RIA Kit; Millipore, St. Charles, Missouri, USA). Ghrelin concentration (pg/ml) was measured in undiluted serum using a competitive ELISA (Bovine Ghrelin Elisa Kit; BlueGene Biotech, Shanghai, China). Total bile acids (µmol/l) were measured in undiluted serum and 2500x diluted bile using a colorimetric total bile acids assay kit (BQ Kits, San Diego, California, USA). IgA concentration in 500x diluted bile (µg/ml) was obtained using a bovine IgA ELISA quantitation set (Bethyl Laboratories, Montgomery, Texas, USA). Interleukin-6 (IL-6) concentration (pg/ml) was quantified in bile by ELISA (Bovine IL-6 screening kit; Thermo Fisher Scientific, Waltham, Massachusetts, USA). Tumour necrosis factor-α (TNF-α) concentration (pg/ml) in undiluted bile and serum was obtained using an ELISA kit (DuoSet ELISA; R&D Systems, Minneapolis, Minnesota, USA). Biliary cortisol metabolites (ng/g) were analysed by an 11-oxoaetiocholonolone enzyme immunoassay (Palme and Möstl 1997).
Statistical analysis
To identify plasma and bile components with potential for predicting liver abscesses, we examined variation in several parameters, including metabolic hormones, indicators of hepatic and whole-body metabolism, sexual hormones and ions, with the presence or absence of liver abscesses serving as classification criteria for the two groups. Data were analysed using SAS V.9.4 software (2003). Normality was tested using the UNIVARIATE procedure, and measures that did not meet the normality requirements were transformed using logarithm, square root or reciprocal transformations. The back-transformed data are presented in the Results section along with the confidence limits calculated at 95 per cent. Bulls were classified by the presence or absence of liver abscesses. The least square means of these two groups were compared using independent t tests. The MIXED procedure was used to compare the least square means of blood analytes on the repeated measures collected before slaughter. The GENERAL LINEAR MODEL procedure was used to compare the means between the two categories for bile and blood analytes assessed at slaughter. Results are reported as means for abscessed bulls v normal bulls (mean, lower and upper 95 per cent confidence limits) and were considered statistically significant when P<0.05 and a trend towards significance when 0.05 < P<0.10.
Results
To identify plasma and bile components with potential for predicting liver abscesses, we examined variation in several parameters, including metabolic hormones, indicators of hepatic and whole-body metabolism, sexual hormones and ions, with the presence or absence of liver abscesses serving as classification criteria for the two groups. Of the 29 bulls studied, nine had liver abscesses. Results are reported as means for abscessed bulls v normal bulls (mean, lower and upper confidence limits).
Table 1 describes the least square mean concentrations of blood parameters across the nine sampling occasions before slaughter for bulls with and without liver abscesses. The decreased levels of albumin, cholesterol and testosterone in the bulls with abscessed livers are remarkable, as well as the increased concentration of cortisol and AST in these animals when compared with the bulls with normal livers. Similarly, Fig 1 depicts the mean blood concentrations of plasma AST, testosterone and albumin in abscessed and normal bulls over nine time points and of cortisol at eight of the above time points excluding the day before slaughter. Analysis of blood collected at the abattoir demonstrated no relationship between abscesses and mean serum bile acid levels (42.22 (33.95, 52.50) v 42.64 (36.84, 49.36) µmol/L, P=0.93) or mean serum ghrelin concentration (33.13 (23.59, 42.66) v 31.74 (25.34, 38.13) pg/ml, P=0.81). Serum TNF-α was measurable in only one bull. Bile was also collected at the abattoir, and results suggest that bulls with abscessed livers may have lower levels of biliary cortisol metabolites (168.52 (124.03, 228.97) v 235.75 (191.93, 289.57) ng/g, P=0.07). Mean levels of biliary IgA were not different between groups (82.86 (55.45, 123.82) v 67.94 (51.89, 88.95) µg/ml, P=0.41). Levels of TNF-α and IL-6 (pg/ml) in bile were measurable in few bulls (three for TNF-α and three for IL-6). Of the three bulls with measurable TNF-α in bile, two had abscessed livers. Of the three bulls with measurable bile IL-6, one had an abscessed liver.
Table 1.
Results of independent t tests comparing least square mean (lower and upper 95 per cent confidence limit) blood parameter concentrations between beef bulls with abscessed and normal livers averaged over the 56-day sampling period
| Parameter (unit) | Abscessed (n=9) | Normal (n=20) | P Value |
| Metabolic hormones | |||
| Cortisol (ng/ml) | 91.52 (37.35, 55.02) | 70.13 (30.08, 40.43) | 0.04 |
| Leptin (ng/ml) | 2.89 (2.47, 3.37) | 2.74 (2.46, 3.04) 0.361 |
0.60 |
| IGF-1 (ng/ml) | 486.8 (445.8, 527.8) | 509.0 (481.1, 536.8) | 0.36 |
| T3 (nmol/l) | 2.74 (2.57, 2.92) | 2.76 (2.64, 2.88) | 0.89 |
| T4 (nmol/l) | 85.73 (80.86, 90.61) | 88.60 (85.30, 91.91) | 0.32 |
| Liver and whole-body metabolism | |||
| CO2 (mmol/l) | 23.16 (22.62, 23.71) | 22.65 (22.28, 23.01) | 0.12 |
| Albumin (g/l) | 30.27 (29.24, 31.30) | 32.04 (31.36, 32.73) | 0.01 |
| ALP (U/l) | 120.2 (107.66, 132.80) | 122.08 (113.60, 130.57) | 0.80 |
| AST (U/l) | 64.85 (59.57, 70.58) | 57.48 (54.29, 60.87) | 0.02 |
| Acetate (g/l) | 34.93 (32.41, 37.64) | 35.06 (33.35, 36.86) | 0.93 |
| Cholesterol (mmol/l) | 2.22 (2.03, 2.41) | 2.45 (2.32, 2.58) | 0.05 |
| Glucose (mmol/l) | 2.15 (1.45, 1.48) | 2.18 (1.47, 1.48) | 0.13 |
| Urea (mmol/l) | 3.84 (3.53, 4.16) | 4.11 (3.90, 4.33) | 0.15 |
| Sexual hormones | |||
| FSH (ng/ml) | 0.33 (0.26, 0.41) | 0.28 (0.24, 0.32) | 0.21 |
| LH (ng/ml) | 0.33 (0.25, 0.42) | 0.35 (0.29, 0.41) | 0.69 |
| Testosterone (ng/ml) | 3.96 (3.19, 4.73) | 5.13 (4.61, 5.65) | 0.02 |
| Prolactin (ng/ml) | 56.21 (40.12, 78.74) | 42.26 (33.63, 53.10) | 0.16 |
| Ions | |||
| Chloride (mmol/l) | 94.57 (93.16, 95.97) | 95.02 (94.08, 95.96) | 0.58 |
| Sodium (mmol/l) | 136.0 (134.00, 137.95) | 136.71 (135.39, 138.02) | 0.53 |
| Potassium (mmol/l) | 4.37 (4.20, 4.54) | 4.38 (4.27, 4.50) | 0.88 |
| Calculated osmolality (mmol/l) | 269.4 (265.60, 273.31) | 271.30 (268.72, 273.88) | 0.42 |
ALP, alkaline phosphatase; AST, aspartate aminotransferase; CO2, carbon dioxide; FSH, follicle stimulating hormone; IGF-1, insulin-like growth factor-1; LH, luteinising hormone; T3, triiodothyronine; T4, thyroxine.
FIG 1.
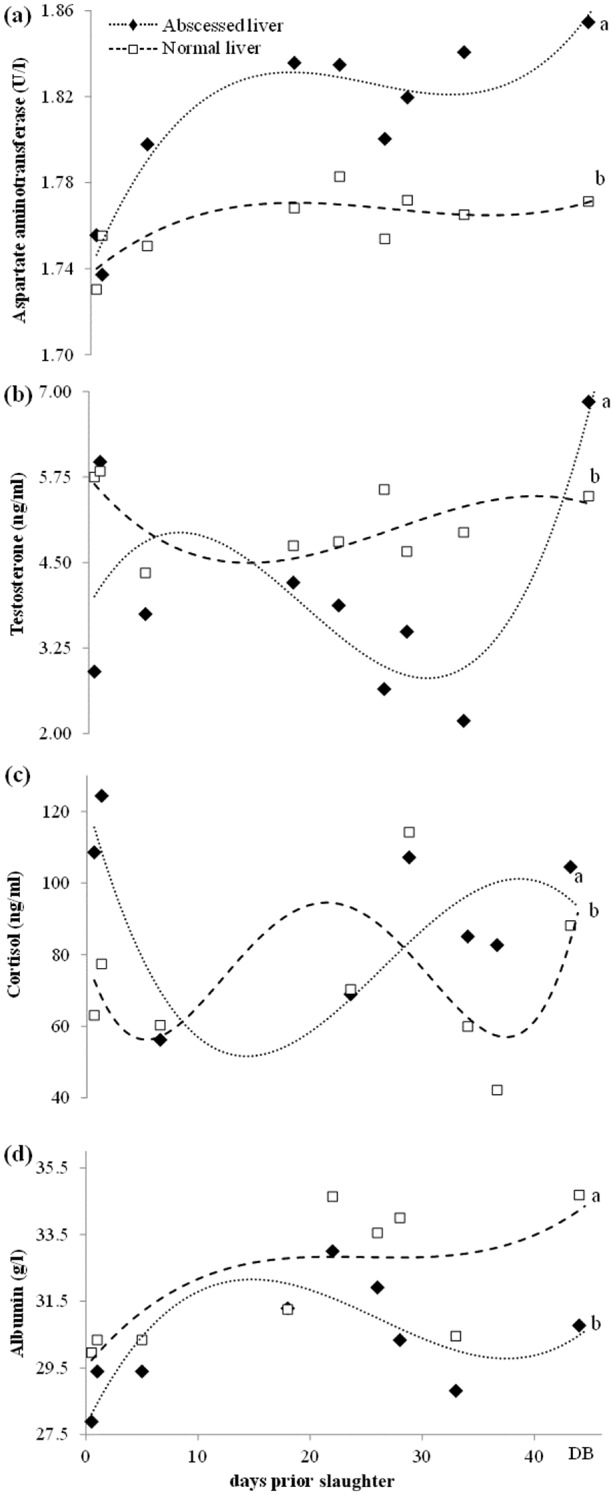
Results of mixed models showing the least square means of blood analytes. (a) Aspartate aminotransferase, (b) testosterone, (c) albumin and (d) cortisol for nine beef bulls with abscessed livers (…♦…) v20 bulls with normal livers (---□---) for each sampling time prior to slaughter (except for cortisol where the last sample immediately prior to slaughter was excluded). DB represents the average number of days (44+8) into the sampling period that corresponded with the day before slaughter. Differing subscripts represent P<0.05 overall the sampling period. The confidence limits for aspartate aminotransferase, testosterone, albumin and cortisol ranged from 54.29 to 70.58, 3.19 to 5.65, 29.24 to 32.73 and 30.08 to 55.02, respectively.
Table 2 describes the means for blood parameters measured at the abattoir. Liver-abscessed bulls had decreased levels of T4, albumin, ALP, cholesterol and glucose in comparison to bulls with normal livers. A trend (P<0.10) was also observed for increased CO2 and decreased osmolality in the animals with abscessed livers when compared with animals with healthy livers. Our results indicate association between the presence and absence of liver abscesses and performance traits, which include FG (5.19 (4.68, 5.69) v 4.60 (4.26, 4.94), P=0.05) and ADG (1.53 (1.38, 1.68) v 1.67 (1.57, 1.78) kg/day, P=0.10). No differences were observed for dry matter intake (7.90 (6.95, 8.85) v 7.66 (7.02, 8.30) kg/day, P=0.67), RFI (0.25 (−0.24, 0.67) v −0.01 (−0.36, 0.30) kg/day, P=0.35) and final body weight (602 (536, 669) v 639 (594, 683) kg, P=0.35). Bulls classified according to presence or absence of liver abscesses also did not differ in age between groups (394.56 (373.91, 415.20) v 401.55 (387.70, 415.40) days, P=0.57).
Table 2.
Comparison of least square means (lower and upper 95 per cent confidence limits) blood parameter concentrations at slaughter between beef bulls with abscessed and normal livers
| Parameter (unit) | Abscessed (n=9) | Normal (n=20) | P value |
| Metabolic hormones | |||
| Cortisol (ng/ml) | 138.55 (96.85, 180.24) | 104.45 (74.11, 134.78) | 0.18 |
| Leptin (ng/l) | 2.89 (2.08, 3.70) | 2.87 (3.32, 3.43) | 0.98 |
| IGF-1 (ng/ml) | 376.78 (328.59, 424.96) | 410.60 (378.28, 442.92) | 0.24 |
| T3 (nmol/l) | 2.77 (2.49, 3.05) | 2.80 (2.61, 2.99) | 0.84 |
| T4 (nmol/l) | 89.43 (81.33, 97.53) | 102.00 (96.57, 107.43) | 0.01 |
| Liver and whole-body metabolism | |||
| CO2 (mmol/l) | 23.78 (22.60, 24.96) | 22.50 (21.71, 23.29) | 0.08 |
| Albumin (g/l) | 32.11 (30.07, 34.15) | 36.35 (34.98, 37.72) | 0.01 |
| ALP (U/l) | 98.11 (82.21, 114.02) | 116.95 (106.28, 127.62) | 0.05 |
| Acetate (g/l) | 33.65 (26.47, 42.77) | 35.88 (30.55, 42.14) | 0.65 |
| Cholesterol (mmol/l) | 2.43 (2.05, 2.80) | 3.04 (2.79, 3.29) | 0.01 |
| Glucose (mmol/l) | 5.14 (4.30, 5.87) | 6.09 (5.64, 6.52) | 0.03 |
| Sexual hormones | |||
| FSH (ng/ml) | 0.35 (0.19, 0.51) | 0.34 (0.23, 0.45) | 0.90 |
| LH (ng/ml) | 0.13 (0.06, 0.20) | 0.14 (0.09, 0.19) | 0.79 |
| Testosterone (ng/ml) | 6.92 (3.17, 10.66) | 6.33 (3.82, 8.84) | 0.79 |
| Prolactin (ng/ml) | 90.73 (1.73, 2.19) | 99.75 (1.84, 2.16) | 0.76 |
| Ions | |||
| Sodium (mmol/l) | 135.11 (131.60, 138.63) | 137.90 (135.54, 140.26) | 0.19 |
| Potassium (mmol/l) | 5.43 (4.86, 6.00) | 5.74 (5.36, 6.12) | 0.37 |
| Calculated osmolality (mmol/l) | 271.11 (264.64, 277.58) | 277.50 (273.16, 281.84) | 0.10 |
ALP, alkaline phosphatase; CO2, carbon dioxide; FSH, follicle stimulating hormone; IGF-1, insulin-like growth factor-1; LH, luteinising hormone; T3, triiodothyronine; T4, thyroxine.
Discussion
Our results indicate that variation in several blood parameters prior to slaughter is associated with the presence of liver abscesses in bulls at slaughter. Of the bulls studied, 31 per cent had liver abscesses. This is comparable to findings of 28 per cent (Brink and others 1990), 24 per cent (Rust and others 1980) and 20.6 per cent (Rezac and others 2014), all of which were noted in larger cattle populations.
Blood collected over multiple time points towards the end of the finishing period contained several constituents which differed between cattle with abscessed and normal livers. Albumin is an abundant serum protein synthesised by the liver (Peters 1995) and low levels of albumin are associated with liver injury (Mitzner and others 2006). We observed diminished levels of albumin in blood of bulls with abscessed livers (Table 1), which suggests that the formation of abscesses compromises liver health. Previous work noted decreased plasma albumin levels in cows displaying indices of hepatic damage (Lechtenberg and Nagaraja 1991). Furthermore, cows with liver disease also have lower levels of blood cholesterol and elevated concentrations of AST (Mudron and others 1999, Bionaz and others 2007), which is also in accordance with our findings. As a diagnostic tool, AST is a consistent and sensitive indicator of hepatic lipidosis in cows (Cebra and others 1997), and while differences in our means were smaller than the ones associated with fatty liver, our observation that levels of AST are significantly higher in plasma of abscessed bulls may reflect some degree of hepatic damage correlated with liver abscesses. Our results also indicate that abscessed bulls have lower levels of blood cholesterol (Table 1), possibly related to changes in hepatic lipoprotein synthesis (Bionaz and others 2007). Cholesterol is the precursor for several steroid hormones including testosterone (Fox 2014); accordingly, our results demonstrate smaller levels of testosterone in abscessed bulls (Table 1). This may also be related to liver abscesses, as liver damage is correlated with diminished blood testosterone, possibly due to decreased hepatic synthesis of sex hormone-binding globulin (Shin and others 2011, Hua and others 2014). Blood cortisol levels were higher in bulls with abscesses (Table 1), which may reflect decreased metabolism of cortisol by the diseased liver (Peterson 1960, McCann and Fulton 1975), or possibly a general inflammatory state initiated by the progression to acidosis and liver abscesses (Gozho and others 2006, Trevisi and Bertoni 2009). Illness in cattle induces a stress response characterised by an increased serum cortisol level (Nahed 2010); thus, our results may also be relevant when considered in context of stress and disease.
The observation of the AST, testosterone, albumin and cortisol profiles over time indicated, in some cases, distinct patterns. For example, AST remained consistently higher in bulls with liver abscesses, particularly through the later sampling stages (Fig 1a). Testosterone appeared consistently lower in abscessed bulls, though some variation exists, possibly due to sampling methods or analysis (Fig 1b). Conversely, cortisol levels did not appear to follow a steady trend (Fig 1c), which may reflect variation in handling and sample collection (Montanholi and others 2013). Albumin levels were lower in abscessed bulls at the majority of time points, and the difference between groups appears to increase with time (Fig 1d). Overall, variation in the above blood components may reflect the disruption of liver tissue integrity due to the formation of liver abscesses.
Blood collected at slaughter was analysed to determine associations between liver abscesses and blood constituents at a single sampling occasion. Again, albumin and cholesterol levels were significantly lower in blood of bulls with abscessed livers, suggesting that the formation of abscesses may result in a measurable response of the liver function (Table 2). Interestingly, blood glucose levels at slaughter were lower in bulls with abscesses. Glucose is used as a marker of hepatic function, as the ability of the liver to regulate blood glucose may be compromised during disease (Cebra and others 1997); thus, further investigation of blood glucose levels in the context of liver abscesses is warranted. We observed lower levels of ALP in cattle with abscessed livers (Table 2). Elevated ALP is an established indicator of cholestasis and hepatitis in humans (Reichling and Kaplan 1988, Myers and others 2003), and may also be elevated in the presence of severe liver disease in cattle (Garner 1952, Bogin and others 1988), which does not agree with our findings. However, its wide range in cattle suggests it may lack accuracy as a predictor (Garner 1952). These findings combined with our results suggest that further study of ALP is required to fully describe its usefulness in diagnosing liver disease in cattle. Blood levels of T4 were lower in abscessed cattle (Table 2). We propose this may be due to the suggested differences observed for FG and rate of weight gain, as both liver abscesses and blood T4 levels are associated with variation in the efficiency of feed utilisation in cattle (Christopherson and others 1979, Pethes and others 1985). Although discrepancies exist in the literature, decreased serum T4 has been observed in patients with liver disease (Green and others 1977), which suggests that liver abscesses might also be involved. We observed no difference in blood cortisol concentrations at slaughter, which may be due to variation induced by the stress of transport, handling and the unfamiliar environment, as mean cortisol levels at slaughter were approximately 33 per cent higher compared with those sampled on farm. This confirms findings from various authors (Andrade and others 2001, Montanholi and others 2013), who noted the variability of cortisol measurements obtained from single samplings in beef cattle subject to handling and restraint stress. Interestingly, our results suggest that concentration of biliary cortisol metabolites may be lower in abscessed bulls (Table 2). Faecal cortisol metabolites are used to study chronic stress in livestock, due to their independence from both hormonal changes and acute stress (Palme 2012). Our results suggest that insight into chronic stress or illness-mediated variation in cortisol levels may be garnered from the analysis of bile, which is an important path for excreting cortisol metabolites into the intestine and then via the faeces (Palme 2012). Finally, our results also suggest that bulls with liver abscesses may have higher levels of blood CO2 (Table 2). This result could be related to the acidotic state which predisposes cattle to liver abscesses (Gianesella and others 2010), but needs further investigation as other authors have found that blood CO2 concentration is decreased in intensively fed cows with ruminal acidosis (Marchesini and others 2013). Overall, it is important to reinforce that blood analysis using abattoir samples is quite distinct from samples collected on farm, as the stress of transportation combined with novel sights and smells can introduce variation in biological indicators (Mitchell and others 1988), which is also evident in the osmolality results obtained on farm and at the abattoir that suggested reduced values in liver abscessed bulls at the abattoir but no differences on farm.
Associations were suggested between liver abscesses and performance traits, including a 13 per cent greater FG, a 9 per cent lower ADG and no differences in feed intake when comparing bulls with and without abscesses. These findings support a lower productive performance in the bulls with liver abscess, given that productivity is affected by health status in the bovine (Brody 1945). Thus, our results are in agreement with other groups, who described negative correlations between severe liver abscesses and traits such as feed efficiency (FG) and ADG (Rust and others 1980, Brink and others 1990, Rezac and others 2014).
Blood indicators of hepatic function such as albumin and cholesterol may offer diagnostic clues both on farm and at slaughter, and could guide decisions on feed bunk management, method of feed processing and choice of additives (Galyean and Eng 1998, Marsh 2012). Future research should involve a larger population of intensely fed cattle from different categories at different physiological stages. Additionally, inducing liver abscess formation and correlating blood indicators with stage and severity of abscess progression would be a useful future direction to refine the findings of this study.
Liver abscesses are a consequence of feeding high-energy rations and have become a widespread problem regarding animal health and production. Several blood parameters related to hepatic function differed in beef bulls diagnosed with liver abscesses at slaughter. Analysis of blood collected over an extended sampling period revealed associations between liver abscesses and elevated levels of blood cortisol and AST, as well as with lower concentrations of blood albumin, cholesterol and testosterone. Results from blood collected at slaughter indicated that abscesses were associated with lower levels of T4, albumin, cholesterol, glucose and ALP, and may be associated with lower biliary cortisol metabolites and higher blood CO2. Of these analytes, albumin and cholesterol were associated with liver abscesses both over time and at slaughter, and their use as predictors warrants further research as they are established markers of liver health. Further defining these parameters and their associations with liver abscesses in intensely fed cattle may enable earlier identification, increase efficacy of livestock management and liver abscess prevention, and ensure food safety.
Footnotes
Funding: Funding for this project was provided by Employment and Social Development Canada (Government of Canada); Ontario Ministry of Agriculture, Food and Rural Affairs and Beef Farmers of Ontario and Agricultural Adaptation Council.
Competing interests: None declared.
Data sharing statement: Data to be shared as per contact and within the context of scientific collaboration.
References
- Andrade O., Orihuela A., Solano J., Galina C. S (2001) Some effects of repeated handling and the use of a mask on stress responses in zebu cattle during restraint. Applied Animal Behaviour Science 71, 175–181 doi:10.1016/S0168-1591(00)00177-5 [DOI] [PubMed] [Google Scholar]
- Beef Cattle Research Council. (2011) National beef quality audit 2010/11 beef carcass audit fact sheet. http://www.beefresearch.ca/files/pdf/fact-sheets/1181_CCA_NBQA_Factsheet_June_15_F.pdf Accessed June 3, 2015
- Bhagat C. I., Garcia-Webb P., Fletcher E., Beilby J. P (1984) Calculated vs measured plasma osmolalities revisited. Clinical Chemistry 30, 1703–1705 [PubMed] [Google Scholar]
- Bionaz M., Trevisi E., Calamari L., Librandi F., Ferrari A., Bertoni G (2007) Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. Journal of Dairy Science 90, 1740–1750 doi:10.3168/jds.2006-445 [DOI] [PubMed] [Google Scholar]
- Bogin E., Avidar Y., Merom M., Soback S., Brenner G (1988) Biochemical changes associated with the fatty liver syndrome in cows. Journal of Comparative Pathology 98, 337–347 doi:10.1016/0021-9975(88)90042-4 [DOI] [PubMed] [Google Scholar]
- Branum G. D., Tyson G. S., Branum M. A., Meyers W. C (1990) Hepatic abscess. changes in etiology, diagnosis, and management. Annals of Surgery 212, 655–662 doi:10.1097/00000658-199012000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U. (2009) Ultrasonography of the liver in cattle. Veterinary Clinics of North America: Food Animal Practice 25, 591–609 doi:10.1016/j.cvfa.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Brink D. R., Lowry S. R., Stock R. A., Parrott J. C (1990) Severity of liver abscesses and efficiency of feed utilization of feedlot cattle. Journal of Animal Science 68, 1201–1207 doi:10.2527/1990.6851201x [DOI] [PubMed] [Google Scholar]
- Brody S. (1945) Bioenergetics and growth with special reference to the efficiency complex in domestic animals. Hafner Press
- Brown T. R., Lawrence T. E (2010) Association of liver abnormalities with carcass grading performance and value. Journal of Animal Science 88, 4037–4043 doi:10.2527/jas.2010-3219 [DOI] [PubMed] [Google Scholar]
- Calkins H. E., Dewey M. L (1968) Quantitative analysis of the microflora of a bovine liver abscess. Journal of Bacteriology 96, 1439–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care. (2009) CCAC guidelines on: the care and use of farm animals in research, teaching and testing. http://www.ccac.ca/Documents/Standards/Guidelines/Farm_Animals.pdf Accessed July 1, 2015
- Canadian Food Inspection Agency. (2015) Ante and post-mortem procedures, dispositions, monitoring and controls—meat species, ostriches, rheas and emus. Section 17.6 Slaughter Operations. http://www.inspection.gc.ca/food/meat-and-poultry-products/manual-of-procedures/chapter-17/eng Accessed June 22, 2015
- Cebra C. K., Garry F. B., Getzy D. M., Fettman M. J (1997) Hepatic lipidosis in anorectic, lactating Holstein cattle: a retrospective study of serum biochemical abnormalities. Journal of Veterinary Internal Medicine 11, 231–237 doi:10.1111/j.1939-1676.1997.tb00096.x [DOI] [PubMed] [Google Scholar]
- Christopherson R. J., Gonyou H. W., Thompson J. R (1979) Effects of temperature and feed intake on plasma concentration of thyroid hormones in beef cattle. Canadian Journal of Animal Science 59, 655–661 doi:10.4141/cjas79-085 [Google Scholar]
- Dixon B. (1973) Is pain the price of farm efficiency? The New Scientist , 170–171 [Google Scholar]
- Doré E., Fecteau G., Hélie P., Francoz D (2007) Liver abscesses in Holstein dairy cattle: 18 cases (1992-2003). Journal of Veterinary Internal Medicine 21, 853–856 [PubMed] [Google Scholar]
- Fox J. T., Thomson D. U., Lindberg N. N., Barling K (2009) A comparison of two vaccines to reduce liver abscesses in natural-fed beef cattle. The Bovine Practitioner 43, 168–174 [Google Scholar]
- Fox S. I. (2014) Chemical Composition of the body In Human Physiology. 12th ed Lange M. McGraw-Hill, New York, USA: pp 24–49 [Google Scholar]
- Galyean M. L., Eng K. S (1998) Application of research findings and summary of research needs: Bud Britton memorial symposium on metabolic disorders of feedlot cattle. Journal of Animal Science 76, 323–327 doi:10.2527/1998.761323x [DOI] [PubMed] [Google Scholar]
- Garner R. J. (1952) Serum alkaline phosphatase in cattle in health and disease. Journal of Comparative Pathology and Therapeutics 62, 287–291 doi:10.1016/S0368-1742(52)80031-1 [DOI] [PubMed] [Google Scholar]
- Gianesella M., Morgante M., Cannizzo C., Stefani A., Dalvit P., Messina V., Giudice E (2010) Subacute ruminal acidosis and evaluation of blood gas analysis in dairy cow. Veterinary Medicine International 2010, 1–4 doi:10.4061/2010/392371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozho G. N., Krause D. O., Plaizier J. C (2006) Rumen lipopolysaccharide and inflammation during grain adaptation and subacute ruminal acidosis in steers. Journal of Dairy Science 89, 4404–4413 doi:10.3168/jds.S0022-0302(06)72487-0 [DOI] [PubMed] [Google Scholar]
- Green J. R., Snitcher E. J., Mowat N. A., Ekins R. P., Rees L. H., Dawson A. M (1977) Thyroid function and thyroid regulation in euthyroid men with chronic liver disease: evidence of multiple abnormalities. Clinical Endocrinology 7, 453–461 doi:10.1111/j.1365-2265.1977.tb01337.x [DOI] [PubMed] [Google Scholar]
- Herenda D., Chambers P. G., Ettriqui A., Seneviratna P., Da Silva, T.J.P (2000) General pathological conditions. Manual on meat inspection for developing countries. http://www.fao.org/docrep/003/t0756e/t0756e02.htm Accessed June 22, 2015
- Hernández J., Benedito J. L., Abuelo A., Castillo C (2014) Ruminal acidosis in feedlot: from aetiology to prevention. The Scientific World Journal , 1–8 doi:10.1155/2014/702572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Sun Y., Zhong Y., Feng W., Huang H., Wang W., Zhang T., Hu Y (2014) Low serum sex hormone-binding globulin is associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Clinical Endocrinology 80, 877–883 doi:10.1111/cen.12360 [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Johnson K. A., Baldwin R. L (1990) Changes in liver and gastrointestinal tract energy demands in response to physiological workload in ruminants. The Journal of Nutrition 120, 649–655 [DOI] [PubMed] [Google Scholar]
- Kent J. R., Scaramuzzi R. J., Lauwers W., Parlow A. F., Hill M., Penardi R., Hilliard J (1973) Plasma testosterone, estradiol, and gonadotrophins in hepatic insufficiency. Gastroenterology 64, 111–115 [PubMed] [Google Scholar]
- Kersting K. W., Thompson J. R., Connolly M. J. pp (2009) Acidosis and rumenitis In Current Veterinary Therapy: Food Animal Practice. Anderson D. E, Rings D. M. Saunders Elsevier: 25–26 [Google Scholar]
- Kumar A., Menon S., Nagaraja T. G., Narayanan S (2015) Identification of an outer membrane protein of Fusobacterium necrophorum subsp. necrophorum that binds with high affinity to bovine endothelial cells. Veterinary Microbiology 176, 196–201 doi:10.1016/j.vetmic.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Lechtenberg K. F., Nagaraja T. G (1991) Hepatic ultrasonography and blood changes in cattle with experimentally induced hepatic abscesses. American Journal of Veterinary Research 52, 803–809 [PubMed] [Google Scholar]
- Liberg P., Jönsson G (1993) Ultrasonography and determination of proteins and enzymes in blood for the diagnosis of liver abscesses in intensively fed beef cattle. Acta Veterinaria Scandinavica 34, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister E. E. (1971) Liver abscesses in intensively reared Holstein-Friesian steers. Canadian Journal of Animal Science 51, 241–242 doi:10.4141/cjas71-033 [Google Scholar]
- Marchesini G., De Nardi R., Gianesella M., Stefani A. L., Morgante M., Barberio A., Andrighetto I., Segato S (2013) Effect of induced ruminal acidosis on blood variables in heifers. BMC Veterinary Research 9, 98 doi:10.1186/1746-6148-9-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. P. (2012) Oats for intensively finished bulls. http://beefandlamb.ahdb.org.uk/wp/wp-content/uploads/2013/04/oats_for_intensive_finishing_cattle_final_report_oct_2012.pdf Accessed June 25, 2014
- McCann V. J., Fulton T. T (1975) Cortisol metabolism in chronic liver disease. The Journal of Clinical Endocrinology & Metabolism 40, 1038–1044 doi:10.1210/jcem-40-6-1038 [DOI] [PubMed] [Google Scholar]
- Mellau L. S. B., Nonga H. E., Karimuribo E. D (2010) A slaughterhouse survey of liver lesions in slaughtered cattle, sheep and goats at Arusha, Tanzania. Research Journal of Veterinary Sciences 3, 179–188 doi:10.3923/rjvs.2010.179.188 [DOI] [PubMed] [Google Scholar]
- Mitchell G., Hattingh J., Ganhao M (1988) Stress in cattle assessed after handling, after transport and after slaughter. Veterinary Record 123, 201–205 doi:10.1136/vr.123.8.201 [DOI] [PubMed] [Google Scholar]
- Mitzner S., Klammt S., Stange J., Schmidt R (2006) Albumin regeneration in liver support—comparison of different methods. Therapeutic Apheresis and Dialysis 10, 108–117 doi:10.1111/j.1744-9987.2006.00351.x [DOI] [PubMed] [Google Scholar]
- Montanholi Y. R., Swanson K. C., Schenkel F. S., McBride B. W., Caldwell T. R., Miller S. P (2009) On the determination of residual feed intake and associations of infrared thermography with efficiency and ultrasound traits in beef bulls. Livestock Science 125, 22–30 doi:10.1016/j.livsci.2009.02.022 [Google Scholar]
- Montanholi Y. R., Swanson K. C., Palme R., Schenkel F. S., McBride B. W., Lu D., Miller S. P (2010) Assessing feed efficiency in beef steers through feeding behavior, infrared thermography and glucocorticoids. Animal 4, 692–701 doi:10.1017/S1751731109991522 [DOI] [PubMed] [Google Scholar]
- Montanholi Y. R., Palme R., Haas L. S., Swanson K. C., Vander Voort G., Miller S. P (2013) On the relationships between glucocorticoids and feed efficiency in beef cattle. Livestock Science 155, 130–136 doi:10.1016/j.livsci.2013.04.002 [Google Scholar]
- Mudron P., Rehage J., Qualmann K., Sallmann H. P., Scholz H (1999) A study of lipid peroxidation and vitamin E in dairy cows with hepatic insufficiency. Journal of Veterinary Medicine Series A 46, 219–224 doi:10.1046/j.1439-0442.1999.00206.x [DOI] [PubMed] [Google Scholar]
- Myers R. P., Tainturier M. H., Ratziu V., Piton A., Thibault V., Imbert-Bismut F., Messous D., Charlotte F., Di Martino V., Benhamou Y., Poynard T (2003) Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. Journal of Hepatology 39, 222–230 doi:10.1016/S0168-8278(03)00171-5 [DOI] [PubMed] [Google Scholar]
- Müller M. J. (1998) Hepatic energy and substrate metabolism: a possible metabolic basis for early nutritional support in cirrhotic patients. Nutrition 14, 30–38 doi:10.1016/S0899-9007(97)00390-0 [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., Laudert S. B., Parrott J. C (1996) Liver abscesses in feedlot cattle. Part 1. Causes, pathogenesis, pathology, and diagnosis.. Compendium on Continuing Education for the Practicing Veterinarian , 230–241 [Google Scholar]
- Nagaraja T. G., Narayanan S. K., Stewart G. C., Chengappa M. M (2005) Fusobacterium necrophorum infections in animals: pathogenesis and pathogenic mechanisms. Anaerobe 11, 239–246 doi:10.1016/j.anaerobe.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., Lechtenberg K. F (2007) Acidosis in feedlot cattle. Veterinary Clinics of North America: Food Animal Practice 23, 333–350 doi:10.1016/j.cvfa.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Nahed S. T. (2010) Investigation of serum insulin and cortisol concentrations in foot and mouth disease-infected cattle in relation to changes in serum biochemical variables and protein electrophoretic fractionation profile. Global Veterinaria 4, 450–455 [Google Scholar]
- Narayanan S., Nagaraja T. G., Okwumabua O., Staats J., Chengappa M. M., Oberst R. D (1997) Ribotyping to compare Fusobacterium necrophorum isolates from bovine liver abscesses, ruminal walls, and ruminal contents. Applied and Environmental Microbiology 63, 4671–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme R., Mostl E (1997) Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. International Journal of Mammalian Biology 62, 192–197 [Google Scholar]
- Palme R. (2012) Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Animal Welfare 21, 331–337 doi:10.7120/09627286.21.3.331 [Google Scholar]
- Peters T. (1995) All about albumin: biochemistry, genetics, and medical applications. Elsevier Inc.
- Peterson R. E. (1960) Adrenocortical steroid metabolism and adrenal cortical function in liver disease. Journal of Clinical Investigation 39, 320–331 doi:10.1172/JCI104043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethes G., Bokori J., Rudas P., Frenyó V. L., Fekete S (1985) Thyroxine, triiodothyronine, reverse-triiodothyronine, and other physiological characteristics of periparturient cows fed restricted energy. Journal of Dairy Science 68, 1148–1154 doi:10.3168/jds.S0022-0302(85)80941-3 [DOI] [PubMed] [Google Scholar]
- Plaizier J. C., Krause D. O., Gozho G. N., McBride B. W (2008) Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. The Veterinary Journal 176, 21–31 doi:10.1016/j.tvjl.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Radostits O. M. (2000) Diseases of the liver and pancreas In Veterinary Medicine: a textbook of diseases of cattle, sheep, pigs, goats and horses, 9th ed Eds Radostits O. M, Gay C. C, Blood D. C, Hinchcliff K. W. Saunders Elsevier: pp 347–359 [Google Scholar]
- Rawlings N. C., Evans A. C (1995) Androgen negative feedback during the early rise in LH secretion in bull calves. Journal of Endocrinology 145, 243–249 doi:10.1677/joe.0.1450243 [DOI] [PubMed] [Google Scholar]
- Reichling J. J., Kaplan M. M (1988) Clinical use of serum enzymes in liver disease. Digestive Diseases and Sciences 33, 1601–1614 doi:10.1007/BF01535953 [DOI] [PubMed] [Google Scholar]
- Rezac D. J., Thomson D. U., Bartle S. J., Osterstock J. B., Prouty F. L., Reinhardt C. D (2014) Prevalence, severity, and relationships of lung lesions, liver abnormalities, and rumen health scores measured at slaughter in beef cattle. Journal of Animal Science 92, 2595–2602 doi:10.2527/jas.2013-7222 [DOI] [PubMed] [Google Scholar]
- Russell J. B. (1999) Excessive grain feeding: acid-resistant bacteria and their impact on cattle. RAAN Conference Proceedings 15, 73 [Google Scholar]
- Rust S. R., Owens F. N., Gill D. R (1980) Liver abscesses and feedlot performance. Oklahoma Agricultural Experiment Station: Animal Science Research Report , 148–150 [Google Scholar]
- Shin J. Y., Kim S. K., Lee M. Y., Kim H. S., Ye B. I., Shin Y. G., Baik S. K., Chung C. H (2011) Serum sex hormone-binding globulin levels are independently associated with nonalcoholic fatty liver disease in people with type 2 diabetes. Diabetes Research and Clinical Practice 94, 156–162 doi:10.1016/j.diabres.2011.07.029 [DOI] [PubMed] [Google Scholar]
- Tadepalli S., Narayanan S. K., Stewart G. C., Chengappa M. M., Nagaraja T. G (2009) Fusobacterium necrophorum: a ruminal bacterium that invades liver to cause abscesses in cattle. Anaerobe 15, 36–43 doi:10.1016/j.anaerobe.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Trevisi E., Bertoni G (2009) Some physiological and biochemical methods for acute and chronic stress evaluation in dairy cows. Italian Journal of Animal Science 8, 265–286 doi:10.4081/ijas.2009.s1.265 [Google Scholar]
- Wagner K. A., Hartmann F. A., Trepanier L. A (2007) Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998-2003. Journal of Veterinary Internal Medicine 21, 417–424 [DOI] [PubMed] [Google Scholar]


