Abstract
Monocyte chemoattractant protein-1 (MCP-1) overproduction from inflamed adipose tissue is a major contributor to obesity-related metabolic syndromes. 3T3-L1 embryonic fibroblasts were cultured and differentiated into adipocytes using an established protocol. Adipocytes were treated with lipopolysaccharide (LPS) to induce inflammation and thus MCP-1 release. At the same time, varying concentrations of chondroitin sulfate (CS) were added in a physiologically relevant range (10-200 µg/mL) to determine its impact on MCP-1 release. Chondroitin sulfate, a natural glycosaminoglycan of connective tissue including the cartilage extracellular matrix, was chosen on the basis of our previous studies demonstrating its anti-inflammatory effect on macrophages. Because the main action of MCP-1 is to induce monocyte migration, cultured THP-1 monocytes were used to test whether CS at the highest physiologically relevant concentration could inhibit cell migration induced by human recombinant MCP-1. Chondroitin sulfate (100-200 µg/mL) inhibited MCP-1 release from inflamed adipocytes in a dose-dependent manner (P < .01, 95% confidence interval [CI]: −5.89 to −3.858 at 100 µg/mL and P < .001, 95% CI: −6.028 to −3.996 at 200 µg/mL) but had no effect on MCP-1–driven chemotaxis of THP-1 monocytes. In summary, CS could be expected to reduce macrophage infiltration into adipose tissue by reduction in adipocyte expression and release of MCP-1 and as such might reduce adipose tissue inflammation in response to pro-inflammatory stimuli such as LPS, now increasingly recognized to be relevant in vivo.
Keywords: Chondroitin sulfate, adipocytes, MCP-1, chemokines, LPS
Introduction
Monocyte chemoattractant protein-1 (MCP-1) is one of the key factors in the initiation of inflammation by inducing the migration of monocytes to inflammatory lesions via its chemotactic effect.1 Once attracted to the lesion, monocytes differentiate into activated macrophages, which are the source of many inflammatory cytokines. Monocyte chemoattractant protein-1 is well known to play a critical role in the pathogenesis of type 2 diabetes and metabolic syndrome in association with abdominal obesity through the recruitment of monocytes by the increased expression of MCP-1 from adipocytes.2 Cullberg recently demonstrated that lipopolysaccharide (LPS) is a potent stimulator of MCP-1 expression in the 3T3-L1 adipocyte cell line.3 One of the key stimuli for the upregulation of MCP-1 is the binding of activated nuclear factor κB (NF-κB) to the MCP-1 gene promoter in multiple cell types including THP-1 macrophages.4,5 Monocyte chemoattractant protein-1 may also play a role in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis by its action on macrophages.6–8 Chondroitin sulfate (CS), a natural glycosaminoglycan of the cartilage extracellular matrix, has clinical benefit in symptomatic osteoarthritis but has never been tested in adipose tissue. Chondroitin sulfate exerts an anti-inflammatory activity and has positive effects on osteoarthritic chondrocytes, synoviocytes, and subchondral bone osteoblasts.9 In addition, we have recently published that CS can attenuate the monosodium urate crystal–mediated THP-1 macrophage inflammatory response reflected by reduced release of pro-inflammatory cytokines interleukin 1β and tumor necrosis factor α (TNF-α).10 We have also recently determined that the CS inhibitory effect is not acting at the inflammasome, but upstream, most likely by inhibiting activation of NF-κB,11 as it has been described for other cell types.12 We sought to determine whether CS had an inhibitory effect on MCP-1 release from LPS-stimulated adipocytes and/or blocked MCP-1–induced chemotaxis of inflammatory cells.
Methods
3T3-L1 adipocyte experiments
3T3-L1 mouse embryonic fibroblasts were obtained from ATCC (Manassas, VA, USA) and were grown to confluence in 5% CO2 on COSTAR 24-well plates (Corning, Kennebunk, ME, USA) with a basal media of Dulbecco’s Modified Eagle’s medium-high glucose containing 10% fetal bovine serum and 1% penicillin/streptomycin (media and supplements from Thermo Fisher, Waltham, MA, USA). Cells were induced to differentiate into adipocytes by the method of Zebisch et al.13 Briefly, at 2 days after confluence (day 1), the following were added to the basal media: 0.5 mM 3-isobutyl-1-methylxanthine, 1 µg/mL insulin, 0.25 µM dexamethasone (Sigma-Aldrich, St Louis, MO, USA), and 2 µM rosiglitazone (Cayman Chemical, Ann Arbor, MI, USA). After 48 hours (day 3), the media were changed to fresh basal media containing 1 µg/mL insulin. After a further 48 hours (day 5), media were changed back to fresh basal media and cells grown for 7 days under these conditions with new media every 2 days. On day 12, cells were washed with phosphate-buffered saline (PBS) and media were changed to serum-free Opti-MEM (Thermo Fisher) without and with varying physiologically relevant12,14 concentrations (10-200 µg/mL) of high-quality and purity, pharmaceutical-grade CS from bovine origin (provided by Bioibérica). After 24 hours (day 13), media were replaced with serum-free Opti-MEM along with the previously mentioned concentrations of CS, without and with 1 µg/mL LPS (Enzo Life Sciences, Farmingdale, NY, USA). After a further 24 hours (day 14), cell viability was measured for each well using PrestoBlue Cell Viability Reagent (Thermo Fisher) and media were tested for MCP-1 by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA). Chondroitin sulfate at the highest concentration used for the experiments (200 µg/mL) was tested against the kit standard to check for assay interference. All experiments were run a single time with each treatment group run in triplicate.
THP-1 monocyte experiments
The THP-1 human monocytic cell line was obtained from ATCC and was grown at 5% CO2 in RPMI 1640 with HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and supplemented with glucose, pyruvate, 2-mercaptoethanol, 10% fetal bovine serum, and penicillin/streptomycin as recommended by ATCC (media and supplements from Thermo Fisher). Cells were washed in PBS and the media changed to serum-free Opti-MEM supplemented with 0.5% bovine serum albumin (Sigma-Aldrich). After 24 hours, cells were resuspended at a density of 1.25 × 106 cells/mL in the same media, with and without 200 µg/mL CS. Monocyte chemotaxis in response to 24-hour exposure to varying concentrations (0, 3.125-100 ng/mL) of recombinant human MCP-1 (R&D Systems) was tested using the CytoSelect 96-well Cell Migration Assay (Cell Biolabs, San Diego, CA, USA). All experiments were run a single time with each treatment group run in triplicate.
Statistical analysis
After normalization for cell viability, cell culture results were expressed as fold change from the media-only negative control (no CS, no LPS). One-way analysis of variance with Bonferroni post hoc test and post hoc linear trend was performed on cell culture results. Unpaired t test was used to assess the effect of LPS on cell viability, and paired t test was used to determine whether CS interfered the MCP-1 ELISA. GraphPad Prism software (La Jolla, CA, USA) was used for all analyses.
Results
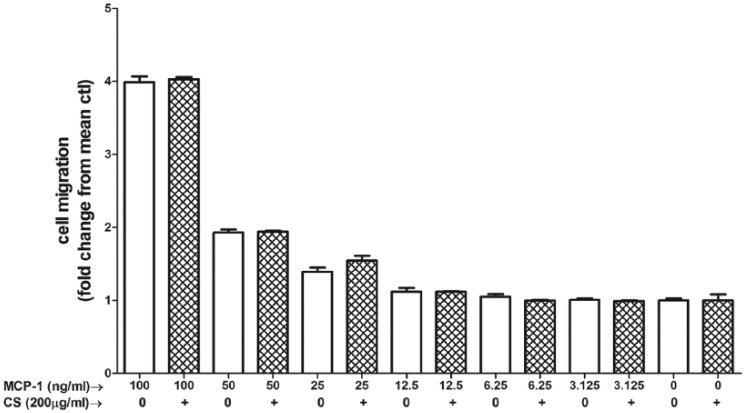
Lipopolysaccharide (1 µg/mL) caused a significant rise in MCP-1 release (P < .0001, 95% confidence interval [CI]: 3.911-5.069) from 3T3-L1 adipocytes. Chondroitin sulfate in physiologically achievable concentrations (100-200 µg/mL) produced a dose-dependent reduction (P < .01, 95% CI: −5.89 to −3.858 at 100 µg/mL and P < .001, 95% CI: −6.028 to −3.996 at 200 µg/mL) of MCP-1 release from 3T3-L1 adipocytes in response to LPS (Figure 1). Cell viability between control and LPS-only groups was unaffected (P = .30, 95% CI: −2333 to 5843) by this dose of LPS (Figure 2). Chondroitin sulfate showed no interference with the MCP-1 ELISA (P = .78, 95% CI: −5.127 to 4.030), producing a standard curve spiked with 200 µg/mL of CS that was identical to an unspiked standard curve (Figure 3). Recombinant MCP-1 (25-100 ng/mL) caused a dose-dependent increase (P < .001, 95% CI: 0.1896-0.5949 at 25 ng/mL and P < .0001, 95% CI: 2.786-3.192 at 100 ng/mL) in cell migration of THP-1 monocytes. Chondroitin sulfate at the highest test concentration (200 µg/mL) had no effect on MCP-1–mediated THP-1 migration (Figure 4).
Figure 1.
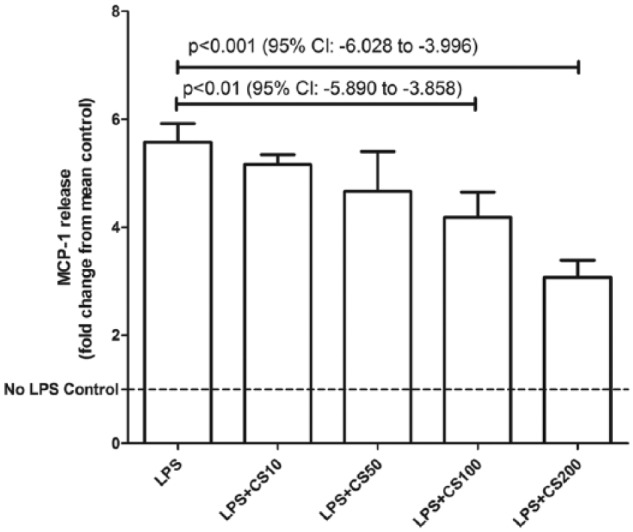
Chondroitin sulfate (CS) reduces MCP-1 release from adipocytes stimulated by LPS. 3T3-L1 cells were differentiated into adipocytes and then stimulated with µg/mL of LPS. CS in physiologically relevant doses (100-200 µg/mL) produced a dose-dependent reduction in MCP-1 release from these cells. Mean MCP-1 (unadjusted for cell viability) was 8.4 ng/mL for control and 39.0 ng/mL for cells treated only with LPS. Bars represent mean + SD. n = 3 for each treatment group. LPS indicates lipopolysaccharides; MCP-1, monocyte chemoattractant protein-1.
Figure 2.
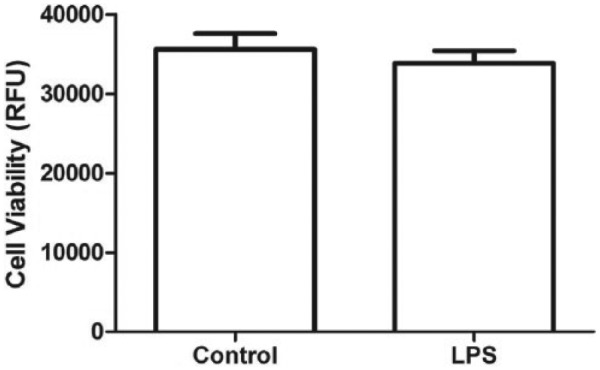
Viability of 3T3-L1 adipocytes was unaffected (P = .30, 95% CI: −2333 to 5843) by the dose of LPS (1 µg/mL) used for experiments. LPS indicates lipopolysaccharides; CI, confidence interval.
Figure 3.
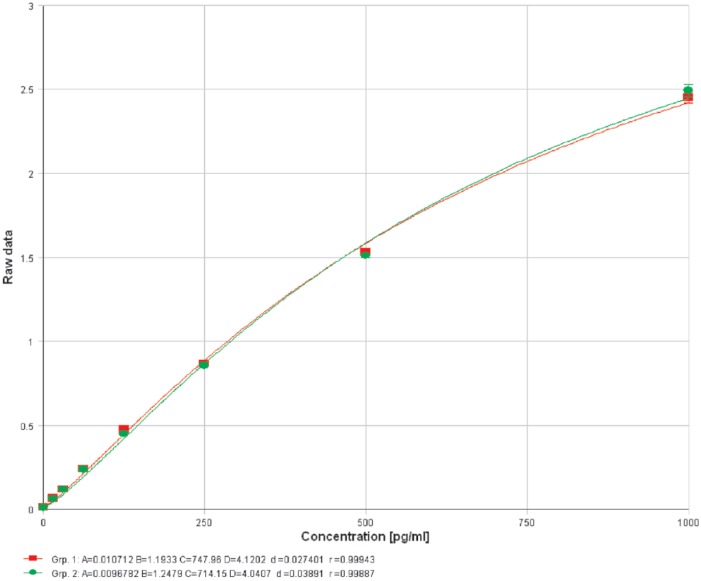
Chondroitin sulfate (CS) showed no interference with the monocyte chemoattractant protein-1 enzyme-linked immunosorbent assay (P = .78, 95% CI: −5.127 to 4.030) producing a standard curve spiked with 200 µg/mL of CS that was identical to an unspiked standard curve. Group 1 (red): kit standard. Group 2 (green): kit standard + 200 µg/mL CS.
Figure 4.
Chondroitin sulfate (CS) has no effect on MCP-1–induced chemotaxis. MCP-1–induced (3.125-100 ng/mL) cell migration of THP-1 monocytes in both the presence and absence of CS. Hatch-marked bars = 200 µg/mL CS. Bars represent mean + SD. n = 3 for each treatment group. MCP-1 indicates monocyte chemoattractant protein-1.
Discussion
It is well established that the binding of activated NF-κB to the MCP-1 gene promoter is a key step in the upregulation of MCP-1 gene expression.4,5 The activation of NF-κB can occur through many receptors including the interleukin-1 receptor, the TNF receptor, and toll-like receptors, such as TLR4, which LPS activate. Although it is still unclear exactly where CS is acting to inhibit NF-κB, whether on its activation11 or on the nuclear translocation of the activated form,12 the inhibition by CS at either point would be expected to reduce the production of MCP-1. The effects of CS on gene expression, at the RNA level and the protein level, have been extensively studied in cells and tissues associated with osteoarthritis, including chondrocytes, synovial fibroblasts, and synovial membrane.15–17 Although CS has been found effective in reducing the production of various inflammatory proteins in these studies, no study has looked at the effect of CS in adipocytes.
Conclusions
Our data demonstrate that CS inhibits the release of MCP-1 from 3T3-L1 adipocytes that have been stimulated with LPS but has no effect on the chemotactic action of MCP-1 on THP-1 monocytes. It has recently been demonstrated that CS can inhibit the release of MCP-1 from human coronary artery endothelial cells inflamed by TNF-α; in this model system, CS also inhibited the migration of THP-1 monocytes toward the inflamed HCAECs.18 Our data strongly suggest that it is the inhibition of MCP-1 release by CS that underlies this effect and not a direct inhibition of the chemotactic action of MCP-1 by CS. Although it is unclear whether CS might accumulate sufficiently in adipose tissue in vivo to have an effect,19 given the in vivo relevance of LPS20 and importance of MCP-1 overproduction in obesity-related metabolic syndromes, inhibiting the release of MCP-1 from adipocytes by CS, and thus blocking the recruitment of macrophages to adipose tissue, could provide a new treatment opportunity for these syndromes. Because CS is so well tolerated and readily accessible, it might provide a safe and clinically effective treatment for reducing inflammation in obesity-related syndromes. Although the more relevant end product of transcription, ie, the translated protein, was studied here, gene expression studies might be a useful future avenue of research. This in vitro work establishes the rationale for evaluation of MCP-1 modulation in future in vivo studies of CS.
Footnotes
Peer review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 447 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: T.V.S. and V.B.K. were supported by an investigator-initiated grant from Bioibérica (Barcelona, Spain).
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.M. and J.V. are employees of Bioibérica. Bioibérica played no role in the decision to submit the manuscript for publication. No nonfinancial conflicts exist for any of the authors.
Author Contributions: TVS conducted most experiments, participated in the design of the study, performed the statistical analysis, and drafted the manuscript. EM participated in the design of the study and helped to draft the manuscript. JV participated in the study design and coordination and manuscript revision. JLH performed some experiments and participated in the manuscript revision. VBK conceived of the study, participated in its design and coordination, and helped to draft the manuscript. EM and JV provided input into the design and manuscript preparation. All authors read and approved the final version of the manuscript.
References
- 1. Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41:998–1001. [DOI] [PubMed] [Google Scholar]
- 2. Panee J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cullberg KB, Larsen JO, Pedersen SB, Richelsen B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages in vitro. Nutr Diabetes. 2014;4:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ueda A, Okuda K, Ohno S, et al. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 5. Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. [DOI] [PubMed] [Google Scholar]
- 6. Alwahsh SM, Gebhardt R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch Toxicol. 2017;91:1545–1563. [DOI] [PubMed] [Google Scholar]
- 7. Huber Y, Gehrke N, Biedenbach J, et al. Voluntary distance running prevents TNF-mediated liver injury in mice through alterations of the intrahepatic immune milieu. Cell Death Dis. 2017;8:e2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alwahsh SM, Xu M, Seyhan HA, et al. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J Gastroenterol. 2014;20:1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. du Souich P. Absorption, distribution and mechanism of action of SYSADOAS. Pharmacol Ther. 2014;142:362–374. [DOI] [PubMed] [Google Scholar]
- 10. Orlowsky EW, Stabler TV, Montell E, Verges J, Kraus VB. Monosodium urate crystal induced macrophage inflammation is attenuated by chondroitin sulphate: pre-clinical model for gout prophylaxis? BMC Musculoskelet Disord. 2014;15:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stabler TV, Huang Z, Montell E, Verges J, Kraus VB. Chondroitin sulphate inhibits NF-κB activity induced by interaction of pathogenic and damage associated molecules. Osteoarthritis Cartilage. 2017;25:166–174. [DOI] [PubMed] [Google Scholar]
- 12. Jomphe C, Gabriac M, Hale TM, et al. Chondroitin sulfate inhibits the nuclear translocation of nuclear factor-kappaB in interleukin-1beta-stimulated chondrocytes. Basic Clin Pharmacol Toxicol. 2008;102:59–65. [DOI] [PubMed] [Google Scholar]
- 13. Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem. 2012;425:88–90. [DOI] [PubMed] [Google Scholar]
- 14. Homandberg GA, Guo D, Ray LM, Ding L. Mixtures of glucosamine and chondroitin sulfate reverse fibronectin fragment mediated damage to cartilage more effectively than either agent alone. Osteoarthritis Cartilage. 2006;14:793–806. [DOI] [PubMed] [Google Scholar]
- 15. Lambert C, Mathy-Hartert M, Dubuc JE, et al. Characterization of synovial angiogenesis in osteoarthritis patients and its modulation by chondroitin sulfate. Arthritis Res Ther. 2012;14:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calamia V, Lourido L, Fernandez-Puente P, et al. Secretome analysis of chondroitin sulfate-treated chondrocytes reveals anti-angiogenic, anti-inflammatory and anti-catabolic properties. Arthritis Res Ther. 2012;14:R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calamia V, Ruiz-Romero C, Rocha B, et al. Pharmacoproteomic study of the effects of chondroitin and glucosamine sulfate on human articular chondrocytes. Arthritis Res Ther. 2010;12:R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melgar-Lesmes P, Garcia-Polite F, Del-Rey-Puech P, et al. Treatment with chondroitin sulfate to modulate inflammation and atherogenesis in obesity. Atherosclerosis. 2016;245:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ronca F, Palmieri L, Panicucci P, Ronca G. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 1998;6:14–21. [DOI] [PubMed] [Google Scholar]
- 20. Huang Z, Kraus VB. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016;12:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]