Abstract
The tissue-engineered heart valve portends a new era in the field of valve replacement. Decellularized heart valves are of great interest as a scaffold for the tissue-engineered heart valve due to their naturally bioactive composition, clinical relevance as a stand-alone implant, and partial recellularization in vivo. However, a significant challenge remains in realizing the tissue-engineered heart valve: assuring consistent recellularization of the entire valve leaflets by phenotypically appropriate cells. Many creative strategies have pursued complete biological valve recellularization; however, identifying the optimal recellularization method, including in situ or in vitro recellularization and chemical and/or mechanical conditioning, has proven difficult. Furthermore, while many studies have focused on individual parameters for increasing valve interstitial recellularization, a general understanding of the interacting dynamics is likely necessary to achieve success. Therefore, the purpose of this review is to explore and compare the various processing strategies used for the decellularization and subsequent recellularization of tissue-engineered heart valves.
Keywords: Tissue-engineered heart valve, recellularization, decellularization
Introduction
Valvular heart disease continues to be a global cause of morbidity and mortality worldwide.1 Approximately 2.5% of the US population suffers from aortic heart valve disease, and that value skyrockets to 13.2% for the population ≥75 years old.2,3 There are many etiologies for heart valve disease, including congenital defects, rheumatic fever, infective endocarditis, and valve calcification, yet after non-surgical options are exhausted, the multiple options for repair or replacement each have limitations. The annual number of patients requiring heart valve surgery is estimated at 290,000 globally, and as the world population continues to grow and age, that number is expected to triple in the next five decades to more than 850,000.4 Despite decades of pursuing the ideal heart valve prosthetic, such a valve still does not exist. Mechanical valves have poor hemocompatibility, requiring a lifetime of anticoagulation therapy. Xenogeneic bioprosthetic valves and biological valves such as homografts lack durability and often require replacement in ~10 years due to calcification and/or degeneration.5–7 Children are even more restricted in the suitable options for heart valve replacement, and although pediatric patients represent only a subset of the total heart valve replacement population, the clinical need for an ideal pediatric valve is far greater.8 The complications inherent to each class of valve substitute currently available for clinical use are amplified in children, which can necessitate reoperation in as little as 2–5 years. The current gold-standard valve replacement for children is the cryopreserved allograft, yet this is not without problems due to the shortage of available grafts and patient-valve size mismatch. Most importantly, no current replacement option allows for somatic growth after implantation, necessitating multiple reoperations in children and impacting surgical strategies such as timing, sequence of surgeries, and serial valve selections.8 Therefore, there is a significant unmet clinical need for a truly successful heart valve replacement option, especially for pediatric patients.
Tissue engineering is a promising approach that may lead to novel constructs that will satisfy this unmet need and overcome the limitations of current valve prosthetics. The tissue-engineered heart valve (TEHV) will be constructed using a combination of a porous scaffold, a cell population, and signaling factors and has the potential to provide (1) excellent hemodynamics, eliminating the need for anticoagulation therapy; (2) active tissue remodeling, preventing degeneration; and (3) growth characteristics, preventing the need for reoperation.
The two primary types of valve scaffolds for the TEHV are natural scaffolds, such as decellularized tissue or biological materials, and synthetic constructs fabricated from degradable polymers.9 Each type has inherent benefits and challenges, but decellularized heart valves are of significant interest. Decellularized heart valves are composed of biological materials that can positively impact cell differentiation and serve as building blocks during the remodeling process.10,11 Additionally, decellularized valves do not necessitate complete biodegradation and often maintain the mechanical anisotropy of the native valves from which they are derived.12–16 Decellularized heart valves have been more clinically relevant than polymeric valves thus far, as they have been implanted as stand-alone valve substitutes and as TEHVs in animals and humans, albeit with mixed results.17–24 However, decellularized heart valves are not without their limitations. Decellularized valves require human or animal tissue for manufacture, which is limited in supply, and necessitates cryopreservation for storage. Freeze-drying of biologic heart valves has been explored to facilitate long-term storage; however, freeze-drying leads to collapse of the extracellular matrix (ECM) structure and disruption of biomolecules. Research with lycoprotectants may overcome this limitation in the future.25 Additionally, the success of decellularized heart valves is highly reliant upon the decellularization process and the potential immune response following implantation.
On the other hand, man-made scaffolds, fabricated from synthetic or biological materials, do not require donor tissue but have struggled to recreate the macro- and micro valve anatomy and mechanical anisotropy of the native valve.16 Fabricated scaffolds must also undergo complete biodegradation in synchrony with the production of ECM to remain functional. Fabricated or synthetic scaffolds have been used as a TEHV in animals, but have seen far less use clinically than decellularized valves.26,27 Therefore, decellularized valve scaffolds have the greatest potential for expeditious development of a TEHV due to the regulatory history, long clinical experience with homografts, as well as a deep research focus by many groups.8,22,28
Before the TEHV is realized, a primary challenge must be overcome: the establishment and growth of a physiologically appropriate cell population within the leaflet tissue. In pursuit of this aim, researchers have employed numerous strategies for recellularization. From these strategies, the two main approaches that have emerged are in vitro recellularization, the traditional tissue engineering approach, and in situ recellularization, also known as guided tissue regeneration.9,29 However, despite the many strategies that have been employed, it is uncertain which has the greatest potential for success, and reliable recellularization of the TEHV has yet to be realized. Therefore, the purpose of this review is to explore and compare the various processing strategies used for the decellularization and subsequent recellularization for TEHVs.
Heart valve decellularization
In the simplest terms, decellularization is the process of removing cellular (including nuclear) material from the ECM of biological tissues. The remaining ECM provides a semi-porous scaffold that retains the complex geometry of the native tissue and is composed of natural components that provide cues for cell migration and differentiation, resulting in constructive remodeling.10,30,31 Despite the clinical use of decellularized tissue for more than a decade, it is only within the last 5 years that quantifiable minimum criteria defining adequate decellularization have been generally accepted in the field.30 These include (1) <50 ng double-stranded DNA (dsDNA) per milligram ECM dry weight, (2) <200 base pair DNA fragment length, and (3) the lack of visible nuclear material in tissue sections stained with 4′,6-diamidino-2-phenylindole (DAPI) or hematoxylin and eosin (H&E).30 These criteria lay out basic metrics to be met as endpoints for decellularized tissues, since ineffective decellularization can affect the immunological response through macrophage polarization and inhibit the constructive remodeling outcome.32–34 While decellularization does preserve the geometry of the native tissue, it has been shown to affect the structure and protein composition, often in a negative manner, depending on the decellularization methods used.30 Additionally, methods of decellularization differ in their effectiveness to remove antigens from the ECM scaffold, such as cellular and nuclear material, as well as lipids and carbohydrates which can also function as antigens.35 Methods of decellularization include the use of detergents, biologic agents, and physical forces. Extensive details on methods of decellularization and the effects on general tissue can be found in the reviews by Crapo et al.30 and Gilbert.31
Methods for heart valve decellularization
In the context of heart valve decellularization, additional consideration must be given to the effects of processing on the mechanics of the heart valve leaflets. Healthy heart valves, particularly the aortic and mitral valves, which control systemic blood flow, are under extreme environmental demands. They experience ~100,000 loading cycles per day, equating to ~3 billion cycles in an average lifetime.9 These physiological forces must be considered during heart valve decellularization since the process can affect the structural and mechanical properties of the remaining ECM. Furthermore, the effect decellularization has on the ECM is process-specific (Table 1, Figure 1).
Table 1.
Various methods for the decellularization of heart valves.
| Decellularization method | Treatments/chemicals | General effectiveness | General effect on valve ECM | Ref. |
|---|---|---|---|---|
| Anionic detergent | SDS or sodium deoxycholate | Lack of visible cell nuclei; ~95% DNA removal | Increased areal strain and peak stretch ratio; decreased flexural stiffness; preservation of GAGs; can disrupt ECM fiber structure | 14,36–42 |
| Non-ionic detergent | Triton X-100 | Lack of visible cell nuclei | Increased areal strain and peak stretch ratio; decreased flexural stiffness; loose ECM network; histological reduction of GAG, laminin, fibronectin, and collagen | 14,39,43–45 |
| Multi-detergent | Triton X-100 + sodium cholate | ~30% DNA removal | Increased extensibility and decreased stiffness; GAG reduction; preservation of elastin and collagen components | 46,47 |
| Triton X-100 + sodium deoxycholate | Lack of visible cell nuclei; 98% DNA removal | Histologic preservation of structure and ECM components | 39,48 | |
| Osmotic shock + Triton X-100 + NLS salt + ethanol | Lack of visible cell nuclei; >97% dsDNA removal | Increased areal strain and peak stretch ratio; decreased stress relaxation; reduced GAG content | 12,49 | |
| Enzymatic | Trypsin + EDTA | Incomplete cell removal | Decreased mechanical properties; histologic tissue damage and loss of basement membrane; histologic reduction of GAG, laminin, fibronectin, and collagen | 14,40,41,43–45,50 |
| Enzymatic combinations | Trypsin + SDS | Lack of visible cell nuclei; 96% DNA removal | Reduction of GAG and α-Gal antigen; preservation of mechanical properties | 51 |
| Trypsin + sodium deoxycholate | Visible cell remnants; 98% DNA removal | Histologic disruption of ECM components | 48 | |
| Trypsin + osmotic shock + Triton X-100 | Lack of visible cells | Misalignment of collagen fibers | 41,52 | |
| Trypsin + osmotic shock | Visible cell remnants | Histologic loss of collagen; GAG reduction; decreased mechanical strength | 45 | |
| Glycol radiation | PEG + gamma irradiation | Lack of visible cell nuclei; >92% cusp DNA removal | Preserved leaflet ultrastructure; removal of α-Gal antigen | 53 |
| Osmotic shock | Hypotonic/hypertonic Tris buffer | Many visible cell remnants | Histologic reduction in MHC antigens; loss of non-collagen proteins | 44,45 |
| Sequential antigen removal | Dithiothreitol, potassium chloride, amidosulfobetaine-14 | Lack of visible cell remnants and reduced antigenicity | Preservation of Young’s modulus and ultimate tensile strength; preservation of collagen and elastin; decreased GAGs | 54–56 |
| Supercritical fluid | CO2; ethanol | Lack of visible cell nuclei; 90% phospholipid removal | Stiffening of tissue; tissue dehydration | 57 |
ECM: extracellular matrix; SDS: sodium dodecyl sulfate; GAG: glycosaminoglycan; NLS: N-lauroylsarcosine sodium salt; dsDNA: double-stranded DNA; EDTA: ethylenediaminetetraacetic acid; α-Gal: galactose-α(1,2)-galactose; PEG: polyethylene glycol; MHC: major histocompatibility complex.
The general effectiveness and general effect on ECM are overall observations and individual results may vary based on the protocol or tissue used.
Figure 1.
(a–d) H&E and (e–h) Movat’s pentachrome staining highlighting the effects of decellularization by SDS (b, f), trypsin (c, g), and Triton X-100 (d, h) compared to native tissue (a, e). All three methods show effective removal of cellular and nuclear material. SDS slides show preservation of leaflet structure and ECM components. Trypsin slides show a “loosening” of the ECM network and loss of structural proteins. Triton X-100 slides show good preservation of leaflet structure but loss of GAGs from the ECM.
Source: Figure reprinted from Liao et al.14 with permission. Copyright 2008 Elsevier: Biomaterials.
Detergent valve decellularization
Of the various decellularization methods, the most common utilize detergents for the removal of cells. Ionic and non-ionic detergents are effective decellularization agents because they are able to solubilize cell membranes, lysing the cells and dissociating DNA. The anionic detergents sodium dodecyl sulfate (SDS) and sodium deoxycholate (SD) are often used in valve decellularization and have proven effective in removing cells and DNA from the ECM; however, prolonged chemical exposure during decellularization has been shown to disrupt collagen fiber structure in valve leaflets.36 The disruption in the ECM leads to an associated deterioration of mechanical properties, observed through increased areal strain and decreased flexural stiffness.14 This is despite preservation of the primary structural proteins found in valves: glycosaminoglycans (GAGs), collagen, and elastin fibers (Figure 1(f)). SDS is also particularly difficult to completely remove from the tissue and residual detergents can adversely affect cell adhesion and repopulation.42 Non-ionic detergents, such as Triton X-100, have also proven effective at cell and DNA removal. In addition to cell removal, Triton X-100 has been shown to delipidize tissue, reducing the concentration of GAGs (Figure 1(h)). GAGs are often attributed to the viscoelastic behavior of tissue and indeed, following decellularization with Triton X-100, a decrease in the stress relaxation of valve leaflet tissue has been observed.12,15 Researchers have also successfully employed multi-detergent decellularization strategies. Studies using a combination of Triton X-100 and SD reported complete cell removal with less damage to the valve ECM, when compared to other methods.39,48 However, neither study employed mechanical testing analysis of the decellularized leaflets and only described the ECM preservation using qualitative histologic methods. A combination of osmotic shock, Triton X-100, and the anionic detergent N-lauroylsarcosine sodium (NLS) salt has been used to successfully decellularize (>97% dsDNA removal) both pulmonary and aortic heart valves from a variety of species, including ovine, porcine, and human.12,15,23 The elastin and collagen components of the ECM were preserved, yet a loss of GAGs associated with Triton X-100 was observed. Biaxial mechanical testing indicated increased areal strain, increased peak stretch ratios, and decreased stress relaxation for ovine pulmonary valves.12 Interestingly, the same trend was observed for ovine aortic valves, yet no change in the areal strain or peak stretch ratio was observed in human aortic valves.15 The authors speculated that the species-specific effect of identical decellularization methods was the result of a loss of collagen crosslinking components and an initial higher cellularity in the ovine valves.15
Enzyme valve decellularization
Enzymatic agents such as nucleases or trypsin have also been utilized in valve decellularization to breakdown biologic molecules and facilitate cell removal. Nucleases cleave nucleic acid sequences into shorter segments, expediting their removal from the ECM and the inclusion of nucleases, such as DNases or RNases, in a decellularization process is so commonplace their use was not specified in Table 1. Trypsin is another common enzyme used in valve decellularization. Trypsin, a serine protease, hydrolytically cleaves proteins and is used to digest cellular proteins in the decellularization process. However, the fibrous structural proteins of the valve ECM have limited resistance to trypsin cleavage and are often affected as well. Decellularization of heart valves using trypsin often results in visible histologic damage to the ECM as well as large alterations to valve mechanics (Figure 1(c)). Compared to detergents, trypsin decellularization is slower to remove cells, causes greater disruption to the elastin and collagen structure, but has a better preservation of GAGs.14,43,45 Complete cell removal by trypsin alone actually requires lengthy decellularization protocols, and the prolonged exposure has been shown to disrupt the remaining ECM beyond practical use.50
Miscellaneous valve decellularization
Additional creative strategies for valve decellularization have been explored, with varying levels of success. Osmotic shock can be used to lyse cells; however, osmotic shock by itself is ineffective at removing the hydrophobic cell membranes and remnants and is therefore not recommended as the sole decellularization technique.44,45 It is often used in combination with detergent or enzymatic-based methods as an initial step, reducing the required detergent/enzyme concentrations and/or exposure times.12,41,45 Mechanical forces such as pressure gradients can also aid in decellularization, as perfusion of decellularizing solutions through the valve conduit has been shown to increase the removal of cellular material from the valve wall compared to immersion protocols.58 Glycol radiation, using a polyethylene glycol (PEG) detergent and gamma irradiation, has proven effective, resulting in >92% DNA removal in the valve cusps while not affecting ultimate tensile strength (UTS) and collagen content.53 Additionally, the gamma irradiation effectively removed the galactose-α(1,2)-galactose (α-Gal) epitope and the porcine endogenous retrovirus.53 Decellularization by sequential antigen solubilization is another effective method for the removal of cellular and antigenic material from xenogeneic tissues.35 Based on shared physiochemical properties, antigens are removed by sequential solubilization techniques targeting hydrophilic and lipophilic proteins.54,55 Using this method, researchers have demonstrated the successful decellularization of bovine pericardium and porcine aortic heart valve leaflets.54–56 In addition to the removal of cellular material, sequential antigen solubilization also successfully removes the α-Gal and major histocompatibility complex I (MHC I) antigens while preserving the structural and mechanical properties of the native, untreated tissue.35,56 Another unique approach to decellularization utilizes supercritical fluid solution of carbon dioxide and ethanol as a cell extraction medium.57 The high permeability and high transfer rate of the supercritical fluid makes this an effective method, resulting in a visible lack of cell nuclei and a 90% reduction in phospholipids, the primary component of cell walls. However, a stiffening and dehydration of the ECM has also been observed, likely attributed to the high ethanol exposure.57
Clinical use of decellularized heart valves
In vivo animal models using stand-alone decellularized valves have seen success, with subject survivability out to 9 months with patent, functioning valves.59,60 However, clinical studies using stand-alone decellularized valves have seen only mixed results. In clinical practice, xenogeneic valves would be preferred to allogeneic valves due to the scarcity of human tissue, yet clinical experience using xenograft, decellularized valves has not been encouraging. The initial iteration of the SynerGraft® valve (CryoLife, Inc., Kennesaw, GA, USA), a porcine xenograft, showed promising preliminary results in adults, but elicited a severe immune response and catastrophic failure in children.17,61 Subsequent studies identified the presence of ECM-associated antigens, particularly the α-Gal epitope, following processing.62 AutoTissue (Berlin, Germany) has developed the other clinical xenograft, the Matrix PTM line of decellularized porcine pulmonary valve replacements, using an SD decellularization method. This valve line has been CE certified since 2004. Both positive and negative clinical outcomes have been reported with freedom from reoperation ranging broadly from 48% at 19 months to 90% at 4 years.63,64 Overall, decellularized xenogeneic valves have performed equivalent to, or worse, than standard cryopreserved allografts, although advances in decellularization protocols or better pre-implant conditioning may increase efficacy.
On the other hand, clinical experience with decellularized human allografts has been more successful, with three options currently available worldwide. The CryoValve® SG valve is the decellularized human allograft from CryoLife, Inc. Comparisons between the CryoValve® SG and standard cryopreserved allografts have not yet found a clear benefit from decellularization.65–68 It is speculated that the CryoValve® SG valve may be more durable, although longer follow-up studies are still necessary to verify these claims.65–70 During a 2014 Food and Drug Administration (FDA) panel to discuss the classification of decellularized valves, CryoLife presented data indicating a 93% freedom from reoperation at 10 years in patients using the CryoValve® SG.71 Other studies have reported freedom from valve dysfunction at approximately 85% at 5 years or 75% at 10 years.67,68 A multi-institutional review by Bibevski et al.72 observed decreased freedom from valve dysfunction and re-intervention in CryoValve® SG valves compared to cryopreserved homografts. However, it is worth noting that within their study, the mean follow-up time was only 5 years and in comparing the decellularized and cryopreserved homograft groups, patients receiving CryoValve® SG valves were significantly older, received significantly larger valves, and more often underwent valve replacement as part of a Ross procedure.72 It is not yet clear whether the durability of the allogeneic version of the SynerGraft valve will be significantly prolonged compared to more classical homografts when human leukocyte antigen (HLA)–ABO donor–recipient matching is optimized and critical conflicts are avoided. The other two clinical uses with decellularized human allografts have seen relative success observing positive early and midterm results. A European group led by Haverich have implanted 131 decellularized allografts since 2005 and they have observed 100% freedom from explant at 10 years of follow-up (compared to 84% freedom from explant for cryopreserved allografts).73,74 Da Costa et al.75 have also seen encouraging results using decellularized aortic allograft replacements, the first of its kind, and have reported 100% freedom from reoperation of the graft at 3 years, although early hospital mortality was 7%. Despite the early success by Haverich and Da Costa using decellularized allografts, long-term results are not yet available (10–20 years), which is the crucial time when the majority of cryopreserved grafts fail. Additionally, without the presence of a viable cell population within the valve leaflets, the decellularized valves are anticipated to suffer the same valve dysfunction fate as standard cryopreserved valves. However, it is worth noting that all three groups using decellularized allografts report an increased freedom from valve dysfunction or replacement compared to standard cryopreserved allografts.67,68,72–75
Limitations of decellularized heart valves
Thus far, complete autologous recellularization of implanted decellularized heart valves has not been realized (see section “Guided tissue regeneration”). Autologous recellularization of decellularized valves has been limited to the valve wall with only endothelial recellularization observed on the leaflet surface (Figure 2). This is far superior compared to cryopreserved valves, which undergo massive degeneration and leukocyte infiltration throughout the valve as evident in Figure 2. However, the fact that recellularization of decellularized valves is limited to the valve surface is problematic since the leaflet is the primary location of valve dysfunction in implanted cryopreserved valves. Without a viable cell population capable of ECM remodeling inside the valve leaflet, decellularized heart valves are anticipated to undergo the same fate as cryopreserved valves and ultimately suffer from valve degeneration.8 Decellularized valves are an important improvement above the standard cryopreserved allograft due to reduced antigenicity, but without restoration of the cell population within the interstitium of the leaflet and the ability for matrix repair and remodeling, the decellularized valve likely will not be the sought after “ideal” heart valve. Therefore, additional processing strategies must be employed to encourage recellularization of the entire valve, including the distal portions of the leaflet.
Figure 2.
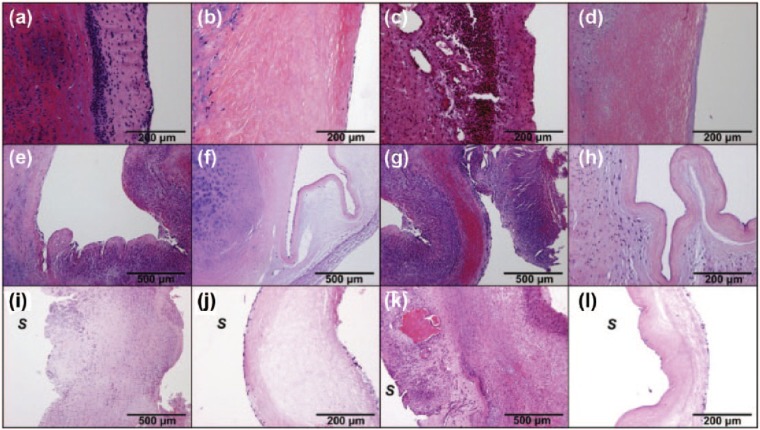
H&E-stained sections highlighting the autologous recellularization of decellularized (dAV) and cryopreserved (cAV) aortic valves after implantation in sheep. Histology of aortic wall: cAV after 3 months (a) and after 9 months (c) with signs of rejection and leukocyte infiltration; dAV after 3 months (b) and after 9 months (d) without any signs of rejection and with partial re-endothelialization and ingrowth of interstitial cells. The same findings are shown in the aortic sinus: cAV degeneration after 3 months (e) and after 9 months (g); dAV sinus without signs of rejection and with partial re-endothelialization and recellularization of leaflet base after 3 months (f) and even more recellularization after 9 months (h). Leaflets from the cAV show massive degeneration and destruction after 3 months (i) and after 9 months (k); dAV distal leaflets show partial re-endothelialization after 3 months (j) and after 9 months (l). (S) shows sinus side of the leaflet.
Source: Figure reprinted from Baraki et al.60 with permission. Copyright 2009 Elsevier: Biomaterials.
Recellularization and conditioning
Ultimately, the recellularization of a tissue-engineered valve should mimic the cell population of a native valve, which is made up of valvular endothelial cells (VECs) and valvular interstitial cells (VICs).76 VECs provide a non-thrombotic monolayer over the valve surface, which plays a key role in valve hemodynamics.77 VICs are active in the remodeling of the ECM of the valve ensuring both tissue durability and growth characteristics.78 Together, these cell populations create a non-thrombotic, durable, living valve. One of the primary challenges to realizing the TEHV is the recellularization of both VICs and VECs to create a physiologically appropriate cell population. There is evidence of a strong relationship between the two cell types, as VECs have been shown to regulate VICs toward a more native-like phenotype when in co-culture compared to VICs in isolated culture.77 However, the exact pathways of valve repair and interaction between the two populations remain largely unknown. Further complexities are introduced since cell populations differ between valves in the heart, so a recellularization method may need to be optimized for each valve type.76 Fortunately, the challenge of recellularization is lessened since there is strong evidence that following implantation, properly prepared valves have the potential for partial recellularization by native cells, particularly re-endothelialization along the valve surface.18,23,24,60,75,79 As demonstrated in Figure 2, re-endothelialization occurs across all valve surfaces while interstitial recellularization is limited to the valve wall and sinus. Therefore, the remaining challenge lies in repopulating the distal leaflet interstitium with VIC-like cells.
Native VICs have been sub-classified into five distinct phenotypes, primarily quiescent VICs (qVICs) and activated VICs (aVICs).78 qVICs are the dominant cell phenotype within the valve leaflet during normal valve function and it is believed they help maintain homeostasis through marginal matrix production and degradation. During valve injury or abnormal stress, qVICs become stimulated by VECs to become aVICs, which actively produce and degrade ECM for valve remodeling.77 aVICs are more easily identified than qVICs with a myofibroblast (MF)-like phenotype that is characterized by positive expression of alpha-smooth muscle actin (αSMA).78 Following injury repair and valve remodeling, the aVICs are eliminated by apoptosis. However, if the activation signals persist or there is dysfunction in the apoptotic process, the aVICs can become osteoblastic VICs (obVICs), which promote angiogenesis, chronic inflammation, fibrosis, and calcification leading to valve disease.78 aVICs are therefore responsible for both valve remodeling and the potential for clinical valve disease. Many heart valve recellularization studies use aVICs, or αSMA+ cells, as the target phenotype for successful recellularization, since aVICs are more easily identified by αSMA and are responsible for valve remodeling. However, it is unknown whether qVICs will ultimately replace the aVIC population and there is a lack of information on the potential for obVIC differentiation. Since initial recellularization is still a primary challenge, no studies have yet investigated the long-term fate of seeded cells, but it must be considered in the future. Regardless, progress in valve recellularization has been made and two main recellularization approaches have emerged: in situ recellularization and in vitro recellularization. Within these two approaches, a number of creative strategies have been employed to address initial cellular repopulation of the decellularized heart valve over the past two decades.
In situ recellularization
In situ recellularization of heart valves relies on the natural regenerative capabilities of the host to repopulate a valve scaffold. In contrast to the typical tissue engineering paradigm, this approach does not attempt to create living tissue ex vivo. Instead, it aims to encourage the tissue healing and remodeling process through the implantation of a biomimetic scaffold capable of stimulating host cell recruitment and self-regeneration. For this reason, the decellularized valve scaffold is well suited, compared to polymer valve scaffolds, as it provides the chemical and mechanical cues of a natural heart valve. The two general approaches for in situ recellularization are implantation of stand-alone decellularized valves or implantation of chemically modified valve grafts.
Guided tissue regeneration
The most obvious, and most attempted, approach to in situ recellularization has been the implantation of stand-alone decellularized valve grafts without any further chemical or mechanical modification. This approach has come to be known as Guided tissue regeneration. As mentioned previously, decellularized valve grafts have been implanted in animal and clinical applications with varying results in terms of survival and growth (see section “Clinical use of decellularized heart valves”). In this section, the focus is the in situ recellularization of the implanted valve scaffolds (Table 2). A more thorough review on the guided tissue regeneration of heart valves is available in Iop and Gerosa.80
Table 2.
Summary of in situ results for the implantation of non-conditioned decellularized valve scaffolds in various animal models and clinical trials.
| Recell method | Tissue | Conditioning | Implant model | Details | Results |
|---|---|---|---|---|---|
| In situ—no conditioning | mPV81 | None | Mouse PV |
Decell valve attached to donor heart and implanted in another mouse | Leaflets were thickened with many αSMA+ cells present early, though less αSMA+ cells present later |
| rbAV82 pAV83 | None | Canine PV,83 aorta82 |
Decell xenogeneic valves implanted in canines | Rabbit valve leaflets degenerated; porcine valves re-endothelialized with minimal cell infiltration near leaflet surface | |
| oAV,60 pAV,84,85 oPV,6,24,86 pPV86 | None | Ovine AV,60 PV,6,24,85,86 aorta84 |
Decell xenogeneic and allogeneic valves implanted in sheep | Re-endothelialization; recell of valve wall; minimal recell of leaflet; xenograft comparable to allograft | |
| pAV80,85,87 | None or stented87 | Porcine AV,87 PV,79 aorta85 |
Decell allogeneic valves implanted in pigs | Aorta implants led to loss of leaflets; PV implants led to good recell of surface and interior of leaflet; AV implants showed recell of conduit wall only | |
| hPV,88,89 hAV,75 pPV17,37,63,64,67,73,74,90,91 | None | Human PV, AV75 |
Decell allogeneic and xenogeneic valves implanted in humans | Allogeneic performed better than xenogeneic; recell of valve wall and endothelialization evident; no evidence of leaflet recell unless by inflammatory cells |
αSMA: alpha-activated smooth muscle actin.
The results are overall observations and the outcome of individual studies may vary. Lower case letters in acronyms denote species (o = ovine, p = porcine, h = human, m = mouse, rb = rabbit).
Currently, the in situ autologous recellularization of implanted decellularized valves is limited and variable. As mentioned previously, many studies have observed complete re-endothelialization and recellularization of the valve wall up to the leaflet base, yet the distal subsurface leaflet remains mostly acellular. This prevents tissue growth and remodeling in the valve leaflets, ultimately leading to valve degeneration. James et al.81 reported an exception to this limited recellularization using a mouse model. At 3 months of implant, they observed a thickening of the decellularized mouse leaflets and repopulation by αSMA+ cells, although by 6 months the leaflets appeared normal and the number of αSMA+ cells decreased.81 This may be the first study demonstrating a state of valve remodeling after implant transitioning into a quiescent, healthy state. While the study by James et al.81 is significant, the implant model presents challenges for clinical translation and the recellularization success is likely due to the thinness of the mouse leaflets, expediting cell infiltration. Additionally, it appears that leaflet recellularization typically decreases as higher order animals are used as implant models (Table 2). Furthermore, it appears that if the decellularized replacement valve is more robust than the original, native valve, the recellularization of the leaflet is better. For example, porcine valves implanted in canines led to good recellularization, as did porcine aortic valves implanted in a porcine pulmonary valve model.79,83
The in situ recellularization of decellularized valves in clinical use has been less successful than in pre-clinical studies. Decellularized allografts have functioned better than decellularized xenografts, as previously mentioned; however, no evidence has been presented demonstrating successful in situ autologous recellularization. Biopsies of the valve conduit taken from decellularized allografts during non-valve related reoperation in humans have shown repopulation of the conduit, although such recellularization is in accordance with similar animal studies.18,75 During the FDA panel meeting in 2014 to re-evaluate decellularized heart valve classification, CryoLife, Inc.71 presented results of their explanted decellularized allografts. The explanted valves had partial recellularization of the distal conduit but an absence of cellularity in the decellularized leaflet up to 11 years in vivo.71 Leaflet repopulation was demonstrated during clinical use of the Matrix PTM line of valves, a decellularized xenograft; however, the repopulating cells appear to be inflammatory rather than phenotypically appropriate valve cells.63,64,90,91
Chemical conditioning for in situ recellularization
As autologous recellularization of decellularized valves is limited, a number of studies have investigated the use of chemical conditioning, or other chemical modifications, before implantation to increase valve recellularization (Table 3). This approach applies cytokine or signaling molecules to modulate the healing response of the host. Specifically, conditioning can be applied that is targeted at increasing cell attachment/migration, decreasing the immune response, increasing biologic activity, or increasing mechanical integrity. One approach designed to increase cell attachment has been the pre-implant conditioning of decellularized valves with fibronectin (FN), either alone or in combination with another growth factor.92–94 In all examples, the valves treated with FN performed better than the respective decellularized or cryopreserved control valve groups.92–94 FN treatment alone led to increased recellularization of the luminal side of the treated graft in a small animal model, although no information was given on specific leaflet recellularization.92 FN in combination with stromal cell–derived factor 1α (SDF-1α) increased the surface and interstitial leaflet recellularization of valves implanted in sheep, as well as decreased the calcification, pannus formation, and immune response.93 FN plus hepatocyte growth factor (HGF) was used to achieve good recellularization in situ.94 Ota et al.94 treated decellularized porcine aortic valves with FN + HGF, and after 1 month of implantation in canines they observed great recellularization of the valve cusps with gene expression of vimentin (VIM) comparable to native valves.
Table 3.
Summary of methods for in situ recellularization of decellularized valve scaffolds with chemical conditioning.
| Recell method | Tissue | Conditioning | Implant model | Details | Results |
|---|---|---|---|---|---|
| In situ—chemical conditioning | rAV,92 oAV,93 pAV94 | Valves treated with FN,92 FN + SDF-1α,93 or FN + HGF94 | rIVC,92 oPV,93 cPV94 | FN-treated valves implanted | FN alone led to luminal recell; FN + SDF-1α led to moderate leaflet recell; FN + HGF led to great recell of the entire leaflet |
| pPV95,96 | CD133 conjugated to valve surface | oPV | Valves conjugated with CD133 and implanted | Early endothelial layer and leaflet interstitial recell with αSMA+ cells; MMP proteins present | |
| bPV,97 hPV,97 oPV23,98 | Valves treated with collagen conditioning solution | bPV,97 oPV23,98 | Valves treated in conditioning solution before implant | Treated valves re-endothelialized but no distal leaflet recell; treated valves decreased antibody production in baboons |
FN: fibronectin; SDF-1α: stromal cell–derived factor 1α; HGF: hepatocyte growth factor; αSMA: alpha-activated smooth muscle actin; MMP: matrix metallopeptidase.
The results are overall observations and the outcome of individual studies may vary. Lower case letters in acronyms denote species (o = ovine, p = porcine, h = human, r = rat, c = canine, b = baboon).
Another approach using chemical conditioning and in situ recellularization has investigated the conjugation of antibodies onto decellularized valve scaffolds to encourage in situ cell attachment. Antibody conjugation is an interesting method because it allows for selective attachment for the cell phenotype of interest. One group has explored conjugating CD133 antibodies onto decellularized valve scaffolds with the purpose of selectively targeting hematopoietic stem cells and endothelial progenitor cells (EPCs) from circulating blood.95,96 After 3 months of implantation in the right ventricular outflow tract (RVOT) of sheep, the antibody-conjugated valves showed increased recellularization throughout the leaflet compared to non-conjugated and cell-seeded controls, including a von Willebrand factor (vWF)+ surface cell layer and an αSMA+ cell population in the leaflet interstitial tissue.95 Additionally, explanted valves showed an increase in collagen, GAG, and matrix metallopeptidase 9 (MMP-9) concentrations compared to non-conjugated valves, indicating both matrix production and remodeling.95 A follow-up study found that CD133+ cells were present by 3 days of implant, but from 30 to 90 days most cells were CD133−, αSMA+, and VIM+, demonstrating the transition and differentiation of the cell population from progenitor cell adhesion to mature valve-like cells (Figure 3).96 However, the increased expression of αSMA+ cells, compared to native controls, is noteworthy since αSMA indicates an aVIC phenotype that may lead to valve disease.96
Figure 3.
Immunological staining demonstrating successful recellularization of leaflets from pulmonary valves conjugated with CD133 and implanted in the pulmonary position in sheep. Texas Red–labeled secondary antibodies show αSMA (top row) and vimentin (bottom row), and the nuclei are DAPI counterstained. Percentage values are the percent of cells with positive expression compared to the total number of cells which represent the mean calculated from all three leaflets. Note the high αSMA expression in the tissue-engineered leaflets compared to native leaflets. L denotes lumen. * indicates p < 0.05. Scale bars are 100 µm.
Source: Figure reprinted from Williams et al.96 with permission. Copyright 2015 Springer Science: Journal of Cardiovascular Translational Research.
Another chemical conditioning strategy has been employed that aims to restore the mechanical properties of the decellularized scaffold and to make the ECM more hospitable for recellularization. This chemical treatment was accomplished by normalizing tissue pH, rehydrating the collagen helix moisture envelope, compacting collagen fibrils, and restoring soluble proteins by treating decellularized valves in a conditioning solution composed of citric acid, hyaluronic acid, lauryl alcohol, and species-specific protein.12,23,97,98 Biomechanical evaluation revealed that the conditioning solution restored the stress relaxation behavior toward that of the native valve properties.12 In an ovine allograft valve implant model, the conditioning process led to better hemodynamic performance, compared to cryopreserved and decellularized valves implanted without conditioning.23,98 Improved re-endothelialization and matrix recellularization were observed following conditioning, although the interstitial subsurface repopulation was limited to the proximal portion of the leaflet.23,98 Similar results were observed using a baboon model and conditioned human valves to simulate human paradigms.97 Re-endothelialization and cusp base recellularization were observed in conditioned human valves after 26 weeks, as well as a decrease in class I and class II antibody production compared to cryopreserved valves, indicating reduced antigenicity.97 In all the above-mentioned animal studies, the conditioned valves performed better than the decellularized and cryopreserved valve controls, indicating they may function better clinically than the current standards of care. However, because the distal portions of the leaflet do not completely recellularize, the potential for a lifelong valve replacement without full leaflet active ECM remodeling is likely limited.
In vitro recellularization
In vitro recellularization, the other proposed method of recellularization for TEHVs, typically follows the traditional paradigm in tissue engineering in which cells are introduced on to a scaffold that is then subjected to in vitro conditioning. This approach relies on using an appropriate cell source and providing conditioning signals to drive cell proliferation and differentiation in a bioreactor. The exact mechanisms that modulate valve cell phenotype are not clear, but there is evidence supporting the role of both mechanical and biochemical cues. In vitro recellularization leverages these cues to drive a seeded cell population into a mature cell population. The approaches that have been explored using in vitro cell seeding can be broken down into three groups, those that did not use any further conditioning, those that applied mechanical conditioning, and those who applied chemical conditioning. Numerous cell sources have been explored for heart valve tissue engineering including VICs, VECs, mesenchymal stem cells (MSCs), bone marrow mononuclear cells (MNCs), MFs, smooth muscles cells (SMCs), ECs, and EPCs. A more thorough review on the seeding cell sources for heart valve tissue engineering is available by Jana et al.99
In vitro seeding without conditioning
Early work in the field of in vitro cell seeding of heart valves investigated the effect of seeding under static culture conditions without added mechanical or chemical conditioning (Table 4). Some of these first efforts studied the potential for leaflet repopulation by isolating the decellularized leaflets and statically seeding cells in culture flasks or well plates. A primary objective of this work was re-creating a healthy endothelial layer; therefore, seeded ECs were often used in these studies.43,100,101 Endothelial cell seeding resulted in a confluent monolayer of cells on the leaflet surface that stained positive for vWF, but lead to little to no cell penetration into the leaflet. Interestingly, the study by Rieder et al.101 found that the decellularization protocol and chemicals used greatly affected the re-endothelialization of decellularized leaflets, with SDS having cytotoxic effects and Triton X-100 leading to the best endothelial coverage. The value of ex vivo (and especially under static conditions) endothelial seeding seems low since proper decellularization and pre-implant conditioning seems to encourage autologous re-endothelialization (presumably effected by circulating EPCs). In addition, without an interstitial cell population, in vitro EC layers typically delaminate and are rapidly lost under physiologic flow conditions.
Table 4.
Summary of in vitro recellularization methods of decellularized valve scaffolds with no conditioning steps applied.
| Recell method | Cell source | Conditioning | Implant model | Details | Results |
|---|---|---|---|---|---|
| In vitro—no conditioning | ECs43,100–102 | None | None | Leaflets seeded statically | EC coverage; no internal leaflet repopulation |
| Fibroblast-like cells103–105 | None | None | Leaflets seeded statically | Mild cell infiltration in leaflet interior; cells αSMA+ and VIM+ | |
| MFs and ECs106,107 | None | cPV106 oPV107 | Valve seeded statically—no conditioning | Complete surface coverage and partial interstitial repopulation | |
| MNC or MSC108 | None | oPV | Cells injected into valve and implanted | Complete cell surface coverage for both groups; MNC leaflets were damaged; MSC leaflets were healthy with αSMA+ cells |
EC: endothelial cell; αSMA: alpha-activated smooth muscle actin; VIM: vimentin; MF: myofibroblast; MNC: mononuclear cell; MSC: mesenchymal stem cell.
The results are overall observations and the outcome of individual studies may vary. Lower case letters in acronyms denote species (o = ovine, c = canine).
Other studies investigating cell seeding on isolated leaflets have attempted to repopulate the leaflet interstitium using fibroblast-like cells such as cardiac stromal cells (CStCs) and neonatal dermal fibroblasts with moderate success.103–105 Dainese et al.103 seeded isolated leaflets with CStCs and observed up to 90% of native cellularity within 50 µm of the leaflet edge, yet they observed only 30% of native cellularity in the inner leaflet regions. These studies demonstrate the efficacy of introducing cells onto decellularized leaflet samples and they led to the seeding of intact decellularized valves. Kim et al.106 and Steinhoff et al.107 both seeded MFs and ECs onto decellularized pulmonary valves and subsequently implanted them into animal models without further in vitro conditioning. Kim et al.106 used an allogeneic model and seeded canine MFs and ECs onto decellularized canine pulmonary valves and implanted them in the pulmonary position of dogs. Steinhoff et al.107 used a xenogeneic animal model and seeded ovine MFs and ECs onto decellularized porcine pulmonary valves before implanting them in the pulmonary position of sheep. Neither group used chemical or mechanical conditioning before implantation, but allowed 6–7 days of static culture after seeding for cell adhesion and proliferation.106,107 Following the longest implant periods evaluated, both studies demonstrated complete endothelialization and partial recellularization of the leaflet interior, although notably less than compared to native leaflet tissue.106,107
An interesting study by Vincentelli et al.108 compared the efficacy of MSCs and MNCs for heart valve tissue engineering by directly injecting the cells into the arterial wall and annulus of decellularized valve scaffold, followed immediately by implantation in lambs. After 7 days in vivo, the injected cells in both groups had scattered throughout the matrix and host cells were also observed. At 4 months, both cell groups showed re-endothelialization but had markedly different recellularization responses. The MNC-seeded valves had thickened and retracted leaflets with calcified nodules and a high presence of CD68+ cells. The MSC-seeded valves had thin leaflets partially recellularized with αSMA+ cells and showed no signs of calcification. While MSC seeding elicited a more favorable in vivo response in this study, it is worth noting that the applicability of MNCs toward heart valve tissue engineering has been explored more recently using polymeric scaffolds in which seeded MNCs induced an inflammation-mediated recellularization response in vivo.109,110 Considering the potential for regenerative paracrine signaling by seeded MNCs, it is not entirely clear whether the CD68+ inflammatory cells present in the MNC-seeded valves in the study by Vincentelli et al.108 were exhibiting an M1 or M2 inflammation response, or whether they would eventually be replaced by VIC-like cells. However, the condition of the leaflets and the presence of calcified nodules in the MNC-seeded valves were not encouraging.
In vitro seeding and mechanical conditioning
Mechanical conditioning of TEHVs utilizes bioreactors and mechanical stimulation with the goal of achieving a mature cell population in vitro (Table 5). Physiologic conditions of high fluid shear and pressure have been shown to encourage the VEC and VIC phenotypes, support cell proliferation, and encourage ECM remodeling.111–117 Mechanical conditioning in bioreactors can simulate the physiological forces of heart valves and drive the differentiation and proliferation of seeded cells down the appropriate pathways.
Table 5.
Various methods for in vitro recellularization of decellularized valve scaffolds with mechanical conditioning.
| Recell method | Cell source | Conditioning | Implant model | Details | Results |
|---|---|---|---|---|---|
| In vitro—mechanical conditioning | MFs and ECs118–120 | Cultured at pulmonary pressure and flow | None | Valves seeded with EC or MF then EC and then cultured | EC seeding led to surface coverage; MF and EC seeding led to great recell with appropriate phenotype |
| MSC121 | Cultured at static, negative, or negative then positive pressure | None | Seeded valves cultured under various pressures | Negative and positive pressures led to EC coverage and moderate cell infiltration of HSP47, VIM+, and αSMA+ cells | |
| oMSC,122 oEC123 | Cultured in pulsatile flow bioreactor then implanted | Ovine aorta122 oAV123 | Seeded valves conditioned before implant | TE valves showed complete endothelium at explant; partial recellularization of leaflets | |
| hMNCs88 | Cultured in perfusion bioreactor | Human PV | Seeded valves conditioned before implant in two patients | In vitro seeding led to complete EC monolayer; both patients showed somatic growth, valve growth, and no valve degradation at 3.5 years |
MF: myofibroblast; EC: endothelial cell; MSC: mesenchymal stem cell; HSP47: heat shock protein 47; VIM: vimentin; αSMA: alpha-activated smooth muscle actin; TE: tissue-engineered; MNC: mononuclear cell.
The results are overall observations and the outcome of individual studies may vary. Lower case letters in acronyms denote species (o = ovine, h = human).
Using a dynamic bioreactor, Lichtenberg et al.119,120 explored the effects of mechanical conditioning after seeding pulmonary heart valves with vascular ECs. They demonstrated that culturing seeded valves under physiological pulmonic conditions (2.0 L/min, 60 bpm, 25 mmHg mean system pressure) can lead to complete re-endothelialization; however, care should be taken when introducing the seeded valve scaffolds to increased shear stress.120 Rapid increases in the pulsatile bioreactor flow (0.1–2.0 L/min; 0.35 L/min increases) led to significant interruptions of the endothelium.119 Conversely, a stepwise, gradual increase in pulsatile flow (0.1–0.5 L/min; 0.1 L/min increases) resulted in a nearly complete endothelial layer.119 These studies showed no recellularization of the leaflet interior, although it was not expected since they seeded ECs. A study by Schenke-Layland et al.118 also investigated physiologic mechanical conditioning on valve recellularization. MFs were seeded onto trypsin decellularized porcine pulmonary valves and cultured under pulmonic conditions (3.0 L/min, 60 bpm, 60/40 mmHg), followed by subsequent seeding with ECs. After culture, the tissue-engineered leaflets were very well recellularized, to an extent comparable with native valves. Cells repopulating the leaflet interior were αSMA+ and VIM+ while the cells on the leaflet surface were vWF+. After recellularization and conditioning, tissue-engineered valves were also mechanically more similar to native valves than their decellularized-only counterparts. Indeed, the only noticeable difference between the tissue-engineered valves and native valves was the presence of αSMA+ cells throughout the tissue-engineered leaflets, likely due to the seeded myofibroblasts. While these results are promising, the high αSMA expression is indicative of aVICs, which are responsible for tissue remodeling but their prolonged activation can also lead to valve disease.78
A different approach using mechanical conditioning under non-physiological conditions has been investigated as a means of repopulating the interstitium of the decellularized leaflet. Converse et al.121 studied the effects of cyclic negative and positive pressures on the recellularization of decellularized valves seeded with bone marrow–derived MSCs. After seeding with MSCs, the valves were cultured in a static bioreactor for 24 h, either conditioned in negative cyclic pressure (5 to −20 mmHg) for 72 h or conditioned at the same negative cyclic pressure for 72 h followed by an additional 10 days conditioning at positive pressure up to 50 mmHg. Static cultured resulted in clumping of the cells on the valve surface and minimal cell infiltration. Mechanical conditioning under negative pressures resulted in a more even distribution of cell coverage on the surface of the leaflet and limited infiltration of cells into the interstitium of the leaflet. Valves subjected to a combination of negative and positive pressures conditioning exhibited further improved cell coverage and displayed moderate cell infiltration, although still less than native. The repopulated interior cells stained positive for CD90, CD29, heat shock protein 47 (HSP47), VIM, and αSMA. Biaxial mechanical testing also revealed that increased culture under positive pressure resulted in mechanical properties more similar to cryopreserved valves.121
Despite encouraging bench-top studies, mechanically conditioned, in vitro seeded valves implanted in animal and pediatric models have only seen moderate recellularization success.122,123 Tudorache et al.123 seeded ovine aortic valves with ovine ECs followed by pulsatile flow conditioning up to 1.0 L/min over the course of a week. After conditioning, the tissue-engineered valves showed a complete endothelial layer on the leaflet surface although no interstitial cells were present. The conditioned valves were implanted in the descending aorta of sheep for 3 months, at which time all valves exhibited normal function. A complete endothelial layer was observed, as expected; however, there was very minimal leaflet interstitial recellularization.123 In this study, mechanical conditioning resulted in endothelialization in the bioreactor, yet the benefit remains unclear since implanted decellularized valves will re-endothelialize autologously, especially in sheep.23,24 One case study investigated pediatric clinical use of mechanically conditioned tissue-engineered valves. Cebotari et al.88 implanted tissue-engineered valves in two pediatric patients using decellularized human pulmonary valve scaffolds seeded with autologous MNCs isolated from peripheral blood. After seeding, the valves were cultured in a continuous perfusion bioreactor using a very low flow rate (15 mL/min) for 21 days.88 Pre-implant histology revealed a confluent monolayer of cells along the leaflet surface with markers positive for EC types. At 3.5 years of follow-up, both patients had recovered normally and had experienced somatic growth, valve annulus growth, and no signs of degradation, stenosis, or cusp thickening. These are highly encouraging results; however, the sample size is very limited and no further follow-up data have been provided so the long-term outcome remains unclear.
In vitro seeding and chemical modification
Another common approach to increase the recellularization of in vitro seeded TEHVs has been to chemically modify the decellularized valve scaffolds prior to seeding (Table 6). Chemical modification utilizes cytokines, chemokines, growth factors, antibodies, and polymers to support phenotype-specific cellular attachment, bolster valve mechanics, and drive cell differentiation.
Table 6.
Various methods for in vitro recellularization of decellularized valve scaffolds with chemical conditioning.
| Recell method | Cell source | Conditioning | Implant model | Details | Results |
|---|---|---|---|---|---|
| In vitro—chemical conditioning | rMSC,124 mMF,125 hMF,125 hEC125 | Valves coated with P3/4HB before seeding | None | Hybrid valves seeded in vitro | Hybrid valves had increased mechanics; in vitro seeding led to cell coverage, but no infiltration |
| rMF,126 hECs,127,128 hMF129 | Leaflets modified with PEG plus TGF-β1, VEGF, or RGD peptides | None | PEG-peptide-modified leaflets seeded with cells | PEGylation increased mechanics and cell surface density, regardless of additional peptide; no cellular infiltration | |
| gMSC130 | Encapsulation of cells in PEG before seeding | Goat aorta | PEG encapsulated cells seeded on decell scaffold and then implanted in goats | PEG cell seeding increased tensile strength, increased ratio of endothelial cells, and decreased thrombosis | |
| rMSC,131 hEPC132,133 | Polyelectrolyte layers of heparin and SDF-1α/chitosan/VEGF adsorbed onto valve | None,132,133 rAorta131 | Valve scaffolds coated with polyelectrolyte multilayers and then seeded with cells | Treated valves led to reduced platelet activation; chitosan and VEGF increased EC adherence and proliferation in vitro; SDF-1α had increased endothelial layer after implant in vivo | |
| oECs134 | Valves coated in CCN1 | oPV | Valves coated in CCN1 and before implant | Treated valves had good recell in vivo; cell coverage higher on ventricularis with mild infiltration of VIM+ and αSMA+ cells | |
| rMSCs135 | Valves conjugated with CD90 antibody | None | Treated leaflets cultured in shear flow chamber with MSCs | Treated leaflets had increased cell attachment distributed across surface | |
| pVIC,136 hMSC11 | Valves treated with fibronectin before seeding | None | Treated leaflets seeded statically | Leaflet surface coverage and mild cell infiltration by appropriate phenotypes | |
| oECs137 | Valves treated with fibronectin before seeding | oPV | Treated valves seeded and then implanted | Treated valves had complete EC layer and good interstitial repopulation | |
| hECs19–21 | Valve treated with ProNectin F before seeding | hPV | Treated valves seeded and implanted in 11 patients | 100% survival at 10 years 3 months biopsy of valve wall showed endothelialization and partial recell by fibroblast cells |
MSC: mesenchymal stem cell; MF: myofibroblast; EC: endothelial cell; P3/4HB: poly(3-hydroxybutyrate-co-4-hydroxybutyrate); PEG: polyethylene glycol; TGF-β1: transforming growth factor beta 1; VEGF: vascular endothelial growth factor; EPC: endothelial progenitor cell; SDF-1α: stromal cell–derived factor 1α; VIM: vimentin; αSMA: alpha-smooth muscle actin; VIC: valvular interstitial cell.
The results are overall observations and the outcome of individual studies may vary. Lower case letters in acronyms denote species (o = ovine, p = porcine, h = human, m = mouse, r = rat, g = goat).
As discussed previously, decellularization can have deleterious effects on the mechanics of the valve leaflet ECM. Chemical crosslinking has been explored as a means of improving the structural and mechanical integrity of the decellularized ECM. Conventional crosslinking methods using glutaraldehyde block immunogenic antigens, yet glutaraldehyde is also cytotoxic preventing cellular ingrowth and therefore is not appropriate for recellularizing valve scaffolds. Other crosslinking methods have been explored using procyanidin, quercetin, and nordihydroguaiaretic acid (NDGA), which have been found to improve the mechanics of the valve leaflets and are not cytotoxic at low concentrations.138–140 After crosslinking, the treated leaflets had increased UTS and elastic modulus compared to untreated decellularized leaflets and in some cases greater than comparable fresh leaflets or glutaraldehyde-treated leaflets.138–140 The recellularization potential after leaflet crosslinking remains largely unknown, however, as only NDGA-treated valves have been briefly seeded with ECs for 24 h, although in that time ECs showed adhesion and proliferation on the crosslinked leaflets.138
Other chemical modifications that can bolster the mechanical integrity of valve leaflets are the incorporation of polymers into the decellularized valves to create hybrid valve scaffolds. These hybrid valves have improved mechanical properties compared to decellularized valves, and the polymer additions can act as a transport mechanism for drug delivery. The biodegradable polymer poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB) has been applied to decellularized porcine aortic valves through impregnation into the tissue or electrospinning onto the valve surface.124,125 Hybrid valves created through P3/4HB electrospinning followed by seeding with MSCs under static conditions had a greater maximum load carrying capacity, UTS, and elastic modulus than their decellularized-only counterparts; however, recellularization was similar between groups and limited to the leaflet surface.124 Impregnated P3/4HB hybrid valves also had increased biomechanics and could support the culture of mouse MFs, human MFs, and human ECs, but only on the valve surface.125 A more common polymer for creating hybrid valves is PEG because it is highly hydrophilic, water soluble, and has active functional groups that facilitate peptide conjugation.126–130 Two groups have investigated modifying decellularized porcine aortic valve leaflets with PEG conjugated with transforming growth factor beta 1 (TGF-β1), vascular endothelial growth factor (VEGF), and/or RGD.126–129 They found that PEGylation of decellularized valves resulted in mechanical properties similar to native valves and that conjugation of various peptides increased surface recellularization after seeding with rat MFs or human umbilical cord vascular ECs.126–128 In fact, in all studies the PEG-peptide-modified valves showed greater surface cell density than valves treated with PEG only or decellularized control valves; however, none of the PEG-peptide-modified valves resulted in repopulation of the leaflet interstitium.126–128
Another group has explored chemical modification of decellularized valves by applying polyelectrolyte multilayers (PEMs) composed of heparin plus VEGF, chitosan, or SDF-1α.131–133 Decellularized porcine aortic valves treated with PEM of heparin–chitosan or heparin–VEGF were subsequently seeded with EPCs, and it was observed that both PEM coatings improved hemocompatibility, showing decreased platelet adhesion and activation.132,133 Although seeding with EPCs did not result in leaflet interstitial recellularization, valves modified with heparin–chitosan were able to support an EC population while valves modified with heparin–VEGF actually had better adhesion, proliferation, and migration of ECs compared to decellularized control valves.132,133 Zhou et al.131 applied a PEM of heparin–SDF-1α to decellularized rat aortic valves prior to seeding with rat bone marrow MSCs and observed increased proliferation and migration of MSCs on the modified scaffolds. Zhou et al.131 also implanted unseeded, heparin–SDF-1α-modified valves in rats and observed luminal re-endothelialization of the aortic wall; however, there was no indication of leaflet recellularization.
Other efforts involving chemical modification of in vitro seeded decellularized valve scaffolds have aimed at increasing the adhesion of both the seeded cells and host cells in vivo. Theodoridis et al.134 coated decellularized ovine pulmonary valves in CCN1, a matricellular protein associated with cell adhesion, proliferation, and differentiation, before seeding them with ovine ECs and implanting them in sheep. At explant, the modified valves had greater cell surface coverage and partial interstitial recellularization by VIM+ and αSMA+ cells.134 Similarly, Ye et al.135 conjugated decellularized porcine aortic valve leaflets with an anti-human CD90 antibody and then cultured them in a shear flow bioreactor with media containing an MSC population. The modified leaflets had a greater cell population distributed evenly across the surface than the un-modified control samples.135 These approaches demonstrate that conjugation of peptides and antibodies can increase the adhesion of cells onto valve surfaces. However, this approach has not yet resulted in the complete repopulation of the leaflet interior. Increasing surface cell density through chemical modification may encourage cell infiltration to a small degree, but other chemokines may be required to repopulate the entire leaflet.
Decellularized valves have also been treated with FN before in vitro cell seeding. FN has been shown to promote differentiation into a VIC phenotype by human MSCs and to reduce the formation of calcific nodules by VICs, particularly in combination with VEGF.141,142 Individual decellularized leaflets treated with FN showed only moderate recellularization when seeded with VICs or MSCs, although immunohistochemistry revealed appropriate differentiation into the correct cell phenotypes (surface cells were vWF+ and interstitial cells were αSMA+ and VIM+).11,136 Dohmen et al.137 treated decellularized porcine pulmonary valves with FN, seeded them with autologous ovine ECs, and implanted them in the pulmonary position of sheep for 6 months. At explant, FN-treated/EC-seeded valves had a complete EC monolayer throughout the valve and good internal recellularization of the leaflet, while valves treated with FN but not seeded had only a partial EC layer and minimal leaflet recellularization.137 The same group also performed a small clinical study using the same in vitro seeding and FN treatment approach. Between 2000 and 2002, 11 patients underwent pulmonary valve replacement with a tissue-engineered pulmonary heart valve treated with ProNectin F (a synthetic FN alternative) and seeded with autologous ECs.19–21 At 10 years of follow-up, there was a 100% survival rate and transthoracic echocardiography showed patent and functioning valves in all patients.20 At 3 months, one patient required a non-related reoperation, during which a small biopsy was taken from the conduit of the TEHV.18 Histology of the biopsy revealed repopulation of the conduit wall by CD31+ and vWF+ surface cells and fibroblast-like interstitial cells.18
Discussion and future directions
Many challenges have been encountered in the pursuit of a TEHV. In the context of engineering a viable TEHV using a decellularized valve scaffold, the most pressing challenge is achieving recellularization of the entire valve, including distal portions of the leaflet, with an appropriate cell population. Upon analysis of the various recellularization strategies that have been employed, it is apparent that all approaches are not equally effective. Chemical conditioning with FN has been one of the more successful approaches and has lead to increased leaflet recellularization in both in situ and in vitro studies.19–21,92–94,137 Other chemical treatments that target increased cell adhesion, including conjugation of CD133 and CCN1 to the decellularized scaffold, have led to increased recellularization of the leaflet surface and the leaflet interstitium by phenotypically appropriate cells.95,96,134 Mechanical conditioning has a positive effect on recellularization in general, although the most effective parameters for mechanical conditioning have yet to be determined and may vary by valve position. Conditioning parameters of pressure and flow similar to native in situ conditions seem to be the most obvious choice, yet mechanical conditioning at pulmonary conditions has had mixed results and conditioning using non-physiological forces has also resulted in limited recellularization.118–123 Complicating matters, the cell population within the TEHV must ultimately mimic that of the native valve. Justifiably so, αSMA is often used as the marker of choice to evaluate the appropriateness of scaffold repopulation since aVICs express αSMA during the remodeling of valve ECM, and matrix remodeling is a basic requirement for a functioning TEHV. However, as discussed previously, the prolonged expression of αSMA and activation of VICs can lead to valve fibrosis, inflammation, and calcification.78 As evidenced above, several groups have shown excellent scaffold repopulation by αSMA+ cells, but the cell population should ultimately transition to a quiescent state. The study by James et al.81 provided one example of this successful transition in a decellularized valve graft implanted in a mouse model; however, similar results have not yet been observed in larger animal implant models. One of the crucial tasks in proving the safety of the TEHV will be proving that a seeded cell population that is initially αSMA+ is capable of transitioning into a quiescent state after valve remodeling is complete.
Successful strategies have been employed for the two main approaches for heart valve recellularization (in situ and in vitro) and have resulted in recellularization of the interstitial tissue of the distal leaflet. In particular, the studies by Ota et al.,94 Jordan et al.,95 Iop et al.,11 and Schenke-Layland et al.118 demonstrate good recellularization of an appropriate valve cell phenotype using a variety of methods. However, as we continue to progress the field of heart valve tissue engineering, we must be mindful of the potential clinical and regulatory challenges associated with successful recellularization strategies. Ex vivo cell seeding maintains the ability to directly influence cell infiltration by the choice of the seeding cells and by applying bioreactor conditioning parameters through mechanical or biochemical means. But the same bioreactor conditioning associated with relatively complete in vitro recellularization requires lengthy valve and cell culture, limiting the potential for off-the-shelf availability and clinical relevance. Short-term in vitro seeding protocols have numerous practical advantages (e.g. sterility) but are challenging in assuring target recellularization populations are achieved. In either case, in vitro seeding will have associated regulatory challenges with assuring correct lineage and phenotype stability of the seeded and subsequent proliferated cells, particularly if MSCs or other stem cells are used. Therefore, the future direction of heart valve tissue engineering may follow the paradigm of guided tissue regeneration and in situ recellularization. That is, the greatest possibility for success lies in developing bioengineered scaffolds and conditioning methods to create “smart,” bioactive heart valves that harness the patient’s own regenerative capabilities to recellularize the construct after implantation. The inherent elegance of in situ recellularization is the simplification between the TEHV product (manufacture, distribution, inventory, and patient allocation) and the clinical event (actual treatment of individual patients). It is worth noting that there are still significant regulatory hurdles associated with in situ recellularization since many of the signaling molecules may be pro-neoplastic and may cause difficulties in sterilization. Finally, it is worth noting that the two recellularization approaches of in vitro and in situ are not mutually exclusive and the benefits of both may be leveraged. For example, short-term seeding of a “pilot” cell population in vitro may provide the signaling cues necessary to induce complete valve repopulation by host cells in situ. Such approaches have already realized recellularization success in vascular tissue engineering and with polymeric heart valve scaffolds.143,144
As discussed previously, decellularized heart valve scaffolds are of significant interest moving forward with the TEHV. However, other scaffold options are being explored that may soon overcome the limitations of decellularized valves. One such scaffold is a polymeric valve under development by XELTIS, a European-based company that started the first clinical trial evaluating a synthetic, biodegradable scaffold in October 2016.145,146 The XELTIS valve is a non-cell-seeded scaffold that allows host cell repopulation and new tissue formation. This valve is promising because of the unlimited supply of a synthetic valve, although long-term follow-up studies are necessary to determine the success of the XELTIS valve implants. Another noteworthy scaffold option comes from decellularizing “man-made” engineered tissue. For example, Syedain et al.147–149 have created valve and leaflet-shaped collagen scaffolds by seeding fibrin gels with dermal cells followed by decellularization. These engineered tissues show potential for both in situ recellularization in sheep and in vitro recellularization after seeding with MSCs.147–149 Similar to synthetic scaffolds, these engineered scaffolds have potential for unlimited supply, but further studies are needed to ensure clinical safety. Until then, the decellularized heart valve is still the scaffold currently in the best position to move forward as a clinically useful TEHV. As advances continue in materials science and in the understanding of heart valve structure, the challenges facing synthetic and engineered scaffolds may be overcome and an artificial scaffold may one day be preferred for bioengineered valve replacements with the attendant manufacturing advantages. However, the timeline for that reality is uncertain, and decellularized valve scaffolds have the greatest potential for the expeditious development of a TEHV due to the regulatory history, long clinical experience with homografts, as well as a deep research focus by many groups.8,22,28 Utilizing the decellularized heart valve as a tissue engineering scaffold offers advantages in terms of both immediate function and overall safety, as the worst-case scenario results in a valve similar to the homograft for which the natural history is well known and clinical advantages are appropriate for specific patient populations.
In addressing the challenges associated with the TEHV, researchers must be mindful of the ultimate goal: improving the clinical management of congenital heart defects and other structural valve diseases. Success in the laboratory does not necessarily equate to successful translation to clinical practice. While the regulatory guidelines for non-viable prosthetic valve substitutes are well defined, the guidelines for FDA approval of a viable combination device (cells + scaffold) such as a TEHV are evolving and no such construct has yet been approved. Valve functional safety will certainly be paramount; however, assurance of consistent recellularization with phenotypically appropriate cell populations could be more challenging to demonstrate in vivo and non-destructively, potentially necessitating new technologies for analyzing recellularization. Ultimately, the challenge remains regarding scaffold repopulation, and the success of the TEHV may be reliant upon developing new performance markers, including in vitro cell population monitoring, not just performance monitoring by echocardiography and traditional magnetic resonance imaging (MRI) methods. Thus, successful heart valve tissue engineering strategies will require elegant science to meet the design criteria of the TEHV, survive regulatory scrutiny, and achieve clinical success eclipsing current valve replacement options.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. D’Arcy JL, Prendergast BD, Chambers JB, et al. Valvular heart disease: the next cardiac epidemic. Heart 2011; 97: 91–93. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 4. Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med 2005; 2: 60–61. [DOI] [PubMed] [Google Scholar]
- 5. Bourguignon T, Bergoend E, Mirza A, et al. Risk factors for valve-related complications after mechanical heart valve replacement in 505 patients with long-term follow up. J Heart Valve Dis 2011; 20: 673–680. [PubMed] [Google Scholar]
- 6. Hopkins RA, Jones AL, Wolfinbarger L, et al. Decellularization reduces calcification while improving both durability and 1-year functional results of pulmonary homograft valves in juvenile sheep. J Thorac Cardiovasc Surg 2009; 137: 907–913. [DOI] [PubMed] [Google Scholar]
- 7. Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology 2009; 55: 135–144. [DOI] [PubMed] [Google Scholar]
- 8. Hopkins R. From cadaver harvested homograft valves to tissue-engineered valve conduits. Prog Pediatr Cardiol 2006; 21: 137–152. [Google Scholar]
- 9. Brody S, Pandit A. Approaches to heart valve tissue engineering scaffold design. J Biomed Mater Res B Appl Biomater 2007; 83: 16–43. [DOI] [PubMed] [Google Scholar]
- 10. Li F, Li W, Johnson S, et al. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium 2004; 11: 199–206. [DOI] [PubMed] [Google Scholar]
- 11. Iop L, Renier V, Naso F, et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials 2009; 30: 4104–4116. [DOI] [PubMed] [Google Scholar]
- 12. Converse GL, Armstrong M, Quinn RW, et al. Effects of cryopreservation, decellularization and novel extracellular matrix conditioning on the quasi-static and time-dependent properties of the pulmonary valve leaflet. Acta Biomater 2012; 8: 2722–2729. [DOI] [PubMed] [Google Scholar]
- 13. Korossis SA, Booth C, Wilcox HE, et al. Tissue engineering of cardiac valve prostheses II: biomechanical characterization of decellularized porcine aortic heart valves. J Heart Valve Dis 2002; 11: 463–471. [PubMed] [Google Scholar]
- 14. Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials 2008; 29: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. VeDepo MC, Buse EE, Quinn RW, et al. Species-specific effects of aortic valve decellularization. Acta Biomater 2017; 50: 249–258. [DOI] [PubMed] [Google Scholar]
- 16. Argento G, Simonet M, Oomens CW, et al. Multi-scale mechanical characterization of scaffolds for heart valve tissue engineering. J Biomech 2012; 45: 2893–2898. [DOI] [PubMed] [Google Scholar]
- 17. Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 2003; 23: 1002–1006; discussion 1006. [DOI] [PubMed] [Google Scholar]
- 18. Dohmen PM, Hauptmann S, Terytze A, et al. In-vivo repopularization of a tissue-engineered heart valve in a human subject. J Heart Valve Dis 2007; 16: 447–449. [PubMed] [Google Scholar]
- 19. Dohmen PM, Lembcke A, Holinski S, et al. Mid-term clinical results using a tissue-engineered pulmonary valve to reconstruct the right ventricular outflow tract during the Ross procedure. Ann Thorac Surg 2007; 84: 729–736. [DOI] [PubMed] [Google Scholar]
- 20. Dohmen PM, Lembcke A, Holinski S, et al. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg 2011; 92: 1308–1314. [DOI] [PubMed] [Google Scholar]
- 21. Dohmen PM, Lembcke A, Hotz H, et al. Ross operation with a tissue-engineered heart valve. Ann Thorac Surg 2002; 74: 1438–1442. [DOI] [PubMed] [Google Scholar]
- 22. Dohmen PM. Clinical results of implanted tissue engineered heart valves. HSR Proc Intensive Care Cardiovasc Anesth 2012; 4: 225–231. [PMC free article] [PubMed] [Google Scholar]
- 23. Quinn R, Hilbert S, Converse G, et al. Enhanced autologous re-endothelialization of decellularized and extracellular matrix conditioned allografts implanted into the right ventricular outflow tracts of juvenile sheep. Cardiovasc Eng Techn 2012; 3: 217–227. [Google Scholar]
- 24. Quinn RW, Hilbert SL, Bert AA, et al. Performance and morphology of decellularized pulmonary valves implanted in juvenile sheep. The Ann Thorac Surg 2011; 92: 131–137. [DOI] [PubMed] [Google Scholar]
- 25. Wang S, Goecke T, Meixner C, et al. Freeze-died heart valve scaffolds. Tissue Eng Part C Methods 2012; 18: 517–525. [DOI] [PubMed] [Google Scholar]
- 26. Kluin J, Talacua H, Smits AI, et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant—from material design to 12 months follow-up in sheep. Biomaterials 2017; 125: 101–117. [DOI] [PubMed] [Google Scholar]
- 27. Xue Y, Sant V, Phillippi J, et al. Biodegradable and biomimetic elastomeric scaffolds for tissue-engineered heart valves. Acta Biomater 2017; 48: 2–19. [DOI] [PubMed] [Google Scholar]
- 28. Cheung DY, Duan B, Butcher JT. Current progress in tissue engineering of heart valves: multiscale problems, multiscale solutions. Expert Opin Biol Ther 2015; 15: 1155–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mol A, Smits AI, Bouten CV, et al. Tissue engineering of heart valves: advances and current challenges. Expert Rev Med Devices 2009; 6: 259–275. [DOI] [PubMed] [Google Scholar]
- 30. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32: 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem 2012; 113: 2217–2222. [DOI] [PubMed] [Google Scholar]
- 32. Keane TJ, Londono R, Turner NJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012; 33: 1771–1781. [DOI] [PubMed] [Google Scholar]
- 33. Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng 2014; 42: 1517–1527. [DOI] [PubMed] [Google Scholar]
- 34. Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol 2008; 20: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong ML, Griffiths LG. Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization. Acta Biomater 2014; 10: 1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bloch O, Erdbrugger W, Volker W, et al. Extracellular matrix in deoxycholic acid decellularized aortic heart valves. Med Sci Monit 2012; 18: BR487–BR492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erdbrugger W, Konertz W, Dohmen PM, et al. Decellularized xenogenic heart valves reveal remodeling and growth potential in vivo. Tissue Eng 2006; 12: 2059–2068. [DOI] [PubMed] [Google Scholar]
- 38. Friedrich LH, Jungebluth P, Sjoqvist S, et al. Preservation of aortic root architecture and properties using a detergent-enzymatic perfusion protocol. Biomaterials 2014; 35: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 39. Kasimir MT, Rieder E, Seebacher G, et al. Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs 2003; 26: 421–427. [DOI] [PubMed] [Google Scholar]
- 40. Tudorache I, Cebotari S, Sturz G, et al. Tissue engineering of heart valves: biomechanical and morphological properties of decellularized heart valves. J Heart Valve Dis 2007; 16: 567–573; discussion 574. [PubMed] [Google Scholar]
- 41. Zhou J, Fritze O, Schleicher M, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials 2010; 31: 2549–2554. [DOI] [PubMed] [Google Scholar]
- 42. Cebotari S, Tudorache I, Jaekel T, et al. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif Organs 2010; 34: 206–210. [DOI] [PubMed] [Google Scholar]
- 43. Grauss RW, Hazekamp MG, Oppenhuizen F, et al. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg 2005; 27: 566–571. [DOI] [PubMed] [Google Scholar]
- 44. Meyer SR, Chiu B, Churchill TA, et al. Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res A 2006; 79: 254–262. [DOI] [PubMed] [Google Scholar]
- 45. Somers P, De Somer F, Cornelissen M, et al. Decellularization of heart valve matrices: search for the ideal balance. Artif Cells Blood Substit Immobil Biotechnol 2012; 40: 151–162. [DOI] [PubMed] [Google Scholar]
- 46. Naso F, Gandaglia A, Formato M, et al. Differential distribution of structural components and hydration in aortic and pulmonary heart valve conduits: impact of detergent-based cell removal. Acta Biomater 2010; 6: 4675–4688. [DOI] [PubMed] [Google Scholar]
- 47. Spina M, Ortolani F, El Messlemani A, et al. Isolation of intact aortic valve scaffolds for heart-valve bioprostheses: extracellular matrix structure, prevention from calcification, and cell repopulation features. J Biomed Mater Res A 2003; 67: 1338–1350. [DOI] [PubMed] [Google Scholar]
- 48. Yu BT, Li WT, Song BQ, et al. Comparative study of the Triton X-100-sodium deoxycholate method and detergent-enzymatic digestion method for decellularization of porcine aortic valves. Eur Rev Med Pharmacol Sci 2013; 17: 2179–2184. [PubMed] [Google Scholar]
- 49. VeDepo MC, Buse EE, Armstrong M, et al. Poster: inter-species variation in the decellularization of aortic valves. In: Society for biomaterials 2015 annual meeting and exposition, Charlotte, NC, 15–19 April 2015. [Google Scholar]
- 50. Schenke-Layland K, Vasilevski O, Opitz F, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol 2003; 143: 201–208. [DOI] [PubMed] [Google Scholar]
- 51. Luo J, Korossis SA, Wilshaw SP, et al. Development and characterization of acellular porcine pulmonary valve scaffolds for tissue engineering. Tissue Eng Part A 2014; 20: 2963–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jing H, Wang Z, Chang Q. De-endothelialized aortic homografts: a promising scaffold material for tissue-engineered heart valves. Cells Tissues Organs 2014; 200: 195–203. [DOI] [PubMed] [Google Scholar]
- 53. Ota T, Taketani S, Iwai S, et al. Novel method of decellularization of porcine valves using polyethylene glycol and gamma irradiation. Ann Thorac Surg 2007; 83: 1501–1507. [DOI] [PubMed] [Google Scholar]
- 54. Wong ML, Leach JK, Athanasiou KA, et al. The role of protein solubilization in antigen removal from xenogeneic tissue for heart valve tissue engineering. Biomaterials 2011; 32: 8129–8138. [DOI] [PubMed] [Google Scholar]
- 55. Wong ML, Wong JL, Athanasiou KA, et al. Stepwise solubilization-based antigen removal for xenogeneic scaffold generation in tissue engineering. Acta Biomater 2013; 9: 6492–6501. [DOI] [PubMed] [Google Scholar]
- 56. Qiao WH, Liu P, Hu D, et al. Sequential hydrophile and lipophile solubilization as an efficient method for decellularization of porcine aortic valve leaflets: structure, mechanical property and biocompatibility study. J Tissue Eng Regen Med. Epub ahead of print 13 December 2016. DOI: 10.1002/term.2388. [DOI] [PubMed] [Google Scholar]
- 57. Sawada K, Terada D, Yamaoka T, et al. Cell removal with supercritical carbon dioxide for acellular artificial tissue. J Chem Technol Biot 2008; 83: 943–949. [Google Scholar]
- 58. Sierad LN, Shaw EL, Bina A, et al. Functional heart valve scaffolds obtained by complete decellularization of porcine aortic roots in a novel differential pressure gradient perfusion system. Tissue Eng Part C Methods 2015; 21: 1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dohmen PM, da Costa F, Holinski S, et al. Is there a possibility for a glutaraldehyde-free porcine heart valve to grow? Eur Surg Res 2006; 38: 54–61. [DOI] [PubMed] [Google Scholar]
- 60. Baraki H, Tudorache I, Braun M, et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 2009; 30: 6240–6246. [DOI] [PubMed] [Google Scholar]
- 61. Goldstein S, Clarke DR, Walsh SP, et al. Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann Thorac Surg 2000; 70: 1962–1969. [DOI] [PubMed] [Google Scholar]
- 62. Kasimir MT, Rieder E, Seebacher G, et al. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Heart Valve Dis 2006; 15: 278–286; discussion 286. [PubMed] [Google Scholar]
- 63. Konertz W, Angeli E, Tarusinov G, et al. Right ventricular outflow tract reconstruction with decellularized porcine xenografts in patients with congenital heart disease. J Heart Valve Dis 2011; 20: 341–347. [PubMed] [Google Scholar]
- 64. Voges I, Brasen JH, Entenmann A, et al. Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. Eur J Cardiothorac Surg 2013; 44: e272–e279. [DOI] [PubMed] [Google Scholar]
- 65. Bechtel JF, Stierle U, Sievers HH. Fifty-two months’ mean follow up of decellularized SynerGraft-treated pulmonary valve allografts. J Heart Valve Dis 2008; 17: 98–104; discussion 104. [PubMed] [Google Scholar]
- 66. Brown JW, Ruzmetov M, Eltayeb O, et al. Performance of SynerGraft decellularized pulmonary homograft in patients undergoing a Ross procedure. Ann Thorac Surg 2011; 91: 416–422; discussion 422–423. [DOI] [PubMed] [Google Scholar]
- 67. Konuma T, Devaney EJ, Bove EL, et al. Performance of CryoValve SG decellularized pulmonary allografts compared with standard cryopreserved allografts. Ann Thorac Surg 2009; 88: 849–854; discussion 554–555. [DOI] [PubMed] [Google Scholar]
- 68. Ruzmetov M, Shah JJ, Geiss DM, et al. Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: a single-institution comparison. J Thorac Cardiovasc Surg 2012; 143: 543–549. [DOI] [PubMed] [Google Scholar]
- 69. Sievers HH, Stierle U, Schmidtke C, et al. Decellularized pulmonary homograft (SynerGraft) for reconstruction of the right ventricular outflow tract: first clinical experience. Z Kardiol 2003; 92: 53–59. [DOI] [PubMed] [Google Scholar]
- 70. Tavakkol Z, Gelehrter S, Goldberg CS, et al. Superior durability of SynerGraft pulmonary allografts compared with standard cryopreserved allografts. Ann Thorac Surg 2005; 80: 1610–1614. [DOI] [PubMed] [Google Scholar]
- 71. CryoLife Inc. CryoLife written submission for docket no. FDA-2014-N-0001-0074. Silver Spring, MD: Food and Drug Administration, 2014. [Google Scholar]
- 72. Bibevski S, Ruzmetov M, Fortuna RS, et al. Performance of SynerGraft decellularized pulmonary allografts compared with standard cryopreserved allografts: results from multiinstitutional data. Ann Thorac Surg 2017; 103: 869–874. [DOI] [PubMed] [Google Scholar]
- 73. Cebotari S, Tudorache I, Ciubotaru A, et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation 2011; 124: S115–S123. [DOI] [PubMed] [Google Scholar]
- 74. Sarikouch S, Horke A, Tudorache I, et al. Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg 2016; 50: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Da Costa FD, Costa AC, Prestes R, et al. The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg 2010; 90: 1854–1860. [DOI] [PubMed] [Google Scholar]
- 76. Della Rocca F, Sartore S, Guidolin D, et al. Cell composition of the human pulmonary valve: a comparative study with the aortic valve—the VESALIO Project. Vitalitate Exornatum Succedaneum Aorticum labore Ingegnoso Obtinebitur. Ann Thorac Surg 2000; 70: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 77. Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng 2006; 12: 905–915. [DOI] [PubMed] [Google Scholar]
- 78. Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 2007; 171: 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Iop L, Bonetti A, Naso F, et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS ONE 2014; 9: e99593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Iop L, Gerosa G. Guided tissue regeneration in heart valve replacement: from preclinical research to first-in-human trials. Biomed Res Int 2015; 2015: 432901 (13 pp.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. James I, Yi T, Tara S, et al. Hemodynamic characterization of a mouse model for investigating the cellular and molecular mechanisms of neotissue formation in tissue engineered heart valves. Tissue Eng Part C Methods 2015; 21: 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Takagi K, Fukunaga S, Nishi A, et al. In vivo recellularization of plain decellularized xenografts with specific cell characterization in the systemic circulation: histological and immunohistochemical study. Artif Organs 2006; 30: 233–241. [DOI] [PubMed] [Google Scholar]
- 83. Iwai S, Torikai K, Coppin CM, et al. Minimally immunogenic decellularized porcine valve provides in situ recellularization as a stentless bioprosthetic valve. J Artif Organs 2007; 10: 29–35. [DOI] [PubMed] [Google Scholar]
- 84. Juthier F, Vincentelli A, Gaudric J, et al. Decellularized heart valve as a scaffold for in vivo recellularization: deleterious effects of granulocyte colony-stimulating factor. J Thorac Cardiovasc Surg 2006; 131: 843–852. [DOI] [PubMed] [Google Scholar]
- 85. Paniagua Gutierrez JR, Berry H, Korossis S, et al. Regenerative potential of low-concentration SDS-decellularized porcine aortic valved conduits in vivo. Tissue Eng Part A 2015; 21: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leyh RG, Wilhelmi M, Rebe P, et al. In vivo repopulation of xenogeneic and allogeneic acellular valve matrix conduits in the pulmonary circulation. Ann Thorac Surg 2003; 75: 1457–1463; discussion 1463. [DOI] [PubMed] [Google Scholar]
- 87. Honge JL, Funder J, Hansen E, et al. Recellularization of aortic valves in pigs. Eur J Cardiothorac Surg 2011; 39: 829–834. [DOI] [PubMed] [Google Scholar]
- 88. Cebotari S, Lichtenberg A, Tudorache I, et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation 2006; 114: I132–I137. [DOI] [PubMed] [Google Scholar]
- 89. Neumann A, Sarikouch S, Breymann T, et al. Early systemic cellular immune response in children and young adults receiving decellularized fresh allografts for pulmonary valve replacement. Tissue Eng Part A 2014; 20: 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Perri G, Polito A, Esposito C, et al. Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur J Cardiothorac Surg 2012; 41: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 91. Ruffer A, Purbojo A, Cicha I, et al. Early failure of xenogenous de-cellularised pulmonary valve conduits—a word of caution! Eur J Cardiothorac Surg 2010; 38: 78–85. [DOI] [PubMed] [Google Scholar]
- 92. Assmann A, Delfs C, Munakata H, et al. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials 2013; 34: 6015–6026. [DOI] [PubMed] [Google Scholar]
- 93. Flameng W, De Visscher G, Mesure L, et al. Coating with fibronectin and stromal cell-derived factor-1alpha of decellularized homografts used for right ventricular outflow tract reconstruction eliminates immune response-related degeneration. J Thorac Cardiovasc Surg 2014; 147: 1398–1404.e2. [DOI] [PubMed] [Google Scholar]
- 94. Ota T, Sawa Y, Iwai S, et al. Fibronectin-hepatocyte growth factor enhances reendothelialization in tissue-engineered heart valve. Ann Thorac Surg 2005; 80: 1794–1801. [DOI] [PubMed] [Google Scholar]
- 95. Jordan JE, Williams JK, Lee SJ, et al. Bioengineered self-seeding heart valves. J Thorac Cardiovasc Surg 2012; 143: 201–208. [DOI] [PubMed] [Google Scholar]
- 96. Williams JK, Miller ES, Lane MR, et al. Characterization of CD133 antibody-directed recellularized heart valves. J Cardiovasc Transl Res 2015; 8: 411–420. [DOI] [PubMed] [Google Scholar]
- 97. Hopkins RA, Bert AA, Hilbert SL, et al. Bioengineered human and allogeneic pulmonary valve conduits chronically implanted orthotopically in baboons: hemodynamic performance and immunologic consequences. J Thorac Cardiovasc Surg 2013; 145: 1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Quinn RW, Bert AA, Converse GL, et al. Performance of allogeneic bioengineered replacement pulmonary valves in rapidly growing young lambs. J Thorac Cardiovasc Surg 2016; 152: 1156–1165.e4. [DOI] [PubMed] [Google Scholar]
- 99. Jana S, Tranquillo RT, Lerman A. Cells for tissue engineering of cardiac valves. J Tissue Eng Regen Med 2016; 10: 804–824. [DOI] [PubMed] [Google Scholar]
- 100. Bin F, Yinglong L, Nin X, et al. Construction of tissue-engineered homograft bioprosthetic heart valves in vitro. ASAIO J 2006; 52: 303–309. [DOI] [PubMed] [Google Scholar]
- 101. Rieder E, Kasimir MT, Silberhumer G, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg 2004; 127: 399–405. [DOI] [PubMed] [Google Scholar]
- 102. Rieder E, Seebacher G, Kasimir MT, et al. Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation 2005; 111: 2792–2797. [DOI] [PubMed] [Google Scholar]
- 103. Dainese L, Guarino A, Burba I, et al. Heart valve engineering: decellularized aortic homograft seeded with human cardiac stromal cells. J Heart Valve Dis 2012; 21: 125–134. [PubMed] [Google Scholar]
- 104. Knight RL, Booth C, Wilcox HE, et al. Tissue engineering of cardiac valves: re-seeding of acellular porcine aortic valve matrices with human mesenchymal progenitor cells. J Heart Valve Dis 2005; 14: 806–813. [PubMed] [Google Scholar]
- 105. Zeltinger J, Landeen LK, Alexander HG, et al. Development and characterization of tissue-engineered aortic valves. Tissue Eng 2001; 7: 9–22. [DOI] [PubMed] [Google Scholar]
- 106. Kim SS, Lim SH, Hong YS, et al. Tissue engineering of heart valves in vivo using bone marrow-derived cells. Artif Organs 2006; 30: 554–557. [DOI] [PubMed] [Google Scholar]
- 107. Steinhoff G, Stock U, Karim N, et al. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation 2000; 102: III50–III55. [DOI] [PubMed] [Google Scholar]
- 108. Vincentelli A, Wautot F, Juthier F, et al. In vivo autologous recellularization of a tissue-engineered heart valve: are bone marrow mesenchymal stem cells the best candidates? J Thorac Cardiovasc Surg 2007; 134: 424–432. [DOI] [PubMed] [Google Scholar]
- 109. Emmert MY, Weber B, Wolint P, et al. Stem cell-based transcatheter aortic valve implantation: first experiences in a pre-clinical model. JACC Cardiovasc Interv 2012; 5: 874–883. [DOI] [PubMed] [Google Scholar]
- 110. Weber B, Scherman J, Emmert MY, et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J 2011; 32: 2830–2840. [DOI] [PubMed] [Google Scholar]
- 111. Thayer P, Balachandran K, Rathan S, et al. The effects of combined cyclic stretch and pressure on the aortic valve interstitial cell phenotype. Ann Biomed Eng 2011; 39: 1654–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yamamoto K, Takahashi T, Asahara T, et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol 2003; 95: 2081–2088. [DOI] [PubMed] [Google Scholar]
- 113. Ahsan T, Nerem RM. Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng Part A 2010; 16: 3547–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Merryman WD, Lukoff HD, Long RA, et al. Synergistic effects of cyclic tension and transforming growth factor-beta1 on the aortic valve myofibroblast. Cardiovasc Pathol 2007; 16: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Merryman WD, Youn I, Lukoff HD, et al. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol 2006; 290: H224–H231. [DOI] [PubMed] [Google Scholar]
- 116. Rath S, Salinas M, Villegas AG, et al. Differentiation and distribution of marrow stem cells in flex-flow environments demonstrate support of the valvular phenotype. PLoS ONE 2015; 10: e0141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Balachandran K, Konduri S, Sucosky P, et al. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann Biomed Eng 2006; 34: 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schenke-Layland K, Opitz F, Gross M, et al. Complete dynamic repopulation of decellularized heart valves by application of defined physical signals-an in vitro study. Cardiovasc Res 2003; 60: 497–509. [DOI] [PubMed] [Google Scholar]
- 119. Lichtenberg A, Cebotari S, Tudorache I, et al. Flow-dependent re-endothelialization of tissue-engineered heart valves. J Heart Valve Dis 2006; 15: 287–293; discussion 293–294. [PubMed] [Google Scholar]
- 120. Lichtenberg A, Tudorache I, Cebotari S, et al. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials 2006; 27: 4221–4229. [DOI] [PubMed] [Google Scholar]
- 121. Converse GL, Buse EE, Neill KR, et al. Design and efficacy of a single-use bioreactor for heart valve tissue engineering. J Biomed Mater Res B Appl Biomater 2017; 105: 249–259. [DOI] [PubMed] [Google Scholar]
- 122. Kajbafzadeh AM, Ahmadi Tafti SH, Mokhber-Dezfooli MR, et al. Aortic valve conduit implantation in the descending thoracic aorta in a sheep model: the outcomes of pre-seeded scaffold. Int J Surg 2016; 28: 97–105. [DOI] [PubMed] [Google Scholar]
- 123. Tudorache I, Calistru A, Baraki H, et al. Orthotopic replacement of aortic heart valves with tissue-engineered grafts. Tissue Eng Part A 2013; 19: 1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hong H, Dong N, Shi J, et al. Fabrication of a novel hybrid scaffold for tissue engineered heart valve. J Huazhong Univ Sci Technolog Med Sci 2009; 29: 599–603. [DOI] [PubMed] [Google Scholar]
- 125. Stamm C, Khosravi A, Grabow N, et al. Biomatrix/polymer composite material for heart valve tissue engineering. Ann Thorac Surg 2004; 78: 2084–2092; discussion 92–93. [DOI] [PubMed] [Google Scholar]
- 126. Deng C, Dong N, Shi J, et al. Application of decellularized scaffold combined with loaded nanoparticles for heart valve tissue engineering in vitro. J Huazhong Univ Sci Technolog Med Sci 2011; 31: 88–93. [DOI] [PubMed] [Google Scholar]
- 127. Zhou J, Hu S, Ding J, et al. Tissue engineering of heart valves: PEGylation of decellularized porcine aortic valve as a scaffold for in vitro recellularization. Biomed Eng Online 2013; 12: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhou J, Nie B, Zhu Z, et al. Promoting endothelialization on decellularized porcine aortic valve by immobilizing branched polyethylene glycolmodified with cyclic-RGD peptide: an in vitro study. Biomed Mater 2015; 10: 065014. [DOI] [PubMed] [Google Scholar]
- 129. Hu XJ, Dong NG, Shi JW, et al. Evaluation of a novel tetra-functional branched poly(ethylene glycol) crosslinker for manufacture of crosslinked, decellularized, porcine aortic valve leaflets. J Biomed Mater Res B Appl Biomater 2014; 102: 322–336. [DOI] [PubMed] [Google Scholar]
- 130. Ouyang H, Zhang JB, Liu Y, et al. Research on application of modified polyethylene glycol hydrogels in the construction of tissue engineered heart valve. Zhonghua Wai Ke Za Zhi 2008; 46: 1723–1726. [PubMed] [Google Scholar]
- 131. Zhou J, Ye X, Wang Z, et al. Development of decellularized aortic valvular conduit coated by heparin-SDF-1alpha multilayer. Ann Thorac Surg 2015; 99: 612–618. [DOI] [PubMed] [Google Scholar]
- 132. Ye X, Hu X, Wang H, et al. Polyelectrolyte multilayer film on decellularized porcine aortic valve can reduce the adhesion of blood cells without affecting the growth of human circulating progenitor cells. Acta Biomater 2012; 8: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 133. Ye X, Wang H, Zhou J, et al. The effect of heparin-VEGF multilayer on the biocompatibility of decellularized aortic valve with platelet and endothelial progenitor cells. PLoS ONE 2013; 8: e54622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Theodoridis K, Tudorache I, Calistru A, et al. Successful matrix guided tissue regeneration of decellularized pulmonary heart valve allografts in elderly sheep. Biomaterials 2015; 52: 221–228. [DOI] [PubMed] [Google Scholar]
- 135. Ye X, Zhao Q, Sun X, et al. Enhancement of mesenchymal stem cell attachment to decellularized porcine aortic valve scaffold by in vitro coating with antibody against CD90: a preliminary study on antibody-modified tissue-engineered heart valve. Tissue Eng Part A 2009; 15: 1–11. [DOI] [PubMed] [Google Scholar]
- 136. Bertipaglia B, Ortolani F, Petrelli L, et al. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur). Ann Thorac Surg 2003; 75: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 137. Dohmen PM, da Costa F, Yoshi S, et al. Histological evaluation of tissue-engineered heart valves implanted in the juvenile sheep model: is there a need for in-vitro seeding? J Heart Valve Dis 2006; 15: 823–829. [PubMed] [Google Scholar]
- 138. Lu X, Zhai W, Zhou Y, et al. Crosslinking effect of Nordihydroguaiaretic acid (NDGA) on decellularized heart valve scaffold for tissue engineering. J Mater Sci Mater Med 2010; 21: 473–480. [DOI] [PubMed] [Google Scholar]
- 139. Zhai W, Chang J, Lin K, et al. Crosslinking of decellularized porcine heart valve matrix by procyanidins. Biomaterials 2006; 27: 3684–3690. [DOI] [PubMed] [Google Scholar]
- 140. Zhai W, Lu X, Chang J, et al. Quercetin-crosslinked porcine heart valve matrix: mechanical properties, stability, anticalcification and cytocompatibility. Acta Biomater 2010; 6: 389–395. [DOI] [PubMed] [Google Scholar]
- 141. Huang W, Xiao DZ, Wang Y, et al. Fn14 promotes differentiation of human mesenchymal stem cells into heart valvular interstitial cells by phenotypic characterization. J Cell Physiol 2014; 229: 580–587. [DOI] [PubMed] [Google Scholar]
- 142. Gwanmesia P, Ziegler H, Eurich R, et al. Opposite effects of transforming growth factor-beta1 and vascular endothelial growth factor on the degeneration of aortic valvular interstitial cell are modified by the extracellular matrix protein fibronectin: implications for heart valve engineering. Tissue Eng Part A 2010; 16: 3737–3746. [DOI] [PubMed] [Google Scholar]
- 143. Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 2010; 107: 4669–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Weber B, Scherman J, Emmert MY, et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J 2011; 32: 2830–2840. [DOI] [PubMed] [Google Scholar]
- 145. Prodan Z. Xeltis 1-year in vivo data of a novel bioabsorbable pulmonary heart valved conduit. In: 30th annual meeting of the European Association of Cardio-Thoracic Surgery (EACTS), Barcelona, 1–5 October 2016. [Google Scholar]
- 146. Xeltis. First heart valve that enables cardiovascular restoration successfully implanted in three patients in “Xplore-I” clinical trial. Zurich: Xeltis, 2016. [Google Scholar]
- 147. Syedain Z, Reimer J, Schmidt J, et al. 6-month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials 2015; 73: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Syedain ZH, Bradee AR, Kren S, et al. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng Part A 2013; 19: 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Syedain ZH, Meier LA, Reimer JM, et al. Tubular heart valves from decellularized engineered tissue. Ann Biomed Eng 2013; 41: 2645–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]