Abstract
Background
Blood–nerve barrier disruption is pivotal in the development of neuroinflammation, peripheral sensitization, and neuropathic pain after peripheral nerve injury. Activation of toll-like receptor 4 and inactivation of Sonic Hedgehog signaling pathways within the endoneurial endothelial cells are key events, resulting in the infiltration of harmful molecules and immunocytes within the nerve parenchyma. However, we showed in a previous study that preemptive inactivation of toll-like receptor 4 signaling or sustained activation of Sonic Hedgehog signaling did not prevent the local alterations observed following peripheral nerve injury, suggesting the implication of another signaling pathway.
Methods
Using a classical neuropathic pain model, the infraorbital nerve chronic constriction injury (IoN-CCI), we investigated the role of the Wnt/β-catenin pathway in chronic constriction injury-mediated blood–nerve barrier disruption and in its interactions with the toll-like receptor 4 and Sonic Hedgehog pathways. In the IoN-CCI model versus control, mRNA expression levels and/or immunochemical detection of major Wnt/Sonic Hedgehog pathway (Frizzled-7, vascular endothelial-cadherin, Patched-1 and Gli-1) and/or tight junction proteins (Claudin-1, Claudin-5, and Occludin) readouts were assessed. Vascular permeability was assessed by sodium fluorescein extravasation.
Results
IoN-CCI induced early alterations in the vascular endothelial-cadherin/β-catenin/Frizzled-7 complex, shown to participate in local blood–nerve barrier disruption via a β-catenin-dependent tight junction protein downregulation. Wnt pathway also mediated a crosstalk between toll-like receptor 4 and Sonic Hedgehog signaling within endoneurial endothelial cells. Nevertheless, preemptive inhibition of Wnt/β-catenin signaling before IoN-CCI could not prevent the downregulation of key Sonic Hedgehog pathway readouts or the disruption of the infraorbital blood–nerve barrier, suggesting that Sonic Hedgehog pathway inhibition observed following IoN-CCI is an independent event responsible for blood–nerve barrier disruption.
Conclusion
A crosstalk between Wnt/β-catenin- and Sonic Hedgehog-mediated signaling pathways within endoneurial endothelial cells could mediate the chronic disruption of the blood–nerve barrier following IoN-CCI, resulting in increased irreversible endoneurial vascular permeability and neuropathic pain development.
Keywords: Neuropathic pain, endothelial cells, tight junctions, toll-like receptor 4, vascular permeability, Wnt, β-catenin, Sonic Hedgehog, rat
Introduction
Peripheral nerve injuries induce multiple neuro-immune and neurovascular alterations within the nerve’s parenchyma. Most benign acute peripheral nerve injuries eventually achieve complete recovery, but in some pathological circumstances, however, nerve healing can be unsuccessful, leading to persistent neuroinflammation and the development of neuropathic pain, a type of chronic pain of very difficult and often unsuccessful management.
Recent studies1–6 have underlined the importance of early and sustained macrophage infiltration in this process. This infiltration is possible via the pathological disruption of the endoneurial blood–nerve barrier (BNB),2 a monolayer of nonfenestrated endothelial cells joined together by tight junctions (TJs) and adherens junctions (AJs) serving to protect the neural parenchyma from external insults, such as the infiltration of harmful cells and blood-borne toxins.7
In previous studies, using classical models of post-traumatic neuropathic pain in rats (namely the sciatic nerve chronic constriction injury (SN-CCI) and infraorbital nerve CCI (IoN-CCI)), we implicated the inhibition of endoneurial endothelial Sonic Hedgehog (SHH) signaling (a morphogen implicated in the regulation of blood–brain barrier homeostasis in adults8) or the activation of toll-like receptor 4 (TLR4) signaling (a pathogen-associated molecular pattern/danger-associated molecular pattern sensor of innate immunity) in the decreased production of several key TJ proteins, such as Claudin-1, Claudin-5, and Occludin. This resulted in increased local vascular permeability, local immunocytes infiltration, and neuropathic-like behavior development (mechanical allodynia) in CCI-injured rats.4,5,9 Nevertheless, neither the preemptive inhibition of TLR4 signaling nor the preemptive activation of Hedgehog signaling in healthy nerves could prevent the molecular, vascular, and behavioral changes resulting from CCI, suggesting the possibility of other players in this pathological context.
Recently, a growing body of evidence has underlined the influence and importance of the Wnt/β-catenin morphogenetic signaling pathway in vascular development, pathological angiogenesis, and vascular permeability.10–12 For instance, in mice, the genetic deletion of Frizzled-7 (Fzd-7), a receptor of Wnt proteins and activator of canonical β-catenin signaling, resulted in the disruption of AJ and increased vascular permeability in vitro and in vivo.13
The Wnt pathway is a ubiquitous signaling pathway found in most pluricellular organisms that plays major roles not only in development but also in numerous diseases in adulthood. Three signaling pathways have been described: the Wnt/β-catenin (“canonical”) pathway, the Wnt/Ca2+ pathway, and the planar cell polarity pathway. Briefly, in the Wnt/β-catenin pathway, activation of membrane Frizzled receptors by Wnt proteins induces a downstream signal transduction leading to an inhibition of the Axin/APC/GSK3β “destruction complex” that is responsible—under resting conditions—for sequestration and degradation of the β-catenin nuclear transcription factor. The nondegraded β-catenin can thus accumulate in the cytoplasm and migrate to the nucleus where it acts as a nuclear transcription factor for many essential genes.14
Whereas several recent studies have suggested the implication of Wnt signaling in neuropathic pain development,15–19 there is scarce data regarding the role of Wnt signaling in the disruption of the BNB following peripheral nerve injury.
Using a classical model of neuropathic pain in rats, the IoN-CCI, we investigated not only the possible implications of Wnt/β-catenin signaling in the early pathological disruption of the endoneurial BNB and resulting neuroinflammation but also the potential interactions of this pathway with other key vascular permeability signaling pathways (such as SHH- or TLR-mediated pathways), as previously described.4,5
Methods
Animals
Adult male Sprague Dawley rats (200–250 g) were purchased from Laboratoires Janvier (Le Genest-Saint-Isle, France) and housed in a light- and temperature-controlled environment (22 ± 1℃, 12/12 h light/dark cycle) with food and water available ad libitum. All the animal experiments performed in this study complied with French and international laws and policies regarding the use of animals in neuroscientific research (European Communities Council Directive #87848, Octobre 1987, Ministère de l’Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection animale; Authorisation #00354.02, Comité d’Ethique, Université Paris Descartes) and with the guidelines from the committee for research and ethical issues of the International Association for the Study of Pain.20
Surgery
CCI of the IoN
Rats were deeply anesthetized using 3.6% isoflurane in O2 at 3 L/min for induction and 2.8% isoflurane in O2 at 3 L/min for maintenance. Right IoN was exposed via an intraoral approach as previously described.5,21 Briefly, a 5-mm vestibular incision and full-thickness flap reflection allowed the exposure of the right IoN at the emergence of the infraorbital foramen. The nerve was further exposed using curved forceps, and two ligatures (5-0 chromic catgut) were loosely tied around the nerve without interrupting the epineurial blood flow, 2 mm apart, at approximately 2 mm from the foraminal emergence. The vestibular incision was then sewed up using 5-0 PROLENE sutures. Sham animals were subjected to the same procedure, but the IoN was only exposed without any direct contact or ligature. CCI- and sham-injured rats were sacrificed by isoflurane overdose at indicated time points after surgery (3 h, 6 h, 24 h, seven days, 14 days; n = 5–8 rats per time point), and approximately 7 mm of IoN were harvested at 2 mm from the infraorbital foramen on both injured and contralateral sides. Harvested tissue samples were immediately frozen in liquid nitrogen and stored at −80℃ until further use.
Perineural injections
To investigate the impact of Wnt/β-catenin-mediated pathways on IoN pathophysiology, PKF 118-310 (a Frizzled-independent Wnt pathway antagonist that prevents the nuclear translocation of β-catenin) was injected in the close vicinity of the right IoN using an intraoral transmucosal injection technique as previously described.5 Each injection consisted of 50 µL of either PKF 118-310 (50 μM; Sigma-Aldrich, Saint-Quentin Fallavier, France) or vehicle (NaCl 0.9%). Rats were pretreated 24 h before surgery (three injections spaced 6 h apart), with the last injection occurring during the surgical procedure (one half of injection volume deposited on the exposed nerve and the other half injected transmucosally after suture of the oral mucosa). Evaluation of resulting molecular alterations and/or BNB permeability was performed 3 h after last perineural injection. After sacrifice, IoN samples were harvested and immediately frozen in liquid nitrogen then stored at −80℃, except for BNB permeability assessment (see “Evaluation of BNB permeability” section).
All the procedures described above were performed by the same investigator (NM) so as to ensure the homogeneity and comparability of resulting data.
Evaluation of BNB permeability
BNB permeability was assessed using sodium fluorescein (NaFlu) as previously described.4,5,22 Briefly, in rats subjected to CCI or perineural injections, NaFlu was injected intravenously (NaFlu, MW 376, 10% in saline, 2 mL/kg; Sigma-Aldrich, Saint-Quentin Fallavier, France) into the jugular vein under deep anesthesia (sodium pentobarbital 50 mg/kg, i.p.; Sanofi, Libourne, France) 3 h after the end of the corresponding surgical procedure and allowed to circulate for 30 min. Rats were then sacrificed using a transcardial 100 mL perfusion of 0.1% (w/v) paraformaldehyde (PAF; Sigma-Aldrich, Saint-Quentin Fallavier, France) in 1X phosphate-buffered saline (PBS; Sigma-Aldrich, Saint-Quentin Fallavier, France). Approximately 7 mm of IoN (at 2 mm from its foraminal emergence) from both injured and contralateral sides were harvested and post-fixed in 4% PAF overnight, then transferred to 20% (w/v) sucrose in PBS 1X for cyroprotection and frozen in isopentane at −30℃. Frozen IoN samples were embedded in Tissue-Tek O.C.T compound (Sakura, Villeneuve d'Ascq, France) and sliced both longitudinally and axially into 30 µm-thick sections using a cryostat microtome (Leica CM3050S, Leica Microsystems, Nanterre, France). Sections were then mounted on glass slides and left to dry before being covered with Fluoromount-G mounting medium (Southern Biotech, Nanterre, France) and a glass coverslip. IoN sections were examined using fluorescence microscopy imaging with a Leica TCS SP5 II confocal microscope (Leica Microsystems, Nanterre, France).
Cell cultures
Immortalized human cerebral microvascular endothelial cells (hCMEC/D3) were kindly provided by Dr. P.-O. Couraud (INSERM U1016, France). The cells were cultured in T75 tissue culture flasks (BD Biosciences, Le Pont de Claix, France) in endothelial cell basal medium-2 (Lonza, Levallois-Perret, France) supplemented with 5% heat-inactivated fetal bovine serum (Sigma-Aldrich, Saint-Quentin Fallavier, France), 10 U/mL penicillin, 10 mg/mL streptomycin (Gibco, Villebon sur Yvette, France), 1.4 mM hydrocortisone (Sigma-Aldrich, Saint-Quentin Fallavier, France), 5 mg/mL ascorbic acid (Sigma-Aldrich, Saint-Quentin Fallavier, France), 1:100 chemically defined lipid concentrate (Gibco, Villebon sur Yvette, France), 10 mM HEPES (Sigma-Aldrich, Saint-Quentin Fallavier, France), and 1 ng/mL human basic fibroblast growth factor (Sigma-Aldrich, Saint-Quentin Fallavier, France) and maintained at 37℃ in a humidified incubator under 5% CO2/95% O2. Cells were passaged once weekly or at near 100% confluence: culture medium was removed, and cells were washed with PBS 1X (Gibco, Thermo Fisher Scientific, Villebon-sur-Yvette, France) before adding 5 mL of 0.05% trypsin-EDTA 1X (Gibco, Thermo Fisher Scientific, Villebon-sur-Yvette, France) and left to incubate 5 min at 37℃. Cells were then harvested and centrifuged 5 min at 1000 r/min; the cell pellet was resuspended in 10 mL culture medium for plating in a new T75 flask in a 1:10 ratio with fresh medium. For pharmacological experiments and mRNA expression levels assessment, 6-well plates were coated with 150 mg/mL cultured rat collagen-I (R&D systems, Minneapolis, MN, USA) diluted in water and incubated at 37℃ for 1 h. Cells were then seeded at a density of 80,000 cells per square centimeter and further incubated for 24 h before the experiments. Cells were then incubated for 3 h with Wnt agonist I (5 μM; Calbiochem, Merck, Darmstadt, Germany; a Frizzled-independent agonist of Wnt/β-catenin nuclear signaling), PKF 118-310 (2 μM; Sigma-Aldrich, Saint-Quentin Fallavier, France), or lipopolysaccharide (LPS) (300 ng/mL; Sigma-Aldrich, Saint-Quentin Fallavier, France). To investigate the impact of Wnt signaling on LPS-mediated molecular alterations in vitro, cells were incubated 30 min with PKF 118-310 (2 μM; Sigma-Aldrich, Saint-Quentin Fallavier, France) then challenged 4 h with LPS (200 ng/mL; Sigma-Aldrich, Saint-Quentin Fallavier, France).
Following the treatment period, cells were washed in PBS 1X, and total RNAs were extracted for reverse transcription polymerase chain reaction (RT-PCR) experiments (see below). Cells used for immunohistochemistry experiments were treated as described above then fixed in PAF 4% and processed as described in the “Immunohistochemistry” section.
RNA extraction and semi-quantitative RT-PCR
Total RNAs from IoNs or cultured cells were extracted using NucleoSpin RNA II Purification kit (Macherey-Nagel, Hoerdt, France), and a NanoDrop spectrophotometer (ND-1000, ThermoFisher Scientific, Villebon-sur-Yvette, France) was used to assess quality and RNA concentrations from absorbance measurements. For real-time RT-PCR analysis, first-strand complementary DNA (cDNA) synthesis from extracted RNA was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Courtaboeuf, France) in accordance to manufacturer’s instructions. Reverse transcription was followed by real-time PCR amplification of each sample in triplicate using TaqMan Universal PCR Master Mix (Applied Biosystems, Courtaboeuf, France), on an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Courtaboeuf, France). The following primers, purchased from Life Technologies (Villebon-sur-Yvette, France), were used in the present experiments: ribosomal protein S18 (RPS18; Rn01428913_gH), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Rn99999916_s1), Claudin-1 (Rn00581740_m1), Claudin-5 (Rn01753146_s1), Occludin (Rn00580064_m1, Hs00170162_m1), Patched-1 (Rn01527980_m1), glioma-associated oncogene-1 (Gli-1; Rn01504237_m1), Fzd-7 (Rn01441541_m1, HS00275833_s1), β-catenin (Rn01504237_m1, Hs00258305_m1), vascular endothelial-cadherin (VE-cadherin; Rn01405893_m1, Hs00170986_m1), vascular endothelial growth factor-A (VEGF-A;Rn01511601_m1), toll-like receptor 2 (TLR2; Rn02133647_s1), CD11b (Rn00709342_s1), chemokine (CC motif) ligand 2 (CCL2; Rn00580555_m1), and hypoxia-inducible factor-1α (HIF-1α; Rn005777560_m1). Specific mRNA levels were calculated after normalization of the results for each sample with those for GADPH or RSP18 mRNAs. Data are presented as relative mRNA units with respect to control values (expressed as fold over control value).
Protein extraction and quantification (enzyme-linked immunosorbent assay)
To assess the levels of VEGF-A protein within the IoN, nerves from rats subjected to CCI were harvested at different time points (6 h, 24 h, 48 h, seven days) using the same tissue collection methodology as for RNA extraction (see corresponding sections for details). Total proteins and specific VEGF-A protein levels were assessed in IoN-CCI nerve samples in triplicate using a specific enzyme-linked immunosorbent assay (ELISA) kit (Rat VEGF-A ELISA kit, EKB01917; Biomatik, Wilmington, DE, USA) according to manufacturer’s instructions.
VEGF-A protein levels are expressed as the ratio of the quantity of specific protein (VEGF-A) in picograms (pg) over the quantity of total proteins (in pg).
Immunohistochemistry
For immunohistochemistry experiments, IoNs of sacrificed rats were harvested as previously described (see “Evaluation of BNB permeability” section), or hCMEC/D3 cells were fixed in PAF 4% following specific pretreatment (see “Cell cultures” section for details) and deposited on glass slides. Slide-mounted 30 µm-thick tissue sections (or cells) were incubated in a blocking solution (3% horse serum, 0.3% Triton X-100 in PBS) for 1 h at room temperature. They were then incubated overnight at 4℃ with rabbit anti-Reca 1 antibody (1:250; AbD Serotec, Kidlington, UK) or rabbit anti-vWF antibody (von Willebrand Factor; 1:500; Abcam, Paris, France) as endothelial markers and goat anti-Fzd-7 antibody (1:400; Santa Cruz, Heidelberg, Germany), mouse anti-VE-cadherin antibody (1:250; Santa Cruz, Heidelberg, Germany), or mouse anti-β-catenin antibody (1:800; BD Bioscience, Le Pont de Claix, France). Slides were then washed three times in 1X PBS and subsequently incubated with Alexa 488- or Alexa 555-conjugated donkey anti-rabbit and donkey anti-goat antibodies (Life technologies, Villebon-sur-Yvette) diluted in PBS (1:600) then washed two times, incubated in TO-PRO 3 (nuclear counterstain; 1:5000; ThermoFischer Scientific, Villebon-sur-Yvette, France) for 5 min and washed once again. Slides were then covered with Fluoromount-G mounting medium (Southern Biotech, Nanterre, France) and a glass coverslip and examined by fluorescence microscopy using a Leica TCS SP5 II confocal microscope (Leica Microsystems, Nanterre, France).
Data and statistical analyses
All data are presented as mean ± standard error of the mean. For RT-PCR experiments, the 2−ΔΔCt method23 was used to analyze the relative differences in specific mRNA levels between groups (RQ Study Software 1.2 version; Applied Biosystems, Courtaboeuf, France); data were then analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test (for time-dependent mRNA expression changes), Kruskal–Wallis test followed by Dunn’s multiple comparison test (for other conditional mRNA expression changes), or Mann–Whitney test when appropriate. For protein quantification, specific protein levels at selected time points were compared to control levels using one-way ANOVA followed by Bonferroni post hoc test. GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analyses. Level of significance was set at p < 0.05.
Results
IoN-CCI induces early and transient alterations in the endoneurial endothelial AJ complex in association with a transient increased production of VEGF-A protein in the rat’s IoN
In order to investigate the possible implications of the Wnt/β-catenin signaling pathway in the increased vascular permeability following IoN-CCI, we explored the expression levels of key mRNAs implicated in the endothelial AJ complex, i.e. the functional tripartite association of endothelial membrane receptor Fzd-7 (a key Wnt pathway receptor) with VE-cadherin (the main endothelial AJ protein) and intracellular β-catenin (the main effector of canonical Wnt signaling) attached to the cytoplasmic tail of VE-cadherin.13,24 Present investigations were restricted to the early events following IoN-CCI, i.e. the functional and molecular alterations during the first two weeks following IoN injury as described in the initial model.25
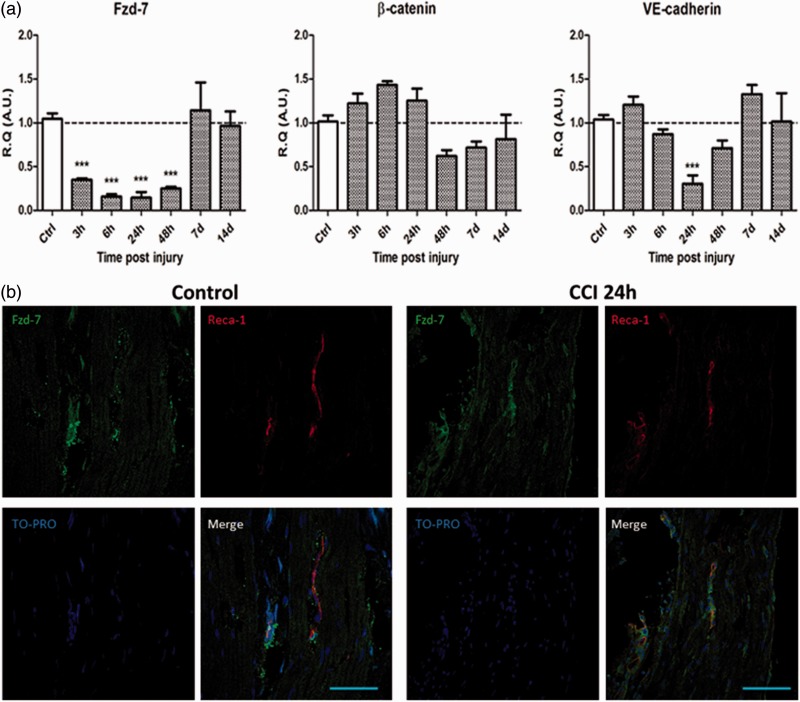
Following IoN-CCI, a significant downregulation of Fzd-7 mRNA could be observed as early as 3 h and sustained till at least 48 h post-CCI. A significant downregulation of VE-cadherin mRNA expression levels could also be observed at 24 h post-CCI. No significant modifications of β-catenin mRNA expression levels could be observed in the studied timeframe (Figure 1(a)).
Figure 1.
Chronic constriction injury of the infraorbital nerve (IoN-CCI) induces early and transient alterations in the Fzd-7/β-catenin/VE-cadherin AJ complex within endoneurial endothelial cells in association with a transient increased production of VEGF-A protein in the infraorbital nerve. (a) IoN-CCI induces early and transient significant downregulation of Fzd-7 and VE-cadherin mRNAs and early upregulation followed by transient downregulation of β-catenin mRNA expression levels. Changes over time of Fzd-7, β-catenin, and VE-cadherin mRNA levels were assessed in the IoN of sham- or CCI-injured animals using semi-quantitative RT-PCR analyses. Data are presented as relative quantification (R.Q.) in arbitrary units (A.U.) corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 5–8 animals for each time point (post-injury); *p < 0.05, **p < 0.01, ***p < 0.001. A one-way ANOVA followed by Bonferroni post hoc test was used. (b, c) IoN-CCI induces a decrease in Fzd-7 immunoreactivity in endoneurial blood vessels compared to control condition (sham-injured animals) at 24 h post-CCI in longitudinal slices of the injured IoN (b) and in axial slices centered on an endoneurial blood vessel (Scale bar = 100 µm) (c). No Fzd-7 immunoreactivity was observed outside of the endoneurial endothelial cells. Paraformaldehyde-fixed slices of IoN from sham- and CCI-injured rats were incubated with TO-PRO (nuclear stain, blue), anti-Reca 1 antibodies (endothelial marker, red), and anti-Fzd-7 antibodies (green) (Scale bar = 10 µm). (d) IoN-CCI induces a decrease in VE-cadherin immunoreactivity in endoneurial blood vessels compared to control condition (sham-injured animals) at 24 h post-CCI in axial slices of the injured IoN. Paraformaldehyde-fixed slices of IoN from sham- and CCI-injured rats were incubated with TO-PRO (nuclear stain, blue), anti-Reca 1 antibodies (endothelial marker, red), and anti-VE cadherin antibodies (green) (Scale bar = 10 µm). (e) IoN-CCI induces a transient increase in VEGF-A mRNA expression levels at 24 h followed by a significant downregulation between 48 h and seven days (left figure) associated with a significant increase in VEGF-A protein production between 24 h and 48 h post-CCI (right figure). Changes over time of VEGF-A mRNA levels were assessed in the IoN of sham- or CCI-injured animals using semi-quantitative RT-PCR analyses. Data are presented as R.Q. in A.U. corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 5–8 animals for each time point (post-injury); *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA followed by Bonferroni post hoc test was used.
Changes over time in VEGF-A protein production in CCI-injured animals (compared to sham-injured animals) were assessed using an enzyme-linked immunosorbent assay (ELISA) technique. Data are presented as the ratio of VEGF-A protein (in pg) over total proteins (in µg). Each bar corresponds to n = 3 rats for each time point (post-injury); *p < 0.05; one-way ANOVA followed by Bonferroni post hoc test was used.
VE: vascular endothelial; AJ: adherens junction; VEGF-A: vascular endothelial growth factor-A; RT-PCR: reverse transcription polymerase chain reaction; SEM: standard error of the mean; Fzd-7: Frizzled-7; ANOVA: analysis of variance.
Fzd-7-like immunoreactive material (in green) could be observed in longitudinal slices of IoNs submitted to CCI (24 h post-injury) or sham injury (control), which colocalized with Reca-1-stained perineurial and endoneurial cells (in red), confirming the endothelial expression of the receptor in the present experimental conditions. Furthermore, global Fzd-7 immunoreactivity was notably lower in the CCI condition (24 h post-CCI) than in sham-injured animals (control) (Figure 1(b)). This was confirmed in axial slices of endoneurial blood vessels, where Fzd-7 immunoreactivity was also clearly lower in the CCI condition (24 h post-CCI) than in sham-injured animals investigated in the same time point (Figure 1(c)). Similar alterations in VE-cadherin immunoreactivity were observed in the CCI condition (24 h post-CCI) compared to sham-injured animals, using the same methodology (Figure 1(d)).
These results suggest a decreased production of Fzd-7 and VE-cadherin, in endoneurial endothelial cells (EECs) of IoNs at 24 h post-CCI.
A study focusing on the role of Fzd-7 as a key regulator of endothelial vascular permeability has suggested that lack of Fzd-7 expression in KO-mice was responsible for the disorganization of endothelial AJ, resulting not only in the separation and spacing of adjacent endothelial cells but also in diminished VE-cadherin and Claudin-5 expression levels13 in accordance with other authors.24,26 In our previous study focusing on the endoneurial endothelial molecular alterations following IoN-CCI, a significant decrease in TJ protein Claudin-5 was evidenced as early as 3 h post-CCI and sustained for at least 14 days.5 Together with the present results, these data provide evidence of significant alterations in AJ and TJ architecture in endoneurial blood vessels of IoNs submitted to CCI.
To further elucidate the mechanisms implicated in the disruption of the AJ complex and subsequent increased vascular permeability, we investigated the potential implication of VEGF-A, a key permeability-inducing factor, known to induce VE-cadherin phosphorylation and internalization, resulting in the dissociation of associated β-catenin,27 allowing its nuclear translocation and subsequent inhibition of Claudin-5 promoter activity.24
Following IoN-CCI, a significant increase in VEGF-A mRNA expression levels was observed at 24 h post-CCI. An increased production of VEGF-A was also observed between 6 h and 48 h (reaching statistical significance at 48 h post-CCI) (Figure 1(e)). The timing for increased VEGF-A protein production coincided with the lowest VE-cadherin and Fzd-7 expression levels (namely, at 24 h post-CCI), coherent with a VEGF-mediated VE-cadherin downregulation.
In vitro, activation of Wnt/β-catenin pathway downregulates VE-cadherin mRNA, whereas inhibition of Wnt/β-catenin pathway upregulates VE-cadherin/Fzd-7 complex in the hCMEC/D3 cell line
To further investigate the implication of Wnt/β-catenin pathway in the increased vascular permeability following IoN-CCI, we explored whether Wnt signaling could modulate the expression levels of key elements of the AJ complex in vitro, in immortalized hCMEC/D3 cell line, a validated blood–brain barrier model.
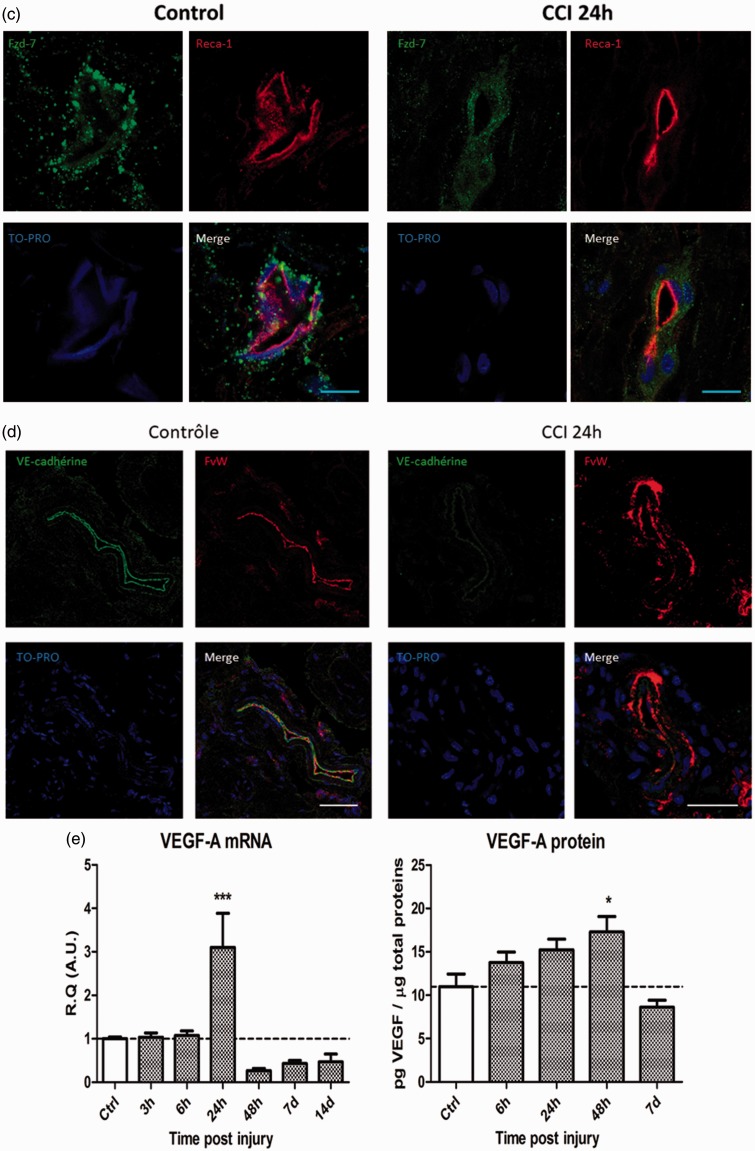
Confocal microscopy imaging of confluent hCMEC/D3 cells showed a cytoplasmic accumulation of Fzd-7-like immunofluorescence (green) in association with a membrane β-catenin immunofluorescence (red) under basal conditions (Figure 2(a)) confirming the presence of Fzd-7 within this endothelial cell line.
Figure 2.
In vitro, in the human cerebral microvascular endothelial cell line (hCMEC/D3), modulation of Wnt/β-catenin pathway induces significant expression changes in the VE-cadherin/Fzd-7/β-catenin complex mRNAs levels. (a) Presence of functional Frizzled-7 within the hCMEC/D3 endothelial cells was assessed using immunofluorescent labeling of Frizzled-7, β-catenin, and TO-PRO observed with confocal microscopy imaging. Paraformaldehyde-fixed cells were incubated with anti-Frizzled-7 antibodies (green), anti-β-catenin (cell membrane surrogate marker, red), and TO-PRO (nuclear stain, blue) (Scale bar = 20 µm). (b) Changes in Frizzled-7, β-catenin, and VE-cadherin mRNAs expression levels were assessed in vitro following stimulation with either Wnt agonist I (5 μM; Wnt pathway agonist) or PKF 118-310 (2 μM; Wnt pathway antagonist). Data are presented as relative quantification (R.Q.) in arbitrary units (A.U.) corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 4 experiments for each condition; *p < 0.05, **p < 0.01, ***p < 0.001; Kruskal–Wallis test followed by Dunn’s multiple comparisons test was used.
VE: vascular endothelial; SEM: standard error of the mean.
Activation of Wnt/β-catenin pathway (using Wnt agonist I; 5 μM) resulted in a trend toward VE-cadherin mRNA downregulation, whereas inhibition of Wnt/β-catenin pathway (using PKF 118-130; 2 μM) induced a significant downregulation of β-catenin mRNA levels. Furthermore, a significant upregulation of Fzd-7 and VE-cadherin mRNA expression levels could be observed in PKF 118-310-treated cells as compared to Wnt agonist I-treated cells (Figure 2(b)).
These results suggest that Wnt/β-catenin pathway could modulate endothelial VE-cadherin/Fzd-7/β-catenin complex mRNA expression levels in vitro. Furthermore, when comparing the molecular alterations observed following in vitro treatment with Wnt agonist I and PKF 118-310, with in vivo mRNA expression levels following IoN-CCI, these results also suggest that IoN-CCI could lead to the activation of Wnt/β-catenin signaling.
In vitro, activation of Wnt/β-catenin pathway in hCMEC/D3 endothelial cells downregulates the mRNAs of key Hedgehog pathway readouts and mediates the molecular changes observed following TLR4 stimulation
Previous studies focusing on the interactions between VE-cadherin and Claudin-5 have suggested close interactions between AJ proteins and TJ proteins in the regulation of vascular permeability.24,26 We therefore sought to investigate whether the Wnt/β-catenin pathway could be implicated in the regulation of TJ proteins expression levels, possibly via interactions with SHH- and/or TLR4-mediated signaling pathways, both of which having previously been implicated in TJ proteins expression changes in vitro (in hCMEC/D3 cells) and in vivo.4
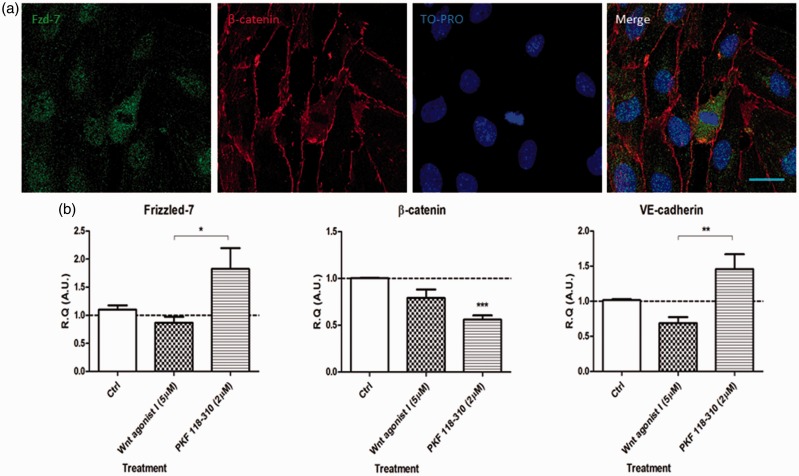
In vitro, in the hCMEC/D3 cell line, activation of Wnt pathway (using Wnt agonist I; 5 μM) resulted in a significant downregulation of key effectors of the Hedgehog pathway mRNAs, namely the transcription factor Gli-1 and the receptor Patched-1 (Figure 3(a)).
Figure 3.
In vitro, in the hCMEC/D3 cell line, activation of Wnt/β-catenin pathway downregulates the mRNAs of key Hedgehog pathway readouts and mediates the molecular changes observed following TLR4 stimulation. (a) Changes in Gli-1 or Patched-1 mRNAs expression levels were assessed in vitro following stimulation with either Wnt agonist I (5 μM; Wnt pathway agonist) or PKF 118-310 (2 μM; Wnt pathway antagonist). Data are presented as relative quantification (R.Q.) in arbitrary units (A.U.) corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 4 experiments for each condition; *p < 0.05, **p < 0.01, ***p < 0.001; Kruskal–Wallis test followed by Dunn’s multiple comparisons test was used. (b) In vitro, following pretreatment with LPS (a TLR4 agonist), an important increase in β-catenin immunoreactivity could be observed at the cell membrane and in the nuclear and perinuclear regions observed in confocal microscopy imaging, suggestive of Wnt pathway activation. Paraformaldehyde-fixed cells were incubated with anti-β-catenin antibodies (green) and TO-PRO (nuclear stain, blue) (Scale bar = 30 µm). (c) Changes in Claudin-5, Gli-1, and TLR2 mRNAs expression levels were assessed in vitro, following LPS stimulation or PKF 118-310 pretreatment followed by LPS stimulation. PKF 118-310 pretreatment strongly mitigated the changes in mRNA expression levels of all three markers, suggesting that TLR4-mediated molecular changes (LPS stimulation) are mediated by active Wnt signaling. Data are presented as R.Q. in A.U. corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 4 experiments for each condition; *p < 0.05, **p < 0.01, ***p < 0.001; Kruskal–Wallis test followed by Dunn’s multiple comparisons test was used.
hCMEC/D3: human cerebral microvascular endothelial cells; TLR4: toll-like receptor 4; SEM: standard error of the mean; LPS: lipopolysaccharide.
Furthermore, following a pretreatment with TLR4 agonist LPS, nuclear and perinuclear accumulation of β-catenin (green) could be observed in hCMEC/D3 cells using immunofluorescence confocal microscopy (Figure 3(b)), suggestive of Wnt/β-catenin pathway activation14 following TLR4-mediated pathway activation. To confirm these results, we investigated whether inhibition of Wnt pathway (using PKF 118-130; 2 μM) could mitigate the molecular alterations observed following TLR4 stimulation (using LPS; 300 ng/mL). Indeed, in vitro, treatment with PKF 118-310 reversed the molecular alterations resulting from LPS stimulation, such as the TJ protein Claudin-5, the SHH pathway readout Gli-1, and the inflammatory marker TLR2 (Figure 3(c)).
Globally, these results indicate that the Wnt/β-catenin pathway could mediate the previously demonstrated crosstalk between TLR4 activation and SHH pathway repression.4 Furthermore, active Wnt signaling could mediate the molecular changes (such as TJ protein downregulation) following an inflammatory challenge (LPS).
In vivo, inhibition of Wnt/β-catenin pathway does not prevent the early molecular and vascular alterations following IoN-CCI
As active Wnt/β-catenin pathway results in SHH pathway effectors downregulation in vitro, and considering our previous results that showed a major contribution of Hedgehog pathway inhibition in the molecular, vascular, and behavioral changes following CCI, we then focused our investigations on the impact of active Wnt/ β-catenin signaling on CCI-mediated molecular alterations in vivo in the IoN-CCI model.
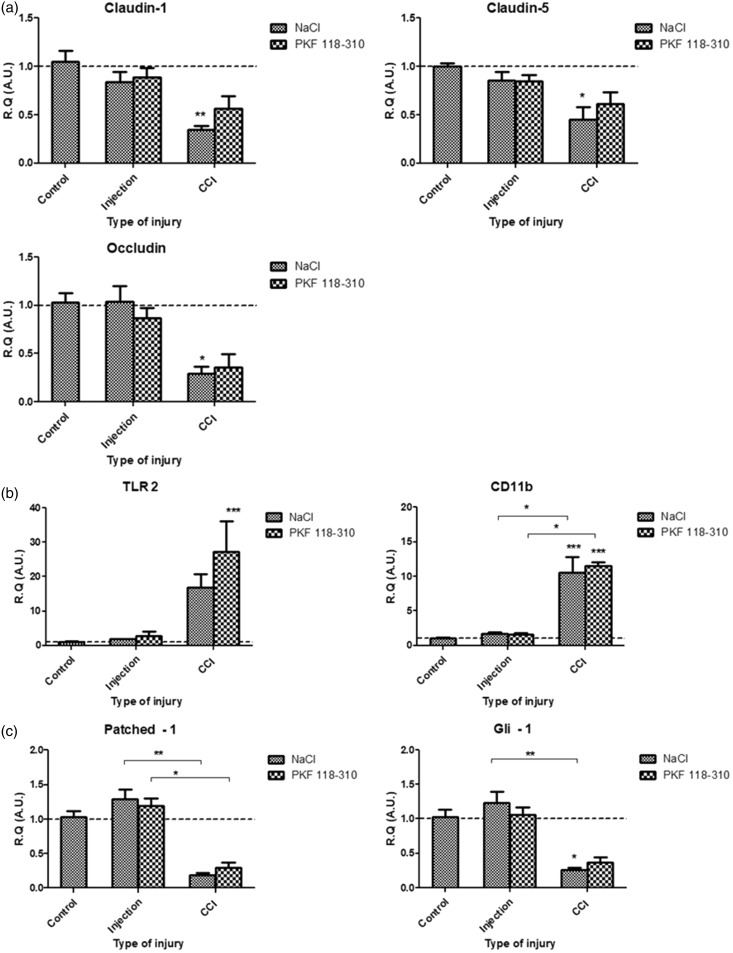
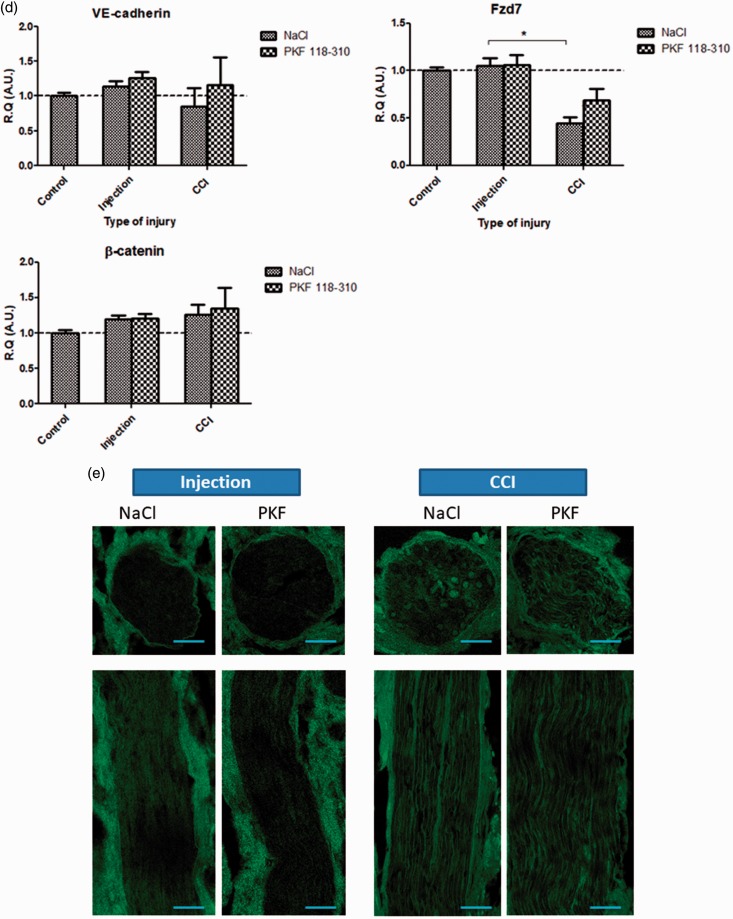
In vivo, at 3 h post-injury, inhibition of Wnt signaling (via preemptive perineural injections of PKF 118-310) could not mitigate the mRNA levels of TJ protein Claudin-1, Claudin-5, and Occludin (Figure 4(a)); inflammatory marker TLR2; macrophage marker CD11b (Figure 4(b)); Hedgehog pathway readouts Patched-1 and Gli-1 (Figure 4(c)); and AJ complex elements VE-cadherin, Fzd-7, and β-catenin (Figure 4(d)) following IoN-CCI. Furthermore, preemptive inhibition of Wnt signaling (via PKF 118-310 perineural injections) could not prevent the increased vascular permeability, evidenced as intraparenchymal accumulation of NaFlu in the IoN, following CCI, as compared to vehicle (NaCl 0.9%) perineural injections (control condition). Noninjured nerves subjected to PKF 118-310/NaCl 0.9% injections (Injection) served as indicators of baseline fluorescence (Figure 4(e)). These results suggest that inhibition of Wnt/β-catenin pathway cannot prevent the molecular and vascular alterations resulting from IoN-CCI.
Figure 4.
In vivo, at 3 h post-injury, inhibition of Wnt/β-catenin signaling could not prevent the molecular and vascular alterations following infraorbital nerve chronic constriction injury (IoN-CCI). (a to d) Changes in mRNAs expression levels of TJ proteins Claudin-1, Claudin-5, Occludin (a), inflammatory markers TLR2 and CD11b (b), Hedgehog pathway markers Patched-1 and Gli-1 (c), and Fzd-7/ β-catenin/VE-cadherin AJ complex proteins (d) were assessed using semi-quantitative RT-PCR following either perineural injections (used as a control condition), or infraorbital nerve chronic constriction injury (CCI) following either PKF 118-310 (50 μM; Wnt pathway antagonist) or NaCl 0.9% injections (three injections, spaced 6 h apart, starting 24 h before injury), as compared to noninjured IoN of naïve rats (serving as baseline values for mRNA levels comparisons). Data are presented as relative quantification (R.Q.) in arbitrary units (A.U.) corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 5–8 animals for each time point (post-injury); *p < 0.05, **p < 0.01, ***p < 0.001. Kruskal–Wallis test followed by Dunn’s multiple comparisons test was used. (e) Fluorescence microscopy observation of paraformaldehyde-fixed axial and longitudinal slices of right IoNs harvested from NaFlu intravenously injected rats submitted to perineural injection or CCI following either PKF 118-310 (Wnt pathway antagonist) or NaCl 0.9% injections (three injections, spaced 6 h apart, starting 24 h before injury) showed an increase in NaFlu fluorescence within the IoN parenchyma following IoN-CCI in both PKF 118-310 and NaCl 0.9% pretreated rats (as compared to control conditions), suggesting that Wnt/β-catenin inhibition could not mitigate the vascular alterations following IoN-CCI (Scale bar = 50 µm).
TJ: tight junction; TLR2: toll-like receptor 2; VE: vascular endothelial; Fzd-7: Frizzled-7; AJ: adherens junction; RT-PCR: reverse transcription polymerase chain reaction; NaFlu: sodium fluorescein.
As Wnt/β-catenin pathway inhibition did not mitigate the downregulation of SHH pathway readouts (Figure 4(c)) and considering the previously shown role of SHH pathway inhibition in BNB disruption,5 both pathways could independently modulate the expression of key TJ proteins (such as Claudin-5) leading to BNB disruption and eventually neuropathic pain development.
Discussion
Wnt/β-catenin pathway is implicated in the disruption of the endoneurial BNB following IoN-CCI
Peripheral nerve injuries mediate numerous local molecular changes implicated not only in the physiological healing process but also in the pathological alterations resulting in neuropathic pain development. Disruption of the endoneurial BNB following peripheral nerve injury has been shown to mediate a local increase in vascular permeability, allowing the passage of harmful molecules and immunocytes, resulting in local neuroinflammation, peripheral sensitization, and neuropathic pain.2,4,5 On a molecular level, this disrupted endothelial barrier stems from a major downregulation of key TJ proteins (such as Claudin-1, Claudin-5, and Occludin) within the EECs, under the regulation of at least two key signaling pathways, namely active TLR4 signaling or inactive SHH signaling.4,5 Nevertheless, as inactive TLR4 signaling and/or active SHH signaling do not mitigate the molecular alterations in the endoneurial BNB following peripheral nerve injury, other signaling pathways could be implicated in such molecular alterations.
The present study focused on the potential implications of the Wnt/β-catenin pathway, a morphogenetic pathway implicated in the regulation of vascular permeability, in the molecular alterations following IoN-CCI, a classical preclinical model of post-traumatic trigeminal neuropathic pain.
Several recent studies have underlined an important role of Wnt signaling in the development of neuropathic pain. For instance, an upregulation of β-catenin and Wnt3a was observed in the spinal dorsal horn following L5 spinal nerve ligation28 and CCI,29 suggesting that Wnt pathway activation in the spinal cord could be a common essential event in neuropathic pain development.15 Furthermore, activation of Wnt pathway could induce an increased production of pro-inflammatory cytokines interleukin-18 and tumor necrosis factor alpha in the spinal cord that participates in the development of neuropathic pain.29 Nevertheless, little attention has been given to the implication of Wnt signaling in the microvascular alterations observed following peripheral nerve injury.
In the present study, following IoN-CCI, an early and sustained downregulation of Fzd-7, a key effector of Wnt signaling and major regulator of vascular permeability,13 could be observed in association with a downregulation of the major AJ protein VE-cadherin within the EECs. Furthermore, a transient increased production of VEGF protein between 6 h and 48 h post-injury could be observed in the parenchyma of IoNs subjected to CCI. This key pro-permeability factor mediates an increase in vascular permeability via the internalization of VE-cadherin, resulting in the disruption of endothelial AJ and TJ.24,27 Interestingly, a previous study has shown the major implication of VEGF signaling in peripheral sensitization and neuropathic pain development,30 whereas another study has shown that the BNB disruption observed following partial ligation of the sciatic nerve could be mediated by a local release of VEGF from resident macrophages.2
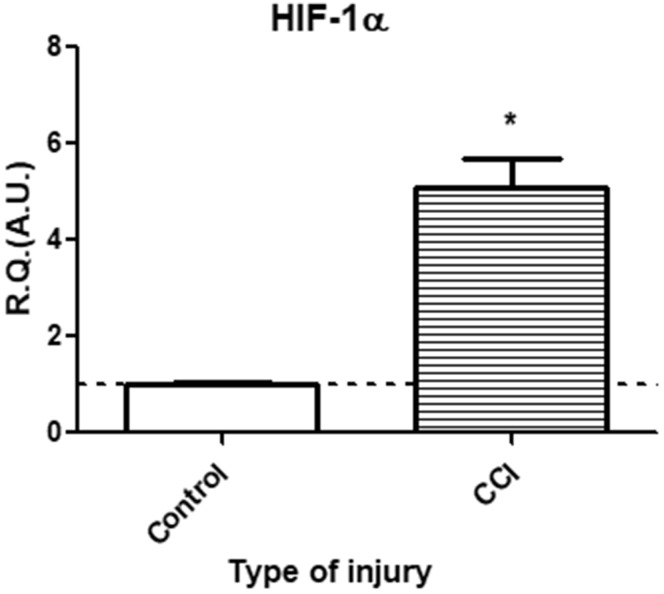
In vitro, in a model of blood–brain barrier endothelial cells, activation of Wnt/β-catenin signaling mediated not only a downregulation in key readouts of the Hedgehog pathway (namely Patched-1 and Gli-1) but also the molecular alterations resulting from TLR4 stimulation, suggesting that, in this model, Wnt/β-catenin signaling could play a major role in the crosstalk between TLR4 and SHH signaling pathways. Furthermore, comparison of in vivo (Figure 1(a)) and in vitro (Figure 2(b)) mRNA expression levels of key Wnt pathway readouts suggests that IoN-CCI could lead to Wnt/β-catenin pathway activation. This activation could result from increased local hypoxia within the IoN, as suggested by a significant increase in HIF-1α mRNA observed at 3 h following IoN-CCI (Figure 5) (also observed in our previous results in a sciatic CCI model4). Indeed, previous studies have shown an activation of Wnt/β-catenin pathway under hypoxic conditions and an increase in vascular permeability in response to an hypoxic insult.31,32 Furthermore, a recent study by Lim et al.33 has underlined the role of endoneurial hypoxia resulting from peripheral nerve injury in the genesis of neuropathic pain.
Figure 5.
In vivo, at 3 h post-injury, CCI of the infraorbital nerve elicited a significant increase in hypoxia marker HIF-1α mRNA expression levels as compared to sham-injured animals. Changes in HIF-1α mRNA levels were assessed in the IoN of CCI-injured animals compared to sham-injured controls, using semi-quantitative RT-PCR analyses. Data are presented as relative quantification (R.Q.) in arbitrary units (A.U.) corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 5–6 animals for each condition; *p < 0.05; Mann–Whitney test was used.
CCI: chronic constriction injury; HIF-1α: hypoxia-inducible factor-1α; IoN: infraorbital nerve; RT-PCR: reverse transcription polymerase chain reaction; SEM: standard error of the mean.
In vivo, inhibition of Wnt/β-catenin signaling could not mitigate the molecular and vascular alterations observed following IoN-CCI, suggesting that active Wnt/β-catenin signaling is not alone sufficient to mediate the molecular and vascular alterations in this neuropathic pain model. This is consistent with our previous research that showed a significant role of the inhibition of endoneurial endothelial SHH signaling following sciatic nerve and IoN-CCI models.4,5 Therefore, activation of Wnt/β-catenin and concurrent inhibition of SHH pathways could be required for CCI-induced infraorbital BNB disruption.
An endoneurial endothelial Wnt/SHH pathways crosstalk could mediate the molecular alterations following IoN-CCI
In an previous study, we had shown that the CCI of the sciatic nerve was associated with a transient increase in SHH protein mRNA expression level associated with a concurrent significant downregulation of key downstream effectors Patched-1 and Gli-1, suggesting that inhibition of Hedgehog signaling following CCI does not result from decreased production of SHH but rather from downregulation of underlying effectors.4 Furthermore, downregulation of VE-cadherin is associated with a disorganization of AJ and TJ proteins at the cell surface24 and subsequent loss of cell polarity,34 leading to loss of primary cilium in the cell,35 a vital component of the endothelial cell membrane, mandatory for functional Hedgehog signaling. Therefore, downregulation of VE-cadherin resulting from CCI could induce the loss of endothelial cell polarity, subsequent loss of primary cilium, and inhibition of Hedgehog signaling.35
VE-cadherin thus seems to play a central role in the regulation of endoneurial endothelial vascular permeability, similarly to that of N-cadherin in neural progenitor cells, which is at the center of the Wnt/Hedgehog crosstalk observed in these cells.36
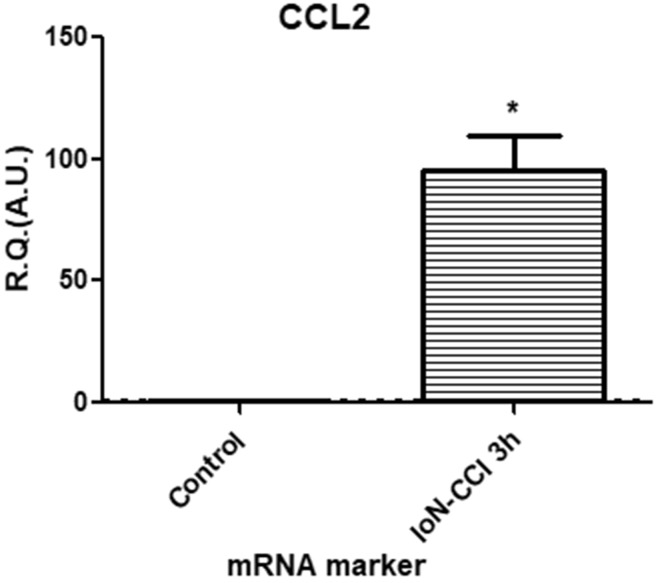
In the CCI model, alterations in the epineural blood flow resulting from the loose ligatures around the nerve induce focal nerve ischemia, an important initial pathogenic mechanism in the development of the painful phenotype.37 The resulting hypoxia, as confirmed in the present study using the HIF-1α marker (Figure 5), could trigger the release of VEGF by resident macrophages2 leading to the phosphorylation and subsequent internalization of VE-cadherin.27,38 Furthermore, the shear stress generated by the altered blood flow could also induce the phosphorylation of VE-cadherin39 also leading to its subsequent internalization (via the mechanosensor complex VE-Cadherin/VEGFR240). Apart from the vascular alterations, the CCI model is also associated with significant hypoxic nerve damage, translating as significant increases in the HIF-1α marker and in the chemokine CCL2 marker (Figure 6), released in peripheral nerve injuries to attract macrophages to the site of injury, as part of the physiological Wallerian degeneration.3,41
Figure 6.
In vivo, at 3 h post-injury, CCI of the infraorbital nerve elicited a significant increase in chemokine marker CCL2 mRNA expression levels as compared to sham-injured animals. Changes in CCL2 mRNA levels were assessed in the IoN of CCI-injured animals compared to sham-injured controls, using semi-quantitative RT-PCR analyses. Data are presented as relative quantification (R.Q.) in arbitrary units (A.U.) corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ± SEM of n = 5–6 animals for each condition; *p < 0.05; Mann–Whitney test was used.
CCI: chronic constriction injury; CCL2: chemokine (CC motif) ligand 2; IoN: infraorbital nerve; RT-PCR: reverse transcription polymerase chain reaction; SEM: standard error of the mean.
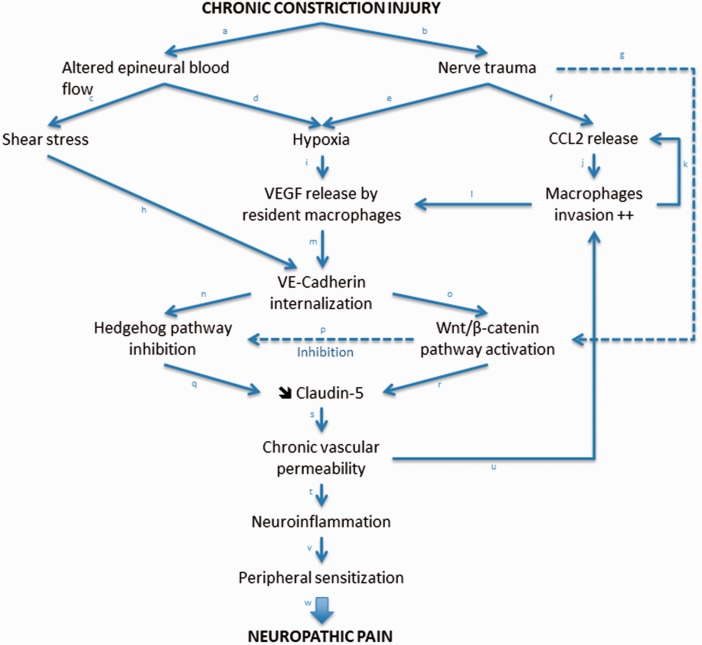
The hypothesized molecular alterations underlying CCI-induced BNB disruption are summarized in Figure 7.
Figure 7.
Simplified modelization of the neuro-immuno-vascular interactions within endoneurial endothelial cells of nerves subjected to chronic constriction injury (CCI). In this model, Claudin-5 was chosen to illustrate the key role of TJ proteins in the disruption of the blood–nerve barrier following CCI, as it has been previously described as the main endoneurial endothelial TJ protein within peripheral nerves.42,43 Other TJ proteins (such as Claudin-1 or Occludin) although not described in this model are of critical importance in the regulation of BNB permeability. Justifications of the proposed model based on past and current results (in italic) and available (nonexhaustive) scientific literature are described hereafter: a, b: Vos et al.,25 Myers et al.,37 Bennett and Xie.44 c: Myers et al.,37 Bennett and Xie,44 Walsh et al.45 d: Myers et al.,37 Nukada et al.,46 Lim et al.33 e: Figure 5; Lim et al.2,33 f: Figure 5; Van Steenwinckel et al.,3 Moreau et al.,4 Sapienza et al.41 g: Figure 3(b) and (c). h: Figure 5; Coon et al.,40 Walsh et al.45 i: Lim et al.2 j: Van Steenwinckel et al.,3 Sapienza et al.41 k: Van Steenwinckel et al.,3 Sapienza et al.41 l: Lim et al.2 m: Figure 1(d) and data not shown (see Results section for details); Gavard,27 Gavard and Gutkind.38 n: Disorganization of AJ/TJ proteins24 induces loss of cell polarity34 resulting in loss of primary cilium35 and subsequent inhibition of Hedgehog pathway.35 o: Gavard and Gutkind,24 Taddei et al.26 p: Figures 3(a) and 4(c) . q: Moreau et al.4,5 r: Figures 3(c) and 4(a) ; Taddei et al.26 s: Figure 4(a) and ( e ); Moreau et al.4,5, Gavard and Gutkind.24 t: Lim et al.,2 Moreau et al.4,5 u: Figure 4(b) ; Lim et al.,2 Moreau et al.4,5 v: Moreau et al.,4,5 Myers and Shubayev.47 w: Kiguchi et al.,30 Xie et al.,48 Basbaum et al.49.
BNB: blood–nerve barrier; TJ: tight junction.
Conclusion
A crosstalk between Wnt/β-catenin- and SHH-mediated signaling pathways within EECs could mediate the chronic disruption of the BNB following peripheral nerve injury (CCI). Internalization of AJ protein VE-cadherin and concurrent downregulation of the key TJ proteins Claudin-1, Claudin-5, and Occludin could drive such BNB disruption, responsible for subsequent blood-borne immunocytes/toxic molecules infiltration resulting in local neuroinflammation, peripheral sensitization, and neuropathic pain development.
Acknowledgment
The authors would like to thank Alexandra Labarthe and Candice Marion for their invaluable help with the experiments.
Author Contributions
The data reported in this paper are completely original. They have not been previously published and are not being considered for publication elsewhere. All the authors have read and approved the paper. They contributed to the present work as follows: experimental design: NM, MP, YB; experimental work: NM, AM, YB; providing the cell lineage: IAR, BBW, POC; analyzing and interpreting data: NM, YB, LV, MP; writing the manuscript: NM, LV, MP, YB.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by institutional funding from INSERM and Université Paris Diderot.
References
- 1.Shi XQ, Zekki H, Zhang J. The role of TLR2 in nerve injury-induced neuropathic pain is essentially mediated through macrophages in peripheral inflammatory response. Glia 2011; 59: 231–241. [DOI] [PubMed] [Google Scholar]
- 2.Lim TKY, Shi XQ, Martin HC, et al. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain 2014; 155: 954–967. [DOI] [PubMed] [Google Scholar]
- 3.Van Steenwinckel J, Auvynet C, Sapienza A, et al. Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve. Brain Behav Immun 2015; 45: 198–210. [DOI] [PubMed] [Google Scholar]
- 4.Moreau N, Mauborgne A, Bourgoin S, et al. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption nerve inflammation and neuropathic pain development. Pain 2016; 157: 827–839. [DOI] [PubMed] [Google Scholar]
- 5.Moreau N, Dieb W, Mauborgne A, et al. Hedgehog pathway-mediated vascular alterations following trigeminal nerve injury. J Dental Res. Epub ahead of print 16 Nov 2016. DOI: 10.1177/0022034516679395. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Li Y, de Carvalho-Barbosa M, et al. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain 2016; 17: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda T. Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry 2013; 84: 208–212. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011; 334: 1727–1731. [DOI] [PubMed] [Google Scholar]
- 9.Chapouly C, Yao Q, Vandierdonck S, et al. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res 2016; 109: 217–227. [DOI] [PubMed] [Google Scholar]
- 10.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev 2009; 19: 476–483. [DOI] [PubMed] [Google Scholar]
- 11.Dejena E. The role of Wnt signaling in physiological and pathological angiogenesis. Circ Res 2010; 107: 943–952. [DOI] [PubMed] [Google Scholar]
- 12.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res 2013; 319: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira TN, Peghaire C, Franzl N, et al. Frizzled7 controls vascular permeability through the Wnt-canonical pathway and cross-talk with endothelial cell junction complexes. Cardiovasc Res 2014; 103: 291–303. [DOI] [PubMed] [Google Scholar]
- 14.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012; 149: 1192–1205. [DOI] [PubMed] [Google Scholar]
- 15.Itokazu T, Hayano Y, Takahashi R, et al. Involvement of Wnt/β-catenin signaling in the development of neuropathic pain. Neurosci Res 2014; 79: 34–40. [DOI] [PubMed] [Google Scholar]
- 16.Simonetti M, Agarwal N, Stösser S, et al. Wnt-Fzd signaling sensitizes peripheral sensory neurons via distinct noncanonical pathways. Neuron 2014; 83: 104–121. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Chen Y, Yu J, et al. TCF4 mediates the maintenance of neuropathic pain through Wnt/β-catenin signaling following peripheral nerve injury in rats. J Mol Neurosci 2015; 56: 397–408. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Thang S, Li L, et al. Involvement of Wnt5a within the cerebrospinal fluid-contacting nucleus in nerve injury-induced neuriopathic pain. Int J Neurosci 2015; 125: 147–153. [DOI] [PubMed] [Google Scholar]
- 19.Xu N, Wu MZ, Deng XT, et al. Inhibition of YAP/TAZ activity in spinal cord suppresses neuropathic pain. J Neurosci 2016; 36: 10128–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 21.Dieb W, Hafidi A. Astrocytes are involved in trigeminal dynamic mechanical allodynia: potential role of D-serine. J Dent Res 2013; 92: 808–813. [DOI] [PubMed] [Google Scholar]
- 22.Yen LF, Wei VC, Kuo EY, et al. Distinct patterns of cerebral extravasation by Evans blue and sodium fluorescein in rats. PLoS One 2013; 8: e68595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 24.Gavard JS, Gutkind JS. VE-cadherin and claudin-5: it takes two to tango. Nat Cell Biol 2008; 10: 883–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci 1994; 14: 2708–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taddei A, Giampietro C, Conti A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin 5. Nat Cell Biol 2008; 10: 923–934. [DOI] [PubMed] [Google Scholar]
- 27.Gavard J. Breaking the VE-cadherin bonds. FEBS Lett 2009; 583: 1–6. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Yuan S, Li B, et al. Regulation of Wnt signaling by nociceptive input in animal models. Mol Pain 2012; 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YK, Huang ZJ, Liu S, et al. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest 2013; 123: 2268–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiguchi N, Kobayashi Y, Kadowaki Y, et al. Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. J Neurochem 2014; 129: 169–178. [DOI] [PubMed] [Google Scholar]
- 31.Mazumdar J, O’Brien WT, Johnson RS, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol 2010; 12: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song X, Zhou Y, Huang Y, et al. Wogonin influences vascular permeability via Wnt/β-catenin pathway. Mol Carcinog 2015; 54: 501–512. [DOI] [PubMed] [Google Scholar]
- 33.Lim TKY, Shi XQ, Johnson JM, et al. Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia contributing to the genesis of neuropathic pain. J Neurosci 2015; 35: 3346–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci 2013; 126: 2545–2549. [DOI] [PubMed] [Google Scholar]
- 35.Das RM, Storey KG. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 2014; 343: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto Y, Sakane F, Hashimoto K. N-cadherin-based adherens junction regulates the maintenance proliferation and differentiation of neural progenitor cells during development. Cell Adh Migr 2015; 9: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers R, Yamamoto T, Yaksh T, et al. The role of focal nerve ischemia and Wallerian degeneration in peripheral nerve injury producing hyperesthesia. Anesthesiology 1993; 78: 308–316. [DOI] [PubMed] [Google Scholar]
- 38.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 2006; 8: 1223–1234. [DOI] [PubMed] [Google Scholar]
- 39.Orsenigo F. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 2012; 3: 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coon BG, Baeyens N, Han J, et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol 2015; 208: 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapienza A, Réaux-Le Goazigo A, Rostène W, et al. [Chemokines and attraction of myeloid cells in peripheral neuropathic pains]. Biol Aujourdhui 2014; 208: 31–44. [DOI] [PubMed] [Google Scholar]
- 42.Hirakawa H, Okajima S, Nagaoka T, et al. Loss and recovery of the blood–nerve barrier in the rat sciatic nerve after crush injury are associated with expression of intercellular junctional proteins. Exp Cell Res 2003; 284: 196–210. [DOI] [PubMed] [Google Scholar]
- 43.Peltonen S, Alanne M, Peltonen J. Barriers of the peripheral nerve. Tissue Barriers 2013; 1: e24956–1–e24956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107. [DOI] [PubMed] [Google Scholar]
- 45.Walsh TG, Murphy RP, Fitzpatrick P, et al. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J Cell Physiol 2011; 226: 3053–3063. [DOI] [PubMed] [Google Scholar]
- 46.Nukada H, Powell HC, Myers RR. Spatial distribution of nerve injury after occlusion of individual major vessels in rat sciatic nerves. J Neuropathol Exp Neurol 1993; 52: 452–459. [DOI] [PubMed] [Google Scholar]
- 47.Myers RR, Shubayev VI. The ology of neuropathy: an integrative review of the role of neuroinflammation and TNF-α axonal transport in neuropathic pain. J Periph Nerv Sys 2011; 16: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie W, Strong JA, Meij JTA, et al. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain 2005; 116: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]