Abstract
Adult stem cells are crucial for tissue homeostasis. These cells reside within exclusive locations in tissues, termed niches, which protect adult stem cell fidelity and regulate their many functions through biophysical-, biochemical- and cellular-mediated mechanisms. There is a growing understanding of how these mechanisms and their components contribute towards maintaining stem cell quiescence, self-renewal, expansion and differentiation patterns. In vitro expansion of adult stem cells is a powerful tool for understanding stem cell biology, and for tissue engineering and regenerative medicine applications. However, it is technically challenging, since adult stem cell removal from their native microenvironment has negative repercussions on their sustainability. In this review, we overview specific elements of the biomimetic niche and how recreating such elements can help in vitro propagation of adult stem cells.
Keywords: Biomimicry, adult stem cells, microenvironment
Introduction
Advances in the knowledge of stem cell biology have resulted in new approaches to treat human disease. This holds enormous potential to revolutionize medicine through solutions for conditions that require tissue regeneration. However, much remains to be understood in terms of stem cell behaviour and technical optimization of protocols to allow proper translation into the clinical setting. The two main fronts of research and clinical application of stem cells comprise (1) stem cell therapies, most commonly based on cell injections or tissue engineered grafts, in which scaffolds are used in combination with stem cells and biologically active molecules and (2) in vitro studies for basic biology research and drug testing.
The application of cell-based strategies is highly limited by difficulties in harvesting and expanding stem cells. For this reason, the most extensively studied cells are embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) that have the ability to differentiate into all adult tissues and to thoroughly proliferate, as well as multipotent mesenchymal stem cells (MSCs), obtained in clinically relevant amounts with potential to differentiate into some mesodermal-derived cell types. Nevertheless, the former present disadvantages such as the risk of teratoma formation and ethical controversies, while the latter cannot be applied to many tissues in the body nor be studied as tissue-specific stem cells in vitro.1,2 Tissue-specific adult stem cells (ASCs), however, give rise to all cell types from their given lineage and have been identified in most tissues of the human body.3 The native three-dimensional (3D) environment that surrounds ASCs is composed of extracellular matrix, neighbouring cells and soluble factors. Within these dynamic microenvironments, denominated niches, ASCs are subjected to biochemical, biophysical and biomechanical phenomena that sustain their ability to both self-renew and differentiate, also known as stemness.4 Functionally, ASCs would be ideal candidates for regenerating injured tissues, since they are natively responsible for maintaining tissue homeostasis throughout life.5 Yet, their isolation and expansion must be substantially improved to allow a better understanding of their identity and behaviour in vitro, as well as to achieve a more effective manipulation for translational use in the clinical setting. Aiming to overcome the challenges of expanding ASCs taken away from their native microenvironment, strategies have been developed to mimic the niches in which these cells reside.
In this review, we will discuss the different features of the biomimetic niche that promote tissue-specific ASC expansion, using haematopoietic, brain, intestinal and muscle tissues as examples. Since the topics of MSC, ESC and iPSC expansion, as well as biomimetic systems promoting differentiation, have been widely covered in the literature, we will not approach such themes, but will review tangible qualities of the biomimetic niche that are necessary for ASC expansion in vitro.
ASCs and their microenvironment
Stem cells reside in vivo within tissue-specific niches where multiple interactions occur. They have the ability to self-renew for long periods and to differentiate into more committed lineages respective of their host tissue.3 Together, cell-to-cell and cell-to-matrix interactions, soluble molecules signalling, physical and mechanical stimuli determine if a stem cell will remain quiescent, divide symmetrically (self-renew) or asymmetrically, producing one cell with same potency and one more differentiated cell.6
When considering the need for stem cells expansion, it is necessary to take into account the native behaviour of the known stem cell populations within a tissue. Tissues in the body have different turnover rates and regenerative potential. For instance, gut, mammary, blood and skin tissues have both a high turnover and regenerative capacity, completely replacing their differentiated cell population within a matter of days or weeks. However, tissues like liver and skeletal muscle present low steady-state turnover rates, but highly effective repair abilities, swiftly recruiting ASCs or progenitor cells in response to injury or disease in order to repair and regenerate. Other tissues such as the brain and heart have low turnover rates and poor repair upon damage, with resident stem cells dividing rarely.7,8
The ASC niches change when ASCs face challenging physiological events, such as mammary gland alterations during pregnancy and hair follicle variations during hair cycle, which require massive cell proliferation, apoptosis and differentiation. Different cell populations are required to adjust to these changes, including more dormant stem cells, usually recruited only during critical need, as well as stem cells with a higher cell cycle rate, more generally associated with tissue homeostasis.9
The existence of a stem cell niche that regulates self-renewal and differentiation was postulated for the human haematopoietic system three decades ago.10 Current knowledge hypothesizes that there are two haematopoietic stem cell (HSC) niches in the adult bone marrow. The osteoblastic niche on the bone surface maintains a quiescent environment for long-term HSCs (LT-HSCs) via endosteal osteoblasts. When mobilized, during haematopoiesis, LT-HSCs give rise to short-term HSCs (ST-HSCs), which are more proliferative and able to generate differentiated blood progenitors. This cell behaviour is promoted by their location, adjacent to sinusoid endothelial cells, within a vascular niche.11,12
The subgranular zone (SGZ) in the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles are the main neurogenic regions in the adult brain. In the SVZ, quiescent neural stem cells (NSCs), known as B-type cells, become activated, generating transit amplifying cells (C-type) which will give rise to type-A neuroblasts or glial cells.13 In the SGZ of the DG, new granule neurons and astrocytes are continuously generated by neural progenitor cells (NPCs).14
The intestinal epithelium is sustained by leucine-rich repeat containing G-protein coupled receptor 5 (Lgr5+) cells that reside at the bottom of the crypts. In the small intestine (SI), they are interspersed by Paneth cells, which function as their niche, providing intestinal stem cells (ISCs) with necessary signals.15 In the colon, the stem cells are intermingled with Reg4+ deep secretory cells (DSCs) that act similarly to Paneth cells.16
Satellite cells (SaCs) are quiescent cells in the muscle, located below the basal lamina of the muscle fibre. Generally considered the muscle stem cells (MuSCs), they are capable of self-renewal, maintaining the SaCs pool and entering the cell cycle when required by external stimuli such as injury and exercise, producing myogenic precursor cells.17
Growing ASCs has been extremely challenging due to the elaborate spatio-temporal arrangement of multiple factors that regulate activation, sustained proliferation and maintenance of an undifferentiated state in vitro. Recreating the critical features to either maintain multipotency or cause controlled specific differentiation is a strategy employed by many researchers in the field.
Two-dimensional (2D) culture is often simplistic and lacks the necessary complexity for stem cell growth, as seen by tentative unsuccessful expansion of cells in these systems.18 3D structures mimicking biological, mechanical and physical properties of ECM are promising systems for advancing such cultures.19,20 Recently, more complex biomaterials for improving ASC proliferation and control of stem-cell fate have been developed, as well as an appreciation of the soluble factors that control these processes.
The importance of niche composition for ASC expansion can be illustrated by parabiotic studies that show SaCs and liver progenitors from aged mice restoring their regeneration capacities when exposed to blood-borne niche factors from younger mice. This indicates that the intrinsic replicative ability of these progenitors may not be the sole cause of tissue degeneration during ageing.21,22 Similar results were observed with NPCs expansion and related cognitive function.23 Furthermore, the niche size regulates the number of ASCs in the bone marrow. An increase in the number of osteoblastic cells to which LT-HSC attach promotes their expansion,24 while niche cell depletion causes LT-HSC diminishment.25
Although stem cell niches vary among tissues, they share common microenvironmental features. Delineating these features and the mechanisms by which cell fate is controlled through biophysical and biochemical parameters within the niche may enhance current ASC expansion strategies.
Microenvironment components of ASC niches
The molecular signalling pathways involved in ASC regulation and self-renewal have been studied extensively. The ASC niche is a dynamic system where the equilibrium between stem cells and their microenvironment is mutually maintained via multiple interrelated communication networks. This multidirectional crosstalk involves not only the biochemical cues exchanged between ASCs, their surrounding cells and ECM, but also a physical relationship. There is a growing appreciation of the biomechanical and biophysical roles in the maintenance of the stem cell niche. Niche homeostasis relies heavily on biophysical properties, such as ECM physicochemistry, pH, fluid dynamics, oxygen tension and mechanical properties of the originating tissue.26,27
Biochemical factors
In the stem cell niche, there is a plethora of morphogens, chemokines, cytokines and growth factors, present either as soluble molecules or as ECM-bound. The communication among ASC and other cells from the niche is mediated by such biomolecules acting via paracrine, autocrine or juxtacrine signalling pathways. Not only the composition of these chemotactic gradients, but also their spatio-temporal distribution, play a significant role in controlling ASC fate.
Wnt
The Wnt pathway is a highly conserved signalling network with crucial functions as a morphogen during development and as a regulator of multiple cellular behaviours throughout life. In mammals, it comprises a family of 19 ligands that interact mainly with the multi-pass membrane receptor Frizzled (diagramatically presented and reviewed in Nusse28). In the canonical pathway, it usually requires the involvement of the LPR5/6 co-receptor, leading to the activation of multiple downstream intracellular molecules, exerting its effects by the nuclear accumulation of β-catenin, which in turn promotes transcription of target genes. The planar cell polarity pathway, which affects cell movement, and the calcium pathway are two non-canonical pathways also activated by Wnt ligands.29 Wnt signalling has been directly implicated in self-renewal, proliferation and differentiation of ASCs in a tissue-specific manner.
For instance, overexpression of β-catenin has been reported to expand HSCs in long-term cultures and in vivo, showing the Wnt pathway involvement in HSC self-renewal and proliferation via the specific ligand Wnt3a.30–32 Furthermore, HSCs have demonstrated high sensitivity to Wnt dosage, indicating that adjustments for in vitro expansion and clinical applications are essential.33
Wnt signalling is considered a central regulator of adult neurogenesis in the hippocampus34,35 and the SVZ.35,36 In a mouse model of β-catenin constitutive activation, Wnt was associated with the expansion of NPCs, resulting in a bigger cortex.37 However, Wnt3a conditioned media caused inhibition of neurosphere formation concomitant with neuronal differentiation in cultured NSCs derived from E11.5 mouse forebrain,38 whereas it promoted proliferation, but not differentiation, of hippocampal NPCs from E15 mice in another study.39 This suggests that Wnt types and protocols should be fine-tuned according to the age and anatomical source of NSCs in order to promote the desired effects in vitro.
Crypt cells in the SI and colon depend on Wnt signalling to proliferate.40,41 R-spondin1 (Rspo1), a Wnt agonist that acts via Lgr4/5 receptors, is an important mitogenic factor for crypt cells.42,43 Hence, Wnt3a and Rspo1 addition to systems aiming to expand ISCs is crucial.44–46
MuSCs also proliferate upon Wnt signalling during regeneration via the canonical pathway, stimulated by Wnt1, Wnt3a, Wnt5a or Wnt11, as opposed to Wnt4 or Wnt6, which inhibits MuSCs proliferation.47 Activation of the planar cell polarity pathway by Wnt7a was also reported as myogenic.48 Conversely, increased Wnt/β-catenin signalling was found to mediate muscle fibrosis acquired with ageing by promotion of fibrogenic conversion in aged myogenic progenitors.22 Taken together, these studies illustrate the importance of precisely tailoring Wnt manipulation to the chosen source of ASCs.
Besides sustaining Wnt signalling via the use of pathway agonists, attention should also be given to inhibitory molecules, such as Dickkopf (Dkk)-related proteins that bind to LRP5/6, preventing the activation of downstream cascades promoted by Wnts.49 Studies using these potent inhibitors of Wnt signalling have helped to build further evidence on the key importance of Wnt for ASCs, via disruption either of stemness or of the ASC proliferative compartment in HSCs,31 NSCs50 and ISCs.41,51 Although Wnt addition to culture seems sufficient for maintaining pathway activation in vitro, the use of DKK inhibitors, like NCI8642,52 especially in complex models utilizing multiple cell types, remains to be tested for a putative enhancement of ASCs expansion. Such experimental strategies could also be applied to the signalling pathways described in the next sections.
Notch
Notch signalling is an evolutionary conserved pathway based on membrane-bound Notch receptors that bind to transmembrane ligands such as Delta (Dll) and Jagged present on adjacent cells, activating transcription of downstream genes via intracellular cascades (comprehensive schematics can be found in in-depth reviews;53,54). There is evidence that Notch signalling is involved in stemness maintenance within the four tissue types discussed in this review.
Notch pathway activation has been reported to promote adult HSCs self-renewal in vitro55 and in vivo,56 regulating both ST-HSCs and LT-HSCs.57 However, its role remains to be further elucidated, as Notch signalling has also been described as dispensable.58,59
High Notch activity has been associated with maintenance of NSCs quiescence,60 NSCs survival and an increase in NPCs accompanying improved functional response to injury. Therefore, Notch is considered to play an essential role in neurogenesis.61,62
The Notch pathway is crucial for preventing ISCs from differentiating.63 Hence, Dll4 signalling is indispensable for ISC culture,45 consonant to in vivo observations, where Notch ablation in mice led proliferative crypt cells to differentiate into post-mitotic goblet cells.64
The quiescent MuSCs pool was diminished when Notch signalling was genetically ablated.65,66 Correspondingly, upregulation of Notch stimulated MuSCs proliferation in response to injury.67
With the exception of HSCs, for which Notch signalling literature reports are conflicting, evidence suggests that this pathway activation generally promotes ASC proliferation.
BMP
Bone morphogenetic proteins (BMPs) belong to the TGF-β superfamily of ligands that interact canonically with type I and type II receptors which, in turn, transduce their signals through the phosphorylation of Smad, resulting ultimately in gene expression regulation (reviewed in Wang et al.68). In ASCs, BMP signalling has been widely implicated in the control of self-renewal.
Ex vivo HSC culture time, for example, was extended by the addition of BMP4 to the media.69 However, the use of a deletion model of the BMP1Ra receptor revealed that BMP signalling disruption led to an increase in LT-HSCs in mice via the expansion of the osteoblastic cells to which they attach.24
In hippocampal NSCs, BMPR1a activation via BMP induced quiescence.70 The addition of the BMP antagonist noggin to in vitro cultures of hippocampal NSCs augmented expressively their cell number and maintenance, while in SVZ NSCs, it only accelerated expansion, without an increase in the cell population.70 Although expansion can be achieved by abrogating BMP signalling with noggin, this may lead to exhaustion of the stem cell pool.71 Nevertheless, inhibition of NPC proliferation mediated by age-related enhanced BMP signalling could be partially restored in mice by the blockade of Smad. Blockade of excessive BMP signalling found during ageing may be, then, beneficial to health, as long as it is not strong enough to extinguish the NSC pool as seen in younger adults.72
Correspondingly, self-renewal of ISCs was suppressed by BMP crosstalk and blockade of the Wnt pathway.73 BMP activity towards intestine cell differentiation has been shown to be abrogated by noggin,74,75 promoting ISCs expansion in culture when added to the media.44,45
Within the TGF-β superfamily pathways, the most studied in MuSCs is the inhibitory effects of TGF-β1 on proliferation, impairing muscle regeneration via Smad3.76–78 However, BMP signalling in vivo and in vitro was shown to stimulate proliferation,79 preventing cell cycle exit of activated SaCs and proliferating myoblasts, resulting in MuSCs expansion.80 Thus, it is critical to consider not only the pathways in which to interfere with when optimizing ASC expansion in vitro but also the multiple molecular targets which may lead to the desired outcomes.
Other morphogens like Sonic hedgehog (Shh) have also been shown to be involved in ASCs regulation. Its actions have been studied in more depth within the brain tissue, where Shh was required for NSC maintenance.61,81,82 Nevertheless, its function remains to be clarified, as Shh has also been shown to promote NSCs specification to neurons.83 In the intestine, crypts of Shh null mice were hyperplastic,84 exhibiting increased proliferation,85 suggesting a role for Shh in ISC proliferation.
It is important to note, though, that all these events take place within cells and their niches in a dynamic fashion. Several studies suggest the existence of communication and crosstalk amongst these major signalling pathways,61,73,86 which may account for the controversies found when studying these molecules separately, as well as during in vitro modelling.
Growth factors, chemokines, cytokines and other molecules
Growth factors within tissues are either bound to the ECM or secreted by stem cells and other niche cells. The regulation of growth factor availability is tightly controlled in vivo. While morphogens tend to sustain stem cells at an undifferentiated state, concomitant action of growth factors generally triggers proliferation via signal transduction cascades using tyrosine kinase pathways.28
In vitro culture and expansion of HSCs is controlled by addition of specific cytokine cocktails, which include stem cell factor (SCF), FMS-like tyrosine kinase-3, thrombopoietin (TPO), different combinations of interleukins (IL),87,88 vascular endothelial growth factor (VEGF),89 platelet-derived growth factor (PDGF)90 and epidermal growth factor (EGF).91 The detection of such growth factors within the niche in vivo has helped to direct development of specific cytokine cocktails. Ligand presentation form may be critical for the outcome, as illustrated by SCF, proven to be more effective in promoting HSCs expansion when bound to the membrane.92
Cultivation of NSCs has been performed with the established mitogen basic fibroblast growth factor (bFGF).93 Addition of the Tie-2 receptor ligand, Angiopoietin (Ang2), to the culture medium in the presence of Dll4 supported self-renewal and precursor expansion.94 Furthermore, cholera toxin,95 K+96 and high doses of insulin growth factor-1 (IGF-1)97 supplementation in the media also enhanced NSCs expansion in vitro.
In addition to the Wnt, Notch and BMP pathways, activation of the EGFR pathway by EGF supplementation is routine in SI and colon organoid cultures.44,45 Optimal combinations of factors for stem cell culture conditions of murine and human intestinal samples have been previously detailed, including gastrin, nicotinamide and TGβ1 and p38 inhibitor inhibitors.45 In vivo, IGF1 and IL-22 have been found to confer proliferative stimuli to ISCs in response to injury.98,99 More recently, clonal ISCs from human foetal colon were grown in two dimensions, in the presence of mouse feeder layers in a similar media to that of 3D organoids, offering an alternative for the study and expansion of ISCs in vitro where the requirement is for homogeneous ISC populations and absence of differentiated cells.100 Moreover, adult colorectal samples were successfully expanded as conditionally reprogrammed cells in the presence of rock inhibitors and 2% oxygen in an approach more adaptable to high-throughput studies.101
Various soluble factors such as hepatocyte growth factor (HGF), IGF-1, bFGF, PDGF-BB, VEGF and nitric oxide have enhanced MuSCs proliferation.102,103 Inhibition p38 associated with enhanced muscle repair in vivo has also promoted MuSCs self-renewal ability in vitro.104
Although finding the optimal combination of soluble factors for ASC expansion can be challenging, progress has been made by high-throughput screenings and by the application of cues from pathways activated during development, when stem cell activity is at its highest.
Neighbouring cells
The cellular components of the ASC may vary according to tissue type, age, steady state or response to injury. Many of the above-mentioned soluble factors are present in the native niches by secretion or membrane-attachment onto ASCs neighbouring cells. These may be other stem cell copies, more committed progenitors, fibroblasts, immune cells, endothelial cells and nerves. Cells associated with ASC niches are therefore likely to produce a host of interactions which regulate ASC function. Some of the essential cell-cell interactions for ASC propagation in specific niches are overviewed below.
Osteoblasts, osteoclasts and fibroblasts comprise the endosteum, considered the osteoblastic niche in the bone marrow. The vascular niche resides within the perivascular space, where endothelial and reticular cells from sinusoids in close proximity to HSCs are considered important players in HSCs homeostasis. Osteoblasts have been implicated as playing a central role in the maintenance of HSCs which reside alongside them, via N-cadherin-mediated cell-cell interactions.24,105 These interactions influence HSCs numbers via BMP signalling24 and HSCs quiescence via secretion of Ang, TPO, SCF-1 and the chemokine CXCL12.106 Endothelial cells (ECs) have been shown to be essential for bone-marrow reconstitution after damage in a VEGFR2-mediated manner.107 In vitro, the secretion of Notch ligands and direct contact with ECs enhanced LT-HSCs expansion without exhausting the compartment,57 an effect also observed in the presence of Ang2 and Ang3.108 Sympathetic nerves and macrophages were also shown to influence HSCs maintenance,109,110 illustrating the array of complex interactions involved in niche homeostasis. Cell-ASC heterotypic interactions critical for HSC expansion remains to be explored as tools for proliferation improvement. Nevertheless, it is likely this will become increasingly important for mimicking such complex niche within ex vivo settings.
Both SGZ and SVZ NSCs are located in close proximity to ECs, a region considered as the neurogenic vascular niche in the central nervous system. Endothelial smooth muscle cells, microglia, mature astrocytes and NPCs are all found neighbouring NSCs.111 As opposed to the SGZ, SVZ harbours noggin-expressing ciliated cells,112 namely, ependymal cells (type-E), which promote cerebrospinal fluid flow, thus allowing NSCs to access multiple neurogenesis modulating factors such as BMPs, Wnt, noggin and retinoic acid (RA).111 Further work is needed to understand the difference between NSCs derived from these two different sources and the role of neighbouring cells in promoting their stemness. In fact, co-culturing studies have shed some light on the mechanisms involved in such interactions. Co-culture of ECs with NSCs promoted self-renewal and neurogenesis,113 possibly through secretion of factors such as VEGF, BMP, Ephrin-B2 and brain-derived neurotrophic factor,111 as could be expected considering the vascular nature of neurogenic areas. Furthermore, a co-culture model investigation aiming to study olfactory bulb putative NSCs has shown that glial astrocytes from the SVZ promoted NSCs expansion via Wnt7a114 indicating once again that heterotypic cell interactions might constitute a successful strategy for ASC long-term culture.
Paneth cells are in direct contact with SI ISCs. Working as their niche in intestinal cultured organoids,15 they secrete essential factors for ISCs self-renewal, proliferation and homeostasis, such as EGF, TGF-α, Dll1, Dll4 and Wnt3a.115 The crucial Wnt3a signal is produced exclusively by Paneth cells and transferred in a membrane-bound, juxtacrine manner.43 More recently, in parallel to Paneth cells in the SI, DCCs were demonstrated to function as an ISC stem cell niche in colon organoids, producing mainly Notch ligands.115 Some of these factors are also secreted by smooth muscle actin-expressing stromal cells from the intestinal lamina propria116 and by other cell types present within ISCs niches. These comprise fibroblasts, ECs, myofibroblasts, mesenchymal cells and immune cells.116 In addition to the established multilineage organoid system, two culture systems in 2D using feeder layers have also been described for ISCs expansion,100,101 supporting the in vitro use of stromal feeders as a form of emulating niche-driven expansion of ASCs. Supporting cells associated with specific ASCs are therefore likely to produce a host of interactions regulating quiescence, self-renewal and cell cycle entry for multiplying the latter. With further studies, more critical interactions for the proliferation of the ASC pool should be uncovered.
SaCs are scattered as single cells associated with post-mitotic multinucleated muscle fibres under the basal lamina, which constitute their niche.17 Myofibres in the SaCs vicinity influence SaCs behaviour via secretion or presentation of membrane-bound factors, such as stromal-derived cell factor 1, a migratory stimulus.117 During repair, SaCs are activated and expanded in response to macrophage-secreted factors, as observed in co-culture experiments.118 It is noteworthy that ECs are found in every vascularized tissue and that the EC-ASC interaction in multiple niches all point to a critical role of ECs in regulating self-renewal potential. In the muscle, the role of ECs is indeed deemed essential for SaCs maintenance. For instance, ECs from capillaries found in close vicinity to SaCs have been shown to stimulate SaC-derived progenitors when co-cultured.102 Although the role of many other cell types has been highlighted in terms of modulating ASC behaviour, it is likely that additional critical interactions will be revealed with further studies addressing more complex multicellular configurations.
Extracellular matrix composition and biophysical aspects
Structural components of the ECM play a critical part in the ASC niche in vivo. The roles that different ECM components play in the self-renewal, quiescence, proliferation and differentiation have been studied in specific niches and may thus be used to control or direct ASC fate.119–122 The ECM that embeds ASCs is composed of structural protein fibres, like collagens and elastin, adhesive proteins, such as fibronectin and laminins, as well as of proteoglycans and polysaccharides. Its shape and composition varies with tissue type, development stage and physiological or pathological processes,123 exerting influence on stem-cell fate.117
The notion that the ECM is a reservoir of growth factors and morphogens has been vastly reported and evidenced. Many of these proteins will bind to the heparin and heparan sulphate (HS) of proteoglycans and get released as soluble signals during cell-mediated ECM degradation, while others utilize proteoglycans as co-factors to bind their ligand making them readily available to cells.124 Aside from growth factor homing and presentation, the ECM has biophysical properties known to play a key role in ASC maintenance. Understanding the interaction between ASCs and their ECM in different niches may enable more successful expansion of these cells in vitro.
Integrin-mediated interactions with ASC niche and ECM components
Cells naturally align to topographical features of their surrounding tissue via contact guidance, using cellular projections termed filopodia.125 Cell contact and adhesion to the ECM is mediated by the integrin family of transmembrane receptors, which are activated upon binding their ligands, like the peptide motif RGD, commonly found in domains of ECM proteins including collagen, laminin and fibronectin. Once integrins bind to their ligands, they trigger intracellular and extracellular responses. These include, but are not limited to, changes in cell shape via cytoskeleton contraction, integrin clustering on the cell surface forming large focal adhesions and ECM remodelling. The capacity for integrin-mediated dictation of cell behaviour is strongly dependent on the density of ligands126 and the number of integrins participating per focal adhesion.127 For example, high ligand density (<70 nm apart) causes large focal adhesions with at least four integrins, which results in cells adopting a stretched conformation, whereas low ligand density (>70 nm apart) leaves cells with small focal adhesions and a round conformation.125 This indicates that in terms of integrin-dependent association with their ECM, ASCs could be more likely to maintain their stemness when they have small focal adhesions as a result of a low density in fibrillar proteins.
Further supporting this concept is the observation of fibronectin, a critical component of cell-ECM adhesion, found as a compact non-functional soluble form in circulation or assembled into extended fibrils after binding its mechanosensitive RGD motif to cell-surface integrins. As fibrillar fibronectin is one of the first ECM proteins actively assembled upon injury, either by recruiting circulating fibronectin or producing their own,124,128 it could be argued that ASC pool expansion to replenish tissue following injury may correlate to fibrillar fibronectin levels.
In the bone-marrow niche, HSC binding to fibronectin via α4- or α5-integrins was essential for replenishing the HSC pool following transplantation129 and prolonged ex vivo cultures of regenerating primate HSCs were achieved in the presence of fibronectin.130 Whether supplemented on its own131 or in combination with laminin,132 fibronectin has generally been found to promote clonogenic growth of HSC and HSC-derived progenitors. It has also been reported that tenascin-C, expressed by stromal and endothelial cells in the bone-marrow niche, plays a crucial role in haematopoietic regeneration after myeloablation, promoting HSC proliferation. Accordingly, in vitro cultures of HSCs on a tenascin-C substrate facilitated α9-integrin-dependent stem cell expansion.133 Furthermore, collagen type I, the main component of bone which also constitutes the HSC osteoblastic niche, favours a higher amplification of CD34+ HSCs from cord blood.134
In the neural SGZ and SVZ of the brain, ECM structures termed fractones make up the NSC niche.116 Laminin, one of the three major components of basal lamina, has been reported to improve NPC expansion mediated by α-integrin when compared to fibronectin and the commercially available ECM mix Matrigel©.135 Chondroitin-sulphate (CS) GAGs were also implicated in NSC proliferation both in vitro and in vivo.136,137 Indeed, CS-GAG hydrogels conditioned with FGF have been shown to prolong neurosphere cultures from postnatal rats in vitro.138 Similarly, endothelium-derived tenascin-C was considered a critical ECM component for proper neurogenesis,139,140 thus possibly facilitating NSC expansion in vitro.
A role for α- and β-integrin-mediated interactions in intestinal culture stemness is suggested by studies which have investigated specific ECM components and ISCs.141 Intestinal primary cultures have been extended when placed in collagen I.121,142 Furthermore, addition of different types of laminin to such collagen I coatings improved growth of Lgr5+ ISCs and progenitors in 2D cultures for later 3D modelling.143 In order to investigate specific ECM components involved in intestinal organoids maintenance, which are normally grown in chemically undefined Matrigel, it was shown that individual addition of fibronectin, laminin 111, collagen IV, hyaluronic acid or perlecan to PEG hydrogels improved ISC proliferation.46 Furthermore, binding to HS was reported to activate regeneration via the Wnt canonical pathway in ISCs.144
The myofibres where SaCs lie within are rich in collagen IV, laminins and proteoglycans.145 Specifically in the muscle niche, fibronectin has been identified as a preferred adhesion substrate for MuSCs, playing a key role in their regenerative potential146 while β1-integrin has been considered essential to SaCs homeostasis and self-renewal.147 Collagen IV was also found to be necessary for SaCs in response to injury.148 Laminin is found not only in myofibrils but also in the juxtavascular niche for SaCs, where it was shown to play a role in the EC-mediated proliferation of SaCs.102 More specifically, laminin 521 offered superior results for long-term expansion.149 Heparan-sulphate proteoglycans form a hydrogel matrix conferring compressive resistance to the muscle while being freely diffusible for soluble signals. Such properties may be directly related to activation and proliferation of SaCs during muscle regeneration mediated by HS-proteoglycans.150
Geometry and mechanotransduction
The ECM protein collagen defines the shape and geometry of a given tissue.151 As variations of collagen-coated substrates are now more regularly used to expand stem cells,152 tracing the collagen signature of the native ASC niche and reproducing that geometry in vitro may offer a significant improvement in ASC expansion. To mention but one example, an individual cell-based computational model of intestinal organoids, which accounted for the bending modulus on the organoid surface, was used to simulate intestinal organoid development in silico.153 The work by Buske et al. revealed that the proliferating stem cells on a flexible basal membrane were able to alter the curvature of the membrane. This altered curvature, forming crypt-like domains mimicking the in vivo conformation of intestinal tissue, resulted in changes to the ISC/Paneth cell ratio.153 The ability to alter cell specification by mimicking the tissue geometry of the intestinal niche provides evidence for encouraging the development of microenvironmental geometrical cues for ASC proliferation.
Indeed, geometrical features such as pore size in 3D matrices have been explored in ASC culture optimization studies. A study in which HSCs were grown on a porous fibronectin-coated scaffold with microcavities varying between 15 and 80 mm in diameter demonstrated that fitting only individual cells in such cavities promoted HSC multipotency, as evidenced by increased HSC marker expression in response to spatial restriction. However, this effect was limited to culture conditions where HSCs became exposed to low cytokine levels, which is a more faithful reflection of the in vivo bone-marrow niche.129 From this model, it could be hypothesized that pore size plays a role in maintaining stem cell potential despite not facilitating expansion, since proliferation rates on porous scaffolds was decreased.
Emerging technologies in the field of tissue engineering and 3D culture can often provide tools by which to develop relevant 3D microenvironments in which to culture cells. Nanofibre technology exploits the sensitivity of cells to the nanotopography of their surrounding matrix, and in particular has been shown to promote self-renewal or differentiation of stem cells in vitro.154 Most of the detailed studies focus on ESCs as opposed to ASC populations. It is worth overviewing these trends, as they are likely to be utilized for ASC expansion and culture. The size of nanofibres generated by electrospinning can be controlled and can range size between 1 and 1000 nm in length and as small as 5 nm in diameter. As such, hybrid meshes of natural (e.g. collagen, gelatin) and synthetic (e.g. polycaprolactone) materials can be generated to mimic topographic patterns and alignment profiles of different native ECM matrices. For instance, polycaprolactone/collagen and polycaprolactone/gelatin nanofibre scaffolds were found to promote human ESC expansion in an undifferentiated state.155 Both composition and fibre diameter can significantly alter proliferation and differentiation of stem cells. For example, laminin-coated electrospun polyethersulfone (PES) fibre mesh of 283 nm diameter increased NSC differentiation by 40% while 749 nm diameter increased neuronal differentiation by only 20% compared to tissue culture plastic.156,157 Functionalized aminated nanofibre PES was also able to largely improve cord blood HSC numbers and engraftment in mice when used as scaffolds for improving such challenging cultures.158 These approaches configure powerful tools to unravel the best-fit nanotopographies for expansion of tissue-specific ASCs.
Mechanotransduction is the method by which mechanical stimuli are sensed by cells after which cells respond biochemically and mechanically. The Hippo pathway is a major signalling pathway involved in the control of organ size in animals through the regulation of cell proliferation and apoptosis by nuclear transduction of mechanical and cytoskeletal signals, mediating cellular responses to mechanical forces.159–161 The known downstream transducers of the Hippo pathway, transcription co-factors yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), behave as sensors and mediators of mechanical cues by altering their localization within a cell.161 When the adhesion surface is small between the cell and its soft extracellular matrix, the YAP/TAZ complex is localized in the cytoplasm of cells causing either apoptosis via proteasome degradation or growth arrest. Mechanical loading and extracellular matrix stiffness cause the translocation of the YAP/TAZ into the nucleus of cells leading to active transcription of genes responsible for cell proliferation and differentiation.162 Recently, it has been shown that matrix stiffness controls ISC expansion by influence on YAP activity.46 If such direct modulation of ASC behaviour is also present in other niches remains to be investigated, especially when considering differences between stiffness and promotion of proliferation in other stem cell compartments, as discussed in the next section.
Although gain of function in YAP has been shown to promote stem cell expansion in intestine and brain tissue of invertebrate models,159,163 such observations were not reproduced in the haematopoietic system of mice.164 In mice intestinal epithelium, YAP/TAZ seems to induce proliferation in the stem cell compartment, while inducing differentiation in other cell types via different transcription factors.165 In muscle, YAP activity is increased in response to injury, resulting in better muscle mass recovery,166 possibly via crosstalk with the Wnt pathway in non-quiescent activated SaCs.167 Furthermore, regulation of stemness by mechanotransduction has been described in non-adult muscle, where YAP activation by muscle contractility promoted the transcription of Notch, maintaining the progenitors pool number.168 Nevertheless, manipulation of this pathway for enhancement of cell propagation remains to be further tested in cultures.
Stiffness and elasticity
Stiffness and elasticity of ECM are universal mechanical cues that cells are capable of sensing and responding to. Changes in ECM elasticity can dictate cell behaviour in terms of proliferation or apoptosis, quiescence or differentiation down to a specific lineage.160 Perhaps, one of the most striking studies has shown how naive MSCs can be directed down different lineages solely in response to matrix elasticity gradients. MSCs cultured on collagen-coated matrices with a stiffness mimicking native brain (Ebrain ~ 0.1 – 1 kPa) exhibit neuron-like features. At increased matrix stiffness, mimicking that of striated muscle (Emuscle ~ 8–17 kPa), cells exert muscle-cell characteristics, whereas at even higher levels of stiffness mimicking bone (Ebone ~ 25–40 kPa), MSCs obtained osteoblast-like morphology.169 This study reveals how cells sense matrix stiffness by first being able to pull on the matrix, and second, by transducing the force required to modify their matrix into a signal for morphological change using cytoskeletal motors non-muscle myosin II linked to focal adhesions complexes.169
It has been proposed that nuclear entry of transcription factors linked to matrix remodelling is generally regulated by stiffness.170 One hypothesis described to explain these observations is that, with increasing collagen content leading to increased ECM stiffness, residing stem cells form a stable actomyosin cytoskeleton, stable focal adhesions and a tension-stable nucleus.166 This could prime cells to TGF-β-mediated activation of ubiquitous transcription factors, such as Smads, to translocate to the nucleus and promote matrix remodelling via collagen synthesis and contractility proteins like α-smooth muscle actin, promoting cells down a differentiation fate.166 However, ASCs residing within soft ECM would not contract nor stabilize their nucleus tension, possibly remaining undifferentiated.166
Furthermore, RA has been reported as a regulator of shuttle transcription factor linked to HSC self-renewal and differentiation.171 Of all the gene targets regulated by RA, lamin-A has attracted the most attention as variations in its expression have a key role in ASCs mechano-responsiveness.172–174 Lamin-A is an intermediate-filament protein that controls nuclear deformability, maintains DNA stability and couples mechanical stimulation to biochemical signalling in stem cells. Its level is proportional to primary tissue matrix stiffness (0.1–40 kPa) and increases in cultured stem cells with increasing matrix/hydrogel stiffness.170,173 Importantly, A-type lamins have also been implicated as intrinsic modulators of ageing in multiple ASC and their niches via the Wnt canonical pathway, the TGF-β pathway and retinoblastoma transcription factor pathway, the latter more extensively studied in SaCs.175 Further studies on mechanical regulation of stemness will allow a deeper understanding on the molecular mechanisms of geometry and stiffness translation to cells. Nevertheless, the relationship between stiffness and ASC propagation has been previously explored in the four niches presently discussed.
Although extracted bone marrow is generally considered a soft tissue (<0.3 kPa),176 bone is rigid (>1000 kPa), whereas endosteal-like ECM secreted by cultured osteoblasts is stiff (20–40 kPa).177 Therefore, this varied range of stiffness found in bone marrow,178 as well as the existence of different niches (e.g. vascular and osteoblastic) and ASC populations (e.g. LT-HSCs and ST-HSCs), could explain non-concordant results in the literature, where both 0.8 kPa179 and 12.2 or 30.4 kPa180 have been reported as the optimal modulus for ex vivo culturing of HSCs.
Variations in stiffness play an important role even in one of the softest tissues in the body, the brain, whose stiffness changes with age and varies from one region to the other. The juvenile or developing brain (~0.04 kPa) is not as stiff as the adult brain (~1.2 kPa). Studies have reported a proliferation peak of NSCs when grown on substrates of 1–4 kPa stiffness, whereas differentiation down the different neural progeny has been observed on both softer (<1 kPa) or stiffer substrates (>7 kPa).172,181
Using intestinal organoids, Gjorevski et al.46 have elucidated that culture within a stiff matrix (1.3 kPa) promotes enhanced proliferation and survival of ISCs, whereas culture within a soft matrix (300 Pa) results in poor proliferation and enhanced differentiation capability, conversely to the hypothesis that lower stiffness would promote stemness.166,170 This has resulted in the development of dynamic 3D matrices for organoid culture in which stiffness changes over time to ideally encourage expansion of cells in the initial stage, followed by differentiation.46
Studies focusing on the MuSC niche have demonstrated that MuSCs retain their capacity for self-renewal in vitro when cultured on 12 kPa hydrogel substrates as opposed to culture in plastic (~106 kPa).18 Despite further evidence showing that primary myoblasts present higher proliferation in stiffer gels (12 and 45 kPa) when compared to softer gels (1 kPa),182 other studies indicate that 2 kPa would be ideal for expanding the proliferative pool of muscle progenitors.183,184 The fact that aged and damaged myofibres are stiff (18 kPa) and that gels presenting such values were inhibitory for proliferative MuSCs should be taken into account during MuSCs growth protocol optimization.183 Increasing stiffness beyond optimal expansion modulus could mimic fibrotic conditions,185 generally considered counterproductive in terms of cell propagation.186 Stiffness related to collagen VI content has also been demonstrated to play a crucial role in SaCs self-renewal, since muscle tissue depleted of collagen VI had significantly reduced stiffness, resulting in impaired tissue regeneration. Nevertheless, this effect was rescued by reinstating the expression of collagen VI.148
There is an emerging body of research focusing on the relationship between not only matrix stiffness and but also elasticity on ASC fate. Controlling matrix elasticity in vitro has been attempted using microfabrication and microfluidic technology to assess cellular responses to matrix stiffness gradients.187 For example, a microfluidic-based lithography channel was developed containing a hydrogel substrate expressing a stiffness gradient that was generated by introducing polyethylene-fibrinogen and different amounts of polethylene glycol diacrylate (PEGDA) into the channel.188 Also, a micro-fabricated post-array made of polymer polydimethylsiloxane (PDMS) and controlled by a magnetic field was used to exert precise elastic forces onto cells in order to investigate how cells would sense these forces.189 This advocates for the mechano-sensitivity of cells and emphasizes that careful selection of in vitro culturing conditions is necessary when growing ASCs, since they exert mechanical forces to their attached ECM and cell-fate decisions are based on the feedback gauge.
Oxygen
There is general consensus that a low oxygen state maintains stem cell quiescence and self-renewal capability. This is based on the observation that mammalian cells in adult tissues undergo aerobic metabolism and thus generate reactive oxygen species resulting in oxidative stress. This in turn leads to DNA damage and mutations, which accumulate over time.190 To maintain stem cells in an undifferentiated and unmutated state, it is hypothesized that the controlled environment of tissue-specific niches protects cells from oxidative stress. There is emerging evidence to suggest that many types of stem cells rely on anaerobic glycolysis to maintain quiescence while limiting DNA damage.191 Stem cells reside in a protective niche where oxygen levels fall below those of surrounding tissue, offering a distinct anatomical compartment in which cells can maintain low rates of proliferation and limit oxidative stress.190 ASCs within hypoxic environments up-regulate hypoxia-inducible factor (HIF)-Iα which in turn promotes glycolysis.191 Low oxygen and transcription factors expressed in response to hypoxia have been found to be involved in maintenance of stemness.191–193 Additionally, HIF-2α has also been implicated in the regulation of stem cell master genes SOX2, NANOG and OCT4.194 All of this has implications for the way in which stem cells are cultured. Maintaining the biomimetic niche characteristics as we study cell populations is paramount to gain meaningful insights into cell behaviour.
The oxygen range accepted to represent physiological hypoxia in tissues within the body is between 2% and 9%, depending on tissue type.195 Compounded by the fact that direct measurement of oxygen tension in tissues is difficult, very often, levels of hypoxia are extrapolated by indirect data which mainly looks at the upregulation of HIF-1α.196 Nevertheless, this may not be a direct reflection of hypoxia, as HIF-Iα may be regulated by other factors.197
Recent direct measurement of the oxygen concentration within the bone-marrow cavity of mice, the niche for HSCs, has revealed that levels of O2 lie between 1.3% and 4.2%.196 Although this may seem surprising given the dense vascularity of the bone marrow, this is countered by the high cellularity and thus high consumption of oxygen by proliferating haematopoietic cells.196 It has thus been suggested that cellular consumption of oxygen dictates the hypoxic niche. Culturing LT-HSCs at 1% oxygen promotes a decrease in the proliferation rate and an accumulation in G0, but increases their engraftment potential.192 This suggests that the low microenvironmental oxygen tension maintains these cells within a protective niche while retaining their self-renewal potential.192 This study adds to the significant body of work on the influence of oxygen tension on stem cell quiescence, where low oxygen tension is considered a niche characteristic essential for the maintenance of the undifferentiated states of embryonic and other stem cell types.
In NSCs, there is evidence to suggest that culturing cells in hypoxic conditions, mainly between 2.5% and 5% promotes self-renewal.198 Interestingly, the oxygen levels in brain vary from 14% to 0.55%.199–201 This large range means cells in different brain compartments are exposed to varying oxygen levels. Moreno and colleagues have elucidated some optimal oxygen ranges to culture NSCs, shown to self-renew and proliferate at 5% oxygen. In this study, the authors showed that the calcium regulated transcription factor Nfatc4 signalling axis acts as a major regulator of NSC self-renewal and proliferation both in vitro and in vivo.202 Levels of hypoxia as low as 1% oxygen tend to lead to apoptosis of NSCs.198
Accordingly, ischaemic preconditioning aiming to protect SI against reperfusion injury has driven upregulation of Lgr5, increasing formation of crypt organoids in vitro while upregulating HIF1α and Wnt/β-catenin.203 Although oxygen levels were not measured in this study, conditionally reprogrammed cells from colon tissue seem to better expand in a 2% controlled oxygen incubator.101
Looking at muscle ASCs, evidence suggests that hypoxic levels as low as 1% oxygen maintain stemness in murine SaC-derived primary myoblasts.204 This study found that culture of primary SaCs at 1% oxygen resulted in the upregulation of Pax7, a key regulator of SaCs self-renewal. Other studies using various MuSCs populations are in agreement with this. In murine muscle myoblasts, 1% hypoxic conditions inhibited the differentiation of these cells, but this process was found to be reversible.205 Similarly, culture of bovine SaCs at 1% oxygen enhanced proliferation rates.206 Hypoxic conditioning at 5% oxygen was also reported to stimulate proliferation of human skeletal precursor cells.207
The importance of the hypoxic element on the ASC niches is thus evident in its direct influence on retention of stemness by cells and prevention of differentiation.
Advances in microenvironment biomimicry for ASC proliferation
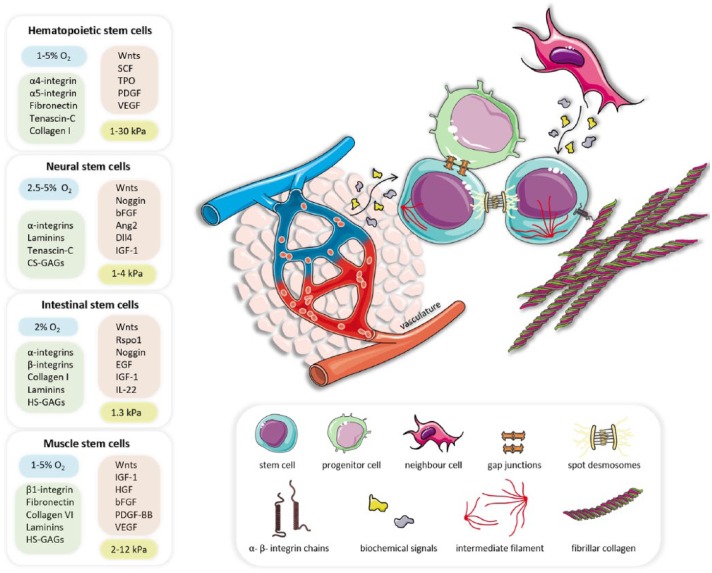
There has been a concerted effort towards recreating biomimetic features in vitro to direct ASC culture. Soluble factors play a critical role in determining ASC self-renewal, proliferation and differentiation, but they play a role alongside and in conjunction with other microenvironmental features. Much recent work has focused on manipulating physical microenvironmental features to modulate ASC fate and these physical components also regulate the release of soluble factors. The main findings highlighted in this review for bone marrow, neural, intestine and muscle tissues ASC niches are overviewed in Figure 1. In addition to biochemical and biophysical factors, other promising advances are likely to impact the field, such as the introduction of more physiological complexity to models by the use of microfluidics, innervation/electric stimuli, high-throughput culture testing and the use of dynamic and nanoscaled matrices.
Figure 1.
Schematic representation of generic adult stem cells (ASCs) niche features, including ASCs, their progenitor cells, neighbour cells, the vasculature and the extracellular matrix. Adult stem cells sense and respond to their surrounding extracellular matrix via integrin-mediated adhesion, depicted as α- and β-integrin chains, together with cytoskeletal intermediate filaments and intercellular spot desmosomes. Paracrine signals and growth factors are secreted by the vasculature and proximal progenitor or neighbouring cells, overall influencing ASC fate. The overarching biochemical, biophysical and biomechanical features discussed and referenced in this review have been distilled down to a list of conditions reported to promote ASC expansion in the four tissue-specific niches of interest. Elements within the boxes comprise general recommendations for oxygen levels (blue), ECM components (green), biochemical factors (orange) and stiffness module (yellow) to be used in specific ASC cultures. This figure was prepared using elements from Servier Medical Art.
2D culture of bone-marrow stromal cells is a well-established technique to support in vitro short-term expansion of non-adherent bone-marrow cells.208 Aiming to improve the necessary expansion of HSCs for clinical applications, engineering strategies have been tested with additional components such as scaffolds, cytokines and supporting stromal cells. PEG-based hydrogels with immobilized SCF and IFN-y enhance the expansion of mouse HSCs and progenitors; however, transplantation assays to confirm the reconstitution ability of expanded HSCs remain to be performed.209 A newly developed scaffold-free automated system with feedback control was developed after in silico prediction of negative regulators of HSC compartment expansion. A tuneable mode of media regulation and a fed-batch feeding strategy that keeps cells at low densities instead of perfusion, promoted an increase in HSCs from cord blood in 11 times after 12 days of culture.210 In another study, PDMS scaffolds filled with collagen I were implanted, and thus conditioned, in mice for 8 weeks and explanted for use as engineered bone marrow in a microfluidic device. These were seeded with HSCs and resulted in functional and proportional expanded HSCs populations within 1 week. This model was successfully tested for toxicology assays and for studying the bone-marrow niche while controlling cell populations.211
Researchers are now able to engineer arrays of micro-wells with robotic protein spotting and topographical micro-patterning of hydrogels, which clearly demonstrate that laminin and fibronectin in specific defined amounts improve mouse NSCs expansion.212 Further methods to expand NSCs include 3D culturing in PDMS-based microfluidic devices.213 These devices hold a central channel, seeded with NSCs and collagen type I hydrogels, which are then surrounded by adjacent channels where cultured brain vascular cells form tube-like structures. This set-up highlights the importance of the presence of vascular cells, or other important cell-cell interactions, resulting in increased NSC survival, neurosphere formation and size.213
Long-term culture of ISCs has been extensively demonstrated by the Matrigel-based organoid systems, which allow the in vitro production of both stem and differentiated intestinal lineages.44,45 The use of synthetic chemically defined 3D matrices generating the same effects as Matrigel could overcome problems imposed by this product’s use, such as its animal origin, unknown precise composition and unsuitability for clinical applications. Recently, synthetic ECM analogues produced by RGD-functionalized PEG hydrogels with different stiffness were found to be appropriate platforms for intestinal organoid cultures. Moreover, ISCs expansion was optimal under higher stiffness while matrix softening could promote differentiation, highlighting the utility of using dynamic matrix strategies for a tight control of ASC fate.46 Such strategies can now be applied to other established organoid cultures in order to substitute Matrigel and define which conditions might increase ASCs number before inducing their commitment to more differentiated lineages.
Aiming to develop a culture model more reflective of the native environment, Serena and colleagues have coupled electrical stimulation to SaCs cultures on 3D porous collagens scaffolds.214 Using this method, both cell proliferation and myogenic potential were improved via nitric oxide release promoted by electrical stimulation before implantation in mice.214
The aforementioned examples illustrate that, despite the accumulated knowledge on ASC niche composition and their role in laboratory-based expansion methods, much remains to be explored in order to overcome the notorious difficulty to achieving long-term propagation of ASCs.
Concluding remarks
Expanding ASCs from tissue samples for longer periods while maintaining their stemness in order to obtain therapeutic and high-throughput testing cell numbers is a much needed tool for stem cell biology and tissue engineering. By reviewing four exemplar ASC niches with different cell turnover rates, we have overviewed how soluble factors, 3D matrix composition, stiffness and oxygen tension all act on ASCs.
In some instances, it is clear to see how the native microenvironment still resonates with cell behaviour, which includes how ASCs react upon stiffness changes and oxygen levels. It is also becoming clearer that representing features of the native microenvironment can be a good starting point to recapitulate in vivo behaviours in vitro. Still, obtaining higher ASCs number from small amounts of starting samples can be challenging, especially for tissues with native low turnover rates and low repair abilities, which may need to be expanded ex vivo to much higher rates than in vivo. Understanding the molecular mechanisms which drive transition between quiescent and proliferative states of ASCs is thus crucial for optimization. Likewise, searching for biological cues from development and injury response may help the development of fitter strategies to bring scientific community closer to effectively use ASCs in basic and applied biomedical research.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: P.A.R. was funded by the Brazilian National Counsel of Technological and Scientific Development and by The Royal Free Charity (grant no. 536995). M.P. was funded by the Bone Cancer Research Trust (grant no. 519149) and by the SCAT Bone Cancer Trust (grant no. 526574). M.L. was funded by the NIHR (i4i programme II-LA-0813-20002) and EPSRC (EP/L020904/1). U.C. was funded by the NIHR (i4i programme II-LA-0813-20002).
References
- 1. Müller R, Lengerke C. Patient-specific pluripotent stem cells: promises and challenges. Nat Rev Endocrinol 2009; 5(4): 195–203. [DOI] [PubMed] [Google Scholar]
- 2. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 2010; 12(1): 87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001; 17(1): 387–403. [DOI] [PubMed] [Google Scholar]
- 4. Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell 2010; 6(2): 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mimeault M, Batra SK. Great promise of tissue-resident adult stem/progenitor cells in transplantation and cancer therapies. Adv Exp Med Biol 2012; 741: 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferraro F, Celso CL, Scadden D. Adult stem cells and their niches. Adv Exp Med Biol 2010; 695: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu Y-C, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol 2012; 13(2): 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rando TA. Stem cells, ageing and the quest for immortality. Nature 2006; 441(7097): 1080–1086. [DOI] [PubMed] [Google Scholar]
- 9. Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 2009; 137(5): 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978; 4(1–2): 7–25. [PubMed] [Google Scholar]
- 11. He N, Zhang L, Cui J, et al. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res 2014; 2014: 128436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borghesi L. Hematopoiesis in steady-state versus stress: self-renewal, lineage fate choice, and the conversion of danger signals into cytokine signals in hematopoietic stem cells. J Immunol 2014; 193(5): 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjornsson CS, Apostolopoulou M, Tian Y, et al. It takes a village: constructing the neurogenic niche. Dev Cell 2015; 32(4): 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ming G, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70(4): 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011; 469(7330): 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sasaki N, Sachs N, Wiebrands K, et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci U S A 2016; 113(37): E5399–E5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuang S, Kuroda K, Le Grand F, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007; 129(5): 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilbert PM, Havenstrite KL, Magnusson KEG, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010; 329(5995): 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 2005; 15(5): 405–412. [DOI] [PubMed] [Google Scholar]
- 20. Edmondson R, Broglie JJ, Adcock AF, et al. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 2014; 12(4): 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005; 433(7027): 760–764. [DOI] [PubMed] [Google Scholar]
- 22. Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007; 317(5839): 807–810. [DOI] [PubMed] [Google Scholar]
- 23. Villeda SA, Luo J, Mosher KI, et al. The aging systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011; 477(7362): 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425(6960): 836–841. [DOI] [PubMed] [Google Scholar]
- 25. Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004; 103(9): 3258–3264. [DOI] [PubMed] [Google Scholar]
- 26. Jiang J, Papoutsakis ET. Stem-cell niche based comparative analysis of chemical and nano-mechanical material properties impacting ex vivo expansion and differentiation of hematopoietic and mesenchymal stem cells. Adv Healthc Mater 2013; 2(1): 25–42. [DOI] [PubMed] [Google Scholar]
- 27. Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol 2013; 23(6): 935–942. [DOI] [PubMed] [Google Scholar]
- 28. Nusse R. Wnt signaling and stem cell control. Cell Res 2008; 18(5): 523–527. [DOI] [PubMed] [Google Scholar]
- 29. Ring A, Kim Y-M, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev 2014; 10(4): 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003; 423(6938): 409–414. [DOI] [PubMed] [Google Scholar]
- 31. Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2008; 2(3): 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luis TC, Weerkamp F, Naber BAE, et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood 2009; 113(3): 546–554. [DOI] [PubMed] [Google Scholar]
- 33. Luis TC, Naber BAE, Roozen PPC, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 2011; 9(4): 345–356. [DOI] [PubMed] [Google Scholar]
- 34. Lie D-C, Colamarino SA, Song H-J, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005; 437(7063): 1370–1375. [DOI] [PubMed] [Google Scholar]
- 35. Qu Q, Sun G, Li W, et al. Orphan nuclear receptor TLX activates Wnt/β-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol 2010; 12(1): 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adachi K, Mirzadeh Z, Sakaguchi M, et al. β-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 2007; 25(11): 2827–2836. [DOI] [PubMed] [Google Scholar]
- 37. Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 2002; 297(5580): 365–369. [DOI] [PubMed] [Google Scholar]
- 38. Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun 2004; 313(4): 915–921. [DOI] [PubMed] [Google Scholar]
- 39. Yoshinaga Y, Kagawa T, Shimizu T, et al. Wnt3a promotes hippocampal neurogenesis by shortening cell cycle duration of neural progenitor cells. Cell Mol Neurobiol 2010; 30(7): 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pinto D, Gregorieff A, Begthel H, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003; 17(14): 1709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuhnert F, Davis CR, Wang H-T, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A 2004; 101(1): 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Binnerts ME, Kim K-A, Bright JM, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A 2007; 104(37): 14700–14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farin HF, Jordens I, Mosa MH, et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 2016; 530(7590): 340–343. [DOI] [PubMed] [Google Scholar]
- 44. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459(7244): 262–265. [DOI] [PubMed] [Google Scholar]
- 45. Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011; 141(5): 1762–1772. [DOI] [PubMed] [Google Scholar]
- 46. Gjorevski N, Sachs N, Manfrin A, et al. Designer matrices for intestinal stem cell and organoid culture. Nature 2016; 539(7630): 560–564. [DOI] [PubMed] [Google Scholar]
- 47. Otto A, Schmidt C, Luke G, et al. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci 2008; 121(17): 2939–2950. [DOI] [PubMed] [Google Scholar]
- 48. Le Grand F, Jones AE, Seale V, et al. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 2009; 4(6): 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bao J, Zheng JJ, Wu D. The structural basis of DKK-mediated inhibition of Wnt/LRP signaling. Sci Signal 2012; 5(224): pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kalani MYS, Cheshier SH, Cord BJ, et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A 2008; 105(44): 16970–16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koch S, Nava P, Addis C, et al. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology 2011; 141(1): 259.e8–268.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iozzi S, Remelli R, Lelli B, et al. Functional characterization of a small-molecule inhibitor of the DKK1-LRP6 interaction. ISRN Mol Biol 2012; 2012: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kopan R, Schroeter EH, Kisslinger JA. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998; 393(6683): 382–386. [DOI] [PubMed] [Google Scholar]
- 54. Kopan R. Notch signaling. Cold Spring Harb Perspect Biol 2012; 4(10): a011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425(6960): 841–846. [DOI] [PubMed] [Google Scholar]
- 56. Varnum-Finney B, Halasz LM, Sun M, et al. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest 2011; 121(3): 1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 2010; 6(3): 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mancini SJC, Mantei N, Dumortier A, et al. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood 2005; 105(6): 2340–2342. [DOI] [PubMed] [Google Scholar]
- 59. Maillard I, Koch U, Dumortier A, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell 2008; 2(4): 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chapouton P, Skupien P, Hesl B, et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci 2010; 30(23): 7961–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006; 442(7104): 823–826. [DOI] [PubMed] [Google Scholar]
- 62. Imayoshi I, Sakamoto M, Yamaguchi M, et al. Essential roles of notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci 2010; 30(9): 3489–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 2013; 340(6137): 1190–1194. [DOI] [PubMed] [Google Scholar]
- 64. Van Es JH, van Gijn ME, Riccio O, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005; 435(7044): 959–963. [DOI] [PubMed] [Google Scholar]
- 65. Bjornson CRR, Cheung TH, Liu L, et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 2012; 30(2): 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mourikis P, Sambasivan R, Castel D, et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 2012; 30(2): 243–252. [DOI] [PubMed] [Google Scholar]
- 67. Conboy IM, Rando TA. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 2012; 11(12): 2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang RN, Green J, Wang Z, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis 2014; 1(1): 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bhatia M, Bonnet D, Wu D, et al. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med 1999; 189(7): 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bonaguidi MA, Peng C-Y, McGuire T, et al. Noggin expands neural stem cells in the adult hippocampus. J Neurosci 2008; 28(37): 9194–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mira H, Andreu Z, Suh H, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 2010; 7(1): 78–89. [DOI] [PubMed] [Google Scholar]
- 72. Yousef H, Morgenthaler A, Schlesinger C, et al. Age-associated increase in BMP signaling inhibits hippocampal neurogenesis. Stem Cells 2015; 33(5): 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. He XC, Zhang J, Tong W-G, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat Genet 2004; 36(10): 1117–1121. [DOI] [PubMed] [Google Scholar]
- 74. Hardwick JCH, Van Den Brink GR, Bleuming SA, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology 2004; 126(1): 111–121. [DOI] [PubMed] [Google Scholar]
- 75. Haramis A-PG, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 2004; 303(5664): 1684–1686. [DOI] [PubMed] [Google Scholar]
- 76. Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 1989; 138(2): 311–315. [DOI] [PubMed] [Google Scholar]
- 77. Cook DR, Doumit ME, Merkel RA. Transforming growth factor-beta, basic fibroblast growth factor, and platelet-derived growth factor-BB interact to affect proliferation of clonally derived porcine satellite cells. J Cell Physiol 1993; 157(2): 307–312. [DOI] [PubMed] [Google Scholar]
- 78. Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 2001; 15(22): 2950–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ono Y, Calhabeu F, Morgan JE, et al. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ 2011; 18(2): 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Friedrichs M, Wirsdöerfer F, Flohé SB, et al. BMP signaling balances proliferation and differentiation of muscle satellite cell descendants. BMC Cell Biol 2011; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 2003; 39(6): 937–950. [DOI] [PubMed] [Google Scholar]
- 82. Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci 2007; 27(22): 5936–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ihrie RA, Shah JK, Harwell CC, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron 2011; 71(2): 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang LC, Nassir F, Liu Z-Y, et al. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology 2002; 122(2): 469–482. [DOI] [PubMed] [Google Scholar]
- 85. Zacharias WJ, Madison BB, Kretovich KE, et al. Hedgehog signaling controls homeostasis of adult intestinal smooth muscle. Dev Biol 2011; 355(1): 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rahman MS, Akhtar N, Jamil HM, et al. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res 2015; 3: 15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gammaitoni L, Bruno S, Sanavio F, et al. Ex vivo expansion of human adult stem cells capable of primary and secondary hemopoietic reconstitution. Exp Hematol 2003; 31(3): 261–270. [DOI] [PubMed] [Google Scholar]
- 88. Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012; 481(7382): 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gerber H-P, Malik AK, Solar GP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 2002; 417(6892): 954–958. [DOI] [PubMed] [Google Scholar]
- 90. Su RJ, Zhang XB, Li K, et al. Platelet-derived growth factor promotes ex vivo expansion of CD34+ cells from human cord blood and enhances long-term culture-initiating cells, non-obese diabetic/severe combined immunodeficient repopulating cells and formation of adherent cells. Br J Haematol 2002; 117(3): 735–746. [DOI] [PubMed] [Google Scholar]
- 91. Doan PL, Himburg HA, Helms K, et al. Epidermal growth factor regulates hematopoietic regeneration following radiation injury. Nat Med 2013; 19(3): 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Doran MR, Markway BD, Aird IA, et al. Surface-bound stem cell factor and the promotion of hematopoietic cell expansion. Biomaterials 2009; 30(25): 4047–4052. [DOI] [PubMed] [Google Scholar]
- 93. Ohkubo Y, Uchida AO, Shin D, et al. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J Neurosci 2004; 24(27): 6057–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Androutsellis-Theotokis A, Rueger MA, Park DM, et al. Angiogenic factors stimulate growth of adult neural stem cells. PLoS ONE 2010; 5(2): e9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Androutsellis-Theotokis A, Walbridge S, Park DM, et al. Cholera toxin regulates a signaling pathway critical for the expansion of neural stem cell cultures from the fetal and adult rodent brains. PLoS ONE 2010; 5(5): e10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Walker TL, White A, Black DM, et al. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J Neurosci 2008; 28(20): 5240–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Aberg MAI, Aberg ND, Palmer TD, et al. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci 2003; 24(1): 23–40. [DOI] [PubMed] [Google Scholar]
- 98. Van Landeghem L, Santoro MA, Mah AT, et al. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J 2015; 29(7): 2828–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015; 528(7583): 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang X, Yamamoto Y, Wilson LH, et al. Cloning and variation of ground state intestinal stem cells. Nature 2015; 522(7555): 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu X, Krawczyk E, Suprynowicz FA, et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat Protoc 2017; 12(2): 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Christov C, Chrétien F, Abou-Khalil R, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 2007; 18(4): 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tatsumi R, Liu X, Pulido A, et al. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol 2006; 290(6): C1487–C1494. [DOI] [PubMed] [Google Scholar]
- 104. Cosgrove BD, Gilbert PM, Porpiglia E, et al. Rejuvenation of the aged muscle stem cell population restores strength to injured aged muscles. Nat Med 2014; 20(3): 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hosokawa K, Arai F, Yoshihara H, et al. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood 2010; 116(4): 554–563. [DOI] [PubMed] [Google Scholar]
- 106. Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007; 1(6): 685–697. [DOI] [PubMed] [Google Scholar]
- 107. Hooper AT, Butler JM, Nolan DJ, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 2009; 4(3): 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med 2006; 12(2): 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 2014; 20(8): 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Spiegel A, Kalinkovich A, Shivtiel S, et al. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell 2008; 3(5): 484–492. [DOI] [PubMed] [Google Scholar]
- 111. Lin R. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res 2015; 1628: 327–342. [DOI] [PubMed] [Google Scholar]
- 112. Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 2000; 28(3): 713–726. [DOI] [PubMed] [Google Scholar]
- 113. Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004; 304(5675): 1338–1340. [DOI] [PubMed] [Google Scholar]
- 114. Moreno-Estellés M, González-Gómez P, Hortigüela R, et al. Symmetric expansion of neural stem cells from the adult olfactory bulb is driven by astrocytes via WNT7A. Stem Cells 2012; 30(12): 2796–2809. [DOI] [PubMed] [Google Scholar]
- 115. Sasaki N, Sachs N, Wiebrands K, et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci U S A 2016; 113(37): E5399–E5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Powell DW, Pinchuk IV, Saada JI, et al. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 2011; 73: 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 2014; 1840(8): 2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chazaud B, Sonnet C, Lafuste P, et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 2003; 163(5): 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nilsson SK, Debatis ME, Dooner MS, et al. Immunofluo-rescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J Histochem Cytochem 1998; 46(3): 371–377. [DOI] [PubMed] [Google Scholar]
- 120. Mercier F. Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell Mol Life Sci 2016; 73(24): 4661–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jabaji Z, Brinkley GJ, Khalil HA, et al. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS ONE 2014; 9(9): e107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bursac N, Juhas M, Rando TA. Synergizing engineering and biology to treat and model skeletal muscle injury and disease. Annu Rev Biomed Eng 2015; 17: 217–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 2008; 121(3): 255–264. [DOI] [PubMed] [Google Scholar]
- 124. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009; 326(5957): 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat Mater 2014; 13(6): 558–569. [DOI] [PubMed] [Google Scholar]
- 126. Cavalcanti-Adam EA, Volberg T, Micoulet A, et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J 2007; 92(8): 2964–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schvartzman M, Palma M, Sable J, et al. Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett 2011; 11(3): 1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li B, Moshfegh C, Lin Z, et al. Mesenchymal stem cells exploit extracellular matrix as mechanotransducer. Sci Rep 2013; 3(1): 2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kurth I, Franke K, Pompe T, et al. Hematopoietic stem and progenitor cells in adhesive microcavities. Integr Biol 2009; 1(5–6): 427–434. [DOI] [PubMed] [Google Scholar]
- 130. Sellers S, Tisdale JF, Agricola BA, et al. The presence of the carboxy-terminal fragment of fibronectin allows maintenance of non-human primate long-term hematopoietic repopulating cells during extended ex vivo culture and transduction. Exp Hematol 2004; 32(2): 163–170. [DOI] [PubMed] [Google Scholar]
- 131. Yokota T, Oritani K, Mitsui H, et al. Growth-supporting activities of fibronectin on hematopoietic stem/progenitor cells in vitro and in vivo: structural requirement for fibronectin activities of CS1 and cell-binding domains. Blood 1998; 91(9): 3263–3272. [PubMed] [Google Scholar]
- 132. Sagar BMM, Rentala S, Gopal PNV, et al. Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem Biophys Res Commun 2006; 350(4): 1000–1005. [DOI] [PubMed] [Google Scholar]
- 133. Nakamura-Ishizu A, Okuno Y, Omatsu Y, et al. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood 2012; 119(23): 5429–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Oswald J, Steudel C, Salchert K, et al. Gene-expression profiling of CD34+ hematopoietic cells expanded in a collagen I matrix. Stem Cells 2006; 24(3): 494–500. [DOI] [PubMed] [Google Scholar]
- 135. Flanagan LA, Rebaza LM, Derzic S, et al. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res 2006; 83(5): 845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sirko S, von Holst A, Wizenmann A, et al. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 2007; 134(15): 2727–2738. [DOI] [PubMed] [Google Scholar]
- 137. Akita K, von Holst A, Furukawa Y, et al. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem Cells 2008; 26(3): 798–809. [DOI] [PubMed] [Google Scholar]
- 138. Karumbaiah L, Enam SF, Brown AC, et al. Chondroitin sulfate glycosaminoglycan hydrogels create endogenous niches for neural stem cells. Bioconjug Chem 2015; 26(12): 2336–2349. [DOI] [PubMed] [Google Scholar]
- 139. Chiquet-Ehrismann R, Orend G, Chiquet M, et al. Tenascins in stem cell niches. Matrix Biol 2014; 37: 112–123. [DOI] [PubMed] [Google Scholar]
- 140. Faissner A, Roll L, Theocharidis U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol Cell Neurosci 2017; 81: 22–31. [DOI] [PubMed] [Google Scholar]
- 141. Lin G, Zhang X, Ren J, et al. Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila. Dev Biol 2013; 377(1): 177–187. [DOI] [PubMed] [Google Scholar]
- 142. Di Claudio F, Muglia C, Smaldini P, et al. Use of a collagen membrane to enhance the survival of primary intestinal epithelial cells. J Cell Physiol 2017; 232: 2489–2496. [DOI] [PubMed] [Google Scholar]
- 143. Scott A, Rouch JD, Jabaji Z, et al. Long-term renewable human intestinal epithelial stem cells as monolayers: a potential for clinical use. J Pediatr Surg 2016; 51(6): 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yamamoto S, Nakase H, Matsuura M, et al. Heparan sulfate on intestinal epithelial cells plays a critical role in intestinal crypt homeostasis via Wnt/β-catenin signaling. Am J Physiol Gastrointest Liver Physiol 2013; 305(3): G241–G249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem 2003; 278(15): 12601–12604. [DOI] [PubMed] [Google Scholar]
- 146. Lukjanenko L, Jung MJ, Hegde N, et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med 2016; 22(8): 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rozo M, Li L, Fan C-M. Targeting β1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med 2016; 22(8): 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Urciuolo A, Quarta M, Morbidoni V, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 2013; 4: 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Penton CM, Badarinarayana V, Prisco J, et al. Laminin 521 maintains differentiation potential of mouse and human satellite cell-derived myoblasts during long-term culture expansion. Skelet Muscle 2016; 6(1): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cornelison DDW, Filla MS, Stanley HM, et al. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol 2001; 239(1): 79–94. [DOI] [PubMed] [Google Scholar]
- 151. Cen L, Liu W, Cui L, et al. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res 2008; 63(5): 492–496. [DOI] [PubMed] [Google Scholar]
- 152. Merten O-W. Advances in cell culture: anchorage dependence. Philos Trans R Soc Lond B Biol Sci 2015; 370(1661): 20140040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Buske P, Przybilla J, Loeffler M, et al. On the biomechanics of stem cell niche formation in the gut – modelling growing organoids. FEBS J 2012; 279(18): 3475–3487. [DOI] [PubMed] [Google Scholar]
- 154. Chen W, Villa-Diaz LG, Sun Y, et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano 2012; 6(5): 4094–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Gauthaman K, Venugopal JR, Yee FC, et al. Nanofibrous substrates support colony formation and maintain stemness of human embryonic stem cells. J Cell Mol Med 2009; 13(9b): 3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Christopherson GT, Song H, Mao H-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009; 30(4): 556–564. [DOI] [PubMed] [Google Scholar]
- 157. Regalado-Santiago C, Juárez-Aguilar E, Olivares-Hernández JD, et al. Mimicking neural stem cell niche by biocompatible substrates. Stem Cells Int 2016; 2016: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chua K-N, Chai C, Lee P-C, et al. Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Exp Hematol 2007; 35(5): 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 2007; 17(23): 2054–2060. [DOI] [PubMed] [Google Scholar]
- 160. Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res 2016; 343(1): 42–53. [DOI] [PubMed] [Google Scholar]
- 161. Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474(7350): 179–183. [DOI] [PubMed] [Google Scholar]
- 162. Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 2012; 13(9): 591–600. [DOI] [PubMed] [Google Scholar]
- 163. Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev 2008; 22(23): 3320–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jansson L, Larsson J. Normal hematopoietic stem cell function in mice with enforced expression of the Hippo signaling effector YAP1. PLoS ONE 2012; 7(2): e32013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol 2015; 17(1): 7–19. [DOI] [PubMed] [Google Scholar]
- 166. Watt KI, Turner BJ, Hagg A, et al. The Hippo pathway effector YAP is a critical regulator of skeletal muscle fibre size. Nat Commun 2015; 6: 6048. [DOI] [PubMed] [Google Scholar]
- 167. Huraskin D, Eiber N, Reichel M, et al. Wnt/β-catenin signaling via Axin2 is required for myogenesis and, together with YAP/Taz and Tead1, active in IIa/IIx muscle fibers. Development 2016; 143(17): 3128–3142. [DOI] [PubMed] [Google Scholar]
- 168. Esteves de Lima J, Bonnin M-A, Birchmeier C, et al. Muscle contraction is required to maintain the pool of muscle progenitors via YAP and NOTCH during fetal myogenesis. Elife 2016; 5: e15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126(4): 677–689. [DOI] [PubMed] [Google Scholar]
- 170. Dingal PCDP, Discher DE. Combining insoluble and soluble factors to steer stem cell fate. Nat Mater 2014; 13(6): 532–537. [DOI] [PubMed] [Google Scholar]
- 171. Purton LE, Dworkin S, Olsen GH, et al. RARγ is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med 2006; 203(5): 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Saha K, Keung AJ, Irwin EF, et al. Substrate modulus directs neural stem cell behavior. Biophys J 2008; 95(9): 4426–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013; 341(6149): 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Banerjee A, Arha M, Choudhary S, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009; 30(27): 4695–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Pekovic V, Hutchison CJ. Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. J Anat 2008; 213(1): 5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Winer JP, Janmey PA, McCormick ME, et al. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A 2009; 15(1): 147–154. [DOI] [PubMed] [Google Scholar]
- 177. Shin J-W, Swift J, Ivanovska I, et al. Mechanobiology of bone marrow stem cells: from myosin-II forces to compliance of matrix and nucleus in cell forms and fates. Differentiation 2013; 86(3): 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Jansen LE, Birch NP, Schiffman JD, et al. Mechanics of intact bone marrow. J Mech Behav Biomed Mater 2015; 50: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Chitteti BR, Kacena MA, Voytik-Harbin SL, et al. Modulation of hematopoietic progenitor cell fate in vitro by varying collagen oligomer matrix stiffness in the presence or absence of osteoblasts. J Immunol Methods 2015; 425: 108–113. [DOI] [PubMed] [Google Scholar]
- 180. Kumar SS, Hsiao J-H, Ling Q-D, et al. The combined influence of substrate elasticity and surface-grafted molecules on the ex vivo expansion of hematopoietic stem and progenitor cells. Biomaterials 2013; 34(31): 7632–7644. [DOI] [PubMed] [Google Scholar]
- 181. Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009; 30(36): 6867–6878. [DOI] [PubMed] [Google Scholar]
- 182. Boontheekul T, Hill EE, Kong H-J, et al. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng 2007; 13(7): 1431–1442. [DOI] [PubMed] [Google Scholar]
- 183. Lacraz G, Rouleau A-J, Couture V, et al. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS ONE 2015; 10(8): e0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Trensz F, Lucien F, Couture V, et al. Increased microenvironment stiffness in damaged myofibers promotes myogenic progenitor cell proliferation. Skelet Muscle 2015; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta 2013; 1832(7): 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Boonen KJM, Post MJ. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue Eng Part B Rev 2008; 14: 419–431. [DOI] [PubMed] [Google Scholar]
- 187. Kshitiz, Kim D-H, Beebe DJ, et al. Micro- and nanoengineering for stem cell biology: the promise with a caution. Trends Biotechnol 2011; 29(8): 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cheung YK, Azeloglu EU, Shiovitz DA, et al. Microscale control of stiffness in a cell-adhesive substrate using microfluidics-based lithography. Angew Chem Int Ed Engl 2009; 48(39): 7188–7192. [DOI] [PubMed] [Google Scholar]
- 189. Sniadecki NJ, Anguelouch A, Yang MT, et al. Magnetic microposts as an approach to apply forces to living cells. Proc Natl Acad Sci U S A 2007; 104(37): 14553–14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 2010; 7(2): 150–161. [DOI] [PubMed] [Google Scholar]
- 191. Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 2014; 15(4): 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Eliasson P, Jönsson J-I. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol 2010; 222(1): 17–22. [DOI] [PubMed] [Google Scholar]
- 193. Tiwari A, Wong CS, Nekkanti LP, et al. Impact of oxygen levels on human hematopoietic stem and progenitor cell expansion. Stem Cells Dev 2016; 25(20): 1604–1613. [DOI] [PubMed] [Google Scholar]
- 194. Forristal CE, Wright KL, Hanley NA, et al. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2010; 139(1): 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 2008; 9(4): 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014; 508(7495): 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014; 505(7483): 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. De Filippis L, Delia D. Hypoxia in the regulation of neural stem cells. Cell Mol Life Sci 2011; 68(17): 2831–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Dings J, Meixensberger J, Jäger A, et al. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 1998; 43(5): 1082–1095. [DOI] [PubMed] [Google Scholar]
- 200. Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 2001; 128(3): 263–276. [DOI] [PubMed] [Google Scholar]
- 201. Ljungkvist ASE, Bussink J, Kaanders JHAM, et al. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res 2007; 167(2): 127–145. [DOI] [PubMed] [Google Scholar]
- 202. Moreno M, Fernández V, Monllau JM, et al. Transcriptional profiling of hypoxic neural stem cells identifies calcineurin-NFATc4 signaling as a major regulator of neural stem cell biology. Stem Cell Reports 2015; 5(2): 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Chen Y, Lee S-H, Tsai Y-H, et al. Ischemic preconditioning increased the intestinal stem cell activities in the intestinal crypts in mice. J Surg Res 2014; 187(1): 85–93. [DOI] [PubMed] [Google Scholar]
- 204. Liu W, Wen Y, Bi P, et al. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 2012; 139(16): 2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Di Carlo A, De Mori R, Martelli F, et al. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J Biol Chem 2004; 279(16): 16332–16338. [DOI] [PubMed] [Google Scholar]
- 206. Kook S, Son Y, Lee K, et al. Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell Biol Int 2008; 32(8): 871–878. [DOI] [PubMed] [Google Scholar]
- 207. Martin SD, Collier FM, Kirkland MA, et al. Enhanced proliferation of human skeletal muscle precursor cells derived from elderly donors cultured in estimated physiological (5%) oxygen. Cytotechnology 2009; 61(3): 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Dexter TM, Wright EG, Krizsa F, et al. Regulation of haemopoietic stem cell proliferation in long term bone marrow cultures. Biomedicine 1977; 27(9–10): 344–349. [PubMed] [Google Scholar]
- 209. Cuchiara ML, Coşkun S, Banda OA, et al. Bioactive poly(ethylene glycol) hydrogels to recapitulate the HSC niche and facilitate HSC expansion in culture. Biotechnol Bioeng 2016; 113(4): 870–881. [DOI] [PubMed] [Google Scholar]
- 210. Csaszar E, Kirouac DC, Yu M, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell 2012; 10(2): 218–229. [DOI] [PubMed] [Google Scholar]
- 211. Torisawa Y, Spina CS, Mammoto T, et al. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat Methods 2014; 11(6): 663–669. [DOI] [PubMed] [Google Scholar]
- 212. Roccio M, Gobaa S, Lutolf MP. High-throughput clonal analysis of neural stem cells in microarrayed artificial niches. Integr Biol 2012; 4(4): 391–400. [DOI] [PubMed] [Google Scholar]
- 213. Shin Y, Yang K, Han S, et al. Stem cells: reconstituting vascular microenvironment of neural stem cell niche in three-dimensional extracellular matrix (Adv. Healthcare Mater. 9/2014). Adv Healthc Mater 2014; 3(9): 1349. [DOI] [PubMed] [Google Scholar]
- 214. Serena E, Flaibani M, Carnio S, et al. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol Res 2008; 30(2): 207–214. [DOI] [PubMed] [Google Scholar]