Abstract
Cell deletion approaches to pain directed at either the primary nociceptive afferents or second-order neurons are highly effective analgesic manipulations. Second-order spinal neurons expressing the neurokinin 1 (NK1) receptor are required for the perception of many types of pain. To delete NK1+ neurons for the purpose of pain control, we generated a toxin–peptide conjugate using DTNB-derivatized (Cys0) substance P (SP) and a N-terminally truncated Pseudomonas exotoxin (PE35) that retains the endosome-release and ADP-ribosylation enzymatic domains but with only one free sulfhydryl side chain for conjugation. This allowed generation of a one-to-one product linked by a disulfide bond (SP-PE35). In vitro, Chinese hamster ovary cells stably transfected with the NK1 receptor exhibited specific cytotoxicity when exposed to SP-PE35 (IC50 = 5 × 10−11 M), whereas the conjugate was nontoxic to NK2 and NK3 receptor-bearing cell lines. In vivo studies showed that, after infusion into the spinal subarachnoid space, the toxin was extremely effective in deleting NK1 receptor-expressing cells from the dorsal horn of the spinal cord. The specific cell deletion robustly attenuated thermal and mechanical pain sensations and inflammatory hyperalgesia but did not affect motoric capabilities. NK1 receptor cell deletion and antinociception occurred without obvious lesion of non–receptor-expressing cells or apparent reorganization of primary afferent innervation. These data demonstrate the extraordinary selectivity and broad-spectrum antinociceptive efficacy of this ligand-directed protein therapeutic acting via receptor-mediated endocytosis. The loss of multiple pain modalities including heat and mechanical pinch, transduced by different populations of primary afferents, shows that spinal NK1 receptor-expressing neurons are critical points of convergence in the nociceptive transmission circuit. We further suggest that therapeutic end points can be effectively and safely achieved when SP-PE35 is locally infused, thereby producing a regionally defined analgesia.
Keywords: Pain, hyperalgesia, carrageenan, neurokinin 1 receptor, NK1 receptor, NK2 receptor, NK3 receptor, spinal lamina I, analgesia, mechanical pain, intrathecal, inflammation, inflammatory, neuropeptides, receptor endocytosis, G protein-coupled receptor, receptor internalization
Introduction
Development of molecular interventions for therapeutic modification of neural circuits includes biochemical delivery strategies ranging from in vivo gene transfer to receptor-mediated cell-specific deletion approaches.1–5 Neuropeptides represent a large number of bioactive compounds, and their specific regional distribution patterns indicate that peptide neuromodulators could be used to treat a wide number of neurological or psychiatric disorders. Peptides are often difficult to transform into small molecule drugs. 6 However, the receptors for these peptides are often members of the seven transmembrane G protein-coupled receptor superfamily 7 and frequently undergo agonist-mediated endocytosis. 8 Therapeutically, receptor internalization provides a ligand-directed route of entry into specific neurons that can be adapted for therapeutic objectives. Substance P (SP), an amidated 11 amino acid neuropeptide derived from the preprotachykinin gene (TAC1), triggers a prominent agonist-mediated receptor internalization of its cognate neurokinin 1 (NK1) receptor, which is a G protein-coupled receptor. 9 Internalization of the NK1 receptor has been demonstrated in cultured cells and in vivo subsequent to substance P binding.10–12
Because of its high concentration in discrete neural pathways, such as the spinal cord and basal ganglia, 13 substance P has been the target of intensive drug development efforts related to pain, depression 14 alcoholism,15–18 and nausea. 19 In the spinal cord, potential analgesic activity was hypothesized based on the connection between nociceptive primary afferent dorsal root ganglion neurons, which make the substance P precursor, preprotachykinin, at a high level, and second-order neurons in the spinal cord, which express NK1 receptors. 20 The interaction of substance P with its receptor is known to mediate key elements of nociceptive signaling, consistent with temporal summation or wind up21,22 and disruption or modulation of substance P signaling has formed the basis of numerous experimental analgesic manipulations including peptide and nonpeptide antagonists, 23 antisense oligonucleotide knockdown, 24 and ligand-directed neurotoxins.3,17 In mice, homologous recombination to delete the gene encoding the NK1 receptor can modulate inflammation-induced hyperalgesia, morphine analgesia, and descending inhibition.25–27 Thus, manipulation of the substance P system, either presynaptically or postsynaptically, supports the idea that the spinal tachykinin system is a fundamental link in pain transmission from periphery to spinal cord as well as higher central nervous system centers.3,28–31
Since its discovery, the process of receptor-mediated endocytosis has been extensively explored for its utility in therapeutic applications. 32 Pseudomonas exotoxin, Diphtheria toxin, and certain plant toxins, including ricin and saporin, are members of a protein family functionally characterized as translation inhibitor proteins. 33 All act in the cytosol of mammalian cells to inhibit protein synthesis and cause cell death at very low concentrations. These toxins use the endocytosis pathway to gain entry into intracellular sorting vesicles; from there, a variety of pathways are available to mediate unfolding and translocation to the cell cytosol. Once refolded in the cytosol, the toxins enzymatically inhibit protein synthesis. Because of the high enzymatic catalytic rate and the apparent resistance of the toxins to proteosome degradation, only a few molecules need to reach the cytosol to be effective. Toxins, therefore, have become agents of choice for experimental immunotherapy for a wide range medical problems including hairy cell leukemia, 34 glioblastoma multiforme, 35 and HIV. 36
Early conjugates between ligands and toxins were constructed using chemical cross-linking agents. Depending on the number of potential reactive groups on each component, this often results in a mixed product possessing a spectrum of activity. Recombinant DNA strategies allow for the generation of gene fusions and the production of chimeric proteins with uniform primary structure. However, several types of molecules cannot easily be made by recombinant DNA expression. For example, small peptide ligands are released from larger precursor proteins and are frequently posttranslationally modified at either their N or C terminus (e.g., amidated). Because these modifications are not easily duplicated upon expression in Escherichia coli, chemical conjugation using a synthetically produced peptide is a potential alternative. One solution to the problem of multiple conjugation sites and the resulting mixed products is to design molecules that have only one reactive group each. CysPE35 is a truncated version of Pseudomonas exotoxin A (PE) engineered to have only one reactive cysteine residue. 37 Likewise, synthetic peptides can be designed to have only one cysteine residue, and the reactive sulfhydryl group can be derivatized with several reagents (e.g., 5′, 5′-dithio-bis-[2-nitrobenzoic acid] (DTNB) or Ellman’s reagent) for cross-linking. Thus, for molecules like SP, where the amidated C-terminus is responsible for binding to the NK1 receptor, N-terminal modification can be used to form a peptide-protein disulfide bond. The result is a toxin-peptide chimera (SP-PE35) that retains the receptor recognition and internalization properties of a native amidated substance P agonist and the endosomal release and protein synthesis inhibition properties of Pseudomonas exotoxin. 38
The present report evaluates the properties of this conjugate in vitro and in vivo. We observe broad-spectrum analgesic activity concomitant with deletion of spinal NK1 receptor-expressing neurons. The results indicate that these neurons are essential for transmission of multiple pain modalities and suggest that SP-PE35 can be used as a molecular intervention for pain control when administered into the cerebrospinal fluid surrounding the spinal cord.
Materials and methods
Peptide synthesis
SP-amide with a Cys residue added at the amino terminus (CRPKPQQFFGLM-amide) was synthesized by solid phase methods and purified by HPLC (Bachem Biosciences, Torrance, CA). The purified peptide was derivatized with DTNB, yielding [TNB-Cys0]-SP. The [TNB-Cys0]-SP was purified to 95%–98% purity by HPLC. Final yields were between 34 and 54 mg of peptide. DTNB derivatization and purification of [TNB-Cys0]-SP product were performed by Quality Controlled Biochemicals (BioSource International, Hopkinton, MA).
Expression and purification of CysPE35
CysPE35 was expressed from the pLV1 plasmid, which contained the coding region for the 35 kDa carboxy-terminal fragment of PE. This fragment was taken from the parent plasmid pCT11 37 and cloned into the pRB98 plasmid (kindly provided by Dr Richard Beers of NCI, NIH, Bethesda, MD) with a chloramphenicol acetyltransferase reporter gene. BL21 (λDE3) E. coli cells transformed with the pLV1 plasmid were grown at 37℃ in 10 L of SuperBroth (Digene), pH 7.3 with 25 µg/ml chloramphenicol (Sigma Corporation, St Louis, MO) until an OD600 ≥ 2.5 was reached, after which isopropyl β-D-1-thiogalactopyranoside at a final concentration of 1 mM was added for induction of expression. The bacterial cells were harvested by low-speed centrifugation and washed extensively in 50 mM Tris, 20 mM EDTA solution. Lysozyme (Sigma) at a final concentration of 200 µg/ml, 5 M NaCl and 25% Triton X-100 were added to lyse the bacterial cells. Following lysis, crude cysPE35 was recovered in the periplasmic fraction in the form of inclusion bodies. The inclusion bodies were solubilized and refolded in a redox-shuffling urea-containing buffer at pH 9.5, followed by dialysis into a non–urea-containing buffer, pH 9.5. The refolded cysPE35 was clarified through a 0.45-µm bottle-top filter (ZapCap, Schleicher and Schuell, Keene, NH), adsorbed to Q-Sepharose Fast Flow resin (Pharmacia, Piscataway, NJ), eluted stepwise first with 150 mM NaCl, 20 mM Tris, pH 7, and then with 350 mM NaCl, 20 mM Tris pH 7. From this step forward, fractions were checked at each purification step for the presence of PE35 by running samples on a 8%–16% Tris-Glycine PAGE gel (Invitrogen, Carlsbad, CA) followed by immunoblotting with an anti-PE monoclonal antibody, M40-1 (1:2000 dilution). 39 The 350 mM NaCl fractions were pooled and applied to a Mono-Q-Sepharose column (Pharmacia) and then eluted with a linear gradient of NaCl. PE35-containing fractions were concentrated, reduced to monomers with β-mercaptoethanol, and then applied to a TSKgel gel filtration column (TosoHaas, Montgomeryville, PA) and separated by FPLC. Fractions containing cysPE35 were eluted into phosphate-buffered saline (PBS) pH 6.0 to retain cysPE35 in the monomeric form. The lower pH prevents intermolecular disulfide bonds from forming.
Conjugation of [TNB-Cys0]-SP with PE35
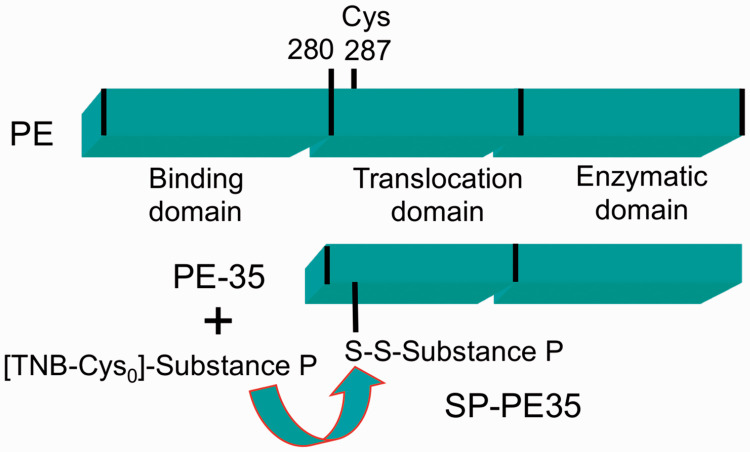
The SP-PE35 conjugate (Figure 1(a)) was made by disulfide exchange. The PE35 molecule has the amino terminal native binding domain removed with the truncation occurring at Gly280 with numbering based on native PE. It has been engineered to contain a single Cys residue at position 287 after removal of a disulfide bridge in the carboxy region. 37 Solutions were first degassed for 1–2 h prior to conjugation. TNB-SP was dissolved in PBS pH 7.4, while cysPE35 was buffer exchanged into PBS pH 7.4. The SP-PE35 conjugate was generated by reacting a 5-fold molar excess of N-terminal TNB-modified Cys0-SP with monomeric cysPE35 in one tube for 1 h at room temperature with constant rocking. Formation of the conjugate was monitored by the release of TNB, which gives off an intense yellow color immediately after mixing at mild alkaline conditions, and measured by spectrophotometry at 412 nm. The SP-PE35 was separated from unreacted TNB-Cys0-SP by a PD-10 Sephadex or Superdex gel filtration column (Pharmacia). To confirm the conjugated product, SP-PE35 samples were run on a 16% Tricine PAGE gel (Invitrogen) up to the 60%–90% window in the gel to resolve the SP and SP-PE35 bands, followed by immunoblotting with the anti-PE monoclonal antibody (1:2000 dilution) and an anti-SP polyclonal antibody (1:5000 dilution, Sigma). Peroxidase-conjugated donkey anti-mouse or anti-rabbit antibodies were used as secondary antibodies and diluted 1:2000 (Jackson Immunoresearch, West Grove, PA)
Figure 1.
Schematic of substance P Pseudomonas exotoxin protein–peptide fusion. A truncated variant (PE35) of pseudomonas exotoxin was used for conjugation. PE35 lacks the binding domain of the full-length Pseudomonas exotoxin and was modified to contain only one native cysteine. 37 Substance P was conjugated to the PE35 toxin by disulfide bonding; this allows the amidated C-terminal methionine of substance P to be free to interact with the NK1 receptor.
Tissue culture and cytotoxicity assays
Chinese hamster ovary (CHO) cells stably transfected with rat NK1, NK2, or NK3 receptors were gifts from Dr James Krause and Mark von Zastrow. Cells were cultured in media containing the aminoglycoside antibiotic, G418 (400 µg/ml), to maintain selection for stable transformants. Cultures of NK1, NK2, and NK3 receptors stably expressed in CHO cells were grown to 90% confluence. For cytotoxicity assays, 1 × 104 cells/well (96-well plate) or 1 × 105 cell/well (24-well plate) were seeded 24 h prior to assay. Cells were treated with SP-PE35 at concentrations of 1 to 10,000 ng/ml for either 24 or 48 h. At the end of the incubation, 3H-leucine was added to a final concentration of 2 µCi/ml for 2 h. The 96-well plates were harvested onto filter mats and counted in a beta plate reader. The 24-well plates were treated with trichloroacetic acid to precipitate cell protein and then dissolved in NaOH and counted in individual scintillation vials. In addition to the assays mentioned above, the cytotoxicity of SP-PE35 was studied in the presence of excess substance P (Figure 2(d)) and anti-substance P antibodies. Similarly, treated plates (35 mm) were stained with methylene blue, fixed for 5 min with 4% paraformaldehyde and photographed to visually demonstrate the dose-dependent loss of the CHO cells expressing NK1 receptors. The staining corroborated the 3H-leucine incorporation studies with cell death occurring upon addition of 5 ng/ml of SP-PE35 to the plates (not shown).
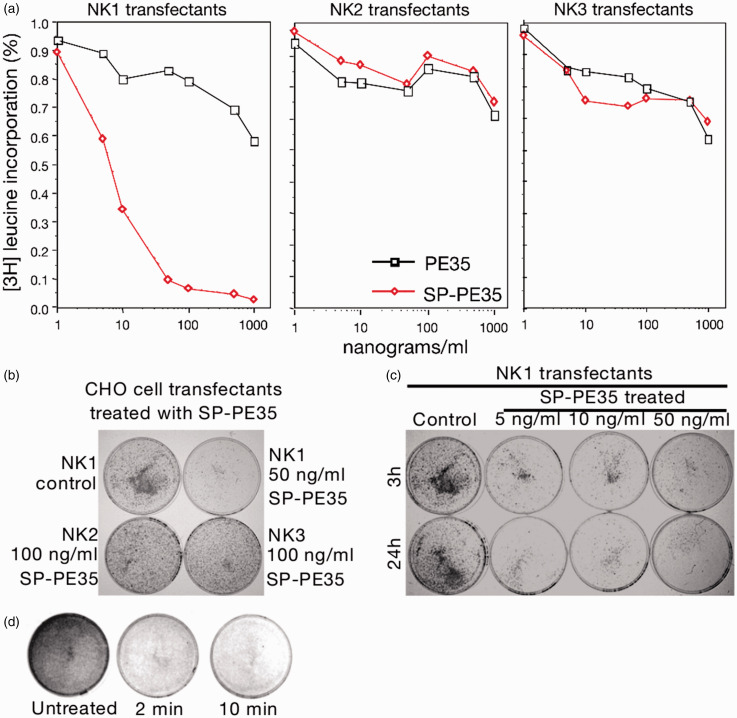
Figure 2.
Assessment of neurokinin receptor subtype specificity and concentration–response of SP-PE35. (a) CHO cells stably expressing NK1, NK2, and NK3 receptor subtypes were labeled with tritiated leucine and exposed to ascending concentrations of SP-P35. Only the NK1 receptor transfectants were lesioned by SP-PE35. (b) Similar data were obtained by staining plates of cells with methylene blue even using twice the dose on the NK2 and NK3 receptor-expressing cells. (c) No difference was seen between 3 h and 24 h incubation with SP-PE35, at any concentration measured. (d) Short pulses of 50 ng/ml SP-PE35 (2 or 10 min) resulted in similar near-total destruction of NK1 receptor-bearing cells .
For the dose response experiment, CHO cells expressing the NK1 receptor were cultured in media with no toxin, 5, 10, 50, and 100 ng/ml of SP-PE35 toxin for 3 and 24 h. The culture media was removed; the cells were washed with fresh media and then cultured for 48 h. For the time-response experiment, the same cell line was used with a concentration of 50 ng/ml of SP-PE 35 toxin. The cells were exposed to the toxin for 0, 2.5, 5, 10, and 100 min. Then, the cells were washed with fresh media and culture for 24 h. In all cases after the incubation time, remaining cells on the plate were fixed and stained with 0.5% Methylene Blue for 5 min, washed in distilled water, and plates were air-dried for analysis.
Intrathecal infusions
All animal studies were approved by the Animal Care and Use Committee of the National Institutes of Health. Rats (300–400 g) were anesthetized with 0.2 ml/100 g of a 1:1 mixture of ketamine:xylazine (stock solutions were 100 and 20 mg/ml, respectively). For intrathecal injection, the rat was placed in a stereotaxic head holder with the nose angled down. The dura overlaying the cisterna magna was exposed by a vertical midline incision starting at the lambda suture. After cleaning the membrane and hemostasis, an incision was made into the dura and arachnoid membranes under a dissecting microscope. The membrane was held with a fine forceps, and a PE10 catheter was advanced 8 cm (lumbar) or 9 cm (sacral injections) into the intrathecal space. 40 The tubing was attached to a 25-µl Hamilton syringe mounted in a syringe infusion pump (Sage Instruments, Cambridge, MA). The tubing was filled with SP-PE35 solution at various concentrations or sterile saline vehicle, and 20 µl was infused over 12 min.
Immunocytochemistry and histology
SP-Pseudomonas exotoxin 35-kDa protein conjugate (SP-PE35) has been used previously to selectively kill striatal NK1 receptor-expressing neurons. 38 Rats were deeply anesthetized with an intraperitoneal injection of pentobarbital (100 mg/kg) and perfused intracardially with ice-cold PBS followed by 4% paraformaldehyde in PBS. The spinal cord was removed and postfixed overnight in 4% paraformaldehyde in PBS and cryopreserved in 30% sucrose. Tissue sections were cut in a cryostat at 30 µm, and immunocytochemistry was performed on free-floating sections. The rat NK1 receptor antibody (Chemicon) was used at a dilution of 1:20,000. Sections were blocked with 3% normal goat serum containing 0.75% Triton X-100. After 1 h, the sections were transferred to 1% normal goat serum containing 0.25% Triton and the indicated concentration of primary antiserum. Sections were incubated overnight at 4℃. Spinal cord sections were developed in nickel-diaminobenzidine using the Elite ABC kit from Vector Labs (Burlingame, CA).
RT-PCR
After intrathecal infusion of the tissue was dissected from the lumbar dorsal enlargement and frozen on dry ice and stored at −80℃ until use. RNA extraction, quantification, and single-tube RT-PCR steps were performed as described. 41
Behavioral assays
Sensitivity to nociceptive thermal stimulation was tested in unrestrained rats with a radiant heat stimulus as described.42,43 Briefly, the rat is placed on an elevated glass plate, with a clear plastic cage (23 × 13 × 12 cm) inverted over the animal. After 5-min habituation to the enclosure, a radiant thermal stimulus is applied under the forepaw, hind paw, or tail. The withdrawal latency is recorded automatically when paw or tail movement interrupts the readings of a photocell in the lamp housing. Sensitivity to nociceptive mechanical stimulation was tested in rats by application of a brief pinch with a toothed forceps; the end point was vocalization and/or withdrawal, and responses were scored as present or absent. Rats were gently held manually during the test. Following lumbar or sacral intrathecal infusions, the body surface was systematically mapped from the tail to the paws and up the back and abdomen following administration of saline vehicle or SP-PE35 by pinching several body regions three times each.
RNA-Seq datasets
Several RNA-Seq datasets were mined from previously published reports, 44 combined with other unpublished datasets, and analyzed using the MAGIC RNA-Seq software suite.45,46
Results
Toxicity and receptor specificity in ectopic expression systems
A series of in vitro experiments were conducted to investigate the specificity of the SP-PE35 conjugate. CHO cells expressing NK1, NK2, and NK3 receptors were incubated with various concentrations SP-PE35. NK1 cells were killed in a concentration-dependent fashion with an IC50 of 2 ng/ml (5 × 10−11M). However, no receptor-selective cell killing was seen, when cells were incubated with unconjugated PE35 or when SP-PE35 was added to cells expressing NK2 and NK3 receptors (Figure 2). We further examined whether toxicity was dependent on receptor binding by competing with excess SP. The NK1 CHO cells were incubated with SP at concentrations ranging from 0.01 to 100 µg/ml and then incubated with SP-PE35. In addition, preincubation of SP-PE35 with an anti-SP antibody also reduced the toxicity of the conjugate (not shown). All three studies indicate that toxicity requires binding to the NK1 receptor.
Immunohistochemical analyses of spinal cord tissue after SP-PE35 infusion
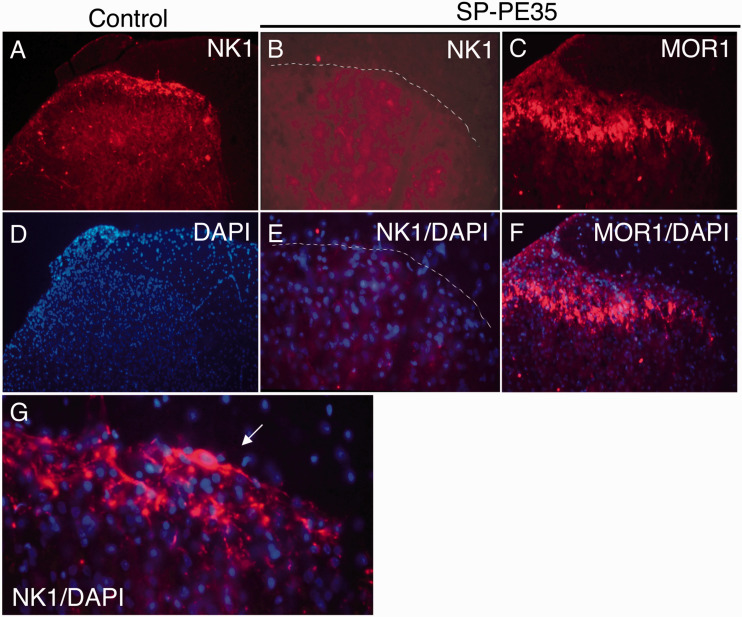
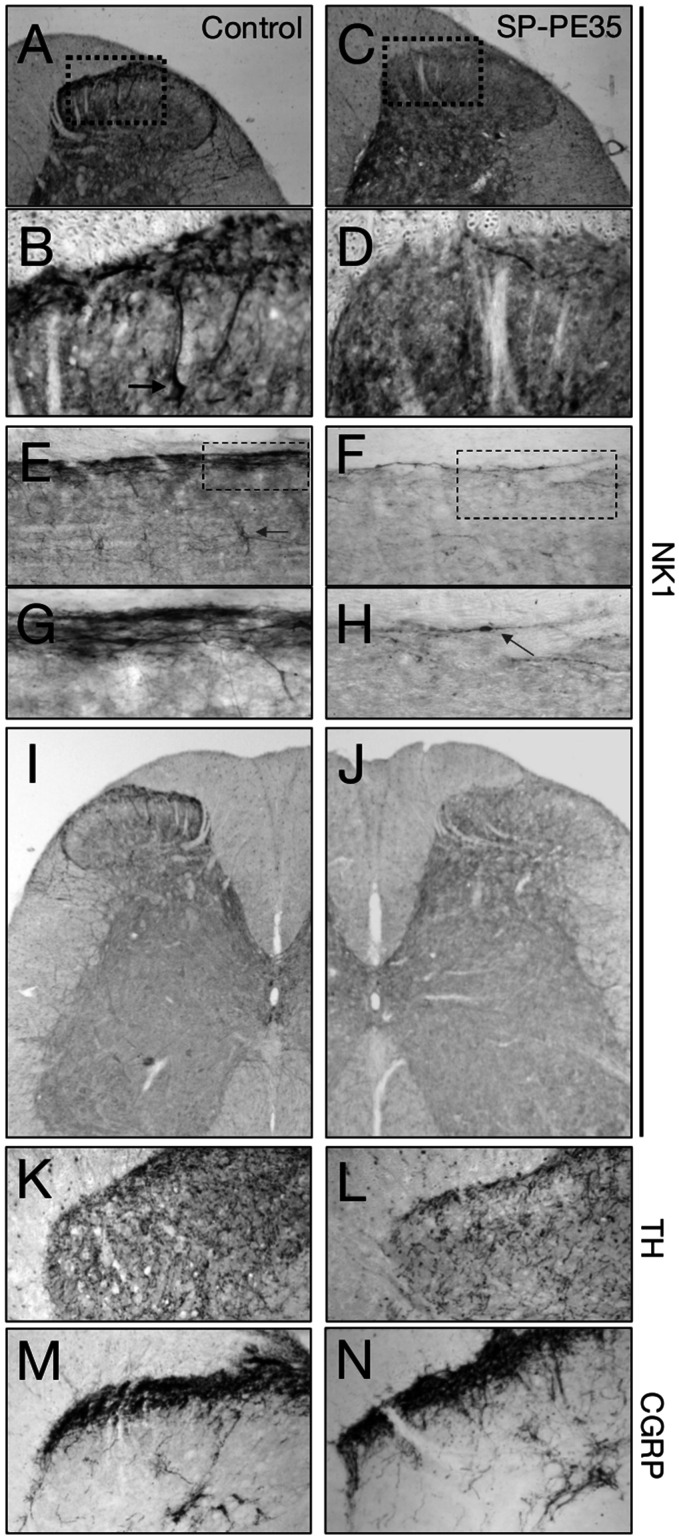
After preliminary dose-ranging studies using NK1 immunoreactive staining as the end point, SP-PE35 was infused in an amount of 15 pmol over 12 min and compared to vehicle-injected controls. In the spinal cord, immunohistochemistry was performed using fluorescent double-labeling for NK1 and DAPI to show nuclei (Figure 3) and with nickel-DAB for enhanced detection of immunopositive cells (Figure 4). In basal conditions, NK1-immunopositivity densely labels the superficial layers of the dorsal spinal cord. This includes a subpopulation of NK1+ Waldeyer neurons, which are large lamina I neurons, 47 many of which are projection neurons. 48 NK1 staining is greatly diminished by intrathecal infusion of SP-PE35. In the same animals, staining for the Mu-opiate receptor is unchanged (Figure 3). Examining coronal and parasagittal sections stained for the NK1 demonstrated near-total ablation of NK1+ cells in the dorsal spinal cord in both the superficial layers (Figure 4). Further, at the 15-pmol dose used, the NK1 neuronal ablation extended from lamina I to the NK1-immunoreactive neurons dorsal to the central canal.
Figure 3.
Dorsal horn NK1 and Mu-opiate receptor after SP-PE35 treatment. Animals were treated intrathecally with SP-PE35 infusion or vehicle control. (a) In the vehicle-treated animals, the NK1 receptor is expressed in the superficial laminae of the dorsal horn, where it is densely expressed in the neuronal perikarya. (b) After SP-PE35 infusion, there was near total loss of NK1 staining in the superficial laminae of the dorsal horn. It is notable that, despite increasing the exposure time for photomicrographs of sections from SP-PE35 treated animals, virtually no NK1+ immunoreactivity is detectable. (c) In contrast, the mu-opiate receptor (MOR1) is readily detectable after SP-PE35 treatment. (d)–(f) DAPI staining was used to show cell nuclei and retention of many viable neurons and supporting cells (d)–(f) is evident after treatment. In untreated animals, a densely NK1+ Waldeyer neuron is visible in lamina 1 (white arrow, (g)). 47 Staining was performed 20 days after injection of SP-PE35.
Figure 4.
NK1 receptor staining after SP-PE35 treatment. Coronal ((a)–(d)) and parasaggital ((e)–(h)) sections through the dorsal spinal cord were stained for the Substance P receptor, NK1 to determine distribution of cell death and retention among NK1+ spinal neurons. In basal conditions, both densely stained cell bodies in superficial dorsal horn ((b), arrow) and neuronal projections express NK1. With SP-PE35 treatment, the presence of neuronal cell bodies is greatly diminished ((c) and (d), (f)–(h)). In parasagittal sections, the superficial layers are densely stained ((e) and (g)). With SP-PE35 treatment, most, but not all NK1+ cells are ablated. An example of a surviving NK1+ cell seen at the margin of the SP-PE35 lesion zone is shown (h). SP-PE35 lesions NK1+ cells in the central canal of the spinal cord in addition to the superficial layers ((i) and (j)). After SP-PE35 treatment, no change was observed in either TH+ cells in the superficial layers of the dorsal horn ((k) and (l)) or in CGRP+ fibers ((m) and (n)). Staining was performed 20 days after injection of SP-PE35.
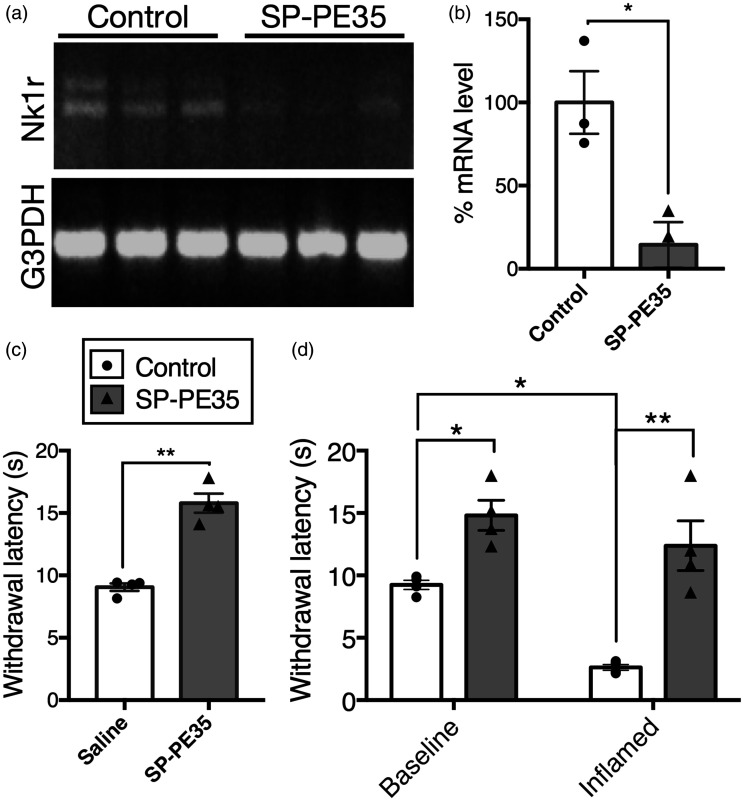
Quantitative measurement of mRNA reduction and behavioral outcomes after SP-PE35 infusion
Efficiency of the toxin was further assayed by measurement of the mRNA encoding the NK1 receptor (Tacr1) in the dorsal spinal cord after the 15-pmol dose of SP-PE35 infused over 12 min. The SP-PE35 infusion reduced the levels of Tacr1 expression by 86% (Figure 5(a) and (b), p < .05, n = 3). Animals were tested for thermal nociceptive responses by measurement of withdrawal latency after stimulation with radiant heat delivered to the plantar surface of the hind paw. Animals treated with intrathecal SP-PE35 showed a 1.8-fold increase in withdrawal latency (Figure 5(c), p < .01, n = 4) indicating reduction in thermal sensitivity.
Figure 5.
Behavioral alterations and reduction of mRNA encoding the NK1 receptor after intrathecal SP-PE35 treatment. Animals were infused with vehicle control or SP-PE35 toxin. SP-PE35 infusion reduced the levels of mRNA encoding the NK1 receptor by 86% ((a) and (b), p < .05, n = 3). Animals were tested for thermal nociceptive responses by stimulating the hind paw with a radiant heat source. Animals treated with intrathecal SP-PE35 showed a 1.8-fold increase in withdrawal latency when stimulated with radiant heat ((c), p < .01, n = 4). In separate experiments, animals were tested for withdrawal latencies before and after induction of inflammatory hyperalgesia by carrageenan injection into the hind paw (d). In baseline testing, SP-PE35 treatment increased withdrawal latency (p < .05, n = 4). Control animals showed strong hyperalgesia after carrageenan injection, as indicated by a 72% reduction in withdrawal latency (p < .05), while SP-PE35-treated animals showed no significant reduction in withdrawal latency with carrageenan treatment. Among inflamed animals, SP-PE35-treated animals showed 4.8-fold higher withdrawal latency on average (p < .01, n = 4) compared to inflamed vehicle treated rats. Testing was between 15 and 46 days after injection of SP-PE35.
In separate experiments, rats were tested for withdrawal latencies before and after induction of inflammatory hyperalgesia by carrageenan injection into the hind paw (Figure 5(d)). Control non–drug-treated animals showed a 72% reduction in withdrawal latency during inflammation, indicating that the inflammatory state produced robust hyperalgesia. SP-PE35 treatment increased withdrawal latency (p < .05, n = 4) without inflammation. After induction of inflammation the animals showed no evidence of hyperalgesia (Figure 5(d)). Among inflamed animals, SP-PE35-treated animals showed 4.8-fold higher withdrawal latency on average relative to animals not treated with SP-PE35 (p < .01, n = 4). Testing was performed as late as 46 days after injection of SP-PE35 indicating that the effects are most likely permanent.
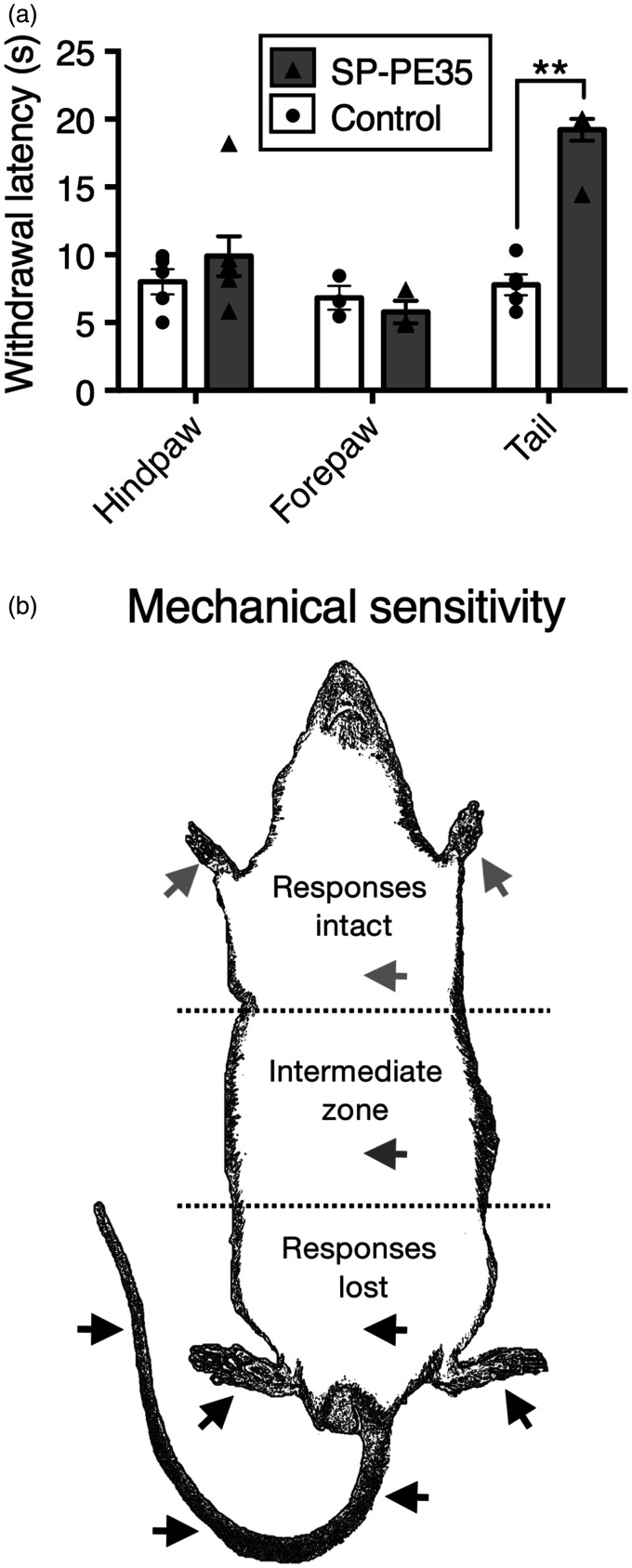
SP-PE35 was able to induce a regionally selective decrease in painful thermal sensation as assessed using nociceptive behavioral responses. Infusion of the 15-pmol dose at the sacralcoccygeal levels of the spinal cord (which receives afferent innervation from the tail) delayed, or eliminated completely, the withdrawal reaction to noxious thermal stimulation of the tail but not the response to stimulation of the hind paws or fore paws (Figure 6(a)). All SP-PE35-treated rats displayed a decrease in thermal sensitivity, and six of seven went to the 20 s cutoff. The confinement of the effect to the tail is likely due to the slow infusion process that restricts the diffusion of the drug substance. The loss of noxious thermal sensibility coincides with a loss of NK1 receptors as assessed by NK1 immunocytochemistry.
Figure 6.
Regional specificity of action after sacral intrathecal infusion of SP-PE35 toxin. (a) To test whether action of SP-PE35 could be confined to a specific set of dermatomes, SP-PE35 was infused into the sacral intrathecal space, and nociceptive withdrawal responses to a radiant thermal stimulus were tested. Rats treated with SP-PE35 showed no significant difference in withdrawal latency when tested on the forepaws or hind paws using this method of administration, but when the tail was tested, the rats showed a 2.5-fold increase in withdrawal latency, with 6/7 animals reaching cutoff (20 s; (a), p < .01, n ≥ 3). (b) Summary figure of mechanonociceptive testing. Multiple animals in this report were tested for mechanical responsiveness by pinching with toothed forceps at the locations indicated by arrowheads (approximate) while gently hand held. Responsiveness was gaged by evocation of withdrawal response or a vocalization. Animals were tested at least three times at each location. SP-PE35-infused rats did not respond to pinch in the posterior section of their body, including the lower abdomen, both hind paws, and three points along the tail. With lumbar intrathecal infusion, in the mid abdomen, there was a variable-sized intermediate zone in which responsiveness could be evoked on some trials. In the anterior portion of the animal, including both forepaws, SP-PE35 showed normal responses to pinch. Control animals withdrew or vocalized at all locations shown.
During testing, we did not observe loss of motor functions in the hind paws or tail, and animals were able to urinate and defecate. No animals showed signs of autotomy, as has been observed in some deafferentation paradigms. 49
Transcriptomic analyses of Tacr1 expression in rat tissue datasets
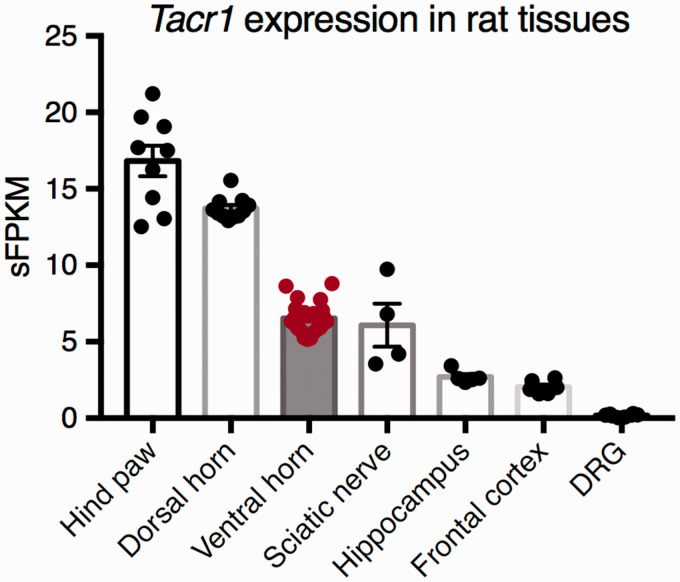
The level of expression of the Tacr1 gene was examined in several RNA-Seq datasets. 44 In the rat, the NK1 receptor is highly expressed in dorsal horn, where it is found on projection neurons and interneurons in the superficial and deeper laminae. However, it is also well expressed in nonneural tissues, such as the hind paw and the sciatic nerve (Figure 7). To examine the potential for off-target effect of SP-PE35, we examined the level of Tacr1 in the lumbar ventral horn, which contains motor neurons. The level of expression in this tissue is approximately half the level in the lumbar dorsal horn and approximately equal to the sciatic nerve, suggesting that this receptor may be expressed in some of the nonneural cells in this tissue and/or in subpopulations of ventral horn motor neurons, although we have not observed strongly NK1-immunoreactive motor neurons in the immunocytochemical material in this report or in two previous studies.50,51 This is consistent with results from other laboratories,52,53 where only diffuse sparse labeling is seen, except around the central canal, and in Onuf’s nucleus, where some neurons are densely labeled.
Figure 7.
RNA-Seq expression of the Tacr1 transcript in several tissues. The Tacr1 gene encoding the Substance P receptor, NK1, was examined in several transcriptomic datasets. This receptor is highly expressed in the lumbar dorsal horn of the spinal cord, where the target projection neurons are located. It is expressed at approximately half this level in the lumbar region of the ventral horn, which contains motor neurons, axon tracts, and connective tissue. It is also expressed in brain tissues such as the frontal cortex and hippocampus. This receptor is highly expressed by nonneural tissues such as the hind paw, which contains skin and muscle, as well as vasculature, and in the sciatic nerve, which consists of Schwann cells and connective tissue. The Tacr1 transcript is expressed at 0.2 sFPKM in the DRG, which is most likely not biologically significant.
Discussion
SP-Pseudomonas exotoxin as a new tool for analgesia research
The data presented reinforce the idea that NK1+ spinal neurons are required for processing nociceptive responses and represent a neuronal point of convergence for heat and mechanical nociception. These data further reinforce the idea that selective deletion of NK1+ spinal neurons leads to profound, regionally selective, multimodal, and permanent analgesia. Similar but not as behaviorally robust results have been obtained with substance P-saporin conjugates.3,16,18 The reagent used in the present study, SP-PE35, is a novel ligand-directed chimeric protein that fuses a recombinant-engineered bacterial cytotoxin with a machine-synthesized, modified neuropeptide (Figure 1). This hybrid approach of synthetic peptide and recombinant protein has advantages over fusion proteins, such as the diphtheria-SP fusion we studied previously. 50 First, the substance P moiety must be C-terminally amidated for receptor recognition. For the fusion protein, amidation is accomplished by an in vitro posttranslational enzymatic reaction using peptidyl-α-amidating monoxygenase, which has limited efficiency. This inefficiency can be overcome by making substance P synthetically as an amide and conjugating it directly to the recombinant protein in a reaction in which the stoichiometry is adjusted to favor formation of the conjugate. Second, our PE35 was engineered to contain only one site for conjugation (Cys287) since a region of native PE containing a disulfide linkage at the carboxy end (amino acids 365–380 including Cys372 and Cys379) of PE was deleted; as was the native binding domain (amino acids 1–279). Thus, the only site for ligand conjugation is Cys287 and following conjugation, substance P provides the sole domain for receptor-mediated cell binding and internalization. The resulting protein–peptide conjugate is a highly selective and efficacious cytotoxin: Cells bearing NK1 receptors were killed, while those bearing NK2 or NK3 receptors exhibited no specific, receptor-mediated cytotoxicity (Figure 2). The selectivity is remarkable, given that the amino acid sequence of substance P (RPKPQQFFGLM-NH2) is similar at the carboxy-terminus to neurokinin A (HKTDSFVGLM-NH2). For all three NK receptor-expressing cell lines, some non–receptor-dependent cell killing was observed which was equivalent to that obtained with underivatized PE35.
Considerations for directed cell deletion approaches to pain control
Previous work has established that, as a group, TRPV1+ dorsal root ganglion neurons are required for most painful sensations, and that the ablation of these cells leads to permanent, multimodal and profound analgesia in rats and companion dogs.1,54–58 Our observation that, in spinal cord, ablation of NK1+ neurons leads to profound multimodal loss of painful sensations suggests that these second order neurons, like the TRPV1+ DRG neurons, are required for nociceptive responses and the maintenance of hyperalgesic states.3,59 The two approaches underscore the importance of novel strategies targeted at inactivation or removal of nociresponsive neurons for analgesia. To date, deafferentation pain syndromes have not been observed with selective chemical axotomy or cell deletion of either TRPV1+ DRG neurons, or NK1+ spinal neurons.17,54 However, dose-limiting ataxia and muscle weakness has been observed with intrathecal injection substance P-toxin conjugates (e.g., substance P-saporin) in the dog17,18; a method of administration which we distinguish from the localized infusion used in the present report. Given the sparse labeling of motor neurons for NK1 receptors (discussed later), the motor side effects most likely represent nonspecific uptake and subsequent toxicity to motor neurons. We have seen motorneurons take up adenovirus much more robustly than dorsal horn neurons 60 despite the low level of coxsackie adenovirus receptor (Cxadr) in ventral horn compared to dorsal horn (6.3 vs. 20.6 sFPKM, respectively). These points are raised to suggest that an effect on motorneurons may not require the presence of an intact substance P moiety and this consideration may be important factor in considerations of dose requirements and route or method of administration. Nonetheless, optimizing the toxin conjugate through enhancement of NK1 receptor binding and the endosomal release of the toxin appears to be a viable strategy for lowering the effective dose. Although we have not made a direct comparison of the efficacy and safety of SP-PE35 to existing SP conjugate toxins, we do observe a very high percentage of NK1+ neuronal loss that includes the neurons around the central canal, and broad-spectrum analgesia without any observations of ataxia or impairment of gait. SP-PE35 is effective on both basal pain and inflammatory hyperalgesia in the rat carrageenan model. This suggests a more complete NK1 neuron elimination was obtained compared to SP-Saporin and that lower doses of SP-PE35 may also yield effective analgesia. For any of these toxin–peptide conjugates, the delivery procedure is another element that can be leveraged to achieve better localization or confinement of the toxin–ligand conjugate. In the present and previous studies 50 we administered the active compound into the cerebrospinal fluid by slow infusion. However, modeling studies of direct intraparenchymal infusions of peptide–toxin conjugate suggest that such infusions may result in a more localized delivery of the toxic conjugate to the dorsal horn.61,62
Insights into clinical applications of SP-Pseudomonas exotoxin
One consideration for clinical use in human spinal cord is that the dorsal horn is much more enveloped by overlying white matter tracts and is may be less accessible to diffusion of SP-PE35 than in the rat. As seen in autoradiograms of medullary dorsal horn after intracisternal [14C]-inulin infusions, even small to intermediate sized molecules do not readily penetrate deeply into the parenchyma from the CSF space. 63 This being the case, then the question of extracellular enzymatic degradation of the ligand portion of the conjugate and access to NK1 receptor expressing neurons may be important considerations. Because of the access problem, a higher dosing in people or large animals than in rats may be needed which may increase the risk of off-target actions. Our experience with resiniferatoxin 64 and the modeling of ligand-toxin infusions suggest that procedure-based, local administration approaches are effective in limiting the distribution of the conjugate and would aid in preventing nonspecific uptake of the toxic moiety. The initial clinical use of resiniferatoxin is to ablate TRPV1+ primary sensory afferents in treatment-resistant pain from advanced cancer.54,65 This indication is also being explored for SP-saporin, 66 and would be a target for SP-PE35 as well. Another indication where ablation of second order NK1+ neurons may be effective is in spinal cord injury pain.67–69 In some of these patients, we hypothesize that ectopic abnormal activity in second order neurons may be reduced by NK1+ neuronal deletion. This is supported by characterization of the NK1+ projection neurons suggesting that these neurons mediate allodynia and hypersensitivity. 70 This concept may be extended to other conditions where interventions at primary afferent nociceptors, such as RTX, may not be effective. Some NK1+ neurons receive inputs that are thought to be nonnociceptive, but which become able to depolarize these neurons during periods of disinhibition. 70 This suggests that the spinal NK1+ neuronal population is required for the transmission of a broad range of painful stimuli, including allodynic sensations originally transduced by nonnociceptive A-fibers. 70 In conjunction with the evidence in the present report that SP-PE35 robustly eliminates responses to pinch, deletion of the NK1+ second order neurons may offer a different spectrum of clinical outcomes and indications than that of deletion TRPV1+ primary afferents.
Potential on-target side effects of SP-PE35
In the rat, the transcript encoding the NK1 receptor is detectable in both the lumbar dorsal and ventral horns (Figure 7). Expression in ventral horn is consistent with the expression of this receptor in both nonneural cell populations, as well as in some subpopulations of ventral horn motor neurons. NK1 is expressed by some neurons in lumbo-sacral cord, including neurons in Onuf’s nucleus, which is involved in micturition, defecation other functions.52,53 Despite this localization, no evidence of impairment in bladder or bowel voiding has been observed following treatment with Substance-P toxin conjugates to date, suggesting that Onuf’s nucleus and NK1+ parasympathetic neurons were not lesioned.16,17 Loss of gait has been observed in some dogs treated with SP-Saporin after cervical, but not lumbar intrathecal injections, 17 although it remains unclear if this effect is mediated by motor neuron loss or lesions in the brain. Further studies are needed to understand the optimal route of administration, and potential cell populations that could be lesioned at different levels of the spinal cord. Confinement of the drug substance is an important consideration given the role of medullary NK1+ neurons in control of the baroreceptor reflex, 71 and in chemosensory-induced ventilation, 72 and as such any route of administration should confine the drug substance below the level of the medulla. Although, other than motor impairment, the intracisternal injections did not appear to produce other serious adverse events.
Conclusions
The utility of toxin conjugates depends upon the specificity of the ligand-directed cytotoxic action and the potential for enzymatic cleavage of the neuropeptide ligand portion is a consideration that has been examined with degradation resistant substance P analogs.73,74 Our finding that this toxin–neuropeptide conjugate can be spatially restricted by local infusion is important given the identification of SP-NK1 systems in brain regions such as the amygdala, 75 striatum, 76 and pontine-medullary autonomic nuclei.77,78 Thus, we propose that SP-PE35 is a strong candidate for permanent regional analgesia, and that further study may demonstrate advantages of this ligand–toxin conjugate in several different pain indications.
Author Contributions
MJI and DJF designed and conceived the project and supervised the experiments. MJI and DJF designed the TNB-Cys0SP and XW, MLVT, RS, and DJF synthesized and purified SP-PE35 and characterized the toxicity in vitro. HC and MJI performed the immunohistochemistry and behavioral experiments in rats. MJI, MRS, XW, HC, MLVT, AJM, and DJF analyzed the data. MJI and MRS wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health Intramural Research Program for the National Cancer Institute, National Institute of Dental and Craniofacial Research, and the Clinical Center.
References
- 1.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 2004; 113: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pleticha J, Heilmann LF, Evans CH, et al. Preclinical toxicity evaluation of AAV for pain: evidence from human AAV studies and from the pharmacology of analgesic drugs. Mol Pain 2014; 10: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantyh PW, Rogers SD, Honore P, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 1997; 278: 275–279. [DOI] [PubMed] [Google Scholar]
- 4.Beutler AS, Reinhardt M. AAV for pain: steps towards clinical translation. Gene Ther 2009; 16: 461–469. [DOI] [PubMed] [Google Scholar]
- 5.Finegold AA, Mannes AJ, Iadarola MJ. A paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic pain. Hum Gene Ther 1999; 10: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 6.Hill R. NK1 (substance P) receptor antagonists—why are they not analgesic in humans? Trends Pharmacol Sci 2000; 21: 244–246. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnadottir TK, Gloriam DE, Hellstrand SH, et al. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 2006; 88: 263–273. [DOI] [PubMed] [Google Scholar]
- 8.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Ann Rev Pharmacol Toxicol 2008; 48: 537–568. [DOI] [PubMed] [Google Scholar]
- 9.Garland AM, Grady EF, Payan DG, et al. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem J 1994; 303(Pt 1): 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantyh PW, Allen CJ, Ghilardi JR, et al. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A 1995; 92: 2622–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantyh PW, DeMaster E, Malhotra A, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 1995; 268: 1629–1632. [DOI] [PubMed] [Google Scholar]
- 12.Bowden JJ, Garland AM, Baluk P, et al. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A 1994; 91: 8964–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungdahl A, Hokfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat—I. Cell bodies and nerve terminals. Neuroscience 1978; 3: 861–943. [DOI] [PubMed] [Google Scholar]
- 14.Kramer MS, Winokur A, Kelsey J, et al. Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. Neuropsychopharmacology 2004; 29: 385–392. [DOI] [PubMed] [Google Scholar]
- 15.George DT, Gilman J, Hersh J, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 2008; 319: 1536–1539. [DOI] [PubMed] [Google Scholar]
- 16.Allen JW, Mantyh PW, Horais K, et al. Safety evaluation of intrathecal substance P-saporin, a targeted neurotoxin, in dogs. Toxicol Sci 2006; 91: 286–298. [DOI] [PubMed] [Google Scholar]
- 17.Brown DC, Agnello K. Intrathecal substance P-saporin in the dog: efficacy in bone cancer pain. Anesthesiology 2013; 119: 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiese AJ, Rathbun M, Butt MT, et al. Intrathecal substance P-saporin in the dog: distribution, safety, and spinal neurokinin-1 receptor ablation. Anesthesiology 2013; 119: 1163–1177. [DOI] [PubMed] [Google Scholar]
- 19.Gan TJ, Apfel CC, Kovac A, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg 2007; 104: 1082–1089. tables of contents. [DOI] [PubMed] [Google Scholar]
- 20.Herbert MK, Holzer P. Why are substance P(NK1)-receptor antagonists ineffective in pain treatment? Anaesthesist 2002; 51: 308–319. [DOI] [PubMed] [Google Scholar]
- 21.Woolf CJ, Thompson SW, King AE. Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J Physiol (Paris) 1988; 83: 255–266. [PubMed] [Google Scholar]
- 22.Henry JL. Participation of substance P in spinal physiological responses to peripheral aversive stimulation. Regul Pept 1993; 46: 138–143. [DOI] [PubMed] [Google Scholar]
- 23.Alvaro G, Di Fabio R. Neurokinin 1 receptor antagonists—current prospects. Curr Opin Drug Discov Devel 2007; 10: 613–621. [PubMed] [Google Scholar]
- 24.Hua XY, Chen P, Polgar E, et al. Spinal neurokinin NK1 receptor down-regulation and antinociception: effects of spinal NK1 receptor antisense oligonucleotides and NK1 receptor occupancy. J Neurochem 1998; 70: 688–698. [DOI] [PubMed] [Google Scholar]
- 25.De Felipe C, Herrero JF, O’Brien JA, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 1998; 392: 394–397. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki R, Hunt SP, Dickenson AH. The coding of noxious mechanical and thermal stimuli of deep dorsal horn neurones is attenuated in NK1 knockout mice. Neuropharmacology 2003; 45: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 27.Rahman W, Sikandar S, Suzuki R, et al. Superficial NK1 expressing spinal dorsal horn neurones modulate inhibitory neurotransmission mediated by spinal GABA(A) receptors. Neurosci Lett 2007; 419: 278–283. [DOI] [PubMed] [Google Scholar]
- 28.Coghill RC, Sang CN, Maisog JM, et al. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 1999; 82: 1934–1943. [DOI] [PubMed] [Google Scholar]
- 29.Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord, 3rd ed. New York, NY: Kluwer Academic/Plenum Publishers, 2004. [Google Scholar]
- 30.Iadarola MJ, Berman KF, Zeffiro TA, et al. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain 1998; 121(Pt 5): 931–947. [DOI] [PubMed] [Google Scholar]
- 31.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010; 11: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232: 34–47. [DOI] [PubMed] [Google Scholar]
- 33.Hartley MR, Lord JM. Cytotoxic ribosome-inactivating lectins from plants. Biochim Biophys Acta 2004; 1701: 1–14. [DOI] [PubMed] [Google Scholar]
- 34.Wayne AS, Fitzgerald DJ, Kreitman RJ, et al. Immunotoxins for leukemia. Blood 2014; 123: 2470–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunwar S, Pai LH, Pastan I. Cytotoxicity and antitumor effects of growth factor-toxin fusion proteins on human glioblastoma multiforme cells. J Neurosurg 1993; 79: 569–576. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhary VK, Mizukami T, Fuerst TR, et al. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature 1988; 335: 369–372. [DOI] [PubMed] [Google Scholar]
- 37.Theuer CP, Kreitman RJ, FitzGerald DJ, et al. Immunotoxins made with a recombinant form of Pseudomonas exotoxin A that do not require proteolysis for activity. Cancer Res 1993; 53: 340–347. [PubMed] [Google Scholar]
- 38.Saka E, Iadarola M, Fitzgerald DJ, et al. Local circuit neurons in the striatum regulate neural and behavioral responses to dopaminergic stimulation. Proc Natl Acad Sci U S A 2002; 99: 9004–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogata M, Chaudhary VK, Pastan I, et al. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem 1990; 265: 20678–20685. [PubMed] [Google Scholar]
- 40.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav 1976; 17: 1031–1036. [DOI] [PubMed] [Google Scholar]
- 41.Yang HY, Mitchell K, Keller JM, et al. Peripheral inflammation increases Scya2 expression in sensory ganglia and cytokine and endothelial related gene expression in inflamed tissue. J Neurochem 2007; 103: 1628–1643. [DOI] [PubMed] [Google Scholar]
- 42.Iadarola MJ, Douglass J, Civelli O, et al. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain Res 1988; 455: 205–212. [DOI] [PubMed] [Google Scholar]
- 43.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 44.Sapio MR, Goswami SC, Gross JR, et al. Transcriptomic analyses of genes and tissues in inherited sensory neuropathies. Exp Neurol 2016; 283: 375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Consortium SM-I. A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat Biotechnol 2014; 32: 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, Yu Y, Hertwig F, et al. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol 2015; 16: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheibel ME, Scheibel AB. Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Res 1968; 9: 32–58. [DOI] [PubMed] [Google Scholar]
- 48.Puskar Z, Polgar E, Todd AJ. A population of large lamina I projection neurons with selective inhibitory input in rat spinal cord. Neuroscience 2001; 102: 167–176. [DOI] [PubMed] [Google Scholar]
- 49.Coderre TJ, Grimes RW, Melzack R. Deafferentation and chronic pain in animals: an evaluation of evidence suggesting autotomy is related to pain. Pain 1986; 26: 61–84. [DOI] [PubMed] [Google Scholar]
- 50.Benoliel R, Eliav E, Mannes AJ, et al. Actions of intrathecal diphtheria toxin-substance P fusion protein on models of persistent pain. Pain 1999; 79: 243–253. [DOI] [PubMed] [Google Scholar]
- 51.Benoliel R, Tanaka M, Caudle RM, et al. Co-localization of N-methyl-D-aspartate receptors and substance P (neurokinin-1) receptors in rat spinal cord. Neurosci Lett 2000; 291: 61–64. [DOI] [PubMed] [Google Scholar]
- 52.Nakaya Y, Kaneko T, Shigemoto R, et al. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol 1994; 347: 249–274. [DOI] [PubMed] [Google Scholar]
- 53.Brown JL, Liu H, Maggio JE, et al. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol 1995; 356: 327–344. [DOI] [PubMed] [Google Scholar]
- 54.Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain 2015; 156: 1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown DC, Iadarola MJ, Perkowski SZ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005; 103: 1052–1059. [DOI] [PubMed] [Google Scholar]
- 56.Bates BD, Mitchell K, Keller JM, et al. Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. Pain 2010; 149: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iadarola MJ, Gonnella GL. Resiniferatoxin for Pain Treatment: An Interventional Approach to Personalized Pain Medicine. Open Pain J 2013; 6: 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iadarola MJ, Mannes AJ. The vanilloid agonist resiniferatoxin for interventional-based pain control. Curr Top Med Chem 2011; 11: 2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols ML, Allen BJ, Rogers SD, et al. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science 1999; 286: 1558–1561. [DOI] [PubMed] [Google Scholar]
- 60.Iadarola MJ, Lee S, Mannes AJ. Gene transfer approaches to pain control. Prog Pain Res Manag 1997; 9: 337–359. [Google Scholar]
- 61.Sarntinoranont M, Iadarola MJ, Morrison PF. A kinetic analysis of substance P trafficking. J Pharm Sci 2003; 92: 232–243. [DOI] [PubMed] [Google Scholar]
- 62.Sarntinoranont M, Iadarola MJ, Lonser RR, et al. Direct interstitial infusion of NK1-targeted neurotoxin into the spinal cord: a computational model. Am J Physiol Regul Integr Comp Physiol 2003; 285: R243–R254. [DOI] [PubMed] [Google Scholar]
- 63.Proescholdt MG, Hutto B, Brady LS, et al. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience 2000; 95: 577–592. [DOI] [PubMed] [Google Scholar]
- 64.Brown JD, Saeed M, Do L, et al. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci Transl Med 2015; 7: 305ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heiss JD, Iadarola M, Oughourli A, et al. Intrathecal Resiniferatoxin for Intractable Cancer Pain. J Neurosurg 2015; 123: A515. [Google Scholar]
- 66.Frankel AE, Lappi DA, Noe C, et al. Preliminary results from a phase I study of Substance P-Saporin in advanced cancer patients with intractable pain. Cancer Res 2016; 76(14 Suppl): Abstract nr CT030. [Google Scholar]
- 67.Cetas JS, Saedi T, Burchiel KJ. Destructive procedures for the treatment of nonmalignant pain: a structured literature review. J Neurosurg 2008; 109: 389–404. [DOI] [PubMed] [Google Scholar]
- 68.Vierck CJ, Baastrup C, Maersk-Moller C, et al. A preclinical model of hyperalgesia following spinal stenosis/compression. Eur J Pain 2015; 19: 1158–1167. [DOI] [PubMed] [Google Scholar]
- 69.Yezierski RP, Liu S, Ruenes GL, et al. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain 1998; 75: 141–155. [DOI] [PubMed] [Google Scholar]
- 70.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 2006; 26: 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helke CJ, Seagard JL. Substance P in the baroreceptor reflex: 25 years. Peptides 2004; 25: 413–423. [DOI] [PubMed] [Google Scholar]
- 72.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 2002; 544: 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiley RG, Lappi DA. Targeting neurokinin-1 receptor-expressing neurons with [Sar9,Met(O2)11 substance P-saporin. Neurosci Lett 1999; 277: 1–4. [DOI] [PubMed] [Google Scholar]
- 74.Wiley RG, Kline RH, 4th, Vierck CJ, Jr. Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience 2007; 146: 1333–1345. [DOI] [PubMed] [Google Scholar]
- 75.Gadd CA, Murtra P, De Felipe C, et al. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J Neurosci 2003; 23: 8271–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garant DS, Iadarola MJ, Gale K. Substance P antagonists in substantia nigra are anticonvulsant. Brain Res 1986; 382: 372–378. [DOI] [PubMed] [Google Scholar]
- 77.Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol (1985) 2006; 101: 1596–1606. [DOI] [PubMed] [Google Scholar]
- 78.Potts JT, Fong AY, Anguelov PI, et al. Targeted deletion of neurokinin-1 receptor expressing nucleus tractus solitarii neurons precludes somatosensory depression of arterial baroreceptor-heart rate reflex. Neuroscience 2007; 145: 1168–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]