Abstract
The heptafluorobutyric anhydride (HFBA), pentafluoropropionic anhydride (PFPA), and trifluoroacetic anhydride (TFAA) are compared as derivatizing reagents to use as the optimal method for the analysis of 10 amphetamines and cathinones in oral fluid. The target compounds were amphetamine (AMP), methamphetamine (MA), 4-methylamphetamine, 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA), 3,4-methylenedioxy-N-ethylamphetamine (MDEA), cathinone (CAT), methcathinone, mephedrone, and ephedrine. Amphetamine-D5, MA-D5, MDA-D5, MDMA-D5, and MDEA-D5 use as internal standards (IS). The analytes and IS were extracted from 0.5 mL of oral fluid by ethyl acetate in the presence of NaOH (0.1 N) as the base and then the dried extracts were derivatized with HFBA, PFPA, or TFAA at 70°C for 30 minutes. The limits of quantification based on signal-to-noise ratios ≥10 were ranged between 2.5 and 10 ng/mL. The calibration graphs were linear in the range of 5 or 10 to 1000 ng/mL for all analytes. Based on sensitivity, the PFPA is proved to be the best for derivatization of the target compounds prior to gas chromatography-mass spectrometry analysis.
Keywords: Amphetamine, drugs, oral fluids, gas chromatography, analysis
Introduction
Amphetamines and related compounds which include amphetamine (AMP), methamphetamine (MA), 4-methylamphetamine (4-MA), 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA), 3,4-methylenedioxy-N-ethylamphetamine (MDEA), cathinone (CAT), and ephedrine (EPH) are widely acknowledged as the drugs of abuse in the market.1–3 More recently, a new synthetic cathinones such as methcathinone (MC) and mephedrone (MEP) are emerging in the market as a bath salt.4 Their stimulant, euphoric, anorectic effects appear to be the main reason for its popularity.5,6
Amphetamines and cathinones are weak bases with relatively low molecular weights. It can diffuse to tissues and biological fluids which have pH lower than blood. In addition to urine and blood, amphetamines and cathinones were detected in alternative biological matrices such as sweat, oral fluid, and hair.7,8
The use of oral fluid for drug testing has many advantages over conventional matrices, it is safe to collect and can offer a quick and noninvasive specimen and condense the potential for adulteration.9,10 Indeed, in many cases, the concentration of drugs in oral fluid represents the physiologically active fraction.11,12 The basic drugs such as the cocaine, amphetamines, and some opioids have similar or higher concentrations in oral fluid than those in plasma; therefore, the use of oral fluid as alternative specimens to blood or urine for testing drugs of abuse has become a great importance in clinical and forensic toxicology.9,13
Practically, gas chromatography-mass spectrometry (GC-MS) analysis of amphetamines and cathinones without derivatization do not confer satisfactory chromatographic behavior. Acylation of the amino or alkylamino groups of amphetamines or cathinones is required to improve the chromatographic shape. Fluorinated anhydrides such as heptafluorobutyric anhydride (HFBA), pentafluoropropionic anhydride (PFPA), and trifluoroacetic anhydride (TFAA) are the most popular derivatizing agents for the derivatization of amphetamines and cathinones prior to GC-MS analysis14–33 but it is not clear which would be the most effective one.
In this work, 3 acylation reagents, HFBA, PFPA, and TFAA, are evaluated for derivatization of AMP, MA, 4-MA, MDA, MDMA, MDEA, CAT, MC, MEP, and EPH after extraction from the oral fluid.
Materials and Methods
Chemicals, reagents, and materials
Stock standards of d-AMP.HCl, d,l-CAT.HCl, d,l-4-MA.HCl, d,l-MA.HCl, d,l-MDA.HCl, d,l-MDMA.HCl, d,l-MDEA.HCl, d,l-AMP-D5.HCl, d,l-MA-D5.HCl, d,l-MDA-D5.HCl, d,l-MDMA-D5.HCl, and d,l-MDEA-D5.HCl at concentrations of 1.0 mg/mL free base in methanol were obtained from Lipomed AG (Arlesheim, Switzerland). Mephedrone HCl, S(−)-MC HCl and 1S,2R(+)-EPH HCl stock standards at a concentration of 1.0 mg/mL free base in methanol were purchased from Cerilliant (Round Rock, TX, USA). The reagents HFBA, PFPA, and TFAA were supplied by United Chemical (UCT, Bristol, PA, USA). Methanol (high-performance liquid chromatography grade, 99.9%), ethyl acetate (99.9%), and sodium hydroxide (≥99.0%) were purchased from Fisher Scientific (Hampton, NH, USA). The GC vials (1.5 mL) and inserts (150 µL) were obtained from Agilent (Santa Clara, CA, USA).
GC-MS conditions
An Agilent GC-MS-7890B with an Agilent autosampler was used for specimen analysis. The GC was equipped with Agilent HP-5MS (5%-phenyl-methylpolysiloxane) column capillary column (30 m × 250 µm × 0.25 µm film thickness). Helium was used as the carrier gas at a flow rate of 1 mL/min. The injection volume was 2.0 µL and injections were made in splitless mode. The injector and interface temperature were maintained at 280°C. The column temperature program was initialized at 80°C and held for 2 minutes, increased to 150°C at a ramp rate of 8°C/min, and then to 280°C with a ramp rate of 30°C/min. Solvent delay time was 6 minutes, giving a total run time of 15.0 minutes. Electron impact ionization mode was used for ionization. The ionizing energy was 70 eV. Qualitative analysis was conducted in the full scan mode (m/z range: 50-500), and quantification was in the in selected ion monitoring (SIM) mode. The deuterated analogues of amphetamines were used as internal standard (IS) for the target compounds, whereas AMP-D5 for AMP, CAT, and 4-MA; MA-D5 for MA, EPH, MC, and MEP; MDA-D5 for MDA; and MDMA-D5 for MDMA and MDMA-D5 for MDEA. Data analysis was performed using the Agilent GC-MS software (MassHunter).
Standards solutions
A mixture of working solution of amphetamines and cathinones at a concentration of 100 µg/mL was prepared by diluting (1:10) of the stock standards with methanol in a volumetric flask. Further working solutions of 10.0, 1.0, and 0.1 μg/mL were obtained and used for the preparation of calibrators. A mixture of IS at a concentration of 5.0 µg/mL for AMP-D5, MA-D5, MDA-D5, MDMA-D5, and MDEA-D5 was prepared by pipetting 50 µL of each compound (100 µg/mL) in 10-mL volumetric flask and made up to 10 mL with methanol.
Spiked samples
For the linearity study, calibration curves at the concentrations of 5, 10, 25, 50, 100, 500, and 1000 ng/mL were prepared in triplicate by fortifying pool of blank oral fluid with appropriate volumes of the mix working solutions.
For the limit of quantification (LOQ) study, a pool of blank oral fluid was fortified with the target compounds at a concentration of 100 ng/mL and then a series of fortified oral fluid (n = 3) at concentrations of 1, 2.5, 5, and 10 ng/mL were prepared.
Sample preparation
To 0.5 mL of oral fluid specimens in 5-mL polypropylene tubes, 50 µL of IS (5.0 µg/mL), 0.5 mL of 0.1 N of NaOH (pH 14), and 3.0 mL of ethyl acetate were added. The tubes were vortex mixed for 3 minutes and centrifuged (3000 rpm) for 5 minutes. The ethyl acetate layer was transferred to 5-mL glass tubes containing 1% HCl in methanol, gently vortexed, and evaporated to dryness using a stream of nitrogen. To the residue, 50 µL of ethyl acetate and 50 µL of HFBA, PFPA, or TFAA were added and heated for 30 minutes at 70°C. Samples were evaporated to dryness under a stream of nitrogen and reconstituted with 50 µL of ethyl acetate.
Measurements procedures
Calibration graphs were established by plotting the peak area ratio of the analyte to the IS versus analyte concentration. The linearity of the method was investigated by evaluation of the correlation coefficient (r2) for each calibration graph and the accuracy (bias) for each calibrator.34 The acceptable value for bias was ±15% and ±20% for LOQ.
Sensitivity for each method was assessed by determining the limit of detection (LOD) and LOQ for all analytes.34 The LOD was defined as the lowest concentration for which the analyte ion signal-to-noise (S/N) ratio was ≥3 (determined by peak height). The LOQ was defined as the lowest concentration for which the analyte ion S/N ratio was ≥10.
Method specificity was evaluated by analysis of 6 different blanks (no analyte or IS) and negative (IS added) oral fluid specimens.34 Co-eluting peaks that might interfere with detection of analytes or IS was examined.
Results and Discussion
Confirmation of unknown amphetamines and cathinones using GC-MS depends on retention time and mass spectra. When SIM is used in place of full scan, at least 3 characteristic ions should be selected and ion ratios must be evaluated.35 In this study, SIM was applied to detect and quantify amphetamines and cathinones using 3 different derivatization methods.
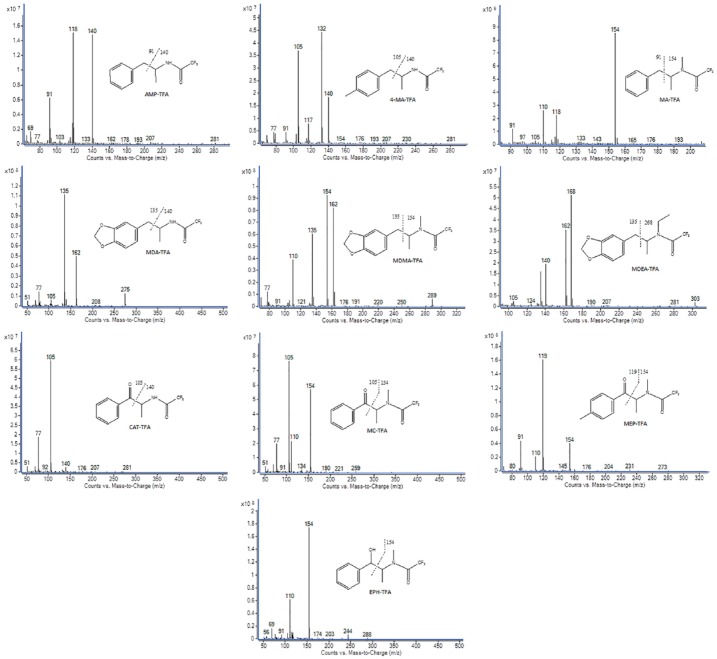
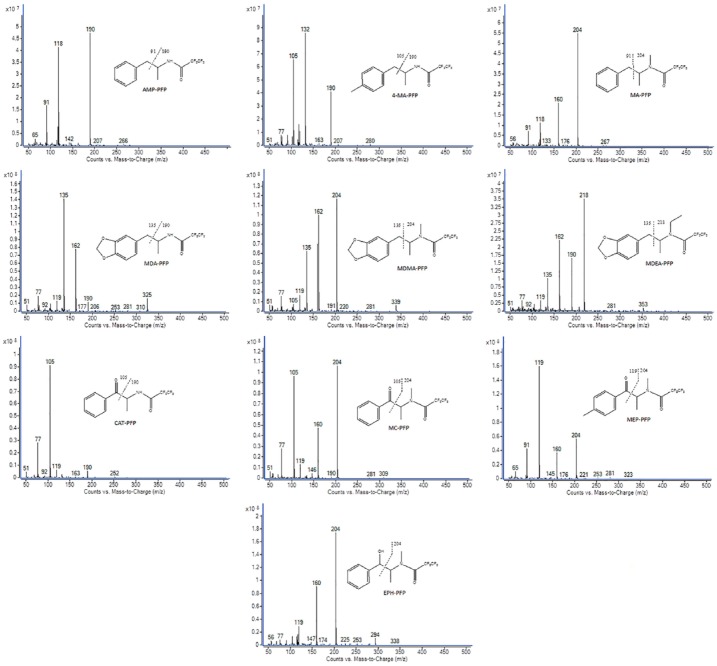
The mass spectra of HFB, PFP, and TFA derivatives of the target amphetamines and cathinones are shown in Figures 1 to 3. The ions with a higher m/z ratio were used as a quantifier and qualifier ions. Based on S/N, the most intense ion was used as a quantifier and 2 characteristic ions were used as a qualifier. The quantifier and qualifier ions for derivatized amphetamines and cathinones by HFBA, PFPA, and TFAA are presented in Table 1. The principal fragmentation occurs by dissociation of the α and β-carbon bonds, as presented in the figure. The fragment ions at m/z 344, 294, 244 for HFB-, PFP-, and TFA-EPH, respectively, are characteristic ions to distinguish between EPH and MA, whereas the retention times for both are close to each other after PFP and TFA derivatization.
Figure 1.
Mass spectra for HFB derivatives of the target amphetamines and cathinones. AMP indicates amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone.
Figure 3.
Mass spectra for TFA derivatives of the target amphetamines and cathinones. AMP indicates amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone.
Table 1.
Ions monitored for gas chromatography-mass spectrometry analysis for HFA, PFP, and TFA derivatives of the target amphetamines and cathinones.
| Name | HFBA |
PFPA |
TFAA |
|||
|---|---|---|---|---|---|---|
| Quantifier ions, m/z | Qualifier ions, m/z | Quantifier ions, m/z | Qualifier ions, m/z | Quantifier ions, m/z | Qualifier ions, m/z | |
| AMP | 240 | 91, 118 | 190 | 91, 118 | 140 | 91, 118 |
| 4-MA | 132 | 105, 240 | 132 | 105, 190 | 132 | 105, 140 |
| MA | 254 | 118, 210 | 204 | 160, 118 | 154 | 110, 118 |
| MDA | 375 | 135, 162 | 162 | 135, 325 | 275 | 135, 162 |
| MDMA | 254 | 162, 210 | 204 | 162, 339 | 154 | 110, 162 |
| MDEA | 268 | 240, 403 | 218 | 190, 353 | 168 | 140, 303 |
| CAT | 105 | 77, 240 | 105 | 77, 190 | 105 | 77, 140 |
| MC | 254 | 105, 210 | 204 | 105, 160 | 154 | 105, 110 |
| MEP | 119 | 210, 254 | 119 | 160, 204 | 119 | 91, 154 |
| EPH | 254 | 210, 344 | 204 | 160, 294 | 154 | 110, 244 |
| AMP-D5 | 244 | 122, 123 | 194 | 122, 123 | 144 | 92, 123 |
| MA-D5 | 258 | 120, 213 | 208 | 119, 163 | 158 | 113, 120 |
| MDA-D5 | 380 | 136, 167 | 167 | 136, 330 | 280 | 136, 167 |
| MDMA-D5 | 258 | 164, 213 | 208 | 164, 344 | 158 | 164, 113 |
| MDEA-D5 | 273 | 241, 408 | 223 | 191, 358 | 173 | 141, 308 |
Abbreviations: AMP, amphetamine; CAT, cathinone; EPH, ephedrine; HFBA, heptafluorobutyric anhydride; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone; PFPA, pentafluoropropionic anhydride; TFAA, trifluoroacetic anhydride.
Figure 2.
Mass spectra for PFP derivatives of the target amphetamines and cathinones. AMP indicates amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone.
The calibration graphs for each analyte showed good linearity over the dynamic range of 5 to 2000 or 10 to 2000 ng/mL within 3 regression curves. Linear correlation coefficients (r2) were calculated from the triplicate analyses at 6 and 7 concentrations. All r2 values were greater than 0.97. The best r2 values were obtained with PFPA (0.99). Linear ranges, accuracy, and precision (n = 3) for each analyte are presented in Tables 2 to 4. Accuracy expressed as a bias and precision expressed as a relative standard deviation were evaluated at each calibration level. The acceptable value for bias was ±15% and ±20% for LOQ. As depicted in the tables, accuracy and precision were within the acceptable limits.
Table 2.
Accuracy and precision data and linearity range (n = 3) for HFA derivatives of the target amphetamines and cathinones.
| Analyte | Concentration, ng/mL |
||||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 100 | 500 | 1000 | |
| AMP | |||||||
| Mean ± SD | — | 10.5 ± 0.9 | 23.6 ± 1.2 | 50.2 ± 3.0 | 98.7 ± 10.4 | 510.3 ± 45.7 | 1017.4 ± 38.3 |
| %RSD | 8.9 | 5.0 | 6.1 | 10.6 | 9.0 | 3.8 | |
| %Bias | 4.7 | −5.7 | 0.3 | −1.3 | 2.1 | 1.7 | |
| 4-MA | |||||||
| Mean ± SD | 5.0 ± 0.3 | 10.6 ± 0.7 | 24.5 ± 0.9 | 52.2 ± 3.6 | 96.8 ± 6.5 | 499.3 ± 15.0 | 1047.7 ± 51.9 |
| %RSD | 5.9 | 6.4 | 3.5 | 6.9 | 6.7 | 3.0 | 5.0 |
| %Bias | 0.3 | 5.8 | −1.9 | 4.5 | −3.2 | −0.1 | 4.8 |
| MA | |||||||
| Mean ± SD | 4.9 ± 0.5 | 9.5 ± 0.9 | 24.9 ± 2.0 | 51.9 ± 5.6 | 99.2 ± 10.9 | 522.1 ± 62.6 | 1064.4 ± 67.5 |
| %RSD | 10.5 | 9.4 | 8.0 | 10.7 | 11.0 | 12.0 | 6.3 |
| %Bias | −2.5 | −5.3 | −0.4 | 3.9 | −0.8 | 4.4 | 6.4 |
| MDA | |||||||
| Mean ± SD | — | 9.9 ± 1.0 | 25.9 ± 1.3 | 52.8 ± 4.0 | 90.8 ± 2.6 | 491.8 ± 58.7 | 1095.3 ± 25.0 |
| %RSD | 10.1 | 5.0 | 7.6 | 2.8 | 11.9 | 2.3 | |
| %Bias | −0.6 | 3.7 | 5.6 | −9.2 | −1.6 | 9.5 | |
| MDMA | |||||||
| Mean ± SD | 4.7 ± 0.5 | 10.4 ± 0.8 | 25.3 ± 0.9 | 53.3 ± 2.2 | 91.0 ± 3.0 | 517.1 ± 64.6 | 1093.8 ± 21.5 |
| %RSD | 11.1 | 7.8 | 3.7 | 4.2 | 3.3 | 12.5 | 2.0 |
| %Bias | −5.8 | 4.1 | 1.2 | 6.6 | −9.1 | 3.4 | 9.4 |
| MDEA | |||||||
| Mean ± SD | 5.1 ± 0.2 | 11.0 ± 0.1 | 24.6 ± 0.7 | 50.7 ± 3.6 | 105.2 ± 7.7 | 465.2 ± 47.8 | 1101.1 ± 131.7 |
| %RSD | 3.1 | 0.6 | 2.9 | 7.0 | 7.3 | 10.3 | 12.0 |
| %Bias | 1.8 | 10.4 | −1.7 | 1.4 | 5.2 | −7.0 | 10.1 |
| EPH | |||||||
| Mean ± SD | 5.1 ± 0.1 | 10.1 ± 0.9 | 24.9 ± 3.0 | 47.9 ± 1.4 | 93.9 ± 7.0 | 498.2 ± 29.5 | 1166.0 ± 42.4 |
| %RSD | 1.8 | 9.0 | 12.0 | 2.9 | 7.5 | 5.9 | 3.6 |
| %Bias | 1.1 | 0.9 | −0.5 | −4.2 | −6.1 | −0.4 | 16.6 |
| CAT | |||||||
| Mean ± SD | 5.3 ± 0.5 | 9.0 ± 0.1 | 24.8 ± 1.0 | 47.4 ± 2.2 | 107.5 ± 4.5 | 545.4 ± 12.0 | 1083.2 ± 56.4 |
| %RSD | 10.4 | 1.7 | 3.9 | 4.7 | 4.2 | 2.2 | 5.2 |
| %Bias | 5.1 | −10.2 | −0.7 | −5.1 | 7.5 | 9.1 | 8.3 |
| MC | |||||||
| Mean ± SD | 4.6 ± 0.4 | 10.0 ± 0.2 | 23.7 ± 1.8 | 51.8 ± 2.1 | 94.3 ± 8.8 | 488.2 ± 48.3 | 1077.0 ± 48.3 |
| %RSD | 8.3 | 2.2 | 7.7 | 4.1 | 9.4 | 9.9 | 4.5 |
| %Bias | −7.2 | −0.4 | −5.2 | 3.5 | −5.7 | −2.4 | 7.7 |
| MEP | |||||||
| Mean ± SD | 5.6 ± 0.6 | 11.0 ± 0.1 | 25.9 ± 1.4 | 47.6 ± 1.9 | 106.4 ± 8.0 | 500.5 ± 16.3 | 1041.3 ± 80.9 |
| %RSD | 11.0 | 0.6 | 5.6 | 4.0 | 7.5 | 3.3 | 7.8 |
| %Bias | 11.0 | 10.1 | 3.4 | −4.9 | 6.4 | 0.1 | 4.1 |
Abbreviations: AMP, amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone; RSD, relative standard deviation.
Table 4.
Accuracy and precision data and linearity range (n = 3) for TFA derivatives of the target amphetamines and cathinones.
| Analyte | Concentration, ng/mL |
||||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 100 | 500 | 1000 | |
| AMP | |||||||
| Mean ± SD | 5.0 ± 0.2 | 11.1 ± 0.1 | 23.9 ± 2.3 | 45.6 ± 0.4 | 90.7 ± 6.6 | 516.8 ± 4.9 | 1000.7 ± 1.3 |
| %RSD | 3.5 | 0.8 | 9.6 | 0.9 | 7.3 | 0.9 | 0.1 |
| %Bias | −0.5 | 10.6 | −4.2 | −8.8 | −9.3 | 3.4 | 0.1 |
| 4-MA | |||||||
| Mean ± SD | 5.2 ± 0.1 | 10.4 ± 0.6 | 25.0 ± 1.5 | 50.9 ± 1.6 | 95.9 ± 8.4 | 526.9 ± 31.4 | 1062.9 ± 4.7 |
| %RSD | 1.5 | 5.6 | 5.9 | 3.1 | 8.8 | 6.0 | 0.4 |
| %Bias | 3.1 | 4.1 | 0.2 | 1.7 | −4.1 | 5.4 | 6.3 |
| MA | |||||||
| Mean ± SD | 5.6 ± 0.1 | 11.0 ± 0.3 | 23.4 ± 1.9 | 44.0 ± 0.8 | 88.9 ± 0.7 | 443.0 ± 5.6 | 1043.7 ± 49.0 |
| %RSD | 1.9 | 3.1 | 8.1 | 1.9 | 0.8 | 1.3 | 4.7 |
| MDA | |||||||
| Mean ± SD | 4.7 ± 0.2 | 9.1 ± 0.1 | 24.8 ± 1.7 | 45.1 ± 2.6 | 93.8 ± 7.4 | 480.6 ± 21.7 | 1047.3 ± 25.5 |
| %RSD | 4.8 | 1.2 | 7.0 | 5.8 | 7.9 | 4.5 | 2.4 |
| %Bias | −5.8 | −9.3 | −0.9 | −9.9 | −6.2 | −3.9 | 4.7 |
| MDMA | |||||||
| Mean ± SD | — | 10.4 ± 0.8 | 26.5 ± 0.7 | 47.9 ± 4.2 | 89.0 ± 6.0 | 480.3 ± 19.3 | 1075.0 ± 8.9 |
| %RSD | 7.5 | 2.6 | 8.7 | 6.7 | 4.0 | 0.8 | |
| %Bias | 4.1 | 6.0 | −4.2 | −11.0 | −3.9 | 7.5 | |
| MDEA | |||||||
| Mean ± SD | 4.5 ± 0.1 | 9.1 ± 0.1 | 24.2 ± 1.2 | 50.7 ± 3.4 | 106.2 ± 7.4 | 478.3 ± 17.4 | 1051.7 ± 142.2 |
| %RSD | 3.0 | 1.1 | 4.9 | 6.7 | 6.9 | 3.6 | 13.5 |
| %Bias | −10.2 | −9.5 | −3.0 | 1.4 | 6.2 | −4.3 | 5.2 |
| EPH | |||||||
| Mean ± SD | 5.1 ± 0.2 | 8.9 ± 0.2 | 26.0 ± 1.3 | 46.1 ± 2.8 | 99.7 ± 13.4 | 523.1 ± 18.4 | 1067.5 ± 60.4 |
| %RSD | 3.5 | 2.5 | 5.1 | 6.1 | 13.5 | 3.5 | 5.7 |
| %Bias | 2.1 | −10.6 | 4.2 | −7.8 | −0.3 | 4.6 | 6.8 |
| CAT | |||||||
| Mean ± SD | 4.9 ± 0.2 | 10.2 ± 0.6 | 23.7 ± 1.8 | 54.6 ± 2.7 | 111.3 ± 1.0 | 466.0 ± 39.0 | 981.8 ± 38.4 |
| %RSD | 3.7 | 5.9 | 7.8 | 4.9 | 0.9 | 8.4 | 3.9 |
| %Bias | −1.2 | 2.3 | −5.2 | 9.2 | 11.3 | −6.8 | −1.8 |
| MC | |||||||
| Mean ± SD | 4.6 ± 0.4 | 10.0 ± 0.2 | 23.7 ± 1.8 | 51.8 ± 2.1 | 94.3 ± 8.8 | 488.2 ± 48.3 | 1077.0 ± 48.3 |
| %RSD | 8.3 | 2.2 | 7.7 | 4.1 | 9.4 | 9.9 | 4.5 |
| %Bias | −7.2 | −0.4 | −5.2 | 3.5 | −5.7 | −2.4 | 7.7 |
| MEP | |||||||
| Mean ± SD | 5.2 ± 0.1 | 11.0 ± 0.1 | 25.9 ± 1.4 | 47.6 ± 1.9 | 106.4 ± 8.0 | 500.5 ± 16.3 | 1041.3 ± 80.9 |
| %RSD | 2.3 | 0.6 | 5.6 | 4.0 | 7.5 | 3.3 | 7.8 |
| %Bias | 3.3 | 10.1 | 3.4 | −4.9 | 6.4 | 0.1 | 4.1 |
Abbreviations: AMP, amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone; RSD, relative standard deviation.
Table 3.
Accuracy and precision data and linearity range (n = 3) for PFP derivatives of the target amphetamines and cathinones.
| Analyte | Concentration, ng/mL |
||||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 100 | 500 | 1000 | |
| AMP | |||||||
| Mean ± SD | 4.7 ± 0.5 | 10.4 ± 0.5 | 26.6 ± 1.6 | 47.1 ± 0.7 | 85.9 ± 0.3 | 525.5 ± 78.3 | 1000.9 ± 47.9 |
| %RSD | 9.9 | 5.1 | 5.8 | 1.6 | 0.3 | 14.9 | 4.8 |
| %Bias | −6.7 | 4.3 | 6.5 | −5.8 | −14.1 | 5.1 | 0.1 |
| 4-MA | |||||||
| Mean ± SD | 5.1 ± 0.2 | 9.5 ± 0.3 | 24.6 ± 0.6 | 46.5 ± 0.1 | 105.6 ± 6.4 | 466.9 ± 32.9 | 956.7 ± 76.5 |
| %RSD | 3.9 | 3.4 | 2.4 | 0.2 | 6.1 | 7.0 | 8.0 |
| %Bias | 1.2 | −5.2 | −1.7 | −7.1 | 5.6 | −6.6 | −4.3 |
| MA | |||||||
| Mean ± SD | 5.4 ± 0.2 | 10.6 ± 0.7 | 25.0 ± 1.5 | 52.7 ± 3.5 | 88.7 ± 0.5 | 493.0 ± 42.1 | 1033.2 ± 39.3 |
| %RSD | 3.2 | 6.1 | 6.0 | 6.6 | 0.6 | 8.5 | 3.8 |
| %Bias | 8.4 | 6.4 | 0.2 | 5.3 | −11.3 | −1.4 | 3.3 |
| MDA | |||||||
| Mean ± SD | 5.2 ± 0.1 | 9.4 ± 0.7 | 26.0 ± 2.7 | 54.1 ± 2.8 | 89.1 ± 1.4 | 474.5 ± 0.7 | 1134.6 ± 13.3 |
| %RSD | 1.9 | 7.2 | 10.3 | 5.1 | 1.6 | 0.1 | 1.2 |
| %Bias | 4.0 | −6.4 | 4.0 | 8.3 | −10.9 | −5.1 | 13.5 |
| MDMA | |||||||
| Mean ± SD | 5.4 ± 0.4 | 9.3 ± 0.9 | 25.1 ± 1.4 | 47.2 ± 3.8 | 90.1 ± 1.7 | 461.5 ± 11.3 | 1064.8 ± 7.7 |
| %RSD | 7.4 | 9.2 | 5.7 | 8.1 | 1.8 | 2.5 | 0.7 |
| %Bias | 7.7 | −7.0 | 0.4 | −5.6 | −9.9 | −7.7 | 6.5 |
| MDEA | |||||||
| Mean ± SD | 4.7 ± 0.1 | 10.0 ± 0.3 | 26.6 ± 0.7 | 52.0 ± 2.5 | 108.1 ± 3.7 | 462.4 ± 12.1 | 1070.1 ± 45.7 |
| %RSD | 1.9 | 3.3 | 2.7 | 4.8 | 3.4 | 2.6 | 4.3 |
| %Bias | −5.3 | −0.5 | 6.4 | 4.0 | 8.1 | −7.5 | 7.0 |
| EPH | |||||||
| Mean ± SD | 5.3 ± 0.1 | 9.0 ± 0.2 | 27.4 ± 0.9 | 54.1 ± 4.4 | 92.5 ± 4.7 | 470.4 ± 46.0 | 1060.6 ± 71.1 |
| %RSD | 1.6 | 2.1 | 3.4 | 8.2 | 5.1 | 9.8 | 6.7 |
| %Bias | 6.8 | −10.3 | 9.5 | 8.3 | −7.5 | −5.9 | 6.1 |
| CAT | |||||||
| Mean ± SD | 4.8 ± 0.1 | 11.0 ± 0.1 | 26.2 ± 1.3 | 49.4 ± 1.3 | 106.7 ± 8.0 | 473.4 ± 19.2 | 1062.0 ± 35.4 |
| %RSD | 2.0 | 1.4 | 4.9 | 2.6 | 7.5 | 4.1 | 3.3 |
| %Bias | −3.4 | 9.9 | 4.7 | −1.2 | 6.7 | −5.3 | 6.2 |
| MC | |||||||
| Mean ± SD | 5.0 ± 0.1 | 9.0 ± 0.1 | 26.2 ± 0.5 | 50.6 ± 5.0 | 103.9 ± 8.2 | 517.2 ± 22.4 | 956.5 ± 43.2 |
| %RSD | 2.0 | 0.8 | 1.8 | 9.9 | 7.9 | 4.3 | 4.5 |
| %Bias | −0.8 | −9.6 | 4.9 | 1.1 | 3.9 | 3.4 | −4.3 |
| MEP | |||||||
| Mean ± SD | 5.0 ± 0.2 | 8.9 ± 0.1 | 24.6 ± 0.7 | 50.3 ± 1.6 | 105.2 ± 6.1 | 473.1 ± 34.0 | 1101.5 ± 14.8 |
| %RSD | 4.3 | 1.0 | 2.8 | 3.1 | 5.8 | 7.2 | 1.3 |
| %Bias | −1.0 | −10.7 | −1.5 | 0.6 | 5.2 | −5.4 | 10.1 |
Abbreviations: AMP, amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone; RSD, relative standard deviation.
A blank sample was analyzed after the highest point of the calibration curve and showed no peaks for the target analytes that the method is free from carryover.
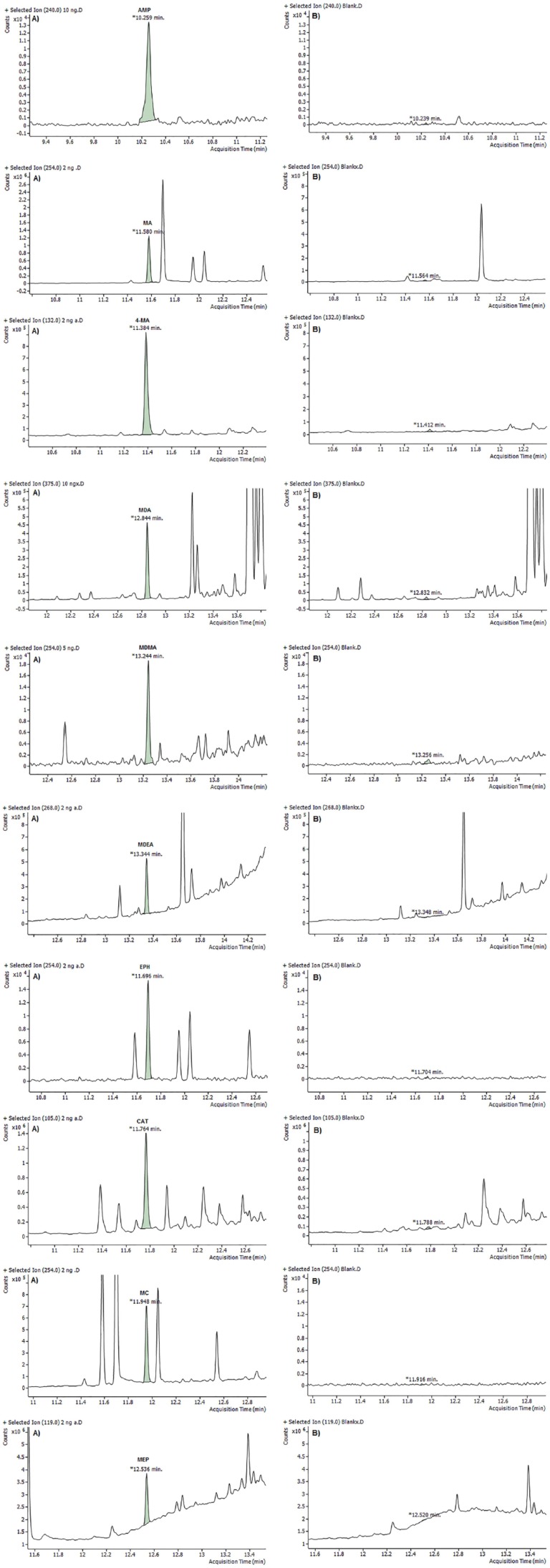
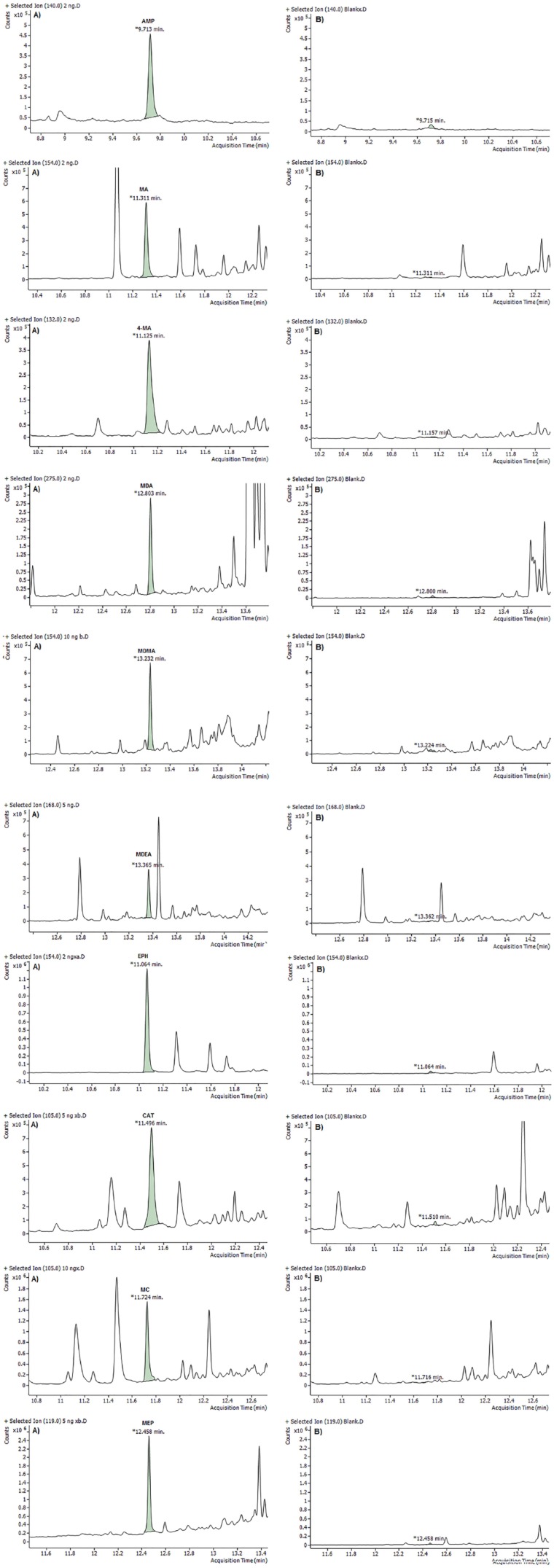
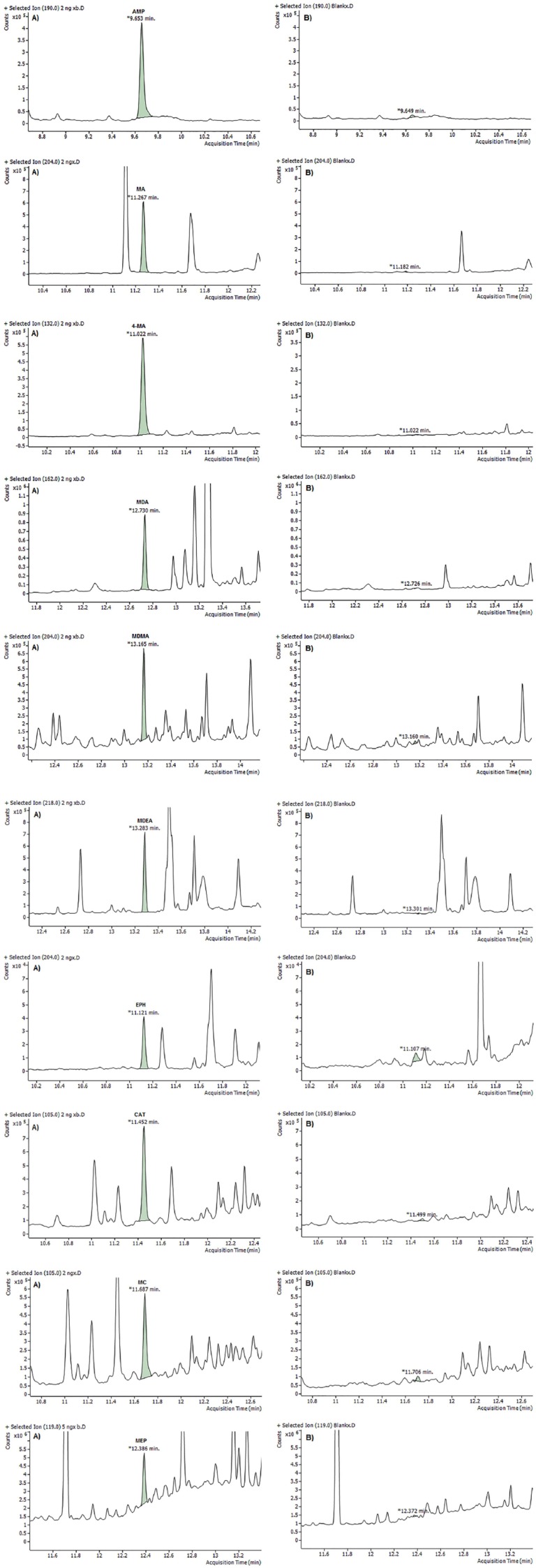
The LOQ was measured in SIM mode using blank oral fluid fortified with all analytes at concentrations of 1, 2.5, 5, and 10 ng/mL. The S/N ratio was calculated from triplicate measurements. The lowest concentration at which the S/N ratio was equal or greater than 10 was considered as the LOQ. Table 5 presents the LOQ for all analytes using different derivatization methods. Selected ion monitoring chromatograms for the analysis of blank oral fluid and fortified sample at LOQ for all analytes are shown in Figures 4 to 6. Based on S/N, the best result was given by PFPA. Moreover, use PFPA as derivatizing reagent allows for very low values of the LOQs (2.5 and 5 ng/mL) for some amphetamines and cathinones compared with the method reported by Mohamed et al29 and Rohrich et al.33 which has LOQs of 20 and 9.8 to 20.2 ng/mL, respectively.
Table 5.
LOQ for HFA, PFP, and TFA derivatives of the target amphetamines and cathinones.
| Analyst | LOQ, ng/mL |
||
|---|---|---|---|
| HFBA | PFPA | TFAA | |
| AMP | 10 | 2.5 | 2.5 |
| 4-MA | 2.5 | 2.5 | 2.5 |
| MA | 2.5 | 2.5 | 2.5 |
| MDA | 10 | 2.5 | 2.5 |
| MDMA | 5 | 2.5 | 10 |
| MDEA | 2.5 | 2.5 | 5 |
| CAT | 2.5 | 2.5 | 5 |
| MC | 2.5 | 2.5 | 5 |
| MEP | 2.5 | 5 | 5 |
| EPH | 2.5 | 2.5 | 2.5 |
Abbreviations: AMP, amphetamine; CAT, cathinone; EPH, ephedrine; HFBA, heptafluorobutyric anhydride; LOQ, limit of quantification; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone; PFPA, pentafluoropropionic anhydride; TFAA, trifluoroacetic anhydride.
Figure 4.
Total ion chromatograms for the gas chromatography-mass spectrometry analysis of HFA derivatives of amphetamines and cathinones at (A) limit of quantification and (B) blank oral fluid. AMP indicates amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone.
Figure 6.
Total ion chromatograms for the gas chromatography-mass spectrometry analysis of TFA derivatives of amphetamines and cathinones at (A) limit of quantification and (B) blank oral fluid. AMP indicates amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone.
Figure 5.
Total ion chromatograms for the gas chromatography-mass spectrometry analysis of PFP derivatives of amphetamines and cathinones at (A) limit of quantification and (B) blank oral fluid. AMP indicates amphetamine; CAT, cathinone; EPH, ephedrine; 4-MA, 4-methylamphetamine; MA, methamphetamine; MC, methcathinone; MDA, 3,4-methylenedioxyamphetamine; MDEA, 3,4-methylenedioxy-N-ethylamphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MEP, mephedrone.
No co-eluting peaks were observed except CAT, and 4-MA could not be separated effectively from EPH if they were derivatized with HFBA or TFAA. However, they have different fragment ions and can be distinguished from each other.
Conclusions
Three acylation reagents, HFBA, PFPA, and TFAA, have been compared with use as derivatizing agents for the analysis of 10 amphetamines and cathinones in oral fluid by GC-MS. The 3 methods have suitable linearity, sensitivity, accuracy, and precision. Based on LOQ, PFPA is proved to be the best for derivatization of AMP, MA, 4-MA, MDA, MDMA, MDEA, CAT, MC, MEP, and EPH after liquid-liquid extraction from the oral fluid samples.
Acknowledgments
The authors would like to thank all staff of The Department of Forensic Chemistry for supporting this work. They also would like to thank Mr Sundaresan Ramachandran for GC-MS technical support.
Footnotes
Peer review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 628 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed equally to this work. KMM and AB conducted the analysis. KMM wrote the main paper, and AB wrote the Supplementary Information. All authors discussed the results and implications and commented on the manuscript at all stages.
References
- 1. Drummer OH, Odell M. The Forensic Pharmacology of Drugs of Abuse. London: Arnold; 2001. [Google Scholar]
- 2. Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. [DOI] [PubMed] [Google Scholar]
- 3. Abourashed EA, El-Alfy AT, Khan IA, Walker L. Ephedra in perspective—a current review. Phytother Res. 2003;17:703–712. [DOI] [PubMed] [Google Scholar]
- 4. Busardò FP, Kyriakou C, Napoletano S, Marinelli E, Zaami S. Mephedrone related fatalities: a review. Eur Rev Med Pharmacol Sci. 2015;19:3777–3790. [PubMed] [Google Scholar]
- 5. Carvalho M, Carmo H, Costa VM, et al. Toxicity of amphetamines: an update. Arch Toxicol. 2012;86:1167–1231. [DOI] [PubMed] [Google Scholar]
- 6. Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. [DOI] [PubMed] [Google Scholar]
- 7. De la, Torre R, Farré M, Navarro M, Pacifici R, Zuccaro P, Pichini S. Clinical pharmacokinetics of amfetamine and related substances. Clin Pharmacokinet. 2004;43:157–185. [DOI] [PubMed] [Google Scholar]
- 8. De la, Torre R, Farré M, Roset PN, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. [DOI] [PubMed] [Google Scholar]
- 9. Drummer OH. Drug testing in oral fluid. Clin Biochem Rev. 2006;27:147–159. [PMC free article] [PubMed] [Google Scholar]
- 10. Schramm W, Smith RH, Craig PA. Methods of simplified saliva collection for the measurement of drugs of abuse, therapeutic drugs, and other molecules. Ann N Y Acad Sci. 1993;694:311–313. [DOI] [PubMed] [Google Scholar]
- 11. Mandel ID. Salivary diagnosis: promises, promises. Ann N Y Acad Sci. 1993;694:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Schramm W, Smith RH, Craig PA, Kidwell DA. Drugs of abuse in saliva: a review. J Anal Toxicol. 1992;16:1–9. [DOI] [PubMed] [Google Scholar]
- 13. Kintz P, Samyn N. Use of alternative specimens: drugs of abuse in saliva and doping agents in hair. Ther Drug Monit. 2002;24:239–246. [DOI] [PubMed] [Google Scholar]
- 14. Ondra P, Válka I, Knob R, et al. Analysis of amphetamine-derived designer drugs by gas chromatography with mass spectrometry. J Anal Toxicol. 2015;40:78–85. [DOI] [PubMed] [Google Scholar]
- 15. de Cássia Mariotti K, Schuh RS, Ferranti P, et al. Simultaneous analysis of amphetamine-type stimulants in plasma by solid-phase microextraction and gas chromatography-mass spectrometry. J Anal Toxicol. 2014;38:432–437. [DOI] [PubMed] [Google Scholar]
- 16. de Souza Eller SC, de Oliveira F, Yonamine M. Measurement uncertainty for the determination of amphetamines in urine by liquid-phase microextraction and gas chromatography-mass spectrometry. Forensic Sci Int. 2016;265:81–88. [DOI] [PubMed] [Google Scholar]
- 17. Hsu MC, Chen D, Liu RH. Detection of abused drugs in urine by GC-MS. J Food Drug Anal. 2009;17:233–245. [Google Scholar]
- 18. Dobos A, Hidvégi E, Somogyi GP. Comparison of five derivatizing agents for the determination of amphetamine-type stimulants in human urine by extractive acylation and gas chromatography-mass spectrometry. J Anal Toxicol. 2012;36:340–344. [DOI] [PubMed] [Google Scholar]
- 19. Lee HH, Lee JF, Lin SY, Chen PH, Chen BH. Simultaneous determination of HFBA-derivatized amphetamines and ketamines in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:162–169. [DOI] [PubMed] [Google Scholar]
- 20. Hidvégi E, Fábián P, Hideg Z, Somogyi G. GC-MS determination of amphetamines in serum using on-line trifluoroacetylation. Forensic Sci Int. 2006;161:119–123. [DOI] [PubMed] [Google Scholar]
- 21. Stout PR, Horn CK, Klette KL. Rapid simultaneous determination of amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine, 3,4-methylenedioxymethamphetamine, and 3,4-methylenedioxyethylamphetamine in urine by solid-phase extraction and GC-MS: a method optimized for high-volume laboratories. J Anal Toxicol. 2002;26:253–261. [DOI] [PubMed] [Google Scholar]
- 22. Lee MR, Song YS, Hwang BH, Chou C-C. Determination of amphetamine and methamphetamine in serum via headspace derivatization solid-phase microextraction–gas chromatography–mass spectrometry. J Chromatogr A. 2000;896:265–273. [DOI] [PubMed] [Google Scholar]
- 23. Marais AA, Laurens JB. Rapid GC-MS confirmation of amphetamines in urine by extractive acylation. Forensic Sci Int. 2009;183:78–86. [DOI] [PubMed] [Google Scholar]
- 24. Villamor JL, Bermejo AM, Fernandez P, Tabernero MJ. A new GC-MS method for the determination of five amphetamines in human hair. J Anal Toxicol. 2005;29:135–139. [DOI] [PubMed] [Google Scholar]
- 25. Valentine JL, Kearns GL, Sparks C, et al. GC-MS determination of amphetamine and methamphetamine in human urine for 12 hours following oral administration of dextro-methamphetamine: lack of evidence supporting the established forensic guidelines for methamphetamine confirmation. J Anal Toxicol. 1995;19:581–590. [DOI] [PubMed] [Google Scholar]
- 26. Jurado C, Gimenez MP, Soriano T, Menéndez M, Repetto M. Rapid analysis of amphetamine, methamphetamine, MDA, and MDMA in urine using solid-phase microextraction, direct on-fiber derivatization, and analysis by GC-MS. J Anal Toxicol. 2000;24:11–16. [DOI] [PubMed] [Google Scholar]
- 27. Mule SJ, Casella GA. Confirmation of marijuana, cocaine, morphine, codeine, amphetamine, methamphetamine, phencyclidine by GC/MS in urine following immunoassay screening. J Anal Toxicol. 1988;12:102–710. [DOI] [PubMed] [Google Scholar]
- 28. Suzuki O, Hattori H, Asano M. Detection of methamphetamine and amphetamine in a single human hair by gas chromatography/chemical ionization mass spectrometry. J Forensic Sci. 1984;29:611–617. [PubMed] [Google Scholar]
- 29. Mohamed KM, Al-Hazmi AH, Alasiri AM, Ali Mel-S. A GC-MS method for detection and quantification of cathine, cathinone, methcathinone and ephedrine in oral fluid. J Chromatogr Sci. 2016;54:1271–1276. [DOI] [PubMed] [Google Scholar]
- 30. Hong WY, Ko YC, Lin MC, et al. Determination of synthetic cathinones in urine using gas chromatography-mass spectrometry techniques. J Anal Toxicol. 2016;40:12–16. [DOI] [PubMed] [Google Scholar]
- 31. Sporkert F, Pragst F, Bachus R, Masuhr F, Harms L. Determination of cathinone, cathine and norephedrine in hair of Yemenite khat chewers. Forensic Sci Int. 2003;133:39–46. [DOI] [PubMed] [Google Scholar]
- 32. Paul BD, Cole KA. Cathinone (khat) and methcathinone (CAT) in urine specimens: a gas chromatographic-mass spectrometric detection procedure. J Anal Toxicol. 2001;25:525–530. [DOI] [PubMed] [Google Scholar]
- 33. Rohrich J, Zorntlein S, Becker J, Urban R. Detection of Δ9-tetrahydrocannabinol and amphetamine-type stimulants in oral fluid using the Rapid Stat™ point-of-collection drug-testing device. J Anal Toxicol. 2010;34:155–161. [DOI] [PubMed] [Google Scholar]
- 34. Guidance for Industry: Bioanalytical method validation. U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Published May 2001.
- 35. American Board of Forensic Toxicology (ABFT). Forensic Toxicology Laboratory Accreditation Checklist. Colorado Springs, CO: ABFT; www.abft.org. Published 2013. [Google Scholar]