Abstract
Background
In view of the increasing heart failure epidemic and awareness of the adverse impact of environmental pollution on human health, we investigated the association of left ventricular structure and function with air pollutants in a general population.
Methods
In 671 randomly recruited Flemish (51.7% women; mean age, 50.4 years) we echocardiographically assessed left ventricular systolic strain and strain rate and the early and late peak velocities of transmitral blood flow and mitral annular movement (2005−2009). Using subject-level data, left ventricular function was cross-sectionally correlated with residential long-term exposure to air pollutants, including black carbon, PM2.5, PM10 (particulate matter) and nitrogen dioxide (NO2), while accounting for clustering by residential address and confounders.
Results
Annual exposures to black carbon, PM2.5, PM10 and NO2 averaged 1.19, 13.0, 17.7, and 16.8 µg/m3. Systolic left ventricular function was worse (p ≤ 0.027) with higher black carbon, PM2.5, PM10 and NO2 with association sizes per interquartile interval increment ranging from −0.339 to −0.458% for longitudinal strain and from −0.033 to −0.049 s−1 for longitudinal strain rate. Mitral E and a′ peak velocities were lower (p ≤ 0.021) with higher black carbon, PM2.5 and PM10 with association sizes ranging from −1.727 to −1.947 cm/s and from −0.175 to −0.235 cm/s, respectively. In the geographic analysis, the systolic longitudinal strain sided with gradients in air pollution. The path analysis identified systemic inflammation as a possible mediator of associations with black carbon.
Conclusions
Long-term low-level air pollution is associated with subclinical impairment of left ventricular performance and might be a risk factor for heart failure.
Keywords: Air pollution, heart failure, NO2, PM2.5, PM10, population science
Introduction
Heart failure is a major public health problem with a prevalence of more than 23 million worldwide.1 Longer life expectancy explains why the incidence of heart failure rises exponentially with aging and over time.1 In industrialized countries, the heart failure prevalence is approximately 1% among all adults and over 10% among the elderly.2 Diastolic heart failure, also referred to as heart failure with preserved ejection fraction, accounts for 40–50% of all cases.3 In view of the increasing awareness of the adverse impact of environmental pollution on human health, identification of air pollution triggers of left ventricular (LV) dysfunction is therefore of major importance.
In population studies, air pollution and exposure to fine and coarse particulate matter (PM) predict the risk of incident heart failure4,5 or are associated with hospitalization6–8 and mortality due to heart failure.7 LV ejection fraction was lower in 50 steel workers exposed to air pollution compared with non-exposed controls,9 but population studies10–12 failed to demonstrate consistent associations of air pollution with LV ejection fraction, assessed by echocardiography11 or magnetic resonance imaging,10 or with diastolic LV function.11,12 Several factors limit the interpretation of the currently available evidence.4–12 First, the heart failure endpoint does not provide information on early subclinical LV dysfunction. Second, LV ejection fraction is observer dependent and is an imprecise index of subtle changes in systolic LV function.13 Finally, to our knowledge, none of the echocardiographic studies applied strain and strain rate as the current state-of-the-art technique14 to assess systolic LV function. To bridge these knowledge gaps, we related, in a population study, systolic and diastolic echocardiographic LV function and LV structure to air pollution data with high spatial resolution averaged over a five-year period.
Methods
Study population
As described in detail elsewhere, enrollment of the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO) participants started in 1985 and continued until 2004.15,16 The initial participation rate was 78.0%. The participants were repeatedly followed-up. The 2005–2009 cycle of fieldwork included an echocardiographic examination. Of 1090 former participants invited by letter, 24 had died, 26 were severely ill and 98 had moved out of the area or did not respond. Of the remaining 942 subjects, 752 renewed consent (participation rate 79.8%). For the current analysis, we excluded 81 participants because of insufficient quality of the echocardiographic images (n = 44), severe valvular disease (n = 22), previous myocardial infarction (n = 8), atrial fibrillation (n = 6), or frequent ventricular ectopic beats (n = 1). The justification of these criteria was their potential impact on the assessment of systolic and diastolic LV function. Thus, the number of participants statistically analysed totalled 671.
The study complied with the Helsinki Declaration for investigation of human subjects.17 The Ethics Committee of the University of Leuven, Belgium, approved the study protocol. Participants gave or renewed written informed consent at each contact.
Echocardiography
A single observer (TK) acquired the echocardiographic images and did the off-line analysis according to current guidelines.14,18 In short, digitally stored echocardiographic images, obtained with a Vivid7 Pro device (GE Vingmed, Horten, Norway) interfaced with a 2.5- to 3.5-MHz phased-array probe, were averaged over three heart cycles. LV structure and diastolic LV function were assessed by EchoPac software, version 4.0.4 (GE Vingmed, Horten, Norway). We determined peak early (E) and peak late (A) diastolic velocities of the transmitral blood flow from the pulsed Doppler signal and peak early (e′) and peak late (a′) velocities of the mitral annular movement by tissue Doppler imaging (TDI) with velocities averaged over four acquisition sites (septal, lateral, inferior, and posterior). The intra-observer reproducibility, defined as the 2-SD interval about the mean of the relative differences across pairwise measurements was 4.6% for LV wall thickness and 4.3% for LV mass,19 and 4.8% for e′ and 4.2% for a′.20 For the assessment of systolic function over and beyond ejection fraction, we applied SPEQLE software (version 4.6.2),21,22 which was specifically designed as a research tool. Using TDI imaging with the sampling volume positioned at the basal LV segments under visual control of the angulation of the ultrasound beam, the software returns longitudinal and radial end-systolic strain and peak systolic and radial strain rate of the inferolateral and inferior walls.21,22 The intra-observer reproducibility was 15.8% and 18.3% for longitudinal and radial strain and 6.7% and 14.0% for longitudinal and radial strain rate, respectively.21
Ambient air pollution
Particulate matter is the sum of all solid and liquid particles suspended in air.23 Fine (PM2.5) and coarse (PM10) particulate matter have an aerodynamic diameter of less than 2.5 µm and 10 µm, respectively. Upon inhalation, these particles, depending on their size, reach the smallest airways and alveoli and can even cross the blood–air barrier and directly penetrate into the blood stream. Black carbon is a component of PM2.5, consists of pure carbon in several bond forms, and finds its origin in the incomplete combustion of fossil fuels, biofuel or biomass. Nitrogen dioxide (NO2) forms from fuel emissions and is an indicator of a larger group of nitrogen oxides that lead to the production of oxide radicals.23
In the current study, black carbon, PM2.5, PM10 and NO2 exposure (µg/m3) were calculated for each participant’s residential address, using a high resolution spatiotemporal interpolation method24 that takes into account land-cover data obtained from satellite images (CORINE database (www.eea.europa.eu/publications/COR0-landcover/at_download/file)) and pollution data of fixed monitoring stations in combination with a dispersion model.25 This approach provides daily exposure values in a dense irregular receptor grid, using input from the Belgian telemetric air quality networks and emissions from point and line sources. The air quality network includes 14 monitoring stations for black carbon, 34 for PM2.5, 65 for PM10, and 44 for NO2. Models covered data averaged over a 5-year period (2010–2014), which reflect the long-term spatial air pollution, and are representative for earlier periods.26–30 Model performance was evaluated by leave-one-out cross-validation across monitoring stations. The spatiotemporal explained variance was more than 74% for black carbon,31 80% for PM2.5,31 70% for PM10,32 and 78% for NO2.32
Other measurements
Blood pressure was the average of five consecutive auscultatory readings obtained after participants had rested for 5 min in the sitting position. Hypertension was a blood pressure of at least 140 mmHg systolic or 90 mmHg diastolic, or use of antihypertensive drugs. Study nurses administered a questionnaire enquiring about each participant’s medical history, smoking and drinking habits, intake of medications and socioeconomic status. Smoking was the current use of any smoking materials on a daily basis. Socioeconomic status was coded according to the complex scales provided by the UK Office of Population Censuses and Surveys,33 and simplified into a linear scale with scores ranging from 1 to 3.34 Fasting blood samples obtained on the day of echocardiography were analysed for plasma glucose and serum creatinine, total and high-density lipoprotein (HDL) cholesterol and γ-glutamyltransferase as index of alcohol intake. Diabetes mellitus was a fasting or random plasma glucose level exceeding 7.0 or 11.1 mmol/l, or use of antidiabetic agents. Randox Laboratories Ltd (County Antrim, Northern Ireland, UK) blindly measured intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin and P-selectin, using Biochip Array Technology according to manufacturer’s instructions (Adhesion Molecule Array EV3519) and a sandwich assay format.35 These biomarkers were selected on the basis that in previous studies they were related to air pollution.36–39
Statistical analysis
For database management and statistical analysis, we used SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Departure from normality was evaluated by Shapiro-Wilk’s statistic. Significance was a two-tailed α-level of .05 or less. Means and proportions were compared using a large sample z-test and Fisher’s exact test, respectively.
We cross-sectionally assessed the association of LV structure and function with air pollution, using a generalized linear mixed model that accounted for resident clustering of participants (sharing the same address) as random effect and with adjustments applied for covariables of possible physiological relevance,22 socioeconomic status, use of lipid-lowering drugs and antihypertensive treatment with diuretics (thiazides, loop diuretics and aldosterone antagonists), inhibitors of the renin–angiotensin system (β-blockers, angiotensin-converting enzyme inhibitors and angiotensin type-1 receptor antagonists) and vasodilators (calcium-channel blockers and α-blockers).
We assessed the geographic associations between multivariable-adjusted LV traits of interest and the PM2.5 exposure, by plotting LV traits of individual participants or LV traits aggregated per municipality over a map showing the contours of pollution. The midterm (2012) annual PM2.5 gradients with a high resolution in the map were interpolated by using a spatiotemporal method that took into account land cover data obtained from satellite images and pollution data of fixed monitoring stations in combination with a dispersion model. Finally, we applied principal component analysis to summarize the highly intercorrelated adhesion molecules into a single normally distributed variable.40 In an attempt to differentiate the direct myocardial from the indirect effects of air pollution, we introduced the so derived summary variable in path analysis as implemented in the PROC CALIS procedure of the SAS package.
Results
Characteristics of participants
All 671 participants were White Europeans, of whom 347 (51.7%) were women. Age averaged (±SD) 50.4 ± 14.7 years (range, 15.1–89.5 years; 1st–99th percentile interval, 18.6–79.9 years). Among 671 participants, 263 (39.2%) had hypertension, of whom 154 (58.6%) were on antihypertensive drug treatment and 21 (3.1%) participants had diabetes. Among 154 patients on antihypertensive drug treatment, 58 (37.7%) took diuretics, 127 (82.5%) inhibitors of the renin system, 30 (19.5%) vasodilators and 56 (36.4%) were on combination therapy with more than one class of blood pressure lowering medication.
Table 1 lists the characteristics of the participants by quartiles of the PM2.5 exposure. From the low to the high quartile, age, plasma glucose and the prevalence of diabetes mellitus decreased from 52.9 to 45.0 years (p < 0.0001), from 4.98 to 4.76 mmol/l (p = 0.011) and from 5.33% to 1.80% (p = 0.044), respectively, whereas the fraction of smokers increased from 16.6% to 26.9% (p = 0.010). The other characteristics listed in Table 1 did not differ across PM2.5 quartiles.
Table 1.
Characteristics of the participants by quartiles of averaged annual PM2.5 exposure.
| Characteristic | Low | Medium, low | Medium, high | High | p value |
|---|---|---|---|---|---|
| Number of participants (%) | |||||
| All in category | 169 | 167 | 168 | 167 | |
| Women | 88 (52.1) | 87 (52.1) | 87 (51.8) | 85 (50.9) | 0.82 |
| Smokers | 28 (16.6) | 34 (20.4) | 44 (26.2) | 45 (26.9) | 0.010 |
| Drinking alcohol ≥ 5 g/day | 72 (42.6) | 69 (41.3) | 64 (38.1) | 77 (46.1) | 0.67 |
| Hypertension | 62 (36.7) | 72 (43.1) | 73 (43.5) | 56 (33.5) | 0.60 |
| Antihypertensive treatment | 38 (22.5) | 42 (25.1) | 42 (25.0) | 32 (19.2) | 0.50 |
| Lipid-lowering treatment | 24 (14.2) | 26 (15.6) | 26 (15.5) | 16 (9.6) | 0.24 |
| Previous CVD | 5 (3.0) | 6 (3.6) | 5 (3.0) | 8 (4.8) | 0.45 |
| Diabetes mellitus | 9 (5.33) | 6 (3.59) | 3 (1.79) | 3 (1.80) | 0.044 |
| Mean ± SD of characteristic | |||||
| Age, years | 52.9 ± 13.9 | 51.9 ± 13.6 | 51.7 ± 15.4 | 45.0 ± 14.8 | <0.0001 |
| Body mass index, kg/m2 | 26.4 ± 4.0 | 26.4 ± 4.2 | 26.6 ± 4.0 | 25.7 ± 4.1 | 0.21 |
| Waist/hip ratio | 0.87 ± 0.08 | 0.88 ± 0.08 | 0.86 ± 0.08 | 0.86 ± 0.08 | 0.31 |
| Office blood pressure | |||||
| Systolic, mmHg | 128.4 ± 16.1 | 129.3 ± 16.1 | 130.5 ± 17.8 | 126.1 ± 15.7 | 0.32 |
| Diastolic, mmHg | 78.8 ± 9.1 | 80.1 ± 9.2 | 80.3 ± 9.7 | 79.6 ± 9.8 | 0.43 |
| Mean, mmHg | 95.3 ± 10.1 | 96.5 ± 10.4 | 97.0 ± 10.7 | 95.1 ± 10.8 | 0.96 |
| Heart rate, beats/min | 59.7 ± 8.7 | 61.1 ± 9.6 | 61.0 ± 9.6 | 61.4 ± 8.9 | 0.11 |
| Socioeconomic status | 1.33 ± 0.52 | 1.34 ± 0.52 | 1.33 ± 0.52 | 1.33 ± 0.52 | 0.12 |
| Biochemical data | |||||
| Total cholesterol, mmol/l | 5.23 ± 0.89 | 5.40 ± 1.00 | 5.40 ± 1.02 | 5.10 ± 0.96 | 0.26 |
| HDL cholesterol, mmol/l | 1.44 ± 0.34 | 1.44 ± 0.36 | 1.45 ± 0.38 | 1.44 ± 0.34 | 0.80 |
| Total/HDL cholesterol ratio | 3.80 ± 1.01 | 3.93 ± 1.02 | 3.93 ± 1.13 | 3.69 ± 1.01 | 0.11 |
| Blood glucose, mmol/l | 4.98 ± 0.94 | 4.98 ± 0.71 | 4.99 ± 0.66 | 4.76 ± 0.41 | 0.011 |
| Serum creatinine, µmol/l | 84.8 ± 15.9 | 83.9 ± 13.0 | 84.8 ± 13.9 | 84.2 ± 12.4 | 0.86 |
| γ-glutamyltransferase, units/l | 21 (16–32) | 22 (15–35) | 22 (15–31) | 22 (14–30) | 0.33 |
Quartile limits (in µg/m3) were: <12.306, 12.307–12.635, 12.636–13.438 and ≥13.439. Hypertension was a blood pressure of ≥140 mmHg systolic or ≥90 mmHg diastolic or use of antihypertensive drugs. Diabetes mellitus was a fasting plasma glucose level of ≥7.0 mmol/l or use of antidiabetic agents. Smoking was the current use of any smoking materials on a daily basis. Socioeconomic status was coded according to the UK Office of Population Censuses and Surveys33 and simplified into a linear scale with scores ranging from 1 to 3. For γ-glutamyltransferase, values are geometric mean (interquartile range). p values are for linear trend.
CVD: cardiovascular disease; HDL: high-density lipoprotein cholesterol
Ambient air pollution
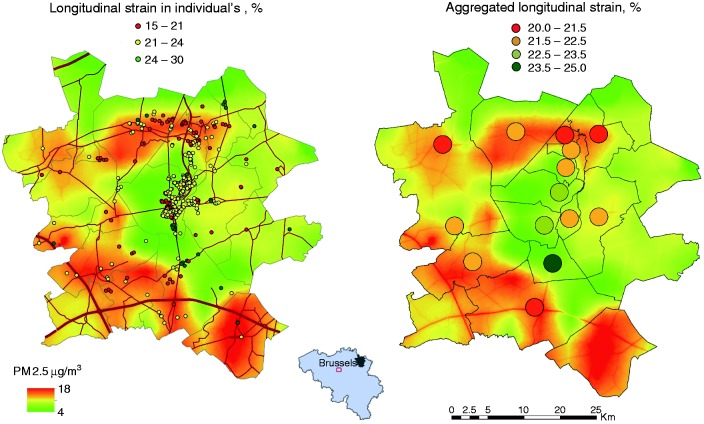
The median interval between echocardiography (2005–2009) and the midpoint assessment of long-term air pollution (30 June 2012) was 5.7 years (5th–95th percentile interval, 3.4–7.0 years). Of the 671 participants, 410 lived alone, while 218, 39 and 4 shared a home with one, two or more participants, respectively. The median long-term air pollution levels (5th–95th percentile interval), to which participants were exposed, amounted to 1.16 µg/m3 (0.98–1.55µg/m3) for black carbon, 12.6 µg/m3 (11.9–14.6 µg/m3) for PM2.5, 16.8 µg/m3 (14.8–22.1 µg/m3) for PM10, and 15.5 µg/m3 (13.2–23.8 µg/m3) for NO2. While accounting for clustering of study participants sharing a home, the levels of these four air pollutants were highly intercorrelated (0.77 ≤ r ≤ 0.94). Figure 1 shows the annual contours of PM2.5 distribution in 2012 in the catchment area of the study.
Figure 1.
Geographical association of multivariable-adjusted longitudinal LV strain at the individual level or aggregated per municipality were associated with PM2.5 air pollution contours. Grey lines indicate borders of municipalities. Red lines represent the air pollution contours of major roads. The Spearman rank correlation between longitudinal LV strain and exposure to PM2.5 was −0.13 (p = 0.0005) and −0.90 (p < 0.0001) in individual and aggregated data, respectively.
LV: left ventricular; PM: particulate matter
LV structure
Standardized to the average age in the whole study population (Table 2), LV mass index, end-diastolic diameter, relative wall thickness or prevalence of LV hypertrophy did not differ across quartiles of the PM2.5 exposure (p ≥ 0.092). Next (Table 3), we applied multivariable-adjusted linear regression, in which we analysed the air pollutants as continuous variables, while accounting for clustering of participants living at the same address and while adjusting for sex, age, mean arterial pressure, heart rate, body mass index, fasting plasma glucose, total-to-HDL cholesterol ratio, serum creatinine, γ-glutamyltransferase, smoking, antihypertensive treatment (by drug class) and socioeconomic status. Consistent with the categorical analyses (Table 2), regression showed no association of LV structure with the air pollutants (p ≥ 0.084; Table 3).
Table 2.
Age-standardized echocardiographic measurements by quartiles of averaged annual PM2.5 exposure
| Characteristic | Low | Medium, low | Medium, high | High | p value |
|---|---|---|---|---|---|
| All in category | 169 | 167 | 168 | 167 | |
| Left ventricular structure | |||||
| Mass index, g/m2 | 92.3 ± 19.3 | 91.1 ± 18.7 | 90.0 ± 18.8 | 90.4 ± 19.0 | 0.29 |
| Hypertrophy, n (%) | 33 (19.5) | 34 (20.4) | 26 (15.5) | 24 (14.4) | 0.092 |
| End-diastolic diameter, cm | 5.05 ± 0.46 | 4.99 ± 0.44 | 5.01 ± 0.47 | 5.01 ± 0.42 | 0.47 |
| Relative wall thickness | 0.37 ± 0.06 | 0.38 ± 0.06 | 0.37 ± 0.06 | 0.37 ± 0.05 | 0.93 |
| Systolic left ventricular function | |||||
| Ejection fraction, % | 70.4 ± 6.0 | 68.6 ± 6.7 | 68.5 ± 6.7 | 68.3 ± 7.2 | 0.0074 |
| Longitudinal strain, % | 22.6 ± 3.6 | 22.3 ± 3.3 | 22.4 ± 3.6 | 21.7 ± 3.5 | 0.020 |
| Longitudinal strain rate, s−1 | 1.34 ± 0.26 | 1.31 ± 0.23 | 1.27 ± 0.24 | 1.26 ± 0.26 | 0.0006 |
| Radial strain, % | 58.1 ± 11.3 | 58.0 ± 12.6 | 56.5 ± 11.1 | 57.1 ± 12.5 | 0.32 |
| Radial strain rate, s−1 | 3.35 ± 0.76 | 3.32 ± 0.83 | 3.29 ± 0.77 | 3.29 ± 0.82 | 0.46 |
| Diastolic left ventricular function | |||||
| Left atrial volume index, ml/m2 | 23.3 ± 5.3 | 22.6 ± 5.4 | 22.2 ± 5.7 | 21.9 ± 5.1 | 0.0094 |
| E peak, cm/s | 78.3 ± 14.8 | 77.0 ± 14.7 | 76.0 ± 13.8 | 73.5 ± 14.3 | 0.0017 |
| A peak, cm/s | 64.9 ± 13.0 | 64.6 ± 12.3 | 64.6 ± 12.2 | 63.6 ± 11.8 | 0.36 |
| E/A ratio | 1.32 ± 0.33 | 1.27 ± 0.28 | 1.28 ± 0.31 | 1.20 ± 0.30 | 0.036 |
| e′, cm/s | 11.8 ± 2.2 | 11.6 ± 2.2 | 11.7 ± 2 | 11.4 ± 2.2 | 0.12 |
| a′, cm/s | 10.2 ± 1.7 | 10.4 ± 1.7 | 10.0 ± 1.7 | 10.0 ± 1.62 | 0.049 |
| E/e′ ratio | 7.06 ± 1.78 | 7.02 ± 1.8 | 6.96 ± 1.59 | 6.84 ± 1.27 | 0.21 |
Quartile limits were: <12.306, 12.307–12.635, 12.636–13.438 and ≥13.439 µg/m3. Values are number of participants (%) or mean ± SD standardized to the average age in the whole study population (50.4 years). Left ventricular hypertrophy was left ventricular mass indexed to body surface area of at least 95 g/m2 in women or 115 g/m2 in men after adjusting for age. Due to image quality, radial strain and strain rate were available in only 610 participants. p values are for linear trend.
PM: particulate matter
Table 3.
Multivariable-adjusted associations of left ventricular structure with averaged annual exposure to air pollutants
| Characteristic | Pollutant (IQR) | Estimate (95% confidence interval) | p value |
|---|---|---|---|
| Left ventricular mass index g/m2 (n = 671) | Black carbon (+0.27 µg/m3) | −0.977 (−2.766 to 0.812) | 0.28 |
| PM2.5 (+1.13 µg/m3) | −1.094 (−2.658 to 0.469) | 0.17 | |
| PM10 (+3.35 µg/m3) | −1.504 (−3.351 to 0.342) | 0.11 | |
| NO2 (+ 4.00 ppm) | −1.234 (−2.634 to 0.166) | 0.084 | |
| End-diastolic diameter, cm (n=671) | Black carbon (+0.27 µg/m3) | −0.008 (−0.050 to 0.033) | 0.69 |
| PM2.5 (+1.13 µg/m3) | −0.010 (−0.046 to 0.027) | 0.61 | |
| PM10 (+3.35 µg/m3) | −0.013 (−0.056 to 0.030) | 0.55 | |
| NO2 (+ 4.00 ppm) | −0.011 (−0.044 to 0.021) | 0.49 | |
| Relative wall thickness (n = 671) | Black carbon (+0.27 µg/m3) | −0.0036 (−0.0098 to 0.0025) | 0.25 |
| PM2.5 (+1.13 µg/m3) | −0.0031 (−0.0085 to 0.0023) | 0.26 | |
| PM10 (+3.35 µg/m3) | −0.0043 (−0.0107 to 0.0021) | 0.19 | |
| NO2 (+ 4.00 ppm) | −0.0034 (−0.0082 to 0.0015) | 0.17 |
Associations accounted for clustering of data among participants living at the same address and were adjusted for sex, age, mean arterial pressure, heart rate, fasting plasma glucose, total-to-HDL cholesterol ratio, serum creatinine, γ-glutamyltransferase, smoking, antihypertensive treatment (by drug class), use of lipid-lowering drugs and socioeconomic status. End-diastolic diameter and relative wall thickness were additionally adjusted for body mass index. Effect sizes are for an IQR increase in the exposure to air pollutants.
IQR: interquartile range; PM: particulate matter; HDL: high-density lipoprotein
Systolic LV function
All participants had a LV ejection fraction over 45% (95% percentile range: 56.8–80.2%) and none had a history of heart failure. Across the PM2.5 quartiles (Table 2), age-standardized LV ejection fraction decreased from 70.4 to 68.3% (p = 0.007) and longitudinal strain and strain rate from 22.6 to 21.7% (p = 0.020) and from 1.34 to 1.26 s−1 (p = 0.0006), respectively. Next, in multivariable-adjusted analyses (Table 4), we expressed effect sizes for an interquartile increment in the air pollutants. In relation to black carbon, longitudinal strain and strain rate were 0.458% (p = 0.019) and 0.043 s−1 (p = 0.0016) lower. The corresponding estimates were 0.380% (p = 0.026) and 0.042 s−1 (p = 0.00052) for PM2.5, 0.454% (p = 0.024) and 0.049 s−1 (p = 0.00059) for PM10, and 0.339% (p = 0.027) and 0.033 s−1 (p = 0.0019) for NO2. Figure 1 shows that LV longitudinal strain segregated with the PM2.5 air pollution contours.
Table 4.
Multivariable-adjusted associations of systolic left ventricular function with averaged annual exposure to air pollutants.
| Characteristics | Pollutant (IQR increase) | Estimate (95% confidence interval) | p value |
|---|---|---|---|
| Ejection fraction, % (n = 671) | Black carbon (+0.27 µg/m3) | −0.403 (−1.148 to 0.342) | 0.29 |
| PM2.5 (+1.13 µg/m3) | −0.547 (−1.196 to 0.101) | 0.098 | |
| PM10 (+3.35 µg/m3) | −0.589 (−1.356 to 0.177) | 0.13 | |
| NO2 (+ 4.00 ppm) | −0.324 (−0.905 to 0.256) | 0.27 | |
| Longitudinal strain, % (n = 671) | Black carbon (+0.27 µg/m3) | −0.458 (−0.840 to −0.077) | 0.019 |
| PM2.5 (+1.13 µg/m3) | −0.380 (−0.714 to −0.045) | 0.026 | |
| PM10 (+3.35 µg/m3) | −0.454 (−0.849 to −0.059) | 0.024 | |
| NO2 (+ 4.00 ppm) | −0.339 (−0.638 to −0.039) | 0.027 | |
| Longitudinal strain rate, s−1 (n = 671) | Black carbon (+0.27 µg/m3) | −0.043 (−0.070 to −0.016) | 0.0016 |
| PM2.5 (+1.13 µg/m3) | −0.042 (−0.065 to −0.018) | 0.00052 | |
| PM10 (+3.35 µg/m3) | −0.049 (−0.076 to −0.021) | 0.00059 | |
| NO2 (+ 4.00 ppm) | −0.033 (−0.054 to −0.012) | 0.0019 | |
| Radial strain, % (n = 610) | Black carbon (+0.27 µg/m3) | −0.187 (−1.540 to 1.165) | 0.79 |
| PM2.5 (+1.13 µg/m3) | −0.392 (−1.558 to 0.775) | 0.51 | |
| PM10 (+3.35 µg/m3) | −0.022 (−1.411 to 1.367) | 0.98 | |
| NO2 (+ 4.00 ppm) | −0.57 (−1.616 to 0.476) | 0.29 | |
| Radial strain rate, s−1 (n = 610) | Black carbon (+0.27 µg/m3) | −0.032 (−0.123 to 0.059) | 0.49 |
| PM2.5 (+1.13 µg/m3) | −0.05 (−0.129 to 0.028) | 0.21 | |
| PM10 (+3.35 µg/m3) | −0.038 (−0.132 to 0.055) | 0.42 | |
| NO2 (+ 4.00 ppm) | −0.049 (−0.120 to 0.021) | 0.17 |
Associations accounted for clustering of data among participants living at the same address and were adjusted for sex, age, mean arterial pressure, heart rate, body mass index, fasting plasma glucose, total-to-HDL cholesterol ratio, serum creatinine, γ-glutamyltransferase, smoking, antihypertensive treatment (by drug class), use of lipid-lowering drugs and socioeconomic status. Effect sizes are for an IQR increase in the exposure to air pollutants. Due to image quality, radial strain and strain rate were available in only 610 participants.
IQR: interquartile range; PM: particulate matter; HDL: high-density lipoprotein
Diastolic LV function
Across the PM2.5 quartiles (Table 2), age-standardized transmitral E peak velocity decreased from 78.3 to 73.5 cm/s (p = 0.0017), E/A ratio from 1.32 to 1.20 (p = 0.036), mitral annular tissue velocity a′ peak from 10.2 to 10.0 cm/s (p = 0.049) and left atrial volume index from 23.3 to 21.9 ml/m2 (p = 0.0094). In adjusted continuous analyses (Table 5), mitral E and a′ peak velocities were 1.727 cm/s (p = 0.021) and 0.195 cm/s (p = 0.019) lower in relation to higher black carbon exposure. The corresponding estimates were 1.913 cm/s (p = 0.0036) and 0.175 cm/s (p = 0.016) for PM2.5 and 1.947 cm/s (p = 0.012) and 0.235 cm/s (p = 0.0063) for PM10, respectively. There was also an inverse association of the E peak velocity (−1.672 cm/s, p = 0.0045) and E/A (−0.026, p = 0.029) with NO2. The associations of transmitral A peak velocity with air pollutants did not reach significance except for PM10 (−1.257, p = 0.046). TDI e′ peak and E/e′ ratio were not significantly associated with air pollutants (p ≥ 0.094).
Table 5.
Multivariable-adjusted associations of diastolic left ventricular function with averaged annual exposure to air pollutants.
| Characteristics | Pollutant (IQR increase) | Estimate (95% confidence interval) | p value |
|---|---|---|---|
| Left atrial volume index, ml/m2 (n = 671) | Black carbon (+0.27 µg/m3) | −0.492 (−1.021 to −0.036) | 0.068 |
| PM2.5 (+1.13 µg/m3) | −0.369 (−0.855 to 0.116) | 0.14 | |
| PM10 (+3.35 µg/m3) | −0.561 (−1.133 to −0.011) | 0.055 | |
| NO2 (+4.00 ppm) | −0.040 (−0.475 to 0.396) | 0.86 | |
| E peak, cm/s (n = 671) | Black carbon (+0.27 µg/m3) | −1.727 (−3.197 to −0.257) | 0.021 |
| PM2.5 (+1.13 µg/m3) | −1.913 (−3.197 to −0.629) | 0.0036 | |
| PM10 (+3.35 µg/m3) | −1.947 (−3.465 to −0.428) | 0.012 | |
| NO2 (+ 4.00 ppm) | −1.672 (−2.823 to −0.521) | 0.0045 | |
| A peak, cm/s (n = 671) | Black carbon (+0.27 µg/m3) | −0.999 (−2.194 to 0.197) | 0.10 |
| PM2.5 (+1.13 µg/m3) | −0.812 (−1.86 to 0.235) | 0.13 | |
| PM10 (+3.35 µg/m3) | −1.257 (−2.493 to −0.022) | 0.046 | |
| NO2 (+ 4.00 ppm) | −0.639 (−1.578 to 0.299) | 0.18 | |
| E/A ratio (n = 671) | Black carbon (+0.27 µg/m3) | −0.028 (−0.058 to 0.002) | 0.066 |
| PM2.5 (+1.13 µg/m3) | −0.025 (−0.051 to 0.001) | 0.064 | |
| PM10 (+3.35 µg/m3) | −0.018 (−0.049 to 0.013) | 0.25 | |
| NO2 (+ 4.00 ppm) | −0.026 (−0.049 to −0.003) | 0.029 | |
| e′, cm/s (n = 671) | Black carbon (+0.27 µg/m3) | −0.173 (−0.376 to 0.029) | 0.094 |
| PM2.5 (+1.13 µg/m3) | −0.103 (−0.281 to 0.075) | 0.26 | |
| PM10 (+3.35 µg/m3) | −0.127 (−0.337 to 0.083) | 0.24 | |
| NO2 (+ 4.00 ppm) | −0.126 (−0.285 to 0.033) | 0.12 | |
| a′, cm/s (n = 671) | Black carbon (+0.27 µg/m3) | −0.195 (−0.358 to −0.032) | 0.019 |
| PM2.5 (+1.13 µg/m3) | −0.175 (−0.318 to −0.032) | 0.016 | |
| PM10 (+3.35 µg/m3) | −0.235 (−0.403 to −0.066) | 0.0063 | |
| NO2 (+ 4.00 ppm) | −0.083 (−0.211 to 0.046) | 0.21 | |
| E/e′ ratio (n = 671) | Black carbon (+0.27 µg/m3) | −0.048 (−0.21 to 0.115) | 0.56 |
| PM2.5 (+1.13 µg/m3) | −0.111 (−0.252 to 0.031) | 0.13 | |
| PM10 (+3.35 µg/m3) | −0.106 (−0.273 to 0.061) | 0.21 | |
| NO2 (+ 4.00 ppm) | −0.089 (−0.216 to 0.038) | 0.17 |
Associations accounted for clustering of data among participants living at the same address and were adjusted for sex, age, mean arterial pressure, heart rate, fasting plasma glucose, total-to-HDL cholesterol ratio, serum creatinine, γ-glutamyltransferase, smoking, antihypertensive treatment (by drug class), use of lipid-lowering drugs and socioeconomic status. The Doppler measurements were additionally adjusted for body mass index. Effect sizes are for an IQR increase in the exposure to air pollutants.
IQR: interquartile range; PM: particulate matter; HDL: high-density lipoprotein
Path analysis
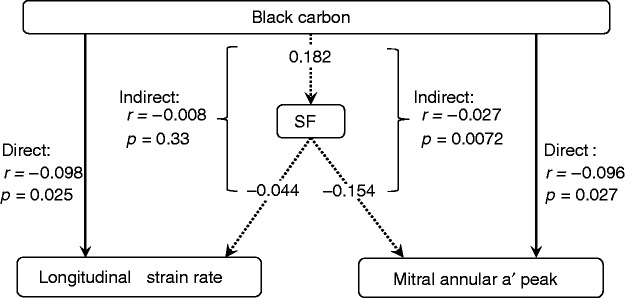
In 532 participants with available data, the serum levels of ICAM-1, VCAM-1, E-selectin and P-selectin averaged 241 ± 88 ng/ml, 512 ± 201 ng/ml, 16.5 ± 7.9 ng/ml and 123 ± 47 ng/ml, respectively. The loadings of these adhesion molecules onto the single factor derived by principal component analyses were 0.88 for ICAM-1, 0.77 for VCAM-1, 0.77 for E-selectin and 0.79 for P-selectin. The single factor explained 64.7% of the variance captured by the four adhesion molecules. In multivariable-adjusted analyses, the direct association of black carbon with longitudinal strain rate was −0.098 (p = 0.025) and −0.096 (p = 0.027) with mitral annular a′ velocity (Figure 2). The indirect associations mediated via the summary factor reflecting the adhesion molecules amounted to −0.008 (p = 0.33) and −0.027 (p = 0.0072), respectively. The other air pollutants were not associated with the adhesion molecules.
Figure 2.
Path analysis differentiated direct associations of black carbon with longitudinal strain rate and mitral annular a′ peak velocity from indirect associations with intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin and P-selectin combined by principal component analysis in a single normally distributed factor (SF). The loadings of these adhesion molecules onto the single factor derived by principal component analyses were 0.88 for ICAM-1, 0.77 for VCAM-1, 0.77 for E-selectin and 0.79 for P-selectin. The single factor explained 64.7% of the variance captured by the four adhesion molecules. Full and dotted lines indicate direct and indirect associations, respectively. Values are multivariable-adjusted correlation coefficients (r) with corresponding p-values. Indirect associations are obtained by multiplying the two correlation coefficients in the path.
Discussion
We aimed to investigate the association of echocardiographically assessed LV structure and function with air pollutants carried by particulate matter, including black carbon, PM2.5, PM10, and gaseous NO2. The key findings can be summarized as follows: (i) longitudinal strain and strain rate decreased with higher levels of all four air pollutants; (ii) the mitral E and a′ peak velocities were inversely associated with black carbon, PM2.5 and PM10; (iii) the geographic analysis confirmed the aforementioned associations; (iv) and the path analysis identified systemic inflammation as a possible mediator of the inverse association of a′ peak velocities with black carbon. On the other hand, our analyses did not reveal any statistically significant association of LV structure, LV ejection fraction, radial LV strain or strain rate with the air pollutants. The null results of LV ejection fraction may be explained as this measurement is not a precise index of systolic LV function,13 so that angulation-controlled TDI is the preferred approach, in particular when LV ejection fraction is normal. Associations of radial LV strain and strain rate with the air pollutants were directionally similar compared with longitudinal LV strain and strain rate, but did not reach statistical significance. The myocardial fibre architecture may explain the latter observation. Indeed, compared with the rotational motion predominantly related to the sub-epicardium and mid-wall, longitudinal LV shortening is mostly driven by the subendocardial wall, which is most vulnerable to noxious agents.41
The World Health Organization recommends that the annual mean levels of PM2.5, PM10 and NO2 be below 10, 20 and 40 µg/m3, respectively.42 The annual exposure thresholds for PM2.5 and PM10 are continuously under revision and currently amount to 25 and 40 µg/m3 in the European Union43 and to 12 and 50 µg/m3 in the United States.44 The clinical implications of our current observations should be gauged against these guidelines43,44 and the heart failure epidemic.1–3 Even at relatively low level of exposure, as observed in our present study, an interquartile interval increment in the air pollutants entailed a 2–3% decrease in longitudinal regional strain and strain rate and in the E and a′ peak velocities. Although apparently tiny when expressed as percentage of the population mean in our predominantly healthy study participants, the long-term effects of lifelong exposure might evoke a substantially worse perspective, certainly in the presence of co-morbidities and other heart failure risk factors.3 Moreover, patients with clinically overt heart failure are more vulnerable to the effects of air pollution, as exemplified by the high mortality associated with high ambient temperature, which increases the concentrations of free radicals in the air.45
Our study moves the field forward, because we analysed subject-level data from people representative of a general population and only exposed to low-level air pollution. Our approach overcomes the limitations of spatial epidemiologic studies using aggregated data, which are prone to ecological bias associated with attempting to deduce individual-level effects from group-level data.46 Few studies10–12 examined the association between LV function and air pollutants in asymptomatic people before the terminal stage of heart failure. Apart from a small case–control study in steel workers,9 our literature search revealed three population studies. In the Multi-Ethnic Study of Atherosclerosis,10 3827 participants, aged 45 to 84 years, underwent cardiac magnetic resonance imaging. In multivariable-adjusted analyses, people living within 50 m of a major roadway compared with those living more than 150 m away had a 1.4 g/m2 (95% confidence interval (CI), 0.3–2.5 g/m2) higher LV mass index with no difference in ejection fraction. Analysis of individual level data did not confirm the increase of LV mass index with higher exposure to PM2.5 (p = 0.45).10 In the Jackson Heart Study,11 4,866 African-Americans underwent echocardiography and had their exposure to air pollution assessed from the distance between their homes and major roadways, categorized as less than 150 m (n = 106), 150–299 m (n = 159), 300–999 m (n = 1,161) and over 1000 m (n = 3,440). In multivariable-adjusted analyses of these aggregate data, there were no residential differences in LV ejection fraction, E-wave velocity, isovolumetric relaxation time and left atrial diameter.11 The Study on the Influence of Air pollution on Lung function, Inflammation and Ageing12 included 264 women (mean age, 74.4 years). The annual median concentrations of air pollutants (2007–2010) were 17.4, 26.4, 25.9 and 37.7 µg/m3 for PM2.5, PM10, NO2 and nitrogen oxide (NOx), respectively.12 In fully adjusted models, left atrial volume index increased by 1.05 ml/m2 (95% CI, 0.99–1.09 ml/m2) and by 1.04 ml/m2 (95% CI, 1.00–1.09 ml/m2) with an interquartile increase in PM2.5 and NOx, respectively.12 As in our current study, E/e′ was not associated with any of the air pollutants.12
Risk factors are known to be associated with air pollution.23,47–49 However, few studies addressed the association of air pollution with heart failure, the terminal stage of a long evolving process with a wide range of aetiologies,2 focusing on acute instead of chronic exposure to air pollution.6–8 The heart failure diagnosis was made from hospital records or registries or death certificates, using aggregate air pollution data without differentiating between systolic and diastolic heart failure.7 A recently published meta-analysis7 reviewed 35 studies that reported associations between four million heart failure events and exposure up to and including lag day.7 Heart failure hospitalizations or deaths increased by 2.12% (95% CI, 1.42–2.82%) and 1.63% (95% CI, 1.20–2.07%) per 10 µg/m3 increments in PM2.5 and PM10, respectively.7 In Beijing, China, over one year, emergency admission for heart failure averaged three per day and the PM2.5 concentration was 102.1 mg/m3 (25th–75th percentile interval, 53.1–82.4ug/m3; range, 6.7–508.5 µg/m3).6 A 10 mg/m3 increment in PM2.5 was associated with a 0.14% (95% CI, 0.01–0.27%) increase in emergency room admissions for any cardiovascular events at lag day 3 and a 1.21% (95% CI, 0.27–2.15%) increase for heart failure at lag day 0.6 Among 6000 hospitalizations for acute cardiovascular syndromes (2004–2007) in Brescia, Italy,8 the hazard ratio for heart failure associated with a 10 µg/m3 increase in PM10 was 1.04 (95% CI, 1.01–1.08) at lag day 0, and 1.02 (95% CI, 0.98–1.06) at day 3. A British cohort study of 836,557 patients, aged 40 to 89 years and registered with 205 English general practices, related heart failure incidence from 2003 until 2007 (ICD10 code of I50) to air pollution data at a 1 km × 1 km resolution.4 The hazard ratios associated with an interquartile interval increment in PM10 (3.0 mg/m3) and NO2 (10.7 mg/m3) were both 1.06 (95% CI, 1.01–1.11).4
The molecular pathways underpinning our current findings remain to be elucidated. In experimental studies systolic50,51 and diastolic51 function decreased in response to PM2.5 exposure. Human studies suggested that circulating adhesion molecules VCAM-1,37,38 ICAM-1,36,38 E-selectin39 and P-selectin52 reflect a systemic inflammatory state and play a role in mediating the adverse cardiovascular effects of air pollution, subsequently leading to coronary heart disease36 or increased susceptibility to thrombotic complications.52 The evidence from these experimental50,51 and human36–39,52 studies justified our path analysis, focusing on circulating adhesion molecules, measured at the time of echocardiography. In our current study, the multivariable-adjusted direct and indirect associations of late diastolic mitral annular velocity (a′) with black carbon were both significant. The inverse associations of E, a′ and the left atrial volume index with air pollution might reflect coupling of the pulmonary circulation to the left atrium53 or direct left atrial toxicity rather than diastolic LV dysfunction. Indeed, inhalation of fine particles leads to endothelial dysfunction,54 reduced NO-dependent vasorelaxation55 and remodelling of the pulmonary arterioles,56 thereby increasing pulmonary vascular resistance and reducing pulmonary venous return to the left atrium. According to the atrial Frank–Starling mechanism,53 these haemodynamic changes in the pulmonary circulation might reduce atrial contractility. Moreover, the left atrium is the target organ first exposed to the toxins carrying nanoparticles that pass the blood–air barrier. Studies showing an increased risk of atrial arrhythmias57 and fibrillation58 in response to air pollution support the latter hypothesis.
Notwithstanding these strong points, our study should also be interpreted within the context of its possible limitations. First, our observational study does not allow inferring causality for the air pollutants as instigators of systolic LV dysfunction. However, two of the Bradford–Hill criteria are fulfilled, including a dose–effect association and plausibility.59 Second, as in previous studies of chronic air pollution,60,61 echocardiographic imaging and collection of the air pollution data was not done simultaneously. On the other hand, we used high-resolution spatial data. Several studies in the Netherlands,27 Italy (Rome),30 the UK28 and Canada (Vancouver)29 demonstrated that the land use models applied in our current study are representative for the air pollution for periods of 10 years or longer prior to the actual modelling.27–30,60 Third, we did not consider applying correction for multiple testing, because the echocardiographic variables (outcome measures) and the air pollution variables (exposure measures) were highly inter-correlated, so that each test did not introduce an independent opportunity for a type-I error.62 Moreover, correcting for multiple testing did not remove the significance of the association of regional longitudinal strain rate with the air pollutants. Finally, we cannot exclude exposure misclassification, for instance because middle-aged people would leave their homes to go to their workplace. Misclassification bias would decrease rather than inflate associations between the LV traits and air pollutants.
In conclusion, using state-of-the-art echocardiographic imaging, we demonstrated that in predominantly healthy people, representative for a general population, chronic low-level air pollution is associated with subclinical but measurable lessening of LV performance, which is a risk factor for heart failure. Satellite observations (2001–2006) at approximately 10 km × 10 km resolution showed excellent agreement with ground-based measurements and indicated a global population-weighted geometric mean PM2.5 concentration of 20.0 ± 6.7 µg/m3.63 The World Health Organization Air Quality PM2.5 Interim Target-1 (35 g/m3 annual average) is exceeded over central and eastern Asia for 38% and for 50% of the world’s population, respectively.63 Keeping in mind that exposure to polluted air, in contrast to smoking, is unintentional and adversely affects health in multiple ways, and that prevention is of paramount importance, global efforts to improve air quality should continue. In the United States alone, a mean reduction in PM2.5 of 3.9 µg/m3 might prevent close to 8000 heart failure hospitalizations and save 3300 million dollars per year.7 What our current study adds is that even low-level chronic exposure to air pollutants introduces early subclinical changes in LV performance in the general population, thereby reinforcing the need for more stringent clean air regulations, because at the subclinical stage, impairment of LV dysfunction is likely to be reversible.
Acknowledgement
The authors gratefully acknowledge the contribution of the nurses working at the examination centre (Linda Custers, Marie-Jeanne Jehoul, Daisy Thijs and Hanne Truyens) and the clerical staff at the Studies Coordinating Centre (Vera De Leebeeck and Renilde Wolfs).
Author contribution
Study concept and design: JAS, TSN and WYY. Acquisition of data: TK, TSN, BGJ, CV, WL, NC and JD. Analysis and interpretation of data: WYY, ZYZ, LT, EMB, BGJ, NC, FFW, AL, PV, EVH, TSN and JAS. Drafting the manuscript: WYY and JAS. Critical revision of the manuscript for intellectual content: all authors. Statistical analysis: WYY, LT and JAS. Obtaining funding: JAS, TSN and AL. Study supervision: JAS. All authors gave final approval and agreed to be accountable of all aspects of the work, ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The European Union (HEALTH-FP7-278249-EUMASCARA, HEALTH-F7-305507 HOMAGE and the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13, G.0880.13, and 11Z0916N) currently support the Studies Coordinating Centre in Leuven. The European Research Council (Starting Grant-2012 310898) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G.0880.13) support the environmental studies at the University of Hasselt. AL is recipient of a KU Leuven Program Financing Grant (PF/10/014).
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur Heart J 2011; 32: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson RW, Carey IW, Kent AJ, et al. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 2013; 24: 44–53. [DOI] [PubMed] [Google Scholar]
- 5.Carey IM, Anderson HR, Atkinson RW, et al. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup Environ Med 2015; 73: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Q, Wang S, Guo Y, et al. Acute exposure to fine particulate matter and cardiovascular hospital emergency room visits in Beijing, China. Environ Pollut 2017; 220: 317–327. [DOI] [PubMed] [Google Scholar]
- 7.Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013; 382: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaduganathan M, De Palma G, Manerba A, et al. Risk of cardiovascular hospitalizations from exposure to coarse Particulate Matter (PM10) below the European Union safety threshold. Am J Cardiol 2016; 117: 1231–1235. [DOI] [PubMed] [Google Scholar]
- 9.Golshahi J, Sadeghi M, Saqira M, et al. Exposure to occupational air pollution and cardiac function in workers of the Esfahan Steel Industry, Iran. Environ Sci Pollut Res Int 2016; 23: 11759–11765. [DOI] [PubMed] [Google Scholar]
- 10.Van Hee VC, Adar SD, Szpiro AA, et al. Exposure to traffic and left ventricular mass and function: The Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med 2009; 179: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver AM, Wellenius GA, Wu W-C, et al. Residential proximity to major roadways is not associated with cardiac function in African Americans: Results from the Jackson Heart Study. Int J Environ Res Public Health 2016; 13: 581–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohlwein S, Klümper C, Vossoughi M, et al. Air pollution and diastolic function in elderly women – Results from the SALIA study cohort. Int J Hyg Environ Health 2016; 219: 356–363. [DOI] [PubMed] [Google Scholar]
- 13.Tops L, Delgado V. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail 2017; 19: 307–313. [DOI] [PubMed] [Google Scholar]
- 14.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications. Endorsed by Japanese Society of Echocardardiography. J Am Soc Echocardiogr 2011; 24: 277–313. [DOI] [PubMed] [Google Scholar]
- 15.Staessen JA, Wang JG, Brand E, et al. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens 2001; 19: 1349–1358. [DOI] [PubMed] [Google Scholar]
- 16.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. on behalf of the European Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension and blood pressure changes in relation to urinary sodium excretion in White Europeans. JAMA 2011; 305: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association. Declaration of Helsinki. JAMA 2013; 227: 184–189. [Google Scholar]
- 18.Gottdiener JS, Bednarz J, Devereux R, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. A report from the American Society of Echocardiography’s Guidelines and Standard Committee and the Task Force on Echocardiography in Clinical Trials. J Am Soc Echocardiogr 2004; 17: 1086–1119. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsova T, Codd V, Brouilette S, et al. Association between left ventricular mass and telomere length in a population study. Am J Epidemiol 2010; 172: 440–450. [DOI] [PubMed] [Google Scholar]
- 20.Kuznetsova T, Herbots L, López B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail 2009; 2: 105–112. [DOI] [PubMed] [Google Scholar]
- 21.Kuznetsova T, Herbots L, Richart T, et al. Left ventricular strain and strain rate in a general population. Eur Heart J 2008; 29: 2014–2023. [DOI] [PubMed] [Google Scholar]
- 22.Yang WY, Zhang ZY, Thijs L, et al. Left ventricular structure and function in relation to environmental exposure to lead and cadmium. J Am Heart Assoc 2017; 6: e004692–e004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brook RD, Rajagopalan S, Pope CA, et al. on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010; 121: 2331–2378. [DOI] [PubMed] [Google Scholar]
- 24.Janssen S, Dumont G, Fierens F, et al. Spatial interpolation of air pollution measurements using CORINE land cover data. Atmos Environ 2008; 42: 4884–4903. [Google Scholar]
- 25.Lefebvre W, Degrawe B, Beckx C, et al. Presentation and evaluation of an integrated model chain to respond to traffic- and health-related policy questions. Environ Model Softw 2013; 40: 160–170. [Google Scholar]
- 26.Cesaroni G, Porta D, Badaloni C, et al. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health 2012; 11: 48–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brauer M, Hoek G, van Vliet P, et al. Estimating long-term average particulate concentrations: Application of traffic indicators and geographic information systems. Epidemiology 2003; 14: 228–239. [DOI] [PubMed] [Google Scholar]
- 28.Briggs D, de Hoogh C, Gullliver J, et al. A regression-based method for mapping traffic-related air pollution: Application and testing in four contrasting urban environments. Sci Total Environ 2000; 253: 151–167. [DOI] [PubMed] [Google Scholar]
- 29.Henderson SB, Beckerman B, Jerrett M, et al. Application of land use regression to estimate long-term of concentrations of traffic-related nitrogen oxides and fine particulate matter. Environ Sci Technol 2007; 41: 2422–2428. [DOI] [PubMed] [Google Scholar]
- 30.Rosenlund M, Forastiere F, Stafoggia M, et al. Comparison of regression models with land-use and emissions data to predict the spatial distribution of traffic-related air pollution in Rome. J Expo Sci Environ Epidemiol 2008; 18: 192–199. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre W, Vercauteren J, Schrooten L, et al. Validation of the MIMOSA-AURORA-IFDM model chain for policy support: Modeling concentrations of elemental carbon in Flanders. Atmos Environ 2011; 45: 6705–6713. [Google Scholar]
- 32.Maiheu A, Veldeman N, Viaene P, et al. Bepaling van de best beschikbare grootschalige concentratiekaarten luchtkwaliteit voor België, http://www.milieurapport.be/Upload/main/0_onderzoeksrapporten/2013/Eindrapport_Concentratiekaarten_29_01_2013_TW.pdf (2012, accessed 19 May 2017).
- 33.Office of Population Censuses and Surveys. Classification of occupations and coding index. London: Government Statistical Service, 1980.
- 34.Nawrot T, Plusquin M, Hogervorst J, et al. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol 2006; 7: 119–126. [DOI] [PubMed] [Google Scholar]
- 35.Masiha S, Sundström J, Lind L. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens 2013; 27: 13–17. [DOI] [PubMed] [Google Scholar]
- 36.Rückerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med 2006; 173: 432–441. [DOI] [PubMed] [Google Scholar]
- 37.Alexeeff SE, Coull BA, Gryparis A, et al. Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect 2011; 119: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bind MA, Baccarelli A, Zanobetti A, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: Associations and epigene-environment interactions in an elderly cohort. Epidemiology 2012; 23: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajat A, Allison M, Diez-Roux AV, et al. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: A repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA). Epidemiology 2015; 26: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jolliffe IT. Principal component analysis. 2nd ed. New York: Springer, 2002.
- 41.Sun JP, Lam Y-Y, Wu C-Q, et al. Effect of age and gender on left ventricular rotation and twist in a large group of normal adults – A multicenter study. Int J Cardiol 2013; 167: 2215–2221. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Global update 2005. Summary of risk assessment, Geneva, Switzerland: World Health Organization, 2006. [Google Scholar]
- 43.European Environment Agency. Air quality in Europe – 2016 report, Luxembourg: European Environment Agency, 2016. [Google Scholar]
- 44.Office of Air Quality Planning and Standards, Health and Environmental Impacts Division, Environmental Protection Agency. Integrated review plan for the national ambient air quality standards for particulate matter. Research Triangle Park, NC: United States Environmental Protection Agency, 2016.
- 45.Nawrot TS, Staessen JA, Fagard RH, et al. Endothelial function and outdoor temperature. Eur J Epidemiol 2005; 20: 407–410. [DOI] [PubMed] [Google Scholar]
- 46.Jarup L, Best N. Editorial comment on geographical differences in cancer incidence in the Belgian province of Limburg by Bruntinx and colleagues. Eur J Cancer 2003; 39: 1973–1975. [DOI] [PubMed] [Google Scholar]
- 47.Kubesch N, De Nazelle A, Guerra S, et al. Arterial blood pressure responses to short-term exposure to low and high traffic-realted air pollution with and without moderate physical activity. Eur J Prev Cardiol 2015; 22: 548–557. [DOI] [PubMed] [Google Scholar]
- 48.Akintoye E, Shi L, Obaitan I, et al. Association between fine particulate matter exposure and subsclinical atherosclerosis: A meta-analysis. Eur J Prev Cardiol 2016; 23: 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li G, Zeng Q, Pan X. Disease burden of ischaemic heart disease fron short-term outdoor air pollution exposure in Tianjin, 2002–2006. Eur J Prev Cardiol 2016; 23: 1774–1782. [DOI] [PubMed] [Google Scholar]
- 50.Gorr MW, Velten M, Nelin TD, et al. Early life exposure to air pollution induces adult cardiac dysfunction. Am J Physiol Heart Circ Physiol 2014; 307: H1353–H1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wold LE, Ying Z, Hutchinson KR, et al. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ Heart Fail 2012; 5: 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wauters A, Esmaeilzadeh F, Bladt S, et al. Pro-thrombotic effect of exercise in a polluted environment: A P-selectin- and CD63-related platelet activation effect. Thromb Haemost 2015; 113: 118–124. [DOI] [PubMed] [Google Scholar]
- 53.Anwar AM, Geleijnse ML, Soliman OII, et al. Left atrial Frank–Starling law assessed by real-time, three-dimensional echocardiographic left atrial function changes. Heart 2007; 93: 1393–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davel AP, Lemos M, Pastro LM, et al. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 2012; 295: 39–46. [DOI] [PubMed] [Google Scholar]
- 55.Courtois A, Andujar P, Ladeiro Y, et al. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: Comparison of urban particulate matter and manufactured nanoparticles. Environ Health Perspect 2008; 116: 1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grunig G, Marsh LM, Esmaeil N, et al. Perspective: Ambient air pollution: Inflammatory response and effects on the lung’s vasculature. Pulm Circ 2014; 4: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao Q, Liu T, Korantzopoulos P, et al. Association between air pollution and development of atrial fibrillation: A meta-analysis of observational studies. Heart Lung 2016; 45: 557–562. [DOI] [PubMed] [Google Scholar]
- 58.Link MS, Luttmann-Gibson H, Schwartz J, et al. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol 2013; 62: 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill AB. The environment and disease: Association or causation? Proc R Soc Med 1965; 58: 295–300. [PMC free article] [PubMed] [Google Scholar]
- 60.Vrijens K, Winckelmans E, Tsamou M, et al. Sex-specific associations between particulate matter exposure and gene expression in independent discovery and validation cohorts of middle-aged men and women. Environ Health Perspect 2017; 125: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruttens D, Verleden SE, Bijnens EM, et al. An association of particulate air pollution and traffic exposure with mortality after lung transplantation in Europe. Eur Respir J 2017; 49: 1600484–1600484. [DOI] [PubMed] [Google Scholar]
- 62.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1: 43–46. [PubMed] [Google Scholar]
- 63.Van Donkelaar A, Martin RV, Brauer M, et al. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: Development and application. Environ Health Perspect 2010; 118: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]