Abstract
Background
We compared the diagnostic performances of two newly introduced fully automated multiple allergen simultaneous tests (MAST) analyzers with two conventional MAST assays.
Methods
The serum samples from a total of 53 and 104 patients were tested for food panels and inhalant panels, respectively, in four analyzers including AdvanSure AlloScreen (LG Life Science, Korea), AdvanSure Allostation Smart II (LG Life Science), PROTIA Allergy-Q (ProteomeTech, Korea), and RIDA Allergy Screen (R-Biopharm, Germany). We compared not only the total agreement percentages but also positive propensities among four analyzers.
Results
Evaluation of AdvanSure Allostation Smart II as upgraded version of AdvanSure AlloScreen revealed good concordance with total agreement percentages of 93.0% and 92.2% in food and inhalant panel, respectively. Comparisons of AdvanSure Allostation Smart II or PROTIA Allergy-Q with RIDA Allergy Screen also showed good concordance performance with positive propensities of two new analyzers for common allergens (Dermatophagoides farina and Dermatophagoides pteronyssinus). The changes of cut-off level resulted in various total agreement percentage fluctuations among allergens by different analyzers, although current cut-off level of class 2 appeared to be generally suitable.
Conclusions
AdvanSure Allostation Smart II and PROTIA Allergy-Q presented favorable agreement performances with RIDA Allergy Screen, although positive propensities were noticed in common allergens.
Keywords: Multiple allergen simultaneous test, Automated analyzer
Highlights
-
•
Two new multiple allergen simultaneous tests (MAST) analyzers are evaluated.
-
•
AdvanSure Smart II and PROTIA presented favorable agreement performance with RIDA.
-
•
Positive propensity of new assays for D. farina and D. pteronyssinus was noticed.
-
•
The changes of cut-off level resulted in total agreement percentage fluctuations.
1. Introduction
The detection of allergen-specific IgE, along with the patient’s chief complaints and medical history, is diagnostically valuable for allergic diseases, such as allergic rhinitis, atopic dermatitis, and asthma [1], [2]. Although in vivo skin test has been traditionally used in the clinical environments, there are several limitations of in vivo skin test including error-prone results in patients with anti-histamine medication or skin diseases such as dermographism, possibility of subjective interpretation, and the lack of standardization for protocols [3], [4]. Therefore, in vitro allergen-specific IgE measurements have been developed using various principles of radioimmunoassay, enzyme immunoassay, fluorescent enzyme immunoassay, immunoblot, and chemiluminescent assay [5], [6], [7].
Among the commercially available in vitro allergy tests, multiple allergen simultaneous tests (MAST) have been continuously developed with the improvements in smaller amounts of serum consumption, shorter turnaround time, and wider spectrum of allergens included in the test [6], [8], [9], [10], [11], [12]. Since the difference in prevalence of allergic diseases according to age, sex, and ethnicity is prominent, the selection of multiple allergen screening panels should be modified in the context of geographical regions and race of the target populations [13], [14], [15]. At the same time, the change of environmental substances in modern society must be considered for the progressive development of MAST assays [16].
Moreover, there is no appropriate medical evidence to define any assay as the standardized reference method due to variability of allergen original materials, extraction methods, attachment processes, and detection techniques [5]. Therefore, it is very difficult to analyze true sensitivity, specificity, positive predictive value, and negative predictive value of a specific MAST assay. Nevertheless, actual comparison of new MAST assay with currently used MAST assays can appropriately provide important information in the practical clinical settings.
Recently, two fully automated analyzers with high-throughput were developed and introduced in the market; AdvanSure Allostation Smart II which is the upgraded version of previous AdvanSure AlloScreen by LG Life Science, and PROTIA Allergy-Q which was newly developed by ProteomeTech. Herein, we compared the diagnostic performances of these assays with two most commonly used MAST assays in Korea, today. In addition, we evaluated propensity of each assay to give positive results for certain allergen, which we defined as “positive propensity”.
2. Methods
2.1. Study participants
We randomly selected the study samples from MAST assay requested serum samples of patients who visited Severance Hospital with symptoms of allergy including urticaria, sneezing, and itching for diagnosis of allergic disease in all age ranges. Additionally, we excluded patients with chronic comorbid diseases such as autoimmunity, malignancy, chronic infection, and other immune-related diseases. Since two different panels were evaluated, we classified patients into two groups so that appropriate panel could be tested based on clinical symptoms and medical records. Due to the variety of allergen types included in the panel of four assays and lack of sufficient sample volume in some patients, different samples were analyzed by different numbers of analyzers with various combinations of allergens. Therefore, only pairs of matched allergens by the same sample were compared among four analyzers.
2.2. In vitro allergen-specific IgE measurements
Serum aliquots were tested by four different systems; AdvanSure AlloScreen (LG Life Science, Seoul, Korea), AdvanSure Allostation Smart II (LG Life Science, Seoul, Korea), PROTIA Allergy-Q (ProteomeTech, Seoul, Korea), and RIDA Allergy Screen (R-Biopharm, Darmstadt, Germany). All the test procedures were performed following the manufacture’s instruction. Although detection ranges were various among four analyzers, results were identically classified into 7 levels and were interpreted as class 0–6 in all analyzers (Table 1).
Table 1.
Specifications of four different MAST analyzers.
| AdvanSure AlloScreen | AdvanSure Allostation Smart II | PROTIA Allergy-Q | RIDA Allergy Screen | |
|---|---|---|---|---|
| Manufacturer | LG Life Science (Korea) | LG Life Science (Korea) | ProteomeTech (Korea) | R-Biopharm (Germany) |
| Instrument | AdvanSure™ Allostation | AdvanSure™ Allostation Smart II | Q-station | AlleRoboT |
| Reagent | AdvanSure™ Alloscreen | AdvanSure™ Alloscreen | PROTIA™ Allergy-Q | AlleisaScreen® |
| Principle | Immunoblot | Immunoblot | Immunoblot | Immunoblot |
| Class stratification | Class 0–6 | Class 0–6 | Class 0–6 | Class 0–6 |
| Degree of automation | Semi automation | Full automation | Full automation | Full automation |
| Number of antigens | ||||
| -Total (common) | 60 (20) | 90 (30) | 70 (18) | 80 (40) |
| -Food panel | 40 | 60 | 44 | 60 |
| -Inhalant panel | 40 | 60 | 44 | 60 |
| Minimal sample volume (μl) | 100 | 250 | 120 | 800 |
| Tested sample volume (μl) | 100 | 100 | 50 | 300 |
| Number of strips utilized | 2 | 2 | 1 | 2 |
| Capacity or number of tests per run | 24 | 30 | 48 | 36 |
| Analysis time (hr) | 3.5 | 4.0 | 4.0 | 3.8 |
| Analysis time per sample (min) | 8.75 | 8 | 5 | 6.3 |
2.3. Inter-method comparison of four analyzers
We compared a pair of analyzers each time in order to maximize the comparison efficiency because different allergen lists are available by four analyzers. Furthermore, we focused on comparison of two specific analyzers (AdvanSure AlloScreen versus AdvanSure Allostation Smart II), because AdvanSure Allostation Smart II is the upgraded version of AdvanSure AlloScreen, both of which are developed by the same manufacturer (LG Life Science). Afterwards, we compared two newly introduced analyzers (i.e. AdvanSure Allostation Smart II and PROTIA Allergy-Q) with currently widely utilized assay (RIDA Allergy Screen) as reference values.
2.4. Comparison among different cut-off levels for positive interpretation
No standardized specific cut-off level for positive result is defined worldwide until today [17]. Moreover, previous studies which compared various MAST assays utilized different cutoff levels. For instance, several studies used class 1 as the cutoff level for positive results [8], [11], [12], whereas class 2 was adopted as the cutoff level for positive results in other studies [6], [10], [32]. Considering the natural characteristics of semi-quantitative results in MAST assays, comparison of different cut-off levels in the paired results might provide clinical clues for more precise diagnostic interpretation. Therefore, we applied cut-off levels of class 1, class 2, and class 3 as minimal requirement for positive results for all comparison analyses.
2.5. Statistical analysis
We analyzed the concordance degree by calculating total agreement percentage following the same methodology used in a previous study [18]; total agreement percentage=(total number of results−number of discrepancies)×100/total number of results. Additionally, concordant positive rates were calculated with the proportions of agreement for positive responses because low frequency of positive results can affect the total agreement percentage. Furthermore, agreement of detection results between two analyzers was determined by Cohen's kappa analysis [19]. Finally, the presence of propensity toward positive results in specific assay for certain allergen was determined when the difference between discrepant results accounted for over 10% of all pairs. For example, when assay A and assay B are compared for allergen C, [(number of samples with A positive, B negative result)−(number of samples with A negative, B positive result)]×100/ total number of results ≥10% can be interpreted as the positive propensity of assay A for allergen C compared to assay B.
For all statistical analyses, we used MedCalc 11.0 (MedCalc Software, Belgium) and SPSS 18.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Characteristics of study participants and paired sets
The serum samples from a total of 53 and 104 patients were tested for food panel and inhalant panel in this study. Characteristics of study participants are summarized in Table 2. Although several patients presented multiple allergic symptoms, urticaria was the most common clinical feature for participants in food panel while allergic rhinitis was the most frequent clinical symptoms for participants in inhalant panel. As mentioned earlier, different numbers of matched pairs were compared in each comparison analysis among four analyzers.
Table 2.
Characteristics of study participants.
| Food panel | Inhalant panel | |
|---|---|---|
| Total number | 53 | 104 |
| Demographic characteristics | ||
| Number of male (%) | 21 (39.6) | 56 (53.8) |
| Age, median [1Q, 3Q] | 32 [9,55] | 35 [20.8, 56.5] |
| Age, range | 1–85 | 5–78 |
| Number of pediatric patients (%)a | 16 (30.2) | 24 (23.1) |
| Clinical symptoms and signs | ||
| Urticaria (%) | 32 (60.4) | 3 (2.9) |
| Dermatitis (%) | 14 (26.4) | 1 (1.0) |
| Allergic rhinitis (%) | 5 (9.4) | 87 (83.7) |
| Allergic bronchitis (%) | 1 (1.9) | 4 (3.8) |
| Asthma (%) | 2 (3.8) | 5 (4.8) |
| Anaphylaxis or angioedema (%) | 6 (11.3) | 1 (1.0) |
| Others (%) | 8 (15.1)b | 4 (3.8)c |
| Matched pairs in each comparison | ||
| AlloScreen vs. Allostation Smart II | 43 | 90 |
| Allostation Smart II vs. RIDA | 30 | 79 |
| Allergy-Q vs. RIDA | 40 | 93 |
Pediatric patients are defined as individuals with age <20 years.
Others include xerotic eczema, erythema multiforme, drug eruption, and insect bite.
Others include adenoid vegetation, chronic sinusitis, and sleep apnea.
3.2. Comparison between AdvanSure AlloScreen and AdvanSure Allostation Smart II for evaluation of upgrade
When we compared qualitative results between AdvanSure AlloScreen and AdvanSure Allostation Smart II, we used class 2 as the cut-off level for positive result since the manufacturer suggested the possibility of class 1 result indicating insufficient clinical significance to trigger allergic progression. A total of 43 and 90 paired serum samples were tested for 39 and 41 allergens in food and inhalant panel, respectively (Table 3). All allergens showed total agreement percentages over 93.0% and 92.2% in food and inhalant panel, respectively, which indicates good concordance between old and new versions of AdvanSure assays. However, 6 allergens (Candida albicans, cheddar cheese, chicken, Cladosporium herbarum, pork, yeast) in food panel and 4 allergens (dog, egg white, mackerel, soy bean) in inhalant panel showed no concordant positive result, possibly due to rare frequency of specific IgE antibodies to these allergens among Koreans and restricted number of paired samples in this study. On the contrary, two most common allergens in both food and inhalant panels which were Dermatophagoides pteronyssinus and Dermatophagoides farina showed high total agreement percentages of over 95.0% and high agreement levels with kappa indices over 0.9. However, total agreement percentage and kappa index decreased to 93.0% and 0.8, respectively, for house dust, which was the third most common allergen.
Table 3.
Comparison between AdvanSure AlloScreen and upgraded AdvanSure Allostation Smart II using cutoff value of class 2.
| Allergen | Food panel | Inhalant panel | ||||||
|---|---|---|---|---|---|---|---|---|
|
N=43 |
N=90 |
|||||||
| Agreement (%) | Kappa index | Kappa index (95% CI) | Concordant positive rate (%) | Agreement (%) | Kappa index | Kappa index (95% CI) | Concordant positive rate (%) | |
| Acacia | 100.0 | 1.00 | (1.00 to 1.00) | 1.1 | ||||
| Alternaria alternata | 100.0 | 1.00 | (1.00 to 1.00) | 11.6 | 100.0 | 1.00 | (1.00 to 1.00) | 3.3 |
| Ash mix | 100.0 | 1.00 | (1.00 to 1.00) | 1.1 | ||||
| Aspergillus fumigatus | 100.0 | 1.00 | (1.00 to 1.00) | 1.1 | ||||
| Bermuda grass | 97.8 | 0.74 | (0.39 to 1.08) | 3.3 | ||||
| Barley meal | 97.7 | 0.66 | (0.03 to 1.28) | 2.3 | ||||
| Beef | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Birch-Alder mix | 97.7 | 0.79 | (0.39 to 1.19) | 4.7 | 96.7 | 0.38 | (−0.17 to 0.93) | 1.1 |
| Buck-wheat | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Candida albicans | 100.0 | NA | 0.0 | |||||
| Cat | 95.3 | 0.64 | (0.18 to 1.10) | 4.7 | 92.2 | 0.50 | (0.18 to 0.81) | 4.4 |
| Cheddar cheese | 100.0 | NA | 0.0 | |||||
| Chicken | 100.0 | NA | 0.0 | |||||
| Citrus mix | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Cladosporium herbarum | 97.7 | 0.00 | (0.00 to 0.00) | 0.0 | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 |
| Cockroach | 97.7 | 0.79 | (0.39 to 1.19) | 4.7 | 97.8 | 0.74 | (0.39 to 1.09) | 3.3 |
| Codfish | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Crab | 93.0 | 0.54 | (0.10 to 0.99) | 4.7 | 96.7 | 0.39 | (−0.15 to 0.93) | 1.1 |
| D. farinae | 95.3 | 0.91 | (0.78 to 1.03) | 48.8 | 100.0 | 1.00 | (1.00 to 1.00) | 35.6 |
| D. pteronyssinus | 100.0 | 1.00 | (1.00 to 1.00) | 51.2 | 95.6 | 0.91 | (0.81 to 1.00) | 35.6 |
| Dandelion | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 | ||||
| Dog | 93.0 | 0.38 | (−0.16 to 0.91) | 2.3 | 97.8 | −0.01 | (−0.03 to 0.00) | 0.0 |
| Egg white | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | 100.0 | NA | 0.0 | |
| Garlic | 97.7 | 0.79 | (0.39 to 1.19) | 4.7 | ||||
| Goldenrod | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 | ||||
| Hazelnut | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 | ||||
| House dust | 93.0 | 0.82 | (0.63 to 1.01) | 23.3 | 93.3 | 0.84 | (0.72 to 0.96) | 26.7 |
| Japanese cedar | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 | ||||
| Japanese Hop | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 |
| Mackerel | 97.7 | 0.66 | (0.03 to 1.28) | 2.3 | 100.0 | NA | 0.0 | |
| Milk | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | 96.7 | 0.81 | (0.59 to 1.02) | 7.8 |
| Mugwort | 95.3 | 0.48 | (−0.14 to 1.10) | 2.3 | 100.0 | 1.00 | (1.00 to 1.00) | 1.1 |
| Oak white | 97.7 | 0.66 | (0.03 to 1.28) | 2.3 | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 |
| Onion | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Orchard grass | 96.7 | 0.56 | (0.12 to 1.00) | 2.2 | ||||
| Oxeye daisy | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 | ||||
| Peach | 97.7 | 0.66 | (0.03 to 1.28) | 2.3 | 97.8 | 0.49 | (−0.11 to 1.09) | 1.1 |
| Peanut | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Penicillium notatum | 100.0 | 1.00 | (1.00 to 1.00) | 2.2 | ||||
| Pigweed | 100.0 | 1.00 | (1.00 to 1.00) | 2.2 | ||||
| Pine | 100.0 | 1.00 | (1.00 to 1.00) | 1.1 | ||||
| Poplar mix | 100.0 | 1.00 | (1.00 to 1.00) | 1.1 | ||||
| Pork | 95.3 | −0.02 | (−0.06 to 0.01) | 0.0 | ||||
| Ragweed | 97.7 | 0.79 | (0.39 to 1.19) | 4.7 | 94.4 | 0.26 | (−0.18 to 0.70) | 1.1 |
| Reed | 97.8 | 0.79 | (0.51 to 1.07) | 4.4 | ||||
| Rice | 97.7 | 0.66 | (0.03 to 1.28) | 2.3 | ||||
| Russian thistle | 96.7 | 0.65 | (0.29 to 1.01) | 3.3 | ||||
| Rye pollens | 97.7 | 0.79 | (0.39 to 1.19) | 4.7 | 95.6 | 0.65 | (0.33 to 0.96) | 4.4 |
| Salmon | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Sallow willow | 94.4 | 0.27 | (−0.15 to 0.70) | 1.1 | ||||
| Shrimp | 93.0 | 0.54 | (0.10 to 0.99) | 4.7 | 95.6 | 0.32 | (−0.16 to 0.80) | 1.1 |
| Soy bean | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | 98.9 | 0.00 | (0.00 to 0.00) | 0.0 |
| Sweet vernal grass | 97.8 | 0.82 | (0.58 to 1.06) | 5.6 | ||||
| Sycamore mix | 98.9 | 0.66 | (0.04 to 1.28) | 1.1 | ||||
| Timothy grass | 97.8 | 0.79 | (0.51 to 1.07) | 4.4 | ||||
| Tomato | 100.0 | 1.00 | (1.00 to 1.00) | 2.3 | ||||
| Tuna | 100.0 | NA | 0.0 | |||||
| Wheat flour | 97.7 | 0.79 | (0.39 to 1.19) | 4.7 | ||||
| Yeast, bakers | 100.0 | NA | 0.0 | |||||
NA: Not available.
3.3. Comparison of AdvanSure Allostation Smart II or PROTIA Allergy-Q with RIDA Allergy Screen applying cut-off level of class 2
We evaluated concordance rate of two newly developed fully automated assays (i.e. AdvanSure Allostation Smart II and PROTIA Allergy-Q) with results by RIDA Allergy Screen considered as the reference values in this study utilizing class 2 for the cut-off level for positive result (Table 4). Total agreement percentages were over 90.0% in most allergens in both assays for food and inhalant panels. However, allergens with the most frequent positive results (i.e. D. farina, D. pteronyssinus, house dust, and storage mite) presented concordance rates ranging from 69.6% to 90.0% for both AdvanSure Allostation Smart II and PROTIA Allergy-Q in food panel as well as inhalant panel.
Table 4.
Comparison of two new fully automated analyzers with RIDA Allergy Screen as a reference using cutoff level of class 2.
| Allergen | AdvanSure Smart II vs. RIDA |
PROTIA Allergy-Q vs. RIDA |
||||||
|---|---|---|---|---|---|---|---|---|
| Food panel | Inhalant panel | Food panel | Inhalant panel | |||||
|
N=30 |
N=79 |
N=40 |
N=93 |
|||||
| Agreement (%) | Concordant positive rate (%) | Agreement (%) | Concordant positive rate (%) | Agreement (%) | Concordant positive rate (%) | Agreement (%) | Concordant positive rate (%) | |
| Acacia | 100.0 | 1.3 | 97.8 | 0.0 | ||||
| Alternaria alternata | 100.0 | 16.7 | 93.7 | 1.3 | 100.0 | 12.5 | 93.5 | 0.0 |
| Anchovy | 100.0 | 0.0 | ||||||
| Ash mix | 100.0 | 1.3 | 96.8 | 0.0 | ||||
| Aspergillus fumigatus | 93.3 | 0.0 | 97.5 | 0.0 | 97.8 | 0.0 | ||
| Banana | 100.0 | 0.0 | ||||||
| Barley meal | 96.7 | 0.0 | 95.0 | 0.0 | ||||
| Beef | 96.7 | 0.0 | 97.5 | 0.0 | ||||
| Bermuda grass | 98.7 | 1.3 | 96.8 | 1.1 | ||||
| Birch-Alder mix | 96.7 | 3.3 | 96.2 | 0.0 | 97.5 | 0.0 | 92.5 | 2.2 |
| Bromelain (CCD) | 93.3 | 0.0 | 92.4 | 1.3 | ||||
| Buck-wheat | 100.0 | 0.0 | 95.0 | 0.0 | ||||
| Candida albicans | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Cat | 96.7 | 0.0 | 88.6 | 2.5 | 97.5 | 0.0 | 93.5 | 2.2 |
| Cheddar cheese | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Chestnut | 96.7 | 0.0 | 100.0 | 0.0 | ||||
| Chicken | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Citrus mix | 100.0 | 0.0 | 97.5 | 0.0 | ||||
| Cladosporium herbarum | 100.0 | 0.0 | 96.2 | 0.0 | 97.8 | 0.0 | ||
| Clam | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Cockroach | 96.7 | 3.3 | 97.5 | 2.5 | 92.5 | 5.0 | 95.7 | 2.2 |
| Codfish | 96.7 | 0.0 | 100.0 | 0.0 | ||||
| Crab | 93.3 | 0.0 | 100.0 | 0.0 | 85.0 | 0.0 | 94.6 | 0.0 |
| Cucumber | 100.0 | 0.0 | ||||||
| D. farinae | 76.7 | 26.7 | 77.2 | 12.7 | 76.7 | 30.0 | 82.8 | 11.8 |
| D. pteronyssinus | 80.0 | 26.7 | 75.9 | 13.9 | 90.0 | 22.5 | 89.2 | 12.9 |
| Dandelion | 100.0 | 1.3 | 96.8 | 2.2 | ||||
| Dog | 96.7 | 0.0 | 97.5 | 0.0 | 87.5 | 5.0 | 97.8 | 2.2 |
| Egg white | 100.0 | 3.3 | 98.7 | 0.0 | 100.0 | 7.5 | 97.8 | 0.0 |
| Garlic | 100.0 | 0.0 | 95.0 | 0.0 | ||||
| Goldenrod | 97.5 | 1.3 | 95.7 | 0.0 | ||||
| Hazelnut | 100.0 | 1.3 | 98.9 | 1.1 | ||||
| House dust | 73.3 | 0.0 | 69.6 | 0.0 | 78.5 | 0.0 | ||
| Japanese cedar | 98.7 | 0.0 | 100.0 | 1.1 | ||||
| Japanese Hop | 100.0 | 0.0 | 98.7 | 1.3 | 100.0 | 0.0 | 96.8 | 0.0 |
| Kiwi | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Latex | 98.7 | 0.0 | 96.8 | 0.0 | ||||
| Mackerel | 96.7 | 0.0 | 100.0 | 0.0 | 95.0 | 0.0 | 98.9 | 0.0 |
| Mango | 100.0 | 0.0 | ||||||
| Milk | 100.0 | 0.0 | 86.1 | 2.5 | 100.0 | 0.0 | 93.5 | 0.0 |
| Mugwort | 96.7 | 0.0 | 98.7 | 0.0 | 100.0 | 0.0 | 98.9 | 1.1 |
| Mussel | 100.0 | 0.0 | ||||||
| Oak white | 100.0 | 3.3 | 98.7 | 0.0 | 100.0 | 2.5 | 96.8 | 0.0 |
| Onion | 100.0 | 0.0 | ||||||
| Orchard grass | 100.0 | 1.3 | 97.8 | 1.1 | ||||
| Oxeye daisy | 100.0 | 1.3 | 100.0 | 2.2 | ||||
| Peach | 96.7 | 0.0 | 97.5 | 1.3 | 100.0 | 0.0 | 97.8 | 0.0 |
| Penicillium notatum | 96.2 | 0.0 | 97.8 | 0.0 | ||||
| Pigweed | 100.0 | 1.3 | 98.9 | 1.1 | ||||
| Pine | 98.7 | 0.0 | 98.9 | 0.0 | ||||
| Poplar mix | 98.7 | 0.0 | 97.8 | 1.1 | ||||
| Peanut | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Pork | 96.7 | 0.0 | 95.0 | 0.0 | ||||
| Potato | 100.0 | 0.0 | 97.5 | 0.0 | ||||
| Pupa, silk cocoon | 90.0 | 0.0 | 90.0 | 0.0 | ||||
| Rabbit | 98.7 | 0.0 | ||||||
| Ragweed | 93.3 | 0.0 | 100.0 | 2.5 | 97.5 | 0.0 | 98.9 | 2.2 |
| Redtop, bent grass | 100.0 | 1.3 | ||||||
| Reed | 96.2 | 0.0 | 97.8 | 0.0 | ||||
| Rice | 100.0 | 0.0 | 95.0 | 0.0 | ||||
| Russian thistle | 98.7 | 1.3 | 97.8 | 1.1 | ||||
| Rye pollens | 100.0 | 0.0 | 93.7 | 2.5 | 95.0 | 0.0 | 98.9 | 2.2 |
| Sallow willow | 100.0 | 1.3 | 97.8 | 2.2 | ||||
| Salmon | 96.7 | 0.0 | 100.0 | 0.0 | ||||
| Shrimp | 96.7 | 6.7 | 94.9 | 0.0 | 95.0 | 0.0 | 100.0 | 0.0 |
| Soy bean | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 |
| Storage mite | 86.7 | 6.7 | 89.9 | 10.1 | 90.3 | 11.8 | ||
| Sweet vernal grass | 94.9 | 1.3 | ||||||
| Sycamore mix | 100.0 | 1.3 | 100.0 | 2.2 | ||||
| Timothy grass | 94.9 | 1.3 | 97.8 | 2.2 | ||||
| Tomato | 100.0 | 0.0 | 97.5 | 0.0 | ||||
| Tuna | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Wheat flour | 93.3 | 0.0 | 90.0 | 0.0 | ||||
| Yeast, bakers | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Yellow jacket (wasp) | 96.2 | 0.0 | ||||||
Furthermore, several allergens which showed propensity toward positive result in specific assay were noticed in both comparison analyses (Table 5). While AdvanSure Allostation Smart II and PROTIA Allergy-Q showed positive propensity for some allergens when compared with RIDA Allergy Screen, RIDA Allergy Screen did not show positive propensity for any allergens with 10% discrepant results. For evaluation of AdvanSure Allostation Smart II, three allergens with the highest positive propensity results were D. farina (23.3%, 22.8%), D. pteronyssinus (20.0%, 24.1%), and house dust (26.7%, 30.4%) in both food and inhalant panels. Similarly three highest positive propensity results for PROTIA Allergy-Q were observed in D. farina (17.2%), D. pteronyssinus (10.8%), and house dust (19.4%) in inhalant panel. However, D. farina (17.5%) and dog (12.5%) showed highest positive propensity results in food panel of PROTIA Allergy-Q. Interestingly, the allergen with largest class difference between AdvanSure Allostation Smart II or PROTIA Allergy-Q and RIDA Allergy Screen was pupa silk cocoon in food panel although it showed positive propensity of 10.0% (i.e. mean difference by class 3 in AdvanSure Allostation Smart II vs. RIDA Allergy Screen comparison and mean difference by class 5 in PROTIA Allergy-Q vs. RIDA Allergy Screen comparison).
Table 5.
List of allergens which present positive propensity in each assay.
| Allergen | AdvanSure SmartII vs. RIDA |
PROTIA Allergy-Q vs. RIDA |
||
|---|---|---|---|---|
| AdvanSure SmartII positive propensity (%) | RIDA positive propensity (%) | PROTIA Allergy-Q positive propensity (%) | RIDA positive propensity (%) | |
| Food panel | ||||
| Cockroach | NS | NS | 7.5 | NS |
| D. farinae | 23.3 | NS | 17.5 | NS |
| D. pteronyssinus | 20.0 | NS | 10.0 | NS |
| Dog | NS | NS | 12.5 | NS |
| House dust | 26.7 | NS | NS | NS |
| Pupa, silk cocoon | 10.0 | NS | 10.0 | NS |
| Storage mite | 13.3 | NS | NS | NS |
| Wheat flour | NS | NS | 10.0 | NS |
| Inhalant panel | ||||
| Birch-alder mix | NS | NS | 7.5 | NS |
| Cat | 8.9 | NS | NS | NS |
| Crab | NS | NS | 5.4 | NS |
| D. farinae | 22.8 | NS | 17.2 | NS |
| D. pteronyssinus | 24.1 | NS | 10.8 | NS |
| House dust | 30.4 | NS | 19.4 | NS |
| Milk | 6.3 | NS | NS | 6.5 |
| Storage mite | 7.6 | NS | NS | NS |
NS: Not significant, defined as discrepant results less than 5%.
3.4. Effects of lowering or raising up the cut-off level for positive result
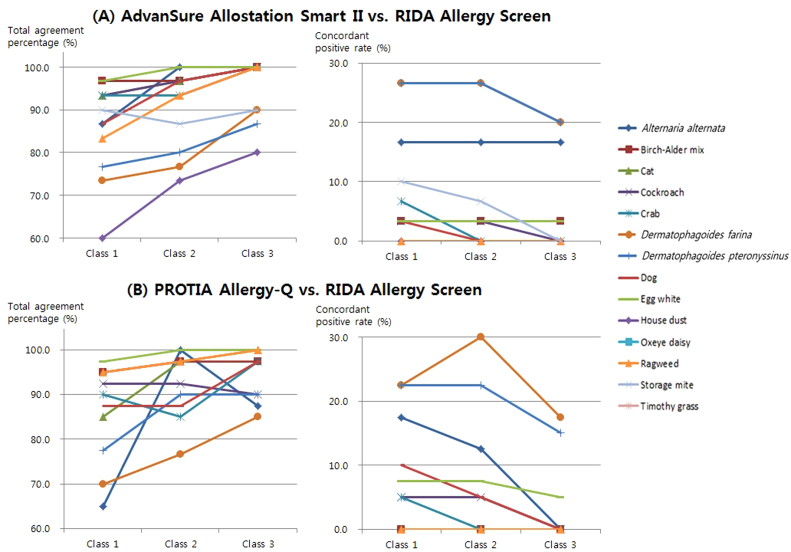
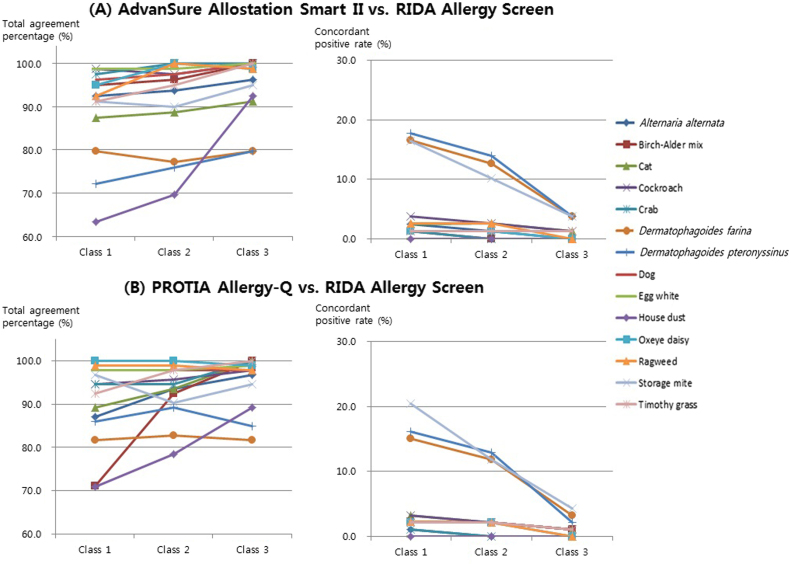
To evaluate the effects of various cut-off levels for positive result determination, we applied two more cut-off levels other than the conventional criteria of class 2 as minimal requirement for positive result; class 1 and class 3 as cut-off levels. Total agreement percentages and concordant positive rates were fairly influenced by application of both higher and lower cut-off levels (Fig. 1, Fig. 2). Since higher cut-off level led to more negative results, concordant positive rates decreased naturally. However, the changes of total agreement percentage according to the increase in cut-off level varied among allergens by different analyzers.
Fig. 1.
Changes of total agreement percentage and concordant positive rate according to three different cut-off levels for positive result determination in the food panels.
Fig. 2.
Changes of total agreement percentage and concordant positive rate according to three different cut-off levels for positive result determination in the inhalant panels.
3.5. Evaluation of positive rates for unique antigens in specific analyzer
Since different analyzers include various allergens, analyzer-specific allergens present diverse frequencies among patients (Table 6). Among the accretional allergens introduced in Advansure Allostation Smart II, Acarus siro and apple in inhalant panel showed significant positive rates of 20.0% and 8.9%, respectively, when class 1 was utilized as cut-off level. When cut-off level was increased to class 2, these positive rates decreased to 12.2% and 4.4%, respectively (data not shown).
Table 6.
List of analyzer-specific allergens and frequencies of positive results using cut-off level of class 1.
| Food panel |
Inhalant panel |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AdvanSure Smart II | PROTIA Allergy-Q | RIDA | AdvanSure Smart II | PROTIA Allergy-Q | RIDA | ||||||
|
N=40 |
N=40 |
N=40 |
N=90 |
N=93 |
N=93 |
||||||
| Allergen | Positivity frequency (rate, %) | Allergen | Positivity frequency (rate, %) | Allergen | Positivity frequency (rate, %) | Allergen | Positivity frequency (rate, %) | Allergen | Positivity frequency (rate, %) | Allergen | Positivity frequency (rate, %) |
| Alder | 2 (5.0) | Almond | 2 (5.0) | Chocolate | 0 | Acarus siro | 18 (20.0) | Weat flour | 3 (3.2) | Anchovy | 0 |
| Celery | 1 (2.5) | Latex | 0 | Alder | 2 (2.2) | Banana | 0 | ||||
| Cacao | 0 | Lilac | 0 | Apple | 8 (8.9) | Chestnut | 1 (1.1) | ||||
| Maize | 1 (2.5) | Redtop, bent grass | 0 | Cacao | 0 | Chocolate | 0 | ||||
| Mushroom | 0 | Wool | 0 | English plantain | 1 (1.1) | Clam | 0 | ||||
| Sesame | 1 (2.5) | Yellow jacket (wasp) | 1 (2.5) | Guinea pig | 0 | Cucumber | 2 (2.2) | ||||
| Squid | 2 (5.0) | Hamster | 0 | Kiwi | 1 (1.1) | ||||||
| Hinoki cypress | 1 (1.1) | Lilac | 1 (1.1) | ||||||||
| Honey bee | 3 (3.3) | Mango | 0 | ||||||||
| Horse | 3 (3.3) | Mussel | 0 | ||||||||
| Maize | 1 (1.1) | Wool | 0 | ||||||||
| Sesame | 1 (1.1) | ||||||||||
| Sheep | 1 (1.1) | ||||||||||
3.6. Frequency of multiple allergen positive results per patient by four different analyzers
Because multiple allergen positive results might indicate cross-reactivity between similar allergens, frequency of patients with two or more positive results was analyzed according to four different analyzers. By application of class 2 as the cut-off level for positive result, AdvanSure AlloScreen and AdvanSure Allostation Smart II presented highest frequency of patients with multiple positive allergens (i.e. AdvanSure AlloScreen: N=30 for food panel and N=43 for inhalant panel, AdvanSure Allostation Smart II: N=23 for food panel and N=37 for inhalant panel) with maximum positive allergen numbers of 23 and 34 in food panel and 28 and 32 in inhalant panel, respectively.
4. Discussion
During the last decade, there have been several remarkable introductions of new MAST assays by different manufacturers into the clinical field of allergic diseases. Accordingly, evaluation and comparison studies of these novel MAST analyzers were reported by few groups [6], [8]. Until today, a total of four MAST assays [i.e. AdvanSure AlloScreen, RIDA Allergy Screen, MAST Optigen (Hitachi), and Polycheck (Biocheck)] were frequently evaluated with each other and showed comparable clinical performances [10], [11], [20]. Recently, Lee and colleagues presented favorable performance of newly developed PROTIA Allergy-Q [12]. In this current trend, we evaluated four MAST analyzers including two newly developed and fully automated assays. This study is the first evaluation report for AdvanSure Allostation Smart II and only the second comparison study for PROTIA Allergy-Q. Also, our study is unique for evaluating upgraded version of specific assay to ensure the improvement by including both AdvanSure AlloScreen and AdvanSure Allostation Smart II.
Based on our results, most results of comparison analyses presented good concordance levels by means of total agreement percentages over 90.0%. Satisfactory agreements were observed not only in the comparison between AdvanSure AlloScreen and AdvanSure Allostation Smart II, but also in the evaluation of AdvanSure Allostation Smart II and PROTIA Allergy-Q compared with RIDA Allergy Screen. Although four allergens with the most frequent positive results, which were D. farina, D. pteronyssinus, house dust, and storage mite, showed slightly lower concordance rates, these different results could be sufficiently overcome by careful interpretation of MAST results in association with clinical manifestations.
One interesting finding we focused on in this study was positive propensity of each analyzer. In the midst of various available MAST analyzers with comparable diagnostic performance, it is important for laboratory physicians to recognize the unique propensity of each analyzer which might easily lead to positive results for particular allergen. Our study suggests that AdvanSure Allostation Smart II and PROTIA Allergy-Q are more sensitive or prone to report positive results for three common allergens (i.e. D. farina, D. pteronyssinus, and house dust) in both food and inhalant panels than RIDA Allergy Screen. Considering the multiple positive result frequencies related with cross-reactivity among similar allergens as possible mechanism for explanation [21], [22], [23], positive propensity of each analyzer should be cautiously understood. Moreover, variations in the allergen extraction method by different manufacturers might have caused this phenomenon of diverse positive propensity in each analyzer.
Adding new allergens in the panel list is another issue for future development of MAST analyzers. Candidate allergens should be assessed based on evidences for continuous and dramatic changes in the environment and socio-behavioral lifestyle of modern individuals [24], [25]. At the same time, cost-effective approach is required for choices of clinically efficient allergen with reference to epidemiologic results of geographically characteristic allergen studies [26], [27], [28], [29]. Our results support the significant positive rate for Acarus siro among Korean population [30], which is unique allergen included only in Advansure Allostation Smart II inhalant panel. Further investigations for Acarus siro as an inhalant allergen in general population might highlight the advantage of Advansure Allostation Smart II.
One of the most important approaches we performed in this study was the re-evaluation of cut-off levels in order to avoid false positive results. Besides the conventional cut-off level of class 2 as the minimal positive result criteria, we analyzed the changes of total agreement percentages and concordant positive rates according to cut-off level decrease to class 1 or increase to class 3. Although the increase of cut-off level seemed to make clinical circumstance more simple and concise by presenting only the definitive positive allergens, this modification resulted in lower concordant positive rates with possibility of missing potentially critical allergens. On the other hand, decrease of cut-off level produced lower total agreement percentages in most allergens, which might obscure physicians from clear identification of clinically relevant allergens. Detailed evaluation with similar approach for optimal cut-off class should be conducted for each analyzer according to specific regional frequency and distribution of allergens in the future.
A critical limitation of this study was the use of RIDA Allergy Screen assay as the reference value for evaluation of newly developed analyzers. Among the comparison studies published until today, most studies included the ImmunoCAP system (Phadia, Uppsala, Sweden) for comparison analyses as an empirically reference method [8], [10], [11], [12], [20]. However, the ImmunoCAP system is neither the official nor the definite reference procedure for measurement of allergen-specific IgE antibodies despite its good reliability and reproducibility. While the ImmunoCAP system might be impractically expensive for efficient clinical service for small to medium sized clinical laboratories [31], RIDA Allergy Screen assay has been continuously evaluated and reported for favorable clinical correlation with not only the ImmunoCAP system, but also serum total IgE [10], [32]. We anticipated that objective comparison between currently available MAST analyzers might provide sufficient information for clinical use in the practical medical field.
In conclusion, AdvanSure Allostation Smart II maintained steady concordant performance in the upgrade process from AdvanSure AlloScreen, with the uniquely extended allergen list including Acarus siro which showed certain positive rates. AdvanSure Allostation Smart II and PROTIA Allergy-Q presented favorable agreement performances with RIDA Allergy Screen, although positive propensities were noticed in some allergens. The conventional cut-off level of class 2 as the minimal positive result criteria appeared to be suitable for current MAST analyzers in the clinical interpretation.
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Burrows B., Martinez F.D., Halonen M., Barbee R.A., Cline M.G. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N. Engl. J. Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Williams P., Sewell W.A., Bunn C., Pumphrey R., Read G., Jolles S. Clinical immunology review series: an approach to the use of the immunology laboratory in the diagnosis of clinical allergy. Clin. Exp. Immunol. 2008;153:10–18. doi: 10.1111/j.1365-2249.2008.03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pipkorn U. Pharmacological influence of antiallergic medication on in vivo allergen testing. Allergy. 1988;43:81–86. doi: 10.1111/j.1398-9995.1988.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 4.Ownby D.R. Allergy testing: in vivo versus in vitro. Pediatr. Clin. N. Am. 1988;35:995–1009. doi: 10.1016/s0031-3955(16)36544-0. [DOI] [PubMed] [Google Scholar]
- 5.Plebani M., Bernardi D., Basso D., Borghesan F., Faggian D. Measurement of specific immunoglobulin E: intermethod comparison and standardization. Clin. Chem. 1998;44:1974–1979. [PubMed] [Google Scholar]
- 6.Lee S., Lim H.S., Park J., Kim H.S. A new automated multiple allergen simultaneous test-chemiluminescent assay (MAST-CLA) using an AP720S analyzer. Clin. Chim. Acta. 2009;402:182–188. doi: 10.1016/j.cca.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Williams P., Onell A., Baldracchini F., Hui V., Jolles S., El-Shanawany T. Evaluation of a novel automated allergy microarray platform compared with three other allergy test methods. Clin. Exp. Immunol. 2015 doi: 10.1111/cei.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.H., Park K.H., Kim H.S. Specific IgE measurement using AdvanSure system: comparison of detection performance with ImmunoCAP system in Korean allergy patients. Clin. Chim. Acta. 2012;413:914–919. doi: 10.1016/j.cca.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Jang W.R., Nahm C.H., Kim J.H. Allergen specific IgE measurement with Polycheck Allergy: comparison of three multiple allergen simultaneous tests. Korean J. Lab. Med. 2009;29:465–472. doi: 10.3343/kjlm.2009.29.5.465. [DOI] [PubMed] [Google Scholar]
- 10.Oh E.J., Lee S.A., Lim J., Park Y.J., Han K., Kim Y. Detection of allergen specific IgE by AdvanSure Allergy Screen test. Korean J. Lab. Med. 2010;30:420–431. doi: 10.3343/kjlm.2010.30.4.420. [DOI] [PubMed] [Google Scholar]
- 11.Han M., Shin S., Park H., Park K.U., Park M.H., Song E.Y. Comparison of three multiple allergen simultaneous tests: RIDA allergy screen, MAST optigen, and polycheck allergy. Biomed. Res. Int. 2013;2013:340513. doi: 10.1155/2013/340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.H., Park H.J., Park K.H., Jeong K.Y., Park J.W. Performance of the PROTIA Allergy-Q System in the Detection of Allergen-specific IgE: A Comparison With the ImmunoCAP System. Allergy Asthma Immunol. Res. 2015;7:565–572. doi: 10.4168/aair.2015.7.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballmer-Weber B.K., Lidholm J., Fernandez-Rivas M. IgE recognition patterns in peanut allergy are age dependent: perspectives of the EuroPrevall study. Allergy. 2015;70:391–407. doi: 10.1111/all.12574. [DOI] [PubMed] [Google Scholar]
- 14.Klemans R.J., van Os-Medendorp H., Blankestijn M., Bruijnzeel-Koomen C.A., Knol E.F., Knulst A.C. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: a systematic review. Clin. Exp. Allergy. 2015;45:720–730. doi: 10.1111/cea.12412. [DOI] [PubMed] [Google Scholar]
- 15.Batard T., Baron-Bodo V., Martelet A. Patterns of IgE sensitization in house dust mite-allergic patients: implications for allergen immunotherapy. Allergy. 2016;71:220–229. doi: 10.1111/all.12796. [DOI] [PubMed] [Google Scholar]
- 16.Allen K.J., Koplin J.J. Why does Australia appear to have the highest rates of food allergy? Pediatr. Clin. N. Am. 2015;62:1441–1451. doi: 10.1016/j.pcl.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Steckelbroeck S., Ballmer-Weber B.K., Vieths S. Potential, pitfalls, and prospects of food allergy diagnostics with recombinant allergens or synthetic sequential epitopes. J. Allergy Clin. Immunol. 2008;121:1323–1330. doi: 10.1016/j.jaci.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y.W., Sohn J.H., Lee J.H., Hong C.S., Park J.W. Allergen-specific IgE measurement with the IMMULITE 2000 system: intermethod comparison of detection performance for allergen-specific IgE antibodies from Korean allergic patients. Clin. Chim. Acta. 2009;401:25–32. doi: 10.1016/j.cca.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 20.Jeong S., Jang G.-C., Cho N.J. Analysis of polycheck allergy results of the recent two years: comparison with skin prick test and ImmunoCAP. Lab. Med. Online. 2012;2:139–147. [Google Scholar]
- 21.Gabriel M.F., Postigo I., Gutierrez-Rodriguez A. Alt a 15 is a new cross-reactive minor allergen of Alternaria alternata. Immunobiology. 2016;221:153–160. doi: 10.1016/j.imbio.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Bublin M., Breiteneder H. Cross-reactivity of peanut allergens. Curr. Allergy Asthma Rep. 2014;14:426. doi: 10.1007/s11882-014-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvinen K.M., Chatchatee P. Mammalian milk allergy: clinical suspicion, cross-reactivities and diagnosis. Curr. Opin. Allergy Clin. Immunol. 2009;9:251–258. doi: 10.1097/ACI.0b013e32832b3f33. [DOI] [PubMed] [Google Scholar]
- 24.Charpin D., Vervloet D. New aero-allergens. Interaction between allergens and the environment. Bull. Acad. Natl. Med. 1997;181:1551–1561. [PubMed] [Google Scholar]
- 25.Muche-Borowski C., Kopp M., Reese I., Sitter H., Werfel T., Schafer T. Allergy prevention. J. Dtsch. Dermatol. Ges. 2010;8:718–724. doi: 10.1111/j.1610-0387.2009.07313.x. [DOI] [PubMed] [Google Scholar]
- 26.Buters J.T., Kasche A., Weichenmeier I. Year-to-year variation in release of Bet v 1 allergen from birch pollen: evidence for geographical differences between West and South Germany. Int. Arch. Allergy Immunol. 2008;145:122–130. doi: 10.1159/000108137. [DOI] [PubMed] [Google Scholar]
- 27.Thomas W.R. Geography of house dust mite allergens. Asian Pac. J. Allergy Immunol. 2010;28:211–224. [PubMed] [Google Scholar]
- 28.Belloni Fortina A., Cooper S.M., Spiewak R., Fontana E., Schnuch A., Uter W. Patch test results in children and adolescents across Europe Analysis of the ESSCA Network feminine 2002–2010. Pediatr. Allergy Immunol. 2015 doi: 10.1111/pai.12397. [DOI] [PubMed] [Google Scholar]
- 29.Ballmer-Weber B.K., Skamstrup Hansen K., Sastre J. Component-resolved in vitro diagnosis of carrot allergy in three different regions of Europe. Allergy. 2012;67:758–766. doi: 10.1111/j.1398-9995.2012.02827.x. [DOI] [PubMed] [Google Scholar]
- 30.Son M., Jeong K.Y., Kim B.J., Lim K.J., Lee J.H., Park J.W. IgE reactivity to Acarus siro extract in Korean dust mite allergic patients. Exp. Appl. Acarol. 2014;63:57–64. doi: 10.1007/s10493-013-9759-6. [DOI] [PubMed] [Google Scholar]
- 31.Hedlin G., Moreno C., Petersson C.J. Allergy diagnosis in children and adults: performance of a new point-of-care device, ImmunoCAP rapid. World Allergy Org. J. 2009;2:138–143. doi: 10.1097/WOX.0b013e3181aed85c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung S.W., Oh E.J., Lee J. Usefulness of total IgE in predicting positive allergen specific IgE Tests in Korean subjects. Korean J. Lab. Med. 2010;30:660–667. doi: 10.3343/kjlm.2010.30.6.660. [DOI] [PubMed] [Google Scholar]