Abstract
Objectives
High sensitivity cardiac troponin T and I (hs-cTnT and hs-cTnI) assays show analytical, diagnostic and prognostic improvement over contemporary sensitive cTn assays. However, given the importance of troponin in the diagnosis of myocardial infarction, implementing this test requires rigorous analytical and clinical verification across the total testing pathway. This was the aim of this study.
Design and methods
Analytical verification included assessment of critical outlier frequency, for hs-cTnI and cTnI assays. Concordance for paired cTnI and hs-cTnI measurements (n=1096) was verified using 99th percentiles for both genders (cTnI: 30 ng/L, hs-cTnI: 25 ng/L) and for men and women separately (hs-cTnI: M: 34;F: 16 ng/L). Discordant data was correlated with clinical and laboratory information. Diagnosis of Acute Coronary Syndrome (ACS) or Non-ACS was adjudicated by two cardiologists independently.
Results
The hs-cTnI assay showed a lower (10-fold) critical outlier rate (0.091%) and more detectable results above the limit of detection (LOD) (23.4%) and 99th percentile (2.4%), compared to cTnI. Analytical concordance between the two assays was high (94.5%) but decreased (91.7%) when gender-specific hs-cTnI cut-offs were used. The hs-cTnI assay gave fewer false negatives (up to 1.0%) but disproportionately more false positives (up to 6.7%) overall, which improved (3.9%) for serial measurements.
Conclusions
Laboratories should analytically and clinically verify hs-cTn assays before use, with attention to performance and the clinical and diagnostic algorithms that support appropriate testing and result interpretation. Work in the pre- and post-analytical phases is necessary to augment the analytical improvement in the new era of troponin testing.
Abbreviations: ACS, Acute Coronary Syndrome; AMI, Acute Myocardial Infarction; CABG, Coronary Artery Bypass Graft; CD, Critical Difference; CV, Coefficient of Variation; CI, Confidence Interval; COPD, Chronic Obstructive Pulmonary Disease; cTn, Cardiac troponin; hs-cTn, High sensitivity cardiac troponin; LOD, Limit of Detection; IQR, Inter-quartile range; FN, False Negative; FP, False Positive; NSTEMI, Non-ST-segment Elevation Myocardial Infarction; TN, True Negative; TP, True Positive; TTP, Total Testing Pathway.
Keywords: Troponin, High sensitivity, Acute Coronary Syndrome
1. Introduction
Over the past 10 years cardiac troponins T and I (cTnT and cTnI) have emerged as the cardiac biomarkers of choice for the diagnosis of Acute Myocardial Infarction (AMI), which is defined biochemically by a rise and/or fall in biomarker concentration with at least one value above the 99th percentile [1]. During this time analytical developments have also resulted in the emergence of high-sensitivity Troponin (hs-cTn) assays into diagnostic use [2], [3] which allow earlier detection of myocardial injury and show improved accuracy over established sensitive assays for diagnosis of AMI [4], [5]. Improved imprecision at concentrations below the 99th percentile of the normal population has permitted measurement of troponin in a greater proportion of healthy individuals [2] and identification of patients with detectable troponin concentrations, less than the 99th percentile, who are at intermediate risk of major adverse cardiac events (MACE) [6]. A possible paradigm shift for troponin use in prognosis, screening and sub-clinical monitoring of myocardial remodelling has therefore emerged [7], [8] and further emphasises the need for laboratories to fully evaluate this test both analytically and clinically.
Although hs-cTn assays are now almost commonplace, with improved diagnostic and prognostic capabilities, much work remains to be done throughout the total testing pathway (TTP). Reports of critical outliers are currently an unexplained feature of high-sensitivity (and contemporary sensitive) assays [9], [10] which may require further analytical improvements for understanding of pre-analytical factors. Assay standardisation for cTnI is also ongoing [11]. There are also post-analytical challenges, including the need to accurately differentiate acute, chronic, cardiac and non-cardiac causes of troponin increases by obtaining serial troponin measurements [12]. A significant troponin change, consistent with an ACS, may be expressed as the absolute (Δ, ng/L) or relative (%) change (delta,δ), where the former shows diagnostic advantage [13], [14], by evaluations against the Reference Change Value (RCV, %) [15] or by using a probability (z-scores) based approach [16]. To enable accurate interpretation of baseline troponin measurements, work continues to establish appropriate troponin reference ranges, accounting for age, race and gender [17]. There are also issues around the appropriateness of troponin requests in specific situations, which may be explained by a knowledge gap in understanding both the utility and limitations of this test by clinical users.
In view of the current challenges that exist for hs-cTn assays, we report our approach, involving analytical and clinical verification, to help transition from the sensitive cTnI in current use to the new hs-cTnI assay on the Abbott Architect and our attempts to addressing the challenges that remain across the TTP.
2. Materials and methods
2.1 Subjects, ethics and study design
The study included 698 patients, comprising 349 men and 349 women (Age median [IQR]: 71 [57–82] years) who attended the Mater Misericordiae University Hospital (MMUH) during March 2013, for whom troponin was requested (n=1119 individual requests, which included repeat measurements [n≥2 requests, n=231 patients] from serial testing) as part of routine clinical management. Most requests were from the emergency department (ED; 45%), presenting mainly with systemic illness (62%) and non-ischaemic chest pain (20%) followed by those with ACS symptoms (17%; of whom almost one third had a NSTEMI). Remaining requests were mainly from general medicine (19%) and surgical sources (14%) with respiratory and cardiology each comprising only 3% of requests. For most patients (61%), the estimated glomerular filtration rate (eGFR) was ≥60 mL/min/1.73 m2 (corresponding to chronic kidney disease [CKD] Stages 1+2), followed by those with CKD Stages 3a and 3b (27%), 4 (10%) and 5 (1%) respectively. The study was approved by the MMUH's research and ethics committee. There were no exclusion criteria. During the one month study period users were asked to complete an audit form, to document the clinical background and reasons for which troponin was requested. For all troponin requests, results for contemporary (sensitive) and high sensitivity assays were obtained in the majority of cases (1096 samples from 684 patients), however only the results from the sensitive assay were reported routinely. Results from paired analysis of samples measured by both assays (n=1096) were compared retrospectively for analytical agreement (linear regression) and concordance against each assay's respective 99th percentiles. The concordance analysis involved consideration of 99th percentiles for both genders (both assays) and gender-specific 99th percentiles (hs-cTnI only). A discordant pair was identified when results from each assay were not either both higher or both lower than the respective assay's 99th percentile (discordant pairs obtained in 94 patients, comprising 115 paired measurements, where two discordant paired measurements [from serial testing] were obtained for 21 of the 94 patients).
hs-cTnI measurements were further evaluated against decision thresholds and absolute change (Δ) criteria, specific to the Abbott Architect, which were proposed recently as part of a 0 h/1 h rule in/rule-out algorithm by the European Society of Cardiology [18].
For discordant pairs of cTnI/hs-cTnI measurements, patient records (electronic and paper) were accessed to obtain relevant information including patient demographics (age, sex, race), laboratory data (C-reactive protein (CRP) concentration, renal function) and cardiology investigations such as. ECG, echocardiography, coronary angiogram, current established diagnoses and past medical history (Table 1). Using the above information, obtained as part of our routine clinical practice, and with criteria described previously to derive prediction rules to triage patients for discharge from the ED, or to admit for further investigations [19], [20], patients were adjudicated to a diagnosis of either Acute Coronary Syndrome (ACS: Definite or Suspected ACS, including Type 1 MI and Angina) or Non-ACS (including Type II MI and non-ischaemic myocardial injury) by two independent cardiologists, blinded to results of troponin analysis and where the second cardiologist adjudicated on a random selection of patients. Findings from this analytical and clinical verification study provided the rationale for the use of algorithms that support appropriate use and interpretation of hs-cTnI measurements in the investigation of ACS.
Table 1.
Demographics, clinical presentation and previous clinical background of patients with cTnI/hs-cTnI data pairs (n=94). Most patients within this discordant cohort had a known cardiac background involving any one or more of: Atrial Fibrillation, Angina, Myocardial Infarction (MI), Congestive Cardiac Failure or previous cardiac procedure (Coronary Artery Bypass Graft ([CABG], Percutaneous Coronary Intervention [PCI], Intra-Cardiac Device [ICD], cardiac transplant or valve repair). For patients with a non-cardiac background, most had respiratory disease. ns=Non-Significant difference in age between males and females.
| Gender | 54 Women/40 Men |
|---|---|
| Age (Median [IQR]) | Women: 82 [70–87] years |
| Men: 79 [68–84] years | |
| (ns) | |
| eGFR ml/min/1.73 m2: | |
| Median [IQR] | 55.5 [31.5–60] |
| % of patients with eGFR: | |
| ≥60 | 45% |
| 30–59 | 33% |
| 15–29 | 19% |
| <15 | 3% |
| CRP concentration (Median[IQR], mg/L) | 38.5 [15–90] |
| % of patients with CRP >7 mg/L | 82% |
| Presenting symptoms | |
| Chest pain | a20 (Cardiac: 5, Pleuritic: 1) |
| Shortness of breath | 39 |
| Palpitations | 3 |
| ECG abnormalities (ST elevation/LBBB/T wave inversion) | 1/2/1 |
| Patients with Cardiac background | 74 |
| Atria fibrillation | 22 |
| Angina | 13 |
| MI (STEMI) | 15(3) |
| Congestive cardiac failure | 2 |
| Cardiac procedure: CABG/PCI/ICD/Transplant/Valve repair | 23 (9/9/3/1/1) |
| Patients without Non-Cardiac background | 20 |
| Respiratory disease (COPD, Pulmonary Fibrosis) | 6 |
| Cerbrovascular disease | 3 |
| Gastro-intestinal disease | 3 |
Type of Chest pain not specified in 14/20 patients. LBBB=Left Bundle Branch Block. STEMI=ST elevation Myocardia Infarction. COPD=Chronic Obstructive Pulmonary Disease.
2.2 Troponin analysis
Cardiac Troponin I (cTnI) was measured using STAT contemporary sensitive and high sensitivity assays on an Abbott ARCHITECT i2000SR analyser (Abbott Laboratories, North Chicago, IL, USA) according to manufacturer's instructions. The manufacturer's reported performance characteristics are shown for both assays in Table 2.
Table 2.
Manufacturer's quoted specifications for the contemporary sensitive (cTnI) and high sensitivity (hs-cTnI) troponin I assays. The concentration units reported for cTnI (ug/L) were converted to ng/L. Imprecision data are based on the manufacturer's single analyte control material.
| Assay | Limit of | Linear | Concentration | 99th percentile | Total imprecision (CV, %) |
|---|---|---|---|---|---|
| detection | range | with CV<10% | concentration | ||
| cTnI (ng/L) | 10 | 10–50,000 | 32 | 30 (Unisex) | 5.5 (117 ng/L) |
| 4.4 (484 ng/L) | |||||
| 3.5 (12,859 ng/L) | |||||
| hs-cTnI (ng/L) | *1.9 | **10–50,000 | 4.7 | 26 (unisex) | 4.0 (20 ng/L) |
| 16 (Female) | 2.7 (194 ng/L) | ||||
| 34 (Male) | 2.5 (37,621 ng/L) |
The quoted (observed) Limit of Detection ranges from 1.1 to 1.9 ng/L.
The quoted (observed) deviation from linearity is <7% (10–50,000 ng/L) for cTnI and ±≤6.8% (10–50,000 ng/L) and ±0.4 ng/L (<10 ng/L) for hs-cTnI. CV=Coefficient of Variation.
For assay verification work, intra- and inter-assay imprecision for the hs-cTnI assay was determined from replicate analysis (n=25) of Internal Quality Control (IQC) material (MAS OmniCardio Level Ultralow [IQC1] [Thermo Scientific, St Albans, UK] and Randox immunoassay Speciality II, Levels 1–3 [IQC2-IQC4] [Randox Laboratories, Crumlin, UK]). To verify inaccuracy, material from the United Kingdom National External Quality Assurance Service (UKNEQAS) cardiac scheme was analysed and results compared to the method group (Abbott Architect) target mean.
For patient sample analysis, blood (10 ml) was collected into lithium heparin gel tubes (Sarstedt, Leicester, UK) and centrifuged at 10,000g for 10 min at room temperature. Plasma was analysed immediately using the same primary tube for both assays.
For outlier studies, plasma samples were obtained from a separate cohort of patients (n=1239), comprising 576 women and 663 men (Age [Median, IQR]: 68 [52–79] years) in whom troponin was requested (ED: 66%, In-patients: 31%, Out-patients and GP: 3%) during routine clinical management. Consecutive duplicate measurements were obtained for each troponin assay on the same day of analysis. Samples with critical outliers were identified as duplicate measurements, from the same assay, which were discordant compared to respective 99th percentiles (both genders) and where the concentration difference (%) between duplicates was greater than the Critical Difference (CD, %). The CD was defined by the formula CD=z×√2×CVa using a pre-defined probability of 0.05, where z=1.96 and CVa represented assay imprecision obtained from IQC data. The CD (%) between duplicate results therefore simplified to 2.8×CVa.
2.3 Statistics
Normality was assessed by the D'Agostino and Pearson omnibus test. The Wilcoxon Signed Rank test (two-tailed) was used for comparison of paired troponin measurements. Spearman Rank was used for all data correlations. For the contemporary sensitive cTnI assay, the manufacturer's quoted 99th percentile of 0.03 μg/L (30 ng/L) was used. For the hs-cTnI assay, data was evaluated using the 99th percentile for both genders of 25 ng/L, which we determined previously [21], and also against the gender-specific reference ranges of 34 and 16 ng/L, quoted by the manufacturer for men and women, respectively, and which were verified previously by Apple et al. [22]. Data were analysed using Prism (Graphpad Software, La Jolla, CA, USA), Excel (Microsoft Office 2007, Microsoft Redmond, WA, USA) and Medcalc (Medcalc, Ostend, Belgium; version 12.1.4).
3. Results
3.1 Analytical verification
3.1.1 Imprecision and inaccuracy
The hs-cTnI assay showed acceptable imprecision (intra-assay and inter-assay CV[%]: 1.9–3.9 and 3.0–6.8, respectively) (Table 3) and inaccuracy (mean[CI] % of target: 96[90–102]) across the analytical range (12.6–16,223 ng/L). Imprecision data collated over two months of routine use corroborated verification studies, where acceptable imprecision was also demonstrated at concentrations below the 99th percentile. Imprecision was superior to that seen for the cTnI assay across the analytical range (Table 3).
Table 3.
Imprecision data for hs-cTnI and cTnI assays. For verification studies (A), IQC material) was analysed at up to four levels (n=25 replicates per level), across the analytical range. Imprecision data (Total CV, %) collated following the analysis of IQC material over 2 months of routine use are also shown for both assays (B). The mean troponin concentration (ng/L) and corresponding intra-assay, inter-assay or total imprecision (CV, %) are shown for each IQC level. NA=not available.
| (A) |
||||
|---|---|---|---|---|
| hs-cTnI | IQC 1 | IQC 2 | IQC3 | IQC 4 |
| Mean [hs-cTnI] (ng/L) | 12.6 | 58.4 | 259 | 16,223 |
| Intra-assay CV (%) | 3.9 | 4.0 | 2.3 | 1.9 |
| Inter-assay CV (%) | 6.8 | 6.5 | 3.2 | 3.0 |
| cTnI | IQC 1 | IQC 2 | IQC3 | |
| Mean [cTnI] (ng/L) | 8.5 | 290.0 | 11,499 | |
| Intra-assay CV (%) | 17.2 | 2.8 | 2.0 | |
| Inter-assay CV (%) | 28.2 | 6.3 | 4.1 | |
| (B) | ||||
| hs-cTnI | IQC1 | IQC 2 | IQC 3 | IQC4 |
| Mean [hs-cTnI] (ng/L) | NA | 48.7 | 230.0 | 930 |
| Total CV (%) | NA | 8.1 | 6.2 | 4.3 |
| cTnI | IQC 1 | IQC 2 | IQC 3 | IQC4 |
| Mean [cTnI] (ng/L) | 6 | 55.7 | 476.0 | 1819 |
| Total CV (%) | 9.4 | 13.7 | 11.5 | 5.2 |
3.1.2 Duplicate analysis: critical outliers
Critical outlier rates of 0.97% and 0.091% were determined for the cTnI and hs-cTnI assays respectively (Fig. 1).
Fig. 1.
Critical outliers for contemporary sensitive (cTnI) and high sensitivity (hs-cTnI) troponin I assays. Data are shown for all duplicate cTnI (A) and hs-cTnI (B) results which were discordant compared to the respective 99th percentile(●). Critical outliers (▲) within this data set are shown for each assay. cTnI results (μg/L) were converted to ng/L before statistical analysis. Dashed lines refer to 99th percentiles of 30 ng/L (cTnI) and 25 ng/L (hs-cTnI) which were used for evaluation of duplicate results. Inset graphs show all duplicate results (<100 ng/L) for each assay.
3.1.3 Concordance studies
3.1.3.1 Analytical comparison
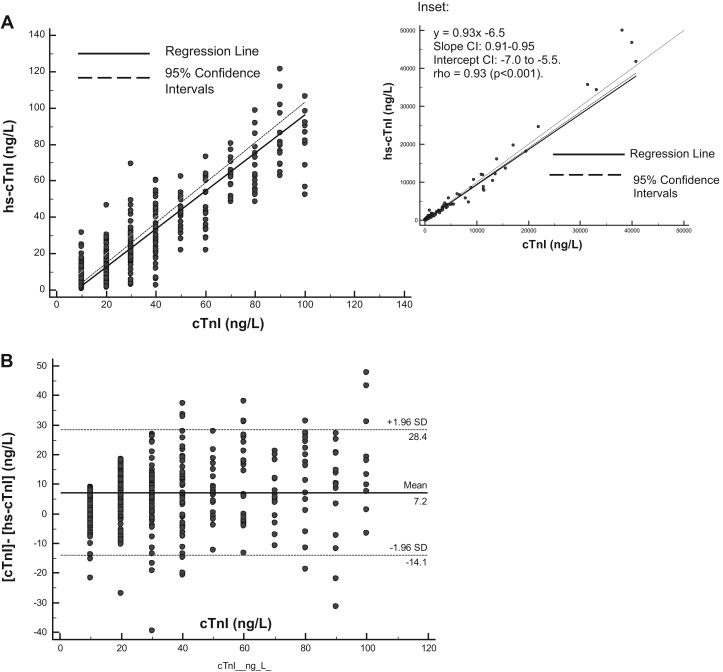
Across the concentration range studied (0.9–88,211 ng/L), hs-cTnI and TnI results were strongly correlated (r=0.93, p<0.0001, n=1096); however hs-cTnI results were significantly lower (p<0.0001) compared to cTnI results (Median [IQR]: 32 [11–125] vs 40 [20–160) (Fig. 2). For paired samples with cTnI concentrations <100 ng/L, results were less strongly correlated (r=0.08, p<0.0001, n=460) with a mean difference of 7.2 ng/L (2SDs:±21.2 ng/L). There were more undetectable results (<LOD) for the cTnI (38%) compared to the hs-cTnI assay (13%) whereas there were more results ≥the 99th percentile for the hs-cTnI than the cTnI assay, particularly when gender-specific cut-offs were considered (Table 4).
Fig. 2.
Analytical agreement between contemporary (cTnI) and high sensitive (hs-cTnI) troponin assays. Paired measurements from the cTnI and hs-cTnI assays were analysed by Passing Bablock (PB) regression (A) and absolute differences between measurements ([cTnI]–hs-cTnI], ng/L) compared using Bland–Altman (B–A) analysis (B), for samples with cTnI concentrations <100 ng/L. Inset: PB regression analysis for paired measurements at all concentrations. cTnI results (μg/L) were converted to ng/L before statistical analysis. PB analysis: y=1.05x−8.3 [Slope CI: 0.99 to 1.10 Intercept CI: −9.5 to −7.1]. Spearman's coefficient of rank correlation (rho)=0.81 (p<0.001). B–A analysis: Mean (± 2SDs) difference=7.2 (± 21.2) ng/L.
Table 4.
Frequency (%) of cTnI and hs-cTnI measurements across the troponin concentration range. For each assay, data were divided into three concentration ranges, defined by the respective Limit of Detection (LOD, cTnI: 0.01 μg/L, hs-cTnI: 1.9 ng/L) and 99th percentile concentrations (cTnI [μg/L]).
| 99th percentiles for data stratification | cTnI % |
hs-cTnI % |
||
|---|---|---|---|---|
| Unisex | Unisex | Gender | Unisex [M] + Gender [F] | |
| <LOD | 38.2 | 12.5 | 12.5 | 12.5 |
| ≥LOD to ≤99th | 29.1a | 52.5b | 51.9c | 48.5d |
| >99th percentile | 32.7a | 35.0b | 35.6c | 39.0d |
| Total | 100% | 100% | 100% | 100% |
0.03, hs-cTnI [ng/L].
25.
Male 34 and female 15.6.
Male 25 and female 15.6.
3.1.3.2 Evaluation of patients using baseline (T0h) and serial hs-cTnI measurements and absolute change (Δ) criteria.
Baseline troponin concentration (hs-cTnI) was <2 ng/L in 18% (n=125) of patients and >52 ng/L in 16% (n=106) of patients. Serial hs-cTnI measurements were obtained in only one third of patients (n=229/684). Of these patients, 61 had a baseline troponin <5 ng/L, of which a change (Δ) >2 ng/L was observed in 12 patients (Δ ≤2 ng/L in 49/61 patients). A Δ >6 ng/L was reported in 102 patients (Fig. 3).
Fig. 3.
Evaluation of patients using baseline and serial hs-cTnI measurements and absolute change (Δ) criteria. hs-cTnI results were stratified using assay-specific Δ values as part of the “rule in/rule out” algorithm proposed by the European Society of Cardiology (ESC) [18], whereby rule out of MI is applicable to patients with baseline measurements (T0h)<2 ng/L or <5 ng/L but changing <2 ng/L (n=158, 23%) and rule in of MI is applicable to patients with T0h>52 ng/L or >6 ng/L (n=162, 24%). Accordingly, 384 patients were stratified using the ESC algorithm. 300 patients with T0h=2–52 ng/L did not have serial measurements and therefore were not stratified. *n=16 had a Δ <2 ng/L, **n=6/56 had a T0h=2–5 ng/L.
3.1.3.3 Concordance compared to assay-specific 99th percentiles
Both assays were highly concordant (94.4%) following result evaluation against respective 99th percentiles for both genders, for each assay, and less so (91.7%) when gender-specific cut-offs were applied to hs-cTnI data (Fig. 4). Discordance was attributed mainly to patients with elevated hs-cTnI but not cTnI results and applicable to both genders similarly (22 men, 22 women), for evaluations involving a single 99th percentile, but attributable disproportionately to females (4 men, 58 women) when hs-cTnI gender-specific cut-offs were used (Fig. 4).
Fig. 4.
Clinical concordance between cTnI and hs-cTnI assays. Results were evaluated against the 99th percentiles of 0.03 μg/L for the cTnI assay and the overall (25 ng/L) and gender-specific (Males/Females: 34/15.6 ng/L) 99th percentiles for the hs-cTnI assay. ACS=Acute Coronary Syndrome, M=Male, F=Female. FN=False Negative, FP=False Positive.
3.2 Clinical verification
3.2.1 Clinical adjudication, correlation and factors affecting diagnosis
For the sub-cohort of patients (n=94) showing discordant results, as assessed using the above approaches (Fig. 4), ACS was diagnosed for 14 patients (15%). In half of these patients (2 men, 5 women), false negative cTnI results were reported. Using gender-specific cut-offs to evaluate hs-cTnI results identified correctly an additional 3 female patients (TPs) but missed 4 male patients with ACS (Fig. 4), and vice versa when applying a single cut-off. Only five out of 8 ACS patients with TP hs-cTnI results had serial measurements, with peak rises >50% (65%, 75%, 19-fold and 27 fold) in 4 patients. For the single male ACS patient presenting with baseline hs-cTnI <99th percentile (<34 ng/L), subsequent measurements were >99th percentile (unisex), rising by 65% (peak). However, for patients with non-ACS presentations, serial measurements (n≥2) were available in only 39 patients; 14/39 patients had 3 or more measurements. Compared to baseline, subsequent measurements showed a rise (11/39), fall (17/39), rise then fall (9/39) or fall then rise (2/39). Peak decreases (%) were >50% for 5 patients, whereas peak increases were >50% (Range: 62%–28 fold) for as many as 13 patients and >100% for 10 patients. In terms of absolute change (Δ, ng/L), the peak (median[IQR]) increase and decrease were 23[4.3–59.0] and 8.2 [4.8–18.8] ng/L, respectively.
Within this sub-cohort, more false positive (FP) patients were reported for the hs-cTnI assay, particularly when the female-specific 99th percentile and the overall cut-off (for males) were used (n=61 patients) (Table 5H). Across all patients (n=698), this represented a 6.7% overall increase in FP patients compared to the cTnI assay (n=14) but only a 1.0% overall decrease in FN patients (n=1), (Table 5A–H). Serial measurements were obtained in almost half (n=30/61) of these FP patients, of whom two thirds (20/30) had at least one FP result from either troponin assay. The overall increase in FP patients due to hs-cTnI measurements was thereby reduced to 3.9% (from 6.7%). Half of these FP patients (10/20) i.e. with non-ACS presentations presented with peak rises to ≥50% (Range: 66–1501%) and raised CRP concentrations (>7 mg/L, n=8: Range 13–93 mg/L) and/or decreased eGFR (<60 ml/min/1.73 m2, n=5: Range 49–27 ml/min/1.73 m2).
Table 5.
A–H Effect of assay type and 99th percentile on diagnostic performance. Data are representative of the sub-cohort of patients (n=94) with discordant cTnI and hs-cTnI results (n=115) whereby paired troponin results, from each assay, were not either both higher or both lower than the respective assay's 99th percentile. Patients were clinically adjudicated to a diagnosis of ACS or Non-ACS to permit diagnostic categorisation (True or False Positive or Negative) of such discordant troponin results. Data in A–D represent discordant results whereas data in E–H represent discordant patients. A and E represent cTnI data (99th percentile=0.03 μg/L [30 ng/L]), B–D (results) and F–H (patients) represent hs-cTnI data, evaluated against 99th percentiles of 25 ng/L (B+F), gender-specific 99th percentiles of 34 (Male) and 15.6 (Female) (C+G) or 99th percentiles of 25 (Male) and (15.6) ng/L (Female) (D+H). FN=False Negative, TN=True Negative, TP=True Positive, FP=False Positive.
| (A) |
|||
|---|---|---|---|
| cTnI (μg/L) | ACS | Non-ACS | Total |
| cTnI ≤0.03 (≤30) | 9 FN | 34 TN | 43 |
| cTnI >0.03 (>30) | 3 TP | 14 FP | 17 |
| Total | 12 | 48 | 60 |
| (B) | |||
| hs-cTnI (ng/L) | |||
| hs-cTnI ≤25 | 4 FN | 13 TN | 17 |
| hs-cTnI >25 | 8 TP | 35 FP | 43 |
| Total | 12 | 48 | 60 |
| (C) | |||
| hs-cTnI (ng/L) | |||
| hs-cTnI ≤15.6/≤34 | 6 FN | 24 TN | 30 |
| hs-cTnI >15.6/>34 | 8 TP | 53 FP | 61 |
| Total | 14 | 77 | 91 |
| (D) | |||
| hs-cTnI (ng/L) | |||
| hs-cTnI ≤15.6/≤25 | 1 FN | 10 TN | 11 |
| hs-cTnI >15.6/>25 | 10 TP | 70 FP | 80 |
| Total | 11 | 80 | 91 |
| (E) | |||
| cTnI (mg/L) | |||
| cTnI ≤0.03 (≤30) | 8 FN | 31 TN | 39 |
| cTnI >0.03 (>30) | 3 TP | 14 FP | 17 |
| Total | 11 | 45 | 56 |
| (F) | |||
| hs-cTnI (ng/L) | |||
| hs-cTnI <25 | 4 FN | 13 TN | 17 |
| hs-cTnI >25 | 8 TP | 31 FP | 39 |
| Total | 12 | 48 | 56 |
| (G) | |||
| hs-cTnI (ng/L) | |||
| hs-cTnI ≤15.6/≤34 | 5 FN | 20 TN | 25 |
| hs-cTnI >15.6/>34 | 6 TP | 47 FP | 53 |
| Total | 11 | 67 | 78 |
| (H) | |||
| hs-cTnI (ng/L) | |||
| hs-cTnI ≤15.6/≤25 | 1 FN | 10 TN | 11 |
| hs-cTnI >15.6/>25 | 8 TP | 61 FP | 69 |
| Total | 7 | 73 | *80 |
n=80 since 2 male patients were included twice; 1 patient with 1 FP+1TN and the other patient with 1TP+1FN.
4. Discussion
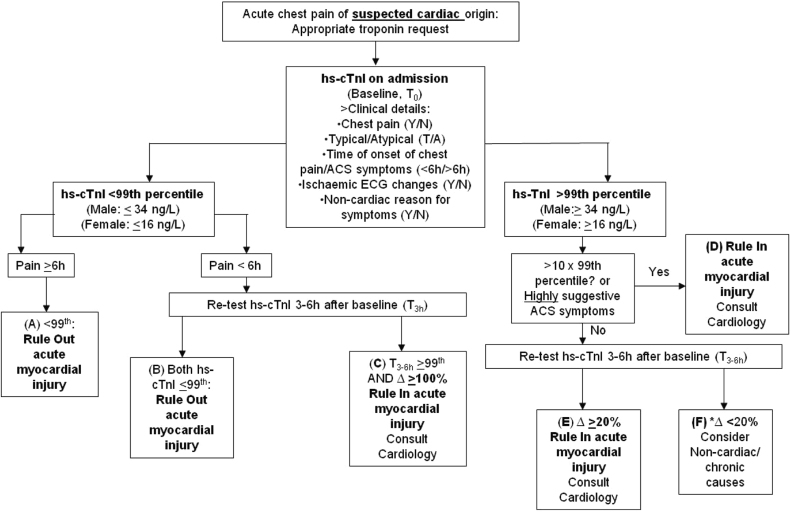
Our approach to implementing the hs-cTnI assay has extended beyond analytical verification to clinical corroboration. This approach looks across the total testing pathway and is particularly necessary for troponin given its importance in diagnosing MI and permitting timely and appropriate management decisions for patients presenting with signs and symptoms suggestive of ACS. In a previous multi-centre study in Ireland, troponin requesting was shown to increase yearly but without an increase in patients presenting with ACS symptoms [23]. Such apparently inappropriate troponin requesting was shown more recently in our own hospital (Khan et al., 2015: personal communication), imposing considerable pressure on cardiology and laboratory resources. Our approach to implementing the hs-cTnI assay has therefore involved development of a clinical algorithm to specify the clinical presentations which merit troponin requesting i.e. to rule in/out ACS (Khan et al., 2015: personal communication). This algorithm also incorporates clinical questions to encourage appropriate troponin ordering. The use of the clinical algorithm, together with a biochemical algorithm for guiding result interpretation (Fig. 5) we have given focus to improving consideration of the pre-test probability of ACS, by ordering clinicians, and the interpretation of troponin results including circumstances when serial testing and/or cardiology input are justified. Such work is necessary to help ensure optimal use of clinical and laboratory resources and selection of appropriate patient pathways.
Fig. 5.
Biochemical algorithm for use and interpretation of hs-cTnI testing in patients with acute chest pain of suspected cardiac origin. Δ refers to a troponin change (Rise or Fall, %). If hs-cTnI is >10×99th percentile, acute myocardial injury may be ruled in with up to 98% specificity (Abbott Diagnostics, personal communication). *Option for repeat troponin when ACS suspicion remains in a non-urgent setting.
Interpretation of troponin results can be challenging, particularly when we consider inappropriate testing (and retesting) in patients with a low pre-test probability of ACS, including those with non-specific (and non-cardiac) signs and symptoms but the possibility of occult cardiac injury. In such circumstances, published biochemical algorithms which utilise baseline and change values for ruling in/out ACS [24] are less useful. However, closing the knowledge gap regarding the effects of certain disease states on troponin measurements may eventually allow design of algorithms that balance simplicity of use with the complexity needed to incorporate different disease-adjusted (and/or age-adjusted) baseline and change thresholds to enhance diagnostic specificity. Until then, obtaining adequate diagnostic accuracy using currently established 99th percentile (gender-specific) and change (e.g. guideline-endorsed) criteria appears only possible using clinical protocols which incorporate judicious use of troponin testing, in the clinical context defined in current universal guidelines [1].
From an analytical verification perspective our outlier study has given the laboratory and its clinical users greater confidence in the new hs-cTnI assay. The lower critical outlier rate which we observed for this assay supports the study by Sawyer et al. [10] who reported no critical outliers. From our clinical correlation studies, patients with raised cTnI but not hs-cTnI results could be explained by the relatively poor imprecision at the 99th percentile for the cTnI assay. However factors beyond analytical imprecision, such as reagent pack size and analyser inactivity have though been shown to contribute to critical outliers [25]. Such factors may be considered logistically, in the routine delivery of the hs-cTnI assay. Our single hs-cTnI critical outlier was reproducible on two different Abbott Architect analysers and followed a period of storage (24 h) at 4 °C. We have therefore implemented duplicate analysis of any retrospective troponin requests to avoid false positive results, as this incident showed. Focusing initially on the analytical and pre-analytical stages of troponin testing, our approach to implementing the Abbott Architect hs-cTnI assay, a level 4 high sensitivity assay [2], agrees with all of the recommendations of a recent national audit of troponin testing [26]. We also strongly endorse the recommendation for assessing imprecision and maintaining control (using 3rd party QC material), particularly at the 99th percentile, as we are cognisant of the potential clinical implications when assay performance is undesirable at such thresholds, as highlighted previously by Apple and Jaffe [27]. Authors of the national audit also describe an approach for reporting troponin results in the presence of haemolysis [28]. Whilst we were amongst the majority of laboratories in that audit who automatically remove the troponin result when haemolysis thresholds have been exceeded, we would also support implementation of the suggested approach whereby significant troponin elevations can be reported in the presence of haemolysis and thereby help to minimise the risk of missing or delaying reports of clinically significant results.
In our assay discordance studies, subsequent clinical verification supported a marginally higher rate of ACS detection using the hs-cTnI assay, particularly when the overall and female 99th percentiles were used for evaluating male and female patient data, respectively. Although work is required to fully assess the added diagnostic and prognostic benefit associated with gender-specific 99th percentiles [29], they have been incorporated into our biochemical algorithm, in an attempt to maximise diagnostic sensitivity, corroborated by our clinical verification. This decision is also supported by Shah et al. [30], who used the Abbott Architect hs-cTnI assay and showed improved diagnosis of type 1 MI in females using the female 99th percentile (16 ng/L), rather than the overall (25 ng/L) threshold. Although our discordance study suggested possible loss of sensitivity using the male-specific 99th percentile (34 ng/L), our study was not designed to fully validate the diagnostic performance of such thresholds. Nevertheless, Shah et al. likewise reported a decrease in MI diagnosis using the higher male-specific threshold. For the 4 male patients with hs-cTnI <34 ng/L but cTnI >0.04 μg/L, results of serial measurements for these patients showed at least one value greater than the male-specific 99th percentile, mirroring the elevations observed with cTnI. Such testing was also in the context of ACS signs and symptoms, including diagnostic ECG changes (e.g. STEMI) and significant cardiac history (e.g. AMI and CABG) whereby one (hs-cTnI) result <34 ng/L would be unlikely to have changed management. Furthermore, men are reported to present with more typical ACS symptoms, reliable ECG changes and are more likely to be referred to a cardiologist, undergo angiography and revascularisation or receive secondary prevention [30]. Our decision to use gender-specific cut-offs therefore remained, with the added benefit of helping to rebalance the reported gender inequality in diagnosis and management of ACS [30]. Whilst the recent national audit on troponin testing [26] does not give specific recommendation for the use of gender-specific thresholds per se, it does recognise a need for assay manufacturers to provide gender-related data so that users can consider any such differences. The audit also commented upon the possible importance of age-related reference values, given the reported relationship of increasing age with higher troponin concentrations [31]. It is noteworthy that within our study cohort (698 patients), the median troponin concentration (hs-cTnI) at baseline increased significantly for each decade, from up to the age of 50 (Median (ng/L) [IQR]: 1.8 [1.1–3.9], n=159) to 10 fold higher levels for patients ≥80 years of age (Median (ng/L)[IQR]: 18.8 [4.9–41.6], n=170). Although age-related reference ranges are not reported by Abbott Laboratories, we would welcome further work in this area to define and clinically validate age-specific thresholds.
Despite the gain in sensitivity using gender-specific cut-offs, our data showed a disproportionate gain in apparent false positive (FP) results i.e. those without ACS but hs-cTnI >99th percentile which concerned our cardiology service, who anticipated a possible increase in unnecessary referrals. This was ameliorated using our biochemical algorithm with cardiology referral in low risk patients limited to those who had a Δ% change of >100% on serial measurement (Fig. 4, group C). This was a conservative decision, following discussions with our cardiologists, and was a preference to the optimal specificity (100%) associated with a 250% change, as reported by Keller et al. [32] who used the Abbott Architect hs-cTnI assay. In the same study, acceptably high specificity (98%) was still achieved using this lower change criterion. Nevertheless, it is important to highlight that such elevations reported with the hs-cTnI assay, but not the cTnI assay, may well reflect significant myocardial injury, which would otherwise have been missed with the contemporary sensitive assay. It is of further importance to highlight that lowering the diagnostic threshold not only increases the diagnosis of MI, particularly for women, but reduces mortality and morbidity when implemented [30], [33], [34]. The ability to use lower thresholds is directly attributable to the superior imprecision of hs-cTn assays and helps to identify more patients at risk of recurrent MI and death that would otherwise not be detected using the less analytically precise cTnI assay [34] and would be unlikely to be considered as appropriate candidates for cardiology referral or secondary prevention therapies. Furthermore, the analytical improvement which underpins hs-cTn assays also translates into improved risk stratification by permitting reliable identification of patients with myocardial injury, irrespective of the pathophysiology, who have troponin concentrations below the overall 99th percentile but inclusive of the female-specific threshold, and worse prognosis than those with undetectable levels [6]. Since our study did not include a period of follow up, we could not confirm whether or not non-ACS patients with troponin elevations (>99th percentile) and probable myocardial injury had worse outcome than those without elevations. However previous studies involving patients in a non-cardiac setting [35] show worse prognosis for patients with troponin elevations above (than below) the 99th percentile. This highlights the clinical importance of such non-ACS elevations and gives possible rationale for a change to routine management and opportunity to modify prognosis even if ACS is ruled out. Through the provision of our clinical algorithm and engagement with our clinical users regarding the appropriate use and limitations of troponin testing, we have further attempted to address any concerns regarding specificity. The clinical implication of using the chosen change criterion, as well as gender-specific thresholds, should be monitored and, accordingly, this has been incorporated into our service's audit cycle and included for on-going discussion and liaison with our service users.
Unnecessary referrals to cardiology often includes patients with troponin increases related to possible renal and/or inflammatory mechanisms e.g. acute kidney injury, pneumonia or sepsis. In a study by de Filippi et al. [36], hs-cTnI (and hs-cTnT) measurements were above the 99th percentile in more than one third (two thirds for hs-cTnT) of patients with CKD and correlated moderately to eGFR, implying renal-specific mechanisms of cardiac injury [36]. In our study, the prevalence of raised CRP (>7 mg/L) and creatinine (M: >107, F: >86 μmol/L) concentrations and reduced eGFR (<60 ml/min/1.73 m2) was greater (1.6–1.7 fold, data not shown) for patients with troponin elevations, using either assay. We also observed significant changes in serial hs-cTnI measurements in patients without ACS but with concurrently changing CRP and/or creatinine results, consistent with renal and inflammatory influences on troponin release. Such troponin changes, in particular in evolving sepsis, were of a magnitude commensurate to a biochemical diagnosis of ACS (e.g. >50%), as previously noted [24]. A higher incidence of creatinine elevations is also reported amongst those with Type 2 MI [37], defined by myocardial injury due to imbalance between oxygen supply and demand, involving varied pathophysiology. In contrast to Type I MI, the prognosis of Type 2 MI is conflicting and treatment recommendations are absent [37]. However the presence of cardiac injury in Type 2 MI or any other secondary mechanism (acute or chronic) remains important given that troponin elevation alone confers poorer prognosis [7]. Whilst clinical presentation, ECG changes and troponin testing must be interpreted in concert, management strategies to improve prognosis in patients with secondary causes of myocardial injury should also be addressed.
In attempt to improve the interpretation of hs-cTn results, particularly in view of the patient groups described above, numerous studies have evaluated serial measurements against significant change criteria, including the Relative Change Value (RCV). Aarke et al.) [38] determined a RCV (−27%/+29%) specifically for haemodialysis patients. Interesting, this RCV is larger the change criterion (20%) proposed previously by the European Society of Cardiology (ESC) [24], which if used would confer loss of diagnostic specificity when such patients are evaluated. By contrast, change criteria may also be inappropriately large and reduce diagnostic sensitivity, particularly when evaluating higher troponin concentrations [39]. This latter observation is apparent for patients presenting very late [14], in the plateau phase of troponin release, especially when %Δ criteria are used, but also when serial results are evaluated using z-scores [16] and Δ criteria. For such patients, with troponin >99th percentile at baseline but rising less than the 20% criterion, a third measurement may be reasonable (Fig. 4, Group F). However, irrespective of the time of presentation from the onset of symptoms, a repeat troponin may be unnecessary when baseline troponin values are very large e.g. 10-fold×99th percentile (Fig. 4, Group D), given high specificity (98%) for ruling in acute myocardial injury at such levels (Abbott Diagnostics: personal communication). It is important to further acknowledge that the algorithm published by the ESC should be used as a template upon which to incorporate method-specific decision thresholds and ideally change criteria whose clinical performance has been validated. In studies involving the Abbott Architect hs-cTnI assay, Keller et al. [32] did, however, show good sensitivity using the 20% change criterion, which importantly included patients who were admitted late (>12 h) after the onset of chest pain. Although there were no such late-presenting patients in our cohort to verify such a change criterion, our clinical users have been reminded regarding such presentations. We have also emphasised the importance of appropriate troponin requesting, careful clinical correlation with cognisance of secondary causes of troponin elevations and the need to understand guidelines and decision thresholds for their use as much as for their limitations.
When baseline values are <99th percentile, lower change criteria e.g. 50% are reported to show high specificity (98%) using the Abbott Architect hs-cTi assay [32]. However we are also aware that such a %∆ is similar if not lower than this assay's RCVs (short-term: 49–69%) [40], [41] and does not discriminate for patients with evolving renal and inflammatory processes, as verified in our own study cohort. By contrast, one of our patients with ACS and troponin <99th percentile at baseline showed an increment of only 29%. However, this was weighted in the context of cardiac chest pain and significant ACS history and exemplifies the obligation for assessing the relative importance of each component of the triad, comprising clinical features, ECG and troponin changes, to enable accurate diagnosis and appropriate management [37].
When we consider troponin thresholds for ruling out acute myocardial injury, the 99th percentile at baseline (only) is appropriate for patients presenting ≥6 h after the onset of chest pain (Fig. 4, Group A), where good diagnostic performance across several hs-cTn assays has been demonstrated [4], [42]. By contrast, there is variable and undesirable performance for early presenters e.g. sensitivity 51–94% at <3 h [42] and conflicting evidence for the Abbott Architect hs-cTnI assay [32], [42]. In line with previous recommendations of the ESC and the UK National Institute for Health and Care Excellence (NICE) [24], [43], protocols for ruling out acute myocardial injury should involve a repeat troponin e.g. 3–6 h after baseline (Fig. 4, Group B: <6 h post-chest pain). Using the Abbott Architect hs-cTnI assay, Kavsak et al. [44] reported optimal sensitivity using this approach, even with repeat measurements taken as early as 90 min from baseline.
Although the Limit of Detection (LOD) for baseline samples shows optimal sensitivity for ruling out acute myocardial injury [32], current NICE guidelines do not support its use, due to undesirable imprecision at such concentrations [43]. The ESC has recently recommended an alternative to the 0 h/3 h ‘dichotomous′ algorithm [18], involving measurements at baseline, 1 h and the diagnostically superior absolute change (Δ) criteria, validated specifically for each assay. A 0/1 h ‘rule in’, ‘rule out’ and ‘observe’ algorithm has been conceived, which involves each assay's unique LOD (e.g. 2 ng/L for Abbott Architect hs-cTnI) at baseline for rule out. Using this threshold, we showed that it could be possible to rule out acute myocardial injury in almost 20% of patients and rule in acute myocardial injury in 15% of patients using baseline measurements alone. However it was not possible to fully evaluate this algorithm in the current study since almost two thirds of patients did not have serial measurements and therefore only a sub-group of study patients could be partitioned to one of the algorithm's three groups. In our cohort, all patients with AMI had baseline values above the LOD concentration, and higher (e.g. 6–7 ng/L), where 10% CVs are achievable as we show from routine internal quality control practices (CV=9.6%, at 6.3 ng/L), and others have corroborated [45]. With current hs-cTn assays, we consider this latter threshold to be more analytically desirable and warranting clinical verification. Our priorities for implementation have been appropriate troponin use, the transition to gender-specific ranges, new units (resulting in larger, whole-number values), an increase in detectable results and the use of established change criteria which have been extensively validated and verified [24], [30], [32]. Such changes alone have resulted in a paradigm shift for our laboratory staff and clinical users. However an evaluation of the ESC algorithm and its comparison to our proposed algorithm, in the context of appropriate troponin testing and retesting relative to the onset of chest pain, merits future research by our service.
5. Conclusions
On a national level, our work may help address inappropriate troponin testing, shown in the previous Irish multi-centre study [23], and in our recent internal study (Khan et al., 2015: personal communication). Our aim to optimise the judicious requesting of troponin testing and the post-analytical clinical actions will require subsequent audit to ascertain any impact on indiscriminate troponin requesting. Implementation of any assay into routine diagnostic use involves work beyond analytical verification procedures, as we have shown. As work continues universally to establish appropriate decision thresholds for interpreting troponin results, routine delivery of this test will require implementation of and adherence to algorithms that are consistent with the assay's analytical and diagnostic capabilities. This can be done most effectively when a laboratory engages continually with its clinical users and can work with them to reduce error throughout the total testing process and maximise patient outcome.
Declarations
Competing interests
None.
Funding
None.
Ethical approval
Mater Misericordiae University Hospital Ethics Committee (Reference: 1/378/1335).
Guarantor
MCF.
Contributorship
GRL wrote the manuscript; MCF and GRL were involved in the study design. BG was involved in analysing patient samples. TCAB and EM were involved in collating patient information. GRL was involved in data analysis. MCF was involved in initiating the study. IK and CMcG were involved in adjudication of ACS. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Acknowledgement
We thank the staff of the Department of Clinical Biochemistry and Diagnostic Endocrinology, MMUH for their support in facilitating investigations during routine diagnostic service.
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D. Third universal definition of myocardial infarction. Eur. Heart J. 2012;33(20):2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 2.Apple F.S. A new season for cardiac troponin assays: it's time to keep a scorecard. Clin. Chem. 2009;55(7):1303–1306. doi: 10.1373/clinchem.2009.128363. [DOI] [PubMed] [Google Scholar]
- 3.Apple F.S., Collinson P.O. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin. Chem. 2012;58(1):54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 4.Keller T., Zeller T., Peetz D., Tzikas S., Roth A., Czyz E. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Eng. J. Med. 2009;361:868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 5.Aldous S.J., Florkowski C.M., Crozier I.G., Elliott J., Georeg P., Lainchbury J.G. Comparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency department. Ann. Clin. Biochem. 2011;48(3):241–248. doi: 10.1258/acb.2010.010219. [DOI] [PubMed] [Google Scholar]
- 6.Schneider H.G. How low can we go: high-sensitivity troponin T in patients presenting with chest pain. Ann. Clin. Biochem. 2011;48(3):198–199. doi: 10.1258/acb.2011.011067. [DOI] [PubMed] [Google Scholar]
- 7.Kavsak P.A., Walsh M., Srinathan S., Thorlacius L., Buse G.L., Botto F. High sensitivity troponin T concentrations in patients undergoing noncardiac surgery: a prospective cohort study. Clin. Biochem. 2011;44(12):1021–1024. doi: 10.1016/j.clinbiochem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Omland T., Pfeffer M.A., Solomon S.D., de Lemos J.A., Røsjø H., Benth J.A. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 2013;61(12):1240–1249. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Pretorius C.J., Dimeski G., O'Rourke P.K., Marquart L., Tyack S.A., Wilgen U. Outliers as a cause of false cardiac troponin results: investigating the robustness of 4 contemporary assays. Clin. Chem. 2011;57(5):710–718. doi: 10.1373/clinchem.2010.159830. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer N., Blennerhassett J., Lambert R., Sheehan P., Vasikaran S.D. Outliers affecting cardiac troponin I measurement: comparison of a new high sensitivity assay with a contemporary assay on the Abbott ARCHITECT analyser. Ann. Clin. Biochem. 2014;51(4):476–484. doi: 10.1177/0004563213499737. [DOI] [PubMed] [Google Scholar]
- 11.Christenson R.H., Bunk D.M., Schimmel H., Tate J.R. Point: put simply, standardization of cardiac troponin I is complicated. Clin. Chem. 2012;58:165–168. doi: 10.1373/clinchem.2011.166140. [DOI] [PubMed] [Google Scholar]
- 12.Apple F.S., Morrow D.A. Delta cardiac troponin values in practice: are we ready to move absolutely forward to clinical routine? Clin. Chem. 2012;58(1):8–12. doi: 10.1373/clinchem.2011.175414. [DOI] [PubMed] [Google Scholar]
- 13.Reichlin T., Irfan A., Twerenbold R., Reiter M., Hochholzer W., Burkhalter H. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011;124:136–145. doi: 10.1161/CIRCULATIONAHA.111.023937. [DOI] [PubMed] [Google Scholar]
- 14.Mueller M., Blener M., Vafaie M., Doerr S., Keller T., Blankenberg S. Absolute and relative kinetic changes of high-sensitivity cardiac troponin T in acute coronary syndrome and in patients with increased troponin in the absence of acute coronary syndrome. Clin. Chem. 2012;58:209–218. doi: 10.1373/clinchem.2011.171827. [DOI] [PubMed] [Google Scholar]
- 15.Wu A.H.B., Lu Q.A., Todd J., Moecks J., Wlans F. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin. Chem. 2009;55(1):52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 16.Pretorious C.J., Cullen L., Parsonage W.A., Greenslade J.H., Tate J.R., Wilgen U., Ungerer J.P.J. Toward a consistent definition of a significant delta troponin with z scores: a way out of chaos? Eur. Heart J. 2014;3(2):149–157. doi: 10.1177/2048872613517084. [DOI] [PubMed] [Google Scholar]
- 17.Sandoval Y., Apple F.S. The global need to define normality: the 99th percentile value of cardiac troponin. Clin. Chem. 2014;60(3):455–462. doi: 10.1373/clinchem.2013.211706. [DOI] [PubMed] [Google Scholar]
- 18.Roffi M., Patrono C., Collette J.-P., Mueller C., Valgimilgi M., Andreotti F. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2015 [Google Scholar]
- 19.Christenson J., Innes G., McKnight D., Thompson C.R., Wong H., Yu E. A clinical prediction rule for early discharge of patients with chest pain. Ann. Emerg. Med. 2006;47(1):1–10. doi: 10.1016/j.annemergmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Hess E.P., Wells G.A., Jaffe A., Stiell I.G. A study to derive a clinical decision rule for triage of emergency department patients with chest pain: design and methodology. BMC Emerg. Med. 2008;8(3):1–10. doi: 10.1186/1471-227X-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGorrian C.M., Lyster S., Roy A., Tarrant H., Codd M., Doran P. Use of a highly-sensitive cardiac troponin I assay in a screening population for hypertrophic cardiomyopathy: a case-referent study. BMC Cardiovasc. Dis. 2013;13(70):1–9. doi: 10.1186/1471-2261-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apple F.S., Ler R., Murakami M.M. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin. Chem. 2012;58(11):1574–1581. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 23.Groarke J.D., Browne L., Margey R., McCann H.A., Blake G.J., Sugrue D.D., Mahon N.G. A multicentre analysis of troponin use in clinical practice. Ir. J. Med. Sci. 2013;182:185–190. doi: 10.1007/s11845-012-0853-2. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K., Mair J., Giannitsis E., Mueller C., Lindahl B., Blankenberg S. How to use high sensitivity troponins in acute cardiac care. Eur. Heart J. 2012;33(18):2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 25.Kavsak P.A., Clark L., Lancaster S., Don-Wauchope A.C. Within-run precision and outlier detection for the Abbott ARCHITECT cardiac troponin I assay. Ann. Clin. Biochem. 2014;51(4):512–514. doi: 10.1177/0004563214534400. [DOI] [PubMed] [Google Scholar]
- 26.McKeeman G.C., Auld P.W. A national survey of troponin testing and recommendations for improved practice. Ann. Clin. Biochem. 2015;52(5):527–542. doi: 10.1177/0004563214568163. [DOI] [PubMed] [Google Scholar]
- 27.Apple F.S., Jaffe F.S. Clinical implications of a recent adjustment to the high-sensitivity cardiac troponin T assay: user beware. Clin. Chem. 2012;58(11):1599–1600. doi: 10.1373/clinchem.2012.194985. [DOI] [PubMed] [Google Scholar]
- 28.Lyon M.E., Ball C.L., Krause R.D., Slotsve G.A., Lyon A.W. Effect of haemolysis on cardiac troponin T determination by the ElecsysR 2010 immunoanalyzer. Clin. Biochem. 2004;37(8):698–701. doi: 10.1016/j.clinbiochem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Jaffe A.S., Apple F.S. High-sensitivity cardiac troponin assays: isn't it time for equality? Clin. Chem. 2014;60(1):7–9. doi: 10.1373/clinchem.2013.217927. [DOI] [PubMed] [Google Scholar]
- 30.Shah A.S.V., Griffiths M., Lee K.K., McAllister D.A., Hunter A.L., Ferry A.V. High-sensitivity cardiac troponin and the under diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKie P.M., Heublein D.M., Scott C.G., Gantzer M.L., Mehta R.A., Rodeheffer R.J. Defining high-sensitivity cardiac troponin concentrations in the community. Clin. Chem. 2013;59(7):1099–1107. doi: 10.1373/clinchem.2012.198614. [DOI] [PubMed] [Google Scholar]
- 32.Keller T., Zeller T., Ojeda F., Tzikas S., Lillpopp L., Sinning C. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 33.Mills N.L., Churchhouse A.M., Lee K.K., Anand A., Gamble D., Shah A.S. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA. 2011;305:1210–1216. doi: 10.1001/jama.2011.338. [DOI] [PubMed] [Google Scholar]
- 34.Mills N.L., Lee K.K., McAllister D.A., Churchhouse A.M., MacLeod M., Stoddart M. Implications of lowering threshold of plasma troponin concentration in diagnosis of myocardial infarction: cohort study. BMJ. 2012;344:e1533. doi: 10.1136/bmj.e1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devereaux P.J., Chan M.T., Onso-Coello P., Walsh M., Berwanger O., Villar J.C. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 36.deFilippi C., Seliger S.L., Kelley W., Duh S.-H., Hise M., Christensen R.H. Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin. Chem. 2012;58(9):1342–1351. doi: 10.1373/clinchem.2012.185322. [DOI] [PubMed] [Google Scholar]
- 37.Collinson P., Lindahl B. Type 2 myocardial infarction: the chimaera of cardiology? Heart. 2015 doi: 10.1136/heartjnl-2014-307122. [DOI] [PubMed] [Google Scholar]
- 38.Aakre K.M., Rørass T., Petersen P.H., Svarstad E., Sellevoll H., Skadberg O. Weekly and 90-minute biological variations in cardiac troponin T and cardiac troponin I in hemodialysis patients and healthy controls. Clin. Chem. 2014;60(6):838–847. doi: 10.1373/clinchem.2013.216978. [DOI] [PubMed] [Google Scholar]
- 39.Khalili H., de Lemos J.A. What constitutes a relevant change in high sensitivity troponin values over serial measurement? Clin. Chem. 2014;60(6):803–805. doi: 10.1373/clinchem.2014.223669. [DOI] [PubMed] [Google Scholar]
- 40.Nordenskjöld A.M., Alström H., Eggers K.M., Fröbert O., Jaffe A.S., Venge P., Lindahl B. Short- and long-term individual variation in cardiac troponin in patients with stable coronary artery disease. Clin. Chem. 2013;59(2):401–409. doi: 10.1373/clinchem.2012.191700. [DOI] [PubMed] [Google Scholar]
- 41.Apple F.S., Murakami M.M., Wians W.F., Ler R., Kaczmarek J.M., Wu A.H.B. Short-term biological variation of cardiac troponin I measured with three high-sensitivity assays. Clin. Chem. 2011;57:A97. [Google Scholar]
- 42.Hoeller R., Giménez M.R., Reichlin T., Twerenbold R., Zellweger C., Moehring B. Normal presenting levels of high-sensitivity troponin and myocardial infarction. Heart. 2013;99(21):1567–1572. doi: 10.1136/heartjnl-2013-303643. [DOI] [PubMed] [Google Scholar]
- 43.Myocardial infarction Early rule out using high sensitivity troponin tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High sensitive Troponin I and AccuTnI+3 assays). National Institute for Health and Care Excellence, 2014. NICE diagnostics guidance 15. 〈https://www.nice.org.uk/guidance/dg15〉 (accessed 15.10.15).
- 44.Kavsak P.A., Worster A., You J.J., Oremus M., Shortt C., Phan K. Ninety-minute vs 3-h performance of high-sensitivity cardiac troponin assays for predicting hospitalization for acute coronary syndrome. Clin. Chem. 2013;59(9):1406–1407. doi: 10.1373/clinchem.2013.208595. [DOI] [PubMed] [Google Scholar]
- 45.Collinson P.O., Gaze D., Goodacre S. The clinical and diagnostic performance characteristics of the high sensitivity Abbott cardiac troponin I assay. Clin. Biochem. 2015;48(4–5):275–281. doi: 10.1016/j.clinbiochem.2014.12.017. [DOI] [PubMed] [Google Scholar]