Abstract
Objectives
Thyroglobulin (Tg) measurements are used to monitor for residual thyroid tissue in patients with differentiated thyroid cancer (DTC) after thyroidectomy and radioiodine ablative therapy. In recent years highly sensitive Tg assays have been developed. In this study the analytical performance of the new Roche Elecsys Tg II assay was evaluated and compared with the well documented Access2 Tg assay (Beckman–Coulter).
Design and methods
Analytical performance was examined using various Clinical and Laboratory Standards Institute (CLSI) evaluation protocols. Tg negative patient sera were used to establish an upper reference limit (URL) for the Elecsys Tg II assay.
Results
Non-linearity, drift and carry-over according to CLSI EP10 and EP6 in a measuring range of 0.04–500 ng/mL were non-significant. Total precision according to CLSI EP5 was 10% at a Tg concentration of 0.08 ng/mL. A patient serum comparison performed according to a modified CLSI EP9 protocol showed a significant difference of a factor of approximately 1.4, despite using an identical CRM calibrator. The Elecsys Tg II assay measured Tg with a two-fold higher sensitivity than the Access2 assay. Finally, using human sera without Tg, an URL of 0.05 ng/mL was determined.
Conclusions
In our hands the highly sensitive Elecsys Tg II assay shows a good analytical performance and a higher sensitivity compared to the Access2 Tg assay. An URL of 0.05 ng/mL for the Elecsys Tg II assay was determined which may improve the clinical utility of the assay for the detection of residual DTC or disease recurrence.
Keywords: Thyroglobulin, Roche Elecsys Tg II assay, validation, reporting limit
1. Introduction
Thyroglobulin (Tg) is a large glycoprotein (about 660 kDa) that is produced exclusively by thyroid follicular cells and therefore is widely used as a tumor marker in differentiated thyroid cancer (DTC) [1]. Depending on the stage of disease, DTC patients are treated by total thyroidectomy and lymph node dissection if necessary, sometimes followed by radioiodine ablation [2]. Tg is measured to monitor patients after this ablative therapy for residual DTC during thyroxine (T4) replacement or, to increase the diagnostic sensitivity of the Tg measurement, after withdrawal of T4 or administration of synthetic TSH [2], [3]. Detectable Tg suggests persistence or recurrence of disease. Second generation highly sensitive Tg immunoassays are now available with improved analytical sensitivity and precision at low concentrations [4]. The term functional sensitivity or functional detection limit is defined as the concentration at which a between-run CV of 20% is observed, and is thus a measure of an assay’s precision at low analyte levels (without considering bias) [5], [6]. It is a recommended parameter for Tg assay characterization and often serves as the reporting limit [7]. However, immunoassays are usually subject to positive or negative bias near the zero point and assay bias may invalidate decision limits of Tg assays [8]. To circumvent this problem, we previously suggested the use of an upper reference limit (URL) that is based on the one-sided (e.g. 99.9%) confidence interval of measurements in sera that are essentially free of Tg and, as such, takes assay bias into account if present [8]. This appears to be a better approach to determination of a decision limit of a Tg assay. To determine such confidence intervals, we measured Tg levels in patient samples selected from treated DTC patients who did not have clinical or imaging evidence of tumor, and who had undetectable serum Tg levels after stimulation with recombinant TSH.
Immunometric Tg sandwich assays may suffer from interference by Tg autoantibodies causing falsely low or negative Tg measurements [9], [10], [11]. These antibodies are detected at diagnosis in approximately 25% of DTC patients and, when present, may complicate Tg monitoring and management of these patients [12]. Guidelines recommend that every Tg test should be accompanied by a Tg autoantibody measurement to validate the Tg result [3]. In addition, quantitative Tg antibody measurements may have potential value as a surrogate post-operative DTC tumor marker in patients with anti-Tg autoantibodies [13]. Therefore, in this study the presence or absence of anti-Tg antibodies was noted in all human samples used in this study.
Roche® have developed a highly sensitive Elecsys Tg II assay and an Elecsys anti-Tg test. In this study we evaluated and compared the Elecsys Tg II assay with the Access2 Tg assay of Beckman Coulter, which is currently used in our laboratory [8]. In addition, anti-Tg autoantibody interferences were examined by using the Roche® Elecsys anti-Tg assay. Finally, a clinical validation using Tg negative patient samples to assess possible assay bias and establish the reporting limit of the Elecsys Tg II assay was performed.
2. Materials and methods
2.1. Materials/patient samples
Patient samples were obtained from a patient population in our academic hospital which serves as a referral institute (level 1) for thyroid carcinoma. Patient samples were selected based on their Tg and anti-Tg values that had been measured previously by luminescence immunoenzymometric assay (LIEMA) (Access2, Beckman Coulter, Brea, CA, USA) and an in-house assay for anti-Tg autoantibodies (see Methods section) [8], respectively. Values in pmol/L were converted to ng/mL by dividing by a factor of 1.5, based on a molecular weight of 660 kDa for Tg. Blood samples were collected in serum gel tubes (Vacutainer blood collection tubes, Becton Dickinson, Franklin Lakes, NJ, USA) and serum was stored in plain tubes at –20 °C until analysis. Samples were anonymized and only used for assay validation and method comparison and therefore approval by the Scientific Ethical Committee was not required. Unless specified otherwise, samples without anti-Tg autoantibodies were selected, as determined with our in-house anti-Tg assay. For most experiments Tg-positive patient samples were used, with Tg concentrations up to 7500 ng/mL (Access2, Beckman Coulter). To determine the URL of the Tg II assay, 12 Tg negative blood samples were selected from DTC patients who underwent complete thyroidectomy followed by radioiodine ablation between 2007 and 2013 (Group A). Blood samples from these patients were collected in 2013 or 2014. At this time these patients did not have clinical or imaging evidence of tumor, and Tg levels after stimulation with recombinant TSH were below the reporting limit of the Access2, and Elecsys Tg II assay (defined as 3 times the within-run SD of duplicate measurements in the lowest concentration range). A second group (Group B) of 10 Tg negative patients was selected from DTC patients who underwent complete thyroidectomy followed by radioiodine ablation between 2004 and 2013, in whom Tg levels before and after stimulation with recombinant TSH were below the reporting limit of the Access2 Tg assay of 0.13 ng/ml [8].
2.2. Methods
Samples were measured on a Modular E170 random access analyzer (Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturer’s specifications. The following assays, calibrators and controls were used according to the manufacturer’s instructions: Elecsys Tg II, Tg II CalSet, PreciControl Thyro Sensitive, Elecsys anti-Tg, anti-Tg CalSet, and PreciControl Thyro AB (Roche). In addition, in-house prepared serum pools were used as controls. A single lot number of all reagents was used for all measurements unless specified otherwise. Samples were measured on an Access2 random access analyzer (Beckman Coulter) according to the manufacturer’s specifications, using the Access2 Thyg reagent pack, Access2 substrate, Access2 washbuffer II, Access2 Thyg sample diluent and Thyg calibrator set (Beckman Coulter). In-house prepared serum pools of patient samples were used as controls. The presence of anti-Tg autoantibodies was assessed by an in-house test in which patient serum is incubated with radiolabelled Tg, after which Tg tracer immune complexes, if present, are precipitated by 6.5% polyethylene glycol (PEG). Results are given in % of total tracer precipitated. For our in-house anti-Tg assay a 1.65% cut-off was established based on the 99.5th percentile of a population of 3000 patient samples (Bhattacharya analysis), which gives an optimal positive predictive value. This cut-off corresponds to the 60 U/L calibrator of the commercially available BRAHMS anti-Tgn RIA (1.9% precipitation in our in-house assay; data not shown), which is calibrated against the International Reference Preparation (IRP) MRC 65/93.
2.3. Protocols
A preliminary evaluation was conducted using the Clinical and Laboratory Standards Institute (CLSI; Wayne, PA, USA) protocol EP10-A3 where 10 samples with low (0.17 ng/ml), medium (234 ng/ml) or high (467 ng/ml) concentration Tg were measured in a predefined order on 5 consecutive days. Linearity was assessed by the CLSI EP6-A protocol. In short, five serum pools were prepared by adding low and high serum pools in 1+3, 1+1 and 3+1 proportions and measured at random and in triplicate. A modified CLSI protocol EP5-A2 was used to investigate precision. Method comparison between the Elecsys Tg II assay and the Access2 Tg assay was performed according to a modified EP9-A2 protocol. The reference interval was verified consistent with CLSI protocol C28-A2. Interference testing of hemolysis, lipemia or icterus was performed according to CLSI protocol EP7-A2.
2.4. Calculations
EP EvaluatorTM (David G. Rhoads Associates Inc. Consultants, South Burlington, VT, USA; release 10) and GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA, USA; version 5.03) were used. For the EP10 preliminary evaluation, the allowable total error (TEA) was set at 20%, in line with the allowable total error for measuring Tg according to Westgard (21.9%) [14]. The allowable bias and coefficient of variation (CV) were defined at 5%, which is even lower than the desirable specifications for inaccuracy (10.4%) and imprecision (7.0%) according to Westgard. For EP6 linearity, the allowable nonlinearity was defined as 5% of the measuring range. EP5 protocols for Tg and anti-Tg were analyzed with the EP Evaluator complex precision module. For the calculation of the imprecision at the lowest Tg concentration of 0.08 ng/mL in the Roche assay, two (out of 15) measurements were excluded as outliers (of >3 standard deviations [SD]). EP9 data were analyzed with the alternative (quantitative) method comparison module. For verification of the reference interval (C28 protocol) three samples were excluded from analysis based on the presence of Tg autoantibodies, as measured with the Elecsys anti-Tg II assay. Comparison of dilution in either Multi Assay diluent or serum was analyzed using Graphpad Prism using a paired t-test.
In order to assess Tg levels below the instrument’s reporting limit of 0.04 ng/mL a supplementary calibration curve was constructed using Relative Light Units (RLUs) obtained in samples with levels slightly above 0.04 ng/mL. Since the RLU’s were in a perfect linear relationship with Tg concentration, Tg concentrations below 0.04 ng/mL could be read from the extrapolated part of this curve. Since the measurements had been done in duplicate, the within-run SD at the lowest range could be estimated. From the extrapolated Tg concentrations the upper limit of the one-sided 99.9% confidence interval, h was adopted as the URL, and was calculated as mean value (=bias)+3.1*SD, in which the SD value was calculated as √(SD2+0.5*within-run SD2) to convert into the total SD for single measurements.
3. Results
3.1. Analytical performance
In the preliminary evaluation by CLSI EP10 there was no significant intercept, slope, carry-over, nonlinearity or drift observed in the Elecsys Tg II assay (data not shown). Linearity of the assay was assessed in more detail using CLSI protocol EP6 covering in total a range of 0.04–500 ng/mL. In a lower range experiment (range 0.05–23 ng/mL) less than 5% non-linearity was observed. In the additional EP6 experiment (range 0.07–474 ng/mL) non-linearity remained <5% for the extended range up to 500 ng/mL (data not shown).
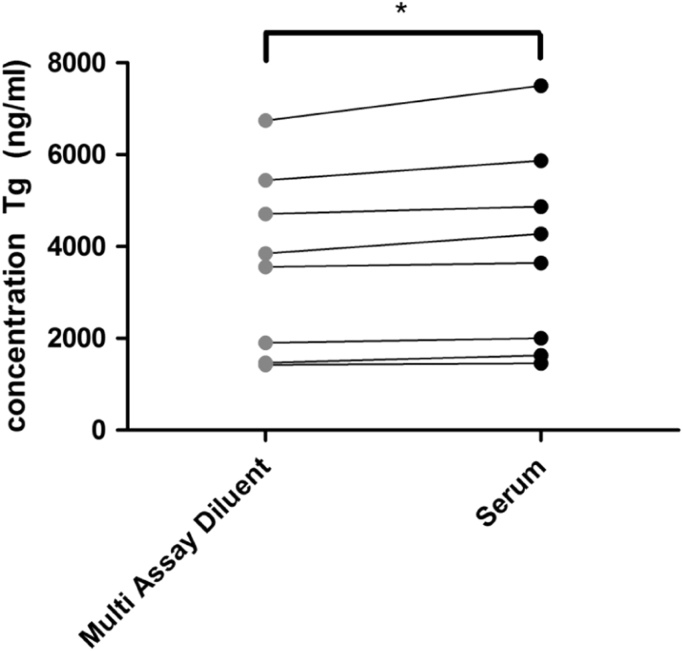
Patients’ samples with Tg levels exceeding the range of the calibration curve must be diluted sometimes up to a hundred fold for more reliable measurement. According to the manufacturer’s instructions and Modular instrument settings, samples with a Tg concentration exceeding the upper measuring limit of 500 ng/mL will be automatically diluted 10-fold with Multi Assay Diluent. To assess whether dilution with diluent behaves identically to matrix-identical human serum, Tg was assayed in 8 patient samples with a Tg concentration higher than 400 ng/mL after manual 10-fold dilution in either Multi Assay Diluent or Tg negative serum. Significantly lower Tg values (up to 10%) in samples diluted with Multi Assay Diluent versus human serum were observed (Fig. 1). Apparently, the Multi Assay Diluent shows non-specific matrix effects.
Fig. 1.
Comparison of sample dilution in either Multi Assay Diluent or Tg negative serum. Results were analyzed using a paired t-test. *p<0.05.
Evaluation of the within-run and total (within-run and between-day) precision performance of the Elecsys Tg II assay was performed according to an adjusted EP5 protocol in which four Tg pools were measured in duplicate during 15 days with low (0.08 and 0.73 ng/mL), medium (2.40 ng/mL) and high (66.5 ng/mL) Tg. The total imprecision (%CV) at these different Tg concentrations was 9.5%, 2.5%, 2.5% and 2.5%, respectively (Table 1).
Table 1.
Imprecision of Elecsys Tg II assay at four different thyroglobulin (Tg) concentrations. The within-run, between-day and total (within-run and between-day) imprecision is expressed as standard deviation (SD) and coefficient of variation (CV).
| Tg concentration (ng/mL) |
Within run |
Between day |
Total |
|||
|---|---|---|---|---|---|---|
|
SD |
CV |
SD |
CV |
SD |
CV |
|
| (ng/mL) | (%) | (ng/mL) | (%) | (ng/mL) | (%) | |
| 0.08 | 0.0063 | 7.6 | 0.0048 | 5.8 | 0.0079 | 9.5 |
| 0.73 | 0.0094 | 1.3 | 0.0157 | 2.2 | 0.0183 | 2.5 |
| 2.40 | 0.036 | 1.5 | 0.047 | 1.9 | 0.059 | 2.5 |
| 66.5 | 0.830 | 1.2 | 1.460 | 2.2 | 1.679 | 2.5 |
To examine possible effects of a change of reagent lot number on Tg results, 10 patient samples were measured with two different reagent lot numbers of the Elecsys Tg II assay. Samples with a Tg concentration below 1 ng/mL were highly comparable, with a non-significant slope of 1.006±0.072 and a non-significant intercept of −0.022±0.029 (data not shown). When samples with higher Tg concentrations up to 77 ng/mL were included, a significant slope was observed of 1.048±0.008 (with a non-significant intercept), which was considered clinically non-relevant and therefore an acceptable difference.
3.2. Method comparison
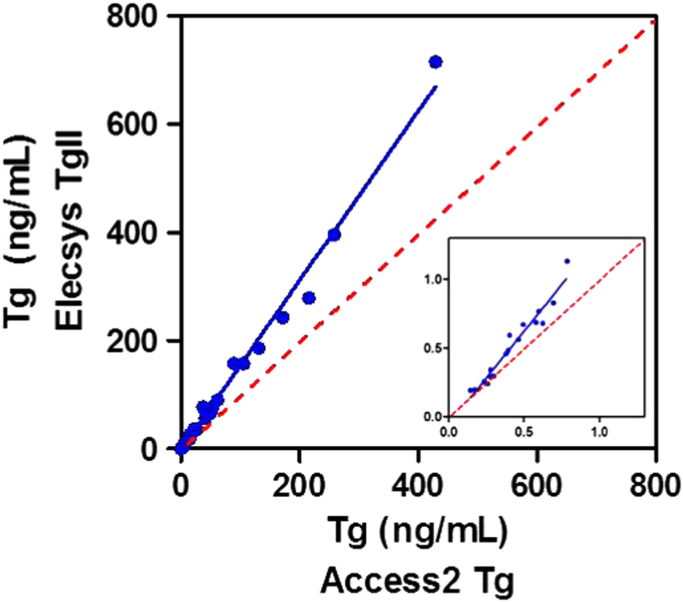
Tg results for patients’ samples determined with the Elecsys Tg II assay and the Access2 Tg assay were compared. For this purpose, 50 patient samples were selected with Tg concentrations ranging from 0.09 to 430 ng/mL that did not contain anti-Tg antibodies, as measured by the Access2 Tg assay and in-house assay, respectively, as currently used in our laboratory. The samples were used for a single measurement with the Elecsys Tg II assay. A correlation coefficient of 0.99 and a significant slope of 1.53 with no significant intercept was observed (Fig. 2). A subset of low concentration Tg samples (up to 1 ng/mL), yielded a correlation coefficient of 0.96 with a significant slope of 1.34 without a significant intercept (Fig. 2, insert). These results indicate that the Elecsys Tg II assay measures Tg by a factor of approximately 1.4 higher in comparison with the Access2 assay.
Fig. 2.
Thyroglobulin (Tg) samples measured either with the Elecsys Tg II assay or Access2 Tg assay. Tg concentrations ranging from 0.02 to 715 ng/mL (n=50) or from 0.02 to 1 ng/mL (n=17; insert). Indicated are the linear regression line (solid line) and 1:1 line (dotted line).
In addition, the Tg 95% reference interval specified by the manufacturer was verified according to CLSI protocol C28, by measuring Tg concentration in 65 subjects deemed to be healthy selected from the laboratory’s patient population (29 men and 36 women, age range 21–60 years). Measured Tg levels ranged from 3.2 to 57.5 ng/mL. As specified by the protocol, less than 10% of the results appeared outside the manufacturer’s reference interval of 3.5–77 ng/mL, verifying this interval (data not shown).
3.3. Interferences
According to the manufacturer’s instructions the Elecsys Tg II assay is unaffected by icterus (bilirubin<1128 μmol/L), hemolysis (hemoglobin<0.373 mmol/L) or lipemia (Intralipid<22.8 mmol/L). These claims were verified by measuring Tg in two serum pools with Tg concentrations of 0.09 ng/mL and 43 ng/mL that were spiked with different concentrations of either lysed red blood cells (maximum concentration of hemoglobin 1.4 mmol/L), conjugated or unconjugated bilirubin (maximum concentration 850 μmol/L), or intralipid (maximum concentration of triglycerides 25 mmol/L). At all concentrations tested, differences in Tg concentration between spiked and unspiked serum were less than 10% suggesting no relevant interference effects of icterus, hemolysis or lipemia on the Elecsys Tg II assay (data not shown).
In general, the most important analytical interference for Tg immunoassays is caused by the possible presence of autoantibodies against Tg, which can falsely lower Tg results in immunometric assays [9], [10], [15]. Therefore, a Tg measurement in a patient’s sample should always be accompanied by measurement of anti-Tg antibodies [3]. The analytical performance of the Roche Elecsys anti-Tg immunoassay was investigated in human serum according to an adjusted CLSI EP5 protocol in which 4 different samples containing antibodies against Tg (with concentrations within the measuring range of 10–4000 IU/mL) were analyzed in duplicate during 15 days. The measured total (within-run and between-day) imprecision (CV) of two serum pools with anti-Tg levels of 47 IU/mL and 544 IU/mL was 15.2% and 5.2%, respectively, and of the Roche PreciControl thyro AB1 (47 IU/ml anti-Tg) and AB2 (152 IU/ml anti-Tg) 16.9% and 8.2%, respectively (Table 2). Notably, on the Levy-Jennings plot a significant (p<0.001) shift in results was observed incidentally after changing the reagent pack (with identical lot number) (data not shown), contributing to the above mentioned imprecision. From these precision data one can estimate that the limit of quantitation (20%CV) lies around 26 IU/mL.
Table 2.
Imprecision of Elecsys anti-Tg II assay at four different anti-Thyroglobulin (anti-Tg) concentrations. The within-run, between-day and total (within-run and between-day) imprecision is expressed as standard deviation (SD) and coefficient of variation (CV).
| Anti-Tg antibody concentration (U/mL) |
Within-run |
Between-day |
Total |
|||
|---|---|---|---|---|---|---|
|
SD |
CV |
SD |
CV |
SD |
CV |
|
| (U/mL) | (%) | (U/mL) | (%) | (U/mL) | (%) | |
| 47 | 3.62 | 7.7 | 7.01 | 15.0 | 7.90 | 16.9 |
| 55 | 4.50 | 8.2 | 7.00 | 12.8 | 8.32 | 15.2 |
| 152 | 6.64 | 4.4 | 10.55 | 7.0 | 12.47 | 8.2 |
| 544 | 15.62 | 2.9 | 23.66 | 4.3 | 28.35 | 5.2 |
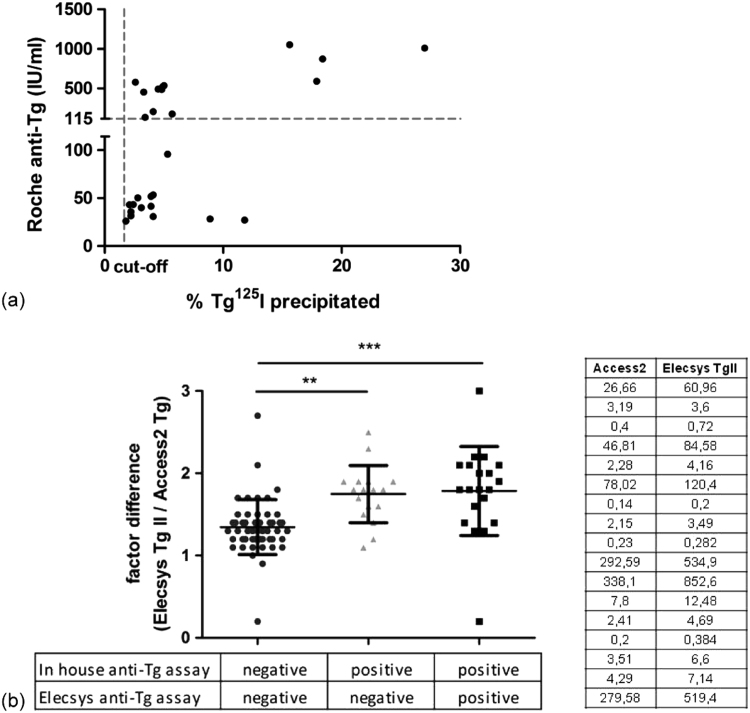
Furthermore, the Roche Elecsys anti-Tg assay was compared with our currently used in-house anti-Tg assay. The Roche assay has been standardized against IRP MRC 65/93, and recommends a threshold value of 115 IU/mL corresponding to the 94th percentile of anti-Tg measurements in healthy, euthyroid subjects. This recommended cut-off level for a ‘positive’ anti-Tg is mainly set for diagnosing autoimmune thyroid disease. For comparison of both anti-Tg assays, anti-Tg antibody-positive patient samples (as determined in our in-house assay) with measurable Tg were selected to measure anti-Tg antibodies with the Elecsys anti-Tg assay. In 17 out of 40 samples anti-Tg levels were below the level of 115 IU/mL in the Roche method (25–96 IU/mL; Fig. 3A). Notably, the ratio of Tg measured in the Roche and Beckman methods in these 17 samples was significantly higher than the ratio in antibody-negative samples and not different from the Tg ratio in the remaining 23 samples that were anti-Tg positive by both methods (Fig. 3B). These results suggest the need to use different cut-off values for either detecting autoimmune thyroid disease or for detection of Tg autoantibodies that potentially affect Tg assays.
Fig. 3.
(a) Comparison of anti-Tg values measured with the Roche Elecsys anti-Tg assay and the in-house assay. The dashed lines indicate the cut-off levels for both methods as defined by the manufacturer (Roche anti-Tg assay; 115 IU/mL) or according to the in house validation report (1.65% Tg 125I precipitated). (b) Comparison of factor difference in Tg measurements (between Elecsys Tg II and Access2 Tg assays) in patient samples with or without anti-Tg antibodies as determined with the in-house PEG precipitation assay or the Elecsys anti-Tg assay. The group in the middle shows the factor difference of those samples in which Elecsys anti-Tg levels were below the cut-off level of 115 IU/mL (anti-Tg antibody negative), but the PEG precipitation was >1.65% (anti-Tg antibody positive).The Tg results of this group are listed in the table. **p< 0.01, ***p<0.001 using one-way ANOVA.
3.4. Upper reference limit of Tg
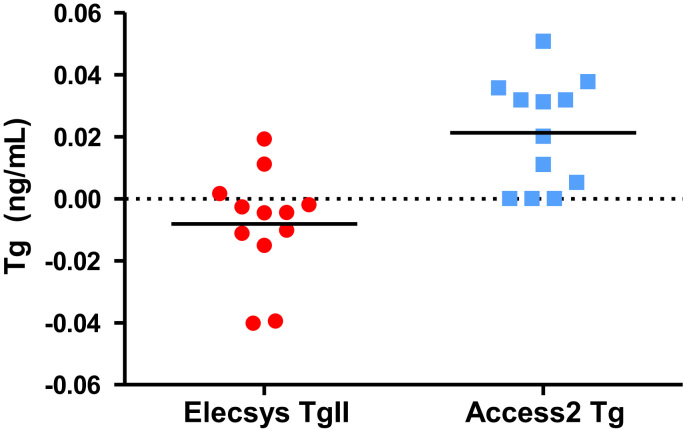
To investigate possible assay bias and establish the upper reference limit of the Elecsys Tg II assay, sera from 12 Tg negative patients (Group A) were measured. As expected, all values were below the reporting limit of the assay (<0.04 ng/mL). By extrapolation from a secondary standard curve the reported RLU were converted to Tg concentrations. A non-significant bias of −0.008 ng/mL was calculated (Fig. 4). The calculated URL from these measurements was 0.052 ng/mL. Similar results were obtained when 7 of these samples were measured using a different lot number of assay reagent. The data were further verified in a different run using a second group of 15 Tg negative patients (group B). Calculating Tg levels in samples of these patients (using a new secondary standard curve) showed a small but statistically significant bias of −0.017 ng/mL (P=0.006) and an URL of 0.054 ng/mL (data not shown). Thus, measuring Tg negative patient samples with the Elecsys Tg II assay in different runs either a small or undetectable negative bias and a maximum calculated URL of 0.054 ng/ml was observed.
Fig. 4.
Assay bias and upper reference limit of Elecsys Tg II assay. Tg negative patient samples were measured using the Elecsys Tg II assay or Access2 Tg assay. Extrapolation of RLU data was used to calculate Tg concentrations below the detection limits of both assays.
4. Discussion
In this study the second generation Elecsys Tg II assay was examined. In a preliminary evaluation according to CLSI protocols EP5, EP6, EP7 and EP10 no deviations were found. Dilution in Multi Assay Diluent demonstrated up to 10% lower values compared to dilution in Tg negative serum. Dilution in Multi Assay Diluent can be performed automatically by the random access analyzer and one can argue that a consistent 10% difference in Tg value at this concentration can be considered clinically irrelevant. Multi Assay Diluent may be used for (automatic) dilutions up to 10 fold, and (if necessary) Tg negative serum should be used for higher (manual) dilutions. In accordance with the manufacturer’s literature, no significant interference by hemolysis, bilirubin or lipemia was detected. Furthermore, no relevant lot to lot variation was observed when testing one additional different lot number of the Elecsys Tg II assay. Analysis of the assay imprecision demonstrated a total CV (%) of 9.5, 2.5, 2.5 and 2.5 at Tg levels of 0.08, 0.73, 2.40 and 67 ng/mL, respectively. These values are lower than the values claimed by the manufacturer (4.5% and 5.9% at Tg concentrations of 1.10 and 0.3 ng/mL, respectively), although a CV below 0.3 ng/ml is not specified, and lower than the allowable total error for measuring Tg according to Westgard of 21.9% [14].
Evaluation of the Elecys Tg II assay was continued with a CLSI EP9 protocol to compare (anti-Tg antibody-negative) patient samples between the Access2 Tg and Elecsys Tg II assays. Interestingly, a difference in the results was observed of approximately factor 1.4 (varying from factor 1.3 for Tg concentrations up to 1 ng/mL, to a factor 1.5 for Tg concentrations up to 439 ng/mL) between the two methods, with the Elecsys Tg II assay reporting higher values. Both assays use calibrators that are standardized against Certified Reference Material (CRM) 457 of the Community Bureau of Reference (BCR) of the European Union [16]. Measurement of the Elecsys Tg II assay calibrators with the Access2 Tg assay and vice versa resulted in differences in Tg values (data not shown), demonstrating non-commutability. Thus, despite the use of certified reference materials, results from various immunoassays can differ from one another, most likely due to different sources and nature of the kit calibrators, the heterogeneity of the analyte and different sets of antibodies with different specificities towards Tg isoforms that are used in each assay. This is further exemplified by a comparison study of various Tg assays (immunoassays, RIA and mass spectrometry) [17]. In light of this observed difference it is important to stress the importance of using the same immunoassay when monitoring individual patients over time, as recommended by the American Thyroid Association guideline [2]. Because of heterogeneity of Tg in individual patients, all patients should be re-baselined when switching Tg methods [2].
Since guidelines mandate that immunometric Tg measurements for follow up of DTC patients are accompanied by quantitative anti-Tg measurements, the Elecsys anti-Tg assay was evaluated and compared with our in-house anti-Tg method. It is known that different anti-Tg assays display wide variability [12,15,18]. For comparison of both anti-Tg assays, anti-Tg antibody-positive patient samples (as determined in our in-house assay) with measurable Tg were selected for measurement of anti-Tg antibodies with the Elecsys anti-Tg assay. Results were categorized according to anti-Tg result, and the ratio of Tg measured by the Roche and Beckman methods was depicted. Notably, the ratio increased in samples which were positive for anti-Tg suggesting less interference in the Elecsys Tg II assay by anti-Tg. Furthermore, in 17 out of 40 samples anti-Tg levels were below the level of 115 IU/Ml in the Roche method, whereas the Tg ratio in these samples did not differ from the ratio in anti-Tg positive samples. This observation, added to the fact that these samples were anti-Tg positive in the in-house assay, suggests the presence of interfering auto-antibodies in these samples also. This underlines the need for a lower cut-off level than that used for diagnosis of autoimmune thyroid disease in order to avoid such high false-negative rates. Based on the estimated limit of quantitation and comparison with the in house anti-Tg assay a reporting limit of 26 IU/mL is proposed, which is in line with the functional sensitivity of this assay reported by others [19].
For the monitoring of DTC patients, the sensitivity of the Tg assay used is of utmost importance, since detection of Tg indicates remnant or recurrence of functional thyroid tissue, which may guide clinical decisions. Immunoassays can be subject to positive or negative bias near the zero point, which may invalidate decision limits of the assay [8]. Therefore, we decided to investigate the URL of the Tg assay by assessing the confidence interval of Tg negative patient samples, as previously described [8]. Using two groups of Tg negative patients the Elecsys Tg II assay showed no or a very small negative bias and a calculated 99.9% confidence limit of up to 0.052 ng/mL. Since all measurements were performed in duplicate, the within-run SD (analytical variation) at the utmost lower range could be estimated. This SD was lower than the SD of the group of Tg negative samples, suggesting the presence of an additional, sample-dependent factor. Comparison of the estimated within-run SD of a sample with very low Tg levels between the Elecsys Tg II (SD 0.0116 ng/mL) and Access2 (SD 0.0185*1.3 ng/mL; personal communication with author of [8], and corrected for a slope of 1.3 as observed in the EP9 comparison), suggests that the Elecsys Tg II assay can measure Tg with a 2-fold higher sensitivity. This improved sensitivity of the Elecsys Tg assay may possibly avoid the need for TSH stimulation for Tg monitoring in DTC patients [4], [11].
5. Conclusion
In our hands the second-generation highly sensitive Elecsys Tg II assay shows good analytical performance and a higher sensitivity in comparison with the Access2 Tg assay. An URL of 0.05 ng/mL for Tg negative patient samples was determined, which may contribute to a clinical decision point concerning residual presence or recurrence of DTC.
Acknowledgments
We would like to acknowledge R.J. Daams, M.L.A. Driessen-van Kol and B.C.B.M. van der Steen for their practical help, and Roche Diagnostics as supplier of materials.
References
- 1.Lin J.D. Thyroglobulin and human thyroid cancer. Clin. Chim. Acta. 2008;388:15–21. doi: 10.1016/j.cca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;2016(26):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kloos R.T., Mazzaferri E.L. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J. Clin. Endocrinol. Metab. 2005;90:5047–5057. doi: 10.1210/jc.2005-0492. [DOI] [PubMed] [Google Scholar]
- 4.Giovanella L., Clark P.M., Chiovato L., Duntas L.H., Elisei R., Feldt-Rasmussen U. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur. J Endocrinol. 2014;171:R33–R46. doi: 10.1530/EJE-14-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay I.D., Bayer M.F., Kaplan M.M., Klee G.G., Larsen P.R., Spencer C.A. American Thyroid Association assessment of current free thyroid hormone and thyrotropin measurements and guidelines for future clinical assays. The Committee on Nomenclature of the American Thyroid Association. Clin. Chem. 1991;37:2002–2008. [PubMed] [Google Scholar]
- 6.Armbruster D.A., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008;29(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 7.Baloch Z., Carayon P., Conte-Devolx B. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 8.Ross H.A., Netea-Maier R.T., Schakenraad E., Bravenboer B., Hermus A.R., Sweep F.C. Assay bias may invalidate decision limits and affect comparability of serum thyroglobulin assay methods: an approach to reduce interpretation differences. Clin. Chim. Acta. 2008;394:104–109. doi: 10.1016/j.cca.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Spencer C.A. Clinical review: Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC) J. Clin. Endocrinol. Metab. 2011;96:3615–3627. doi: 10.1210/jc.2011-1740. [DOI] [PubMed] [Google Scholar]
- 10.Spencer C.A., Bergoglio L.M., Kazarosyan M., Fatemi S., LoPresti J.S. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2005;90:5566–5575. doi: 10.1210/jc.2005-0671. [DOI] [PubMed] [Google Scholar]
- 11.Evans C., Tennant S., Perros P. Thyroglobulin in differentiated thyroid cancer. Clin. Chim. Acta. 2015;444:310–317. doi: 10.1016/j.cca.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Verburg F.A., Luster M., Cupini C., Chiovato L., Duntas L., Elisei R. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23:1211–1225. doi: 10.1089/thy.2012.0606. [DOI] [PubMed] [Google Scholar]
- 13.Feldt-Rasmussen U., Verburg F.A., Luster M., Cupini C., Chiovato L., Duntas L. Thyroglobulin autoantibodies as surrogate biomarkers in the management of patients with differentiated thyroid carcinoma. Curr. Med. Chem. 2014;21:3687–3692. doi: 10.2174/0929867321666140826120844. [DOI] [PubMed] [Google Scholar]
- 14.Ricos C., Alvarez V., Cava F. Current databases on biological variation: pros, cons and progress. Scand. J. Clin. Lab. Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 15.Spencer C., Fatemi S. Thyroglobulin antibody (TgAb) methods-strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best. Pract. Res. Clin. Endocrinol. Metab. 2013;27:701–712. doi: 10.1016/j.beem.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Feldt-Rasmussen U. Purification and assessment of stability and homogeneity of human thyroglobulin reference material (CRM 457) Exp. Clin. Endocrinol. 1994;102:87–91. [Google Scholar]
- 17.Netzel B.C., Grebe S.K., Carranza Leon B.G., Castro M.R., Clark P.M., Hoofnagle A.N. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J. Clin. Endocrinol. Metab. 2015;100:E1074–E1083. doi: 10.1210/jc.2015-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickett A.J., Jones M., Evans C. Causes of discordance between thyroglobulin antibody assays. Ann. Clin. Biochem. 2012;49:463–467. doi: 10.1258/acb.2012.012008. [DOI] [PubMed] [Google Scholar]
- 19.Spencer C., Petrovic I., Fatemi S., LoPresti J. Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications. J. Clin. Endocrinol. Metab. 2014;99:4589–4599. doi: 10.1210/jc.2014-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]