Abstract
Background
Evidence is conflicting regarding the association between Helicobacter pylori infection and diabetes mellitus. The study objective was to examine associations of H. pylori infection, gastric ulcers and duodenal ulcers, with diabetes mellitus.
Methods
This cross-sectional study was undertaken using coded data from the computerized database of Maccabi Health Services in Israel, on 147,936 individuals aged 25–95 years who underwent the urea breath test during 2002–2012. Multiple logistic regression models were conducted, while adjusting for known risk factors for diabetes mellitus.
Results
A H. pylori positive test was recorded for 76,992 (52.0%) individuals and diabetes for 12,207 (8.3%). The prevalence of diabetes was similar in individuals with and without H. pylori infection, but this association was modified (P for heterogeneity 0.049) by body mass index (BMI): adjusted odds ratio (aOR) 1.16 (95% confidence intervals (CI) 1.04–1.29) in persons with BMI<25 kg/m2 versus aOR 1.03 (95% CI 0.98–1.08) in persons with BMI≥25 kg/m2. Diabetes mellitus prevalence was higher in persons with gastric (aOR 1.20 (95% CI 1.06–1.34)) and duodenal ulcers (aOR 1.20 (95% CI 1.12–1.28)) compared to persons without these diagnoses.
Conclusions
In this large population-based study, we demonstrated significant positive associations, albeit of small magnitude, of H. pylori infection and peptic disease with diabetes. The long-term gastric inflammation and associated-damage to the gastric mucosa might play a role in such associations.
Introduction
Diabetes mellitus (DM) is a major public health problem, with increasing prevalence globally [1]. The major burden (90–95%) is caused by type 2 DM, which typically develops in adulthood and is characterized by variable levels of insulin resistance, impaired insulin secretion and increased glucose production [2]. Established risk factors for type 2 DM include sociodemographic factors [3–5] and lifestyle e.g., obesity, physical inactivity, poor diet and smoking [3, 6, 7]. However, these factors do not fully explain the occurrence of the disease.
Infections have been postulated to play a role in the pathogenesis of type 2 DM [8, 9], through the involvement of low grade systemic inflammation. The bacterium Helicobacter pylori is of particular interest, given its chronic course and persistent inflammation.
H. pylori colonizes the human stomach for decades, usually without causing apparent disease. H. pylori causes chronic gastritis, gastric and duodenal ulcers, and in rare occasions gastric cancer and lymphoma, which usually develop during adulthood [10]. H. pylori infection might also play a role in extragastric diseases [11], such as unexplained iron deficiency anemia and idiopathic thrombocytopenia [12].
Several studies have addressed the relationship between H. pylori infection and DM; however, the findings are conflicting [8, 9, 13–17]. Two meta-analyses of observational studies reported significant positive associations between H. pylori infection and DM with pooled odds ratios of 1.33 and 2.0 [18, 19]. However, the crude results of the original studies were combined in these meta-analyses, rather than the adjusted results; therefore, the impact of confounding could not be ruled out. Consideration of age, socioeconomic status and body mass index (BMI) are especially important, given the associations of these factors with both H. pylori infection and DM [4–7, 20, 21]. Adjustment for these and other confounders was limited in many of the previous studies on H. pylori and DM. Moreover, most of the evidence on the association between H. pylori infection and DM is based on small samples. Large population-based studies are essential, since the postulated relationship between H. pylori infection and DM appears to be of small magnitude. Gastric inflammation and damage to the gastric mucosa may be at the base of an association between H. pylori infection and DM. However, the role of peptic ulcers, which develop following persistent gastric inflammation in DM, has rarely been assessed.
The aim of the current large population-based study was to examine associations of H. pylori infection, and of gastric and duodenal ulcers, with DM. We also examined whether such associations differ by age and BMI. Our hypothesis was that positive associations would be found of H. pylori infection, and of related gastroduodenal diseases, with DM prevalence.
Materials and methods
Study design and population
A cross-sectional study was conducted using anonymous data retrieved from the computerized database of Maccabi Health Services (MHS). MHS is the second largest health maintenance organization in Israel, with two million insured persons (~25% of the Israeli population). The source population comprised adults aged ≥25 years insured in MHS who performed the Urea Breath Test (UBT) between 2002 and 2012. Individuals were excluded from the study population if they used anti-H. pylori eradication therapy (according to purchases of medications) or proton pump inhibitors four weeks before the UBT. Persons with a history of bariatric surgery and recent cancer diagnosis (within three years from the UBT) were also excluded.
Data extraction and definitions
Information was obtained on the results of UBT, diagnoses of DM, hypertension, dyslipidemia, gastric ulcers, duodenal ulcers, H. pylori-gastritis, birth year, sex, town of residence, smoking (ever, never, and unknown) and BMI (weight in kilograms (kg)/height2 in meters (m)).
H. pylori positivity was defined based on the UBT result; individuals were classified as H. pylori positive if they had a UBT result >3.5, and negative if the test result was ≤3.5. If more than one UBT was performed, we used the first test to determine H. pylori positivity. The presence of gastroduodenal diseases was defined based on the following codes of the international classification of disease, 9th revision (ICD-9): H. pylori-gastritis (41.86), gastric ulcer (531) and duodenal ulcer (532).
DM was defined based on entry to the MHS DM registry. Persons having at least one of the following criteria were classified as diabetic: 1) HbA1c ≥7.25%; 2) glucose ≥200 mg/dL; 3) diagnosis in the medical chart of DM based on any ICD-9 relevant code, and HbA1c ≥6.5% or glucose>125 mg/dL; 4) purchase of anti-diabetic medications twice in the previous two months [22]. Anti-diabetic medications can be purchased by prescription only, and data are routinely sent to primary care physicians for final validation.
Hypertension was defined based on entry to the MHS hypertension registry [23]. Individuals were classified as hypertensive if they had two or more physician's diagnoses of hypertension or diagnosis from hospital records, and two or more blood pressure measurements with systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. If a diagnosis of hypertension was lacking, four documented abnormal blood pressure measurements were required with ≥50% of systolic and diastolic blood pressure measurements of >160 and >90 mmHg, respectively. Persons with ≥6 dispensed hypertension medications were also classified as hypertensive [23].
Dyslipidemia was determined based on diagnoses codes (ICD-9th code 272 and its subhierarchy, and equivalent MHS internal codes), documented by a physician twice or more in the medical record.
Socioeconomic status was defined based on the socioeconomic rank of place of residence at the level of town, as defined by the Israel Central Bureau of Statistics [24]. The ranks are on a scale from 1 to 10, with higher ranks representing a higher socioeconomic status. This aggregative socioeconomic index reflects a combination of basic characteristics of a specific geographical unit investigated, mainly financial resources of the residents, housing conditions, motorization level, education and employment [24]. Communities with socioeconomic ranks of 1–5, 6–7 and 8–10 were classified as low, intermediate and high, respectively. Country of birth was classified as: Israel, Former Soviet Union (FSU), America/Europe (excluding countries of the FSU), North Africa/Asia and other/unknown.
Statistical analysis
The Chi square test was used to examine associations of DM with H. pylori infection and with related gastroduodenal diseases. The Chi square test was also used to examine differences between H. pylori infected and uninfected individuals in sociodemographic variables, BMI and smoking; as well as differences between diabetic and non-diabetic patients according to these variables. Age-adjusted Mantel-Haenszel Odds Ratios (ORMH) and the corresponding 95% confidence intervals (CIs) were calculated to examine associations of H. pylori infection (based on UBT), gastric ulcer and duodenal ulcer, with DM prevalence.
Multivariable logistic regression models were fitted, while adjusting for age, socioeconomic status, country of birth, hypertension, dyslipidemia, BMI and smoking. Adjusted OR (aOR) and 95% CIs were obtained from these models. The analyses were performed in stratification by age group (25–29, 30–39, 40–49, 50–59, 60–69 and 70–95 years) and BMI (<25 versus ≥25 kg/m2), separately for each exposure variable: H. pylori infection (based on UBT), gastric ulcer, and duodenal ulcer. Additional analysis was done while adding both variables H. pylori infection (based on UBT) and gastric ulcer, as well as a different analysis with the variables H. pylori infection and duodenal ulcer.
Using the chi square test for heterogeneity, the effect modification by age and BMI was assessed.
Two-sided P<0.05 was considered significant.
Data were analysed using SPSS version 23 (IBM, Armonk, New York, USA) and WinPepi [25]
Ethical consideration
The study protocol was approved by the Helsinki committee of Assuta Medical Center and the ethics committee of Tel Aviv University. Since this is a retrospective study in which we used coded (anonymized) administrative data from electronic medical records, exemption from informed consent was granted by the Helsinki committee.
Results
Among 158,059 individuals aged 25–95 years who underwent UBT between 2002 and 2012, 147,936 met study inclusion criteria and their data were analyzed. The mean age of the eligible persons was 42.8 years (standard deviation 12.7); about 39% of them were males, and 24.3% were born in the FSU. In total, 76,965 (52.0%) were H. pylori positive (UBT>3.5). Diagnoses of H. pylori-gastritis, gastric ulcer and duodenal ulcer were documented in 50,035 (33.8%), 3153 (2.1%) and 10,366 (7.0%) individuals, respectively. DM was determined in 12,2207 (8.3%) persons and obesity (BMI≥30 kg/m2) in 25,910 (17.5%).
The prevalence of H. pylori infection (based on UBT results) increased from 48.2% in persons aged 25–29 years to 55.1% and 56.9% in persons aged 30–39 and 40–49 years, respectively; and decreased gradually in older age groups. The prevalence of H. pylori infection differed according to country of birth, being highest in those born in the FSU countries (59%), and lowest (36%) in those born in the Americas/Europe (Table 1).
Table 1. H. pylori infection prevalence according to sociodemographic characteristics.
| Total | H. pylori positive, N (%) | |
|---|---|---|
| Age (years) | ||
| 25–29 | 22,547 | 10,866 (48.2%) |
| 30–39 | 46,357 | 25,542 (55.1%) |
| 40–49 | 39,473 | 22,452 (56.9%) |
| 50–59 | 22,014 | 10,737 (48.8%) |
| 60–69 | 12,116 | 5274 (43.5%) |
| 70–95 | 5429 | 2094 (38.6%) |
| Sex, males | ||
| Men | 58,173 | 30,787 (52.9%) |
| Women | 89,763 | 46,178 (51.4%) |
| Socioeconomic status rank a | ||
| Low (1–5) | 60,282 | 35,652 (59.1%) |
| Intermediate (6–7) | 39,500 | 19,672 (49.8%) |
| High (8–10) | 38,790 | 17,113 (44.1%) |
| Missing | 9364 | 4528 (48.4%) |
| Country of birth | ||
| Israel | 96,180 | 48,831 (50.8%) |
| Former Soviet Union countries | 35,885 | 21,024 (58.6%) |
| Asia/ North Africa | 5379 | 2955 (54.9%) |
| Europeb / America | 7009 | 2550 (36.4%) |
| Other/unknown | 3483 | 1605 (46.1%) |
| BMI (kg/m2) c | ||
| <18.5 | 4139 | 1879 (45.4%) |
| 18.5–24 | 62,053 | 31,131 (50.2%) |
| 25–29 | 49,636 | 26,301 (53.0%) |
| ≥30 | 25,910 | 14,261 (55.0%) |
| Missing | 6198 | 3393 (54.7%) |
| Smoking | ||
| Ever | 20,913 | 11,950 (57.1%) |
| Never | 86,540 | 44,069 (50.9%) |
| Unknown | 40,483 | 20,946 (51.7%) |
| Hypertension | 33,317 | 16,426 (49.3%) |
| No hypertension | 114,619 | 60,539 (52.8%) |
| Dyslipidemia | 46,637 | 22,905 (49.1%) |
| No dyslipidemia | 101,299 | 54,060 (53.4%) |
a Socioeconomic status(SES) rank of city/town of residence; 1–5 represents low SES; 6–7, intermediate SES; and 8–10, high SES.
b Excluding countries of the Former Soviet Union.
c BMI: body mass index; kg: kilogram; m: meter
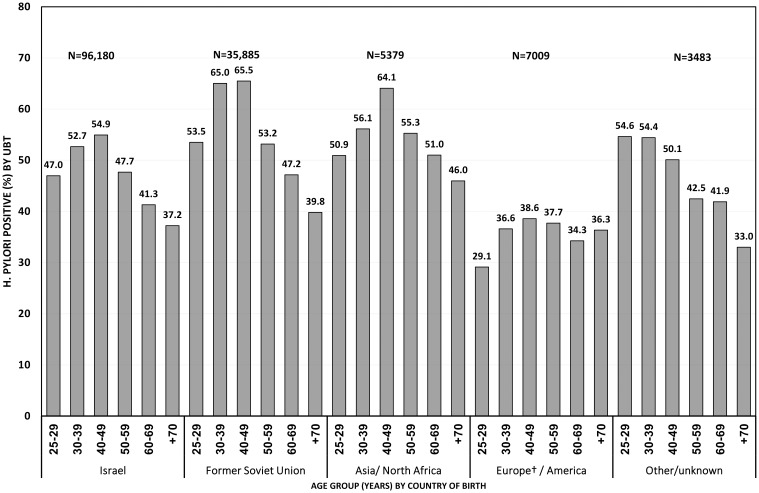
The pattern of the age-specific prevalence of H. pylori infection found in the entire cohort (Table 1) was also evident for subgroups of persons who were born in Israel, in the FSU and in Asia/North Africa, but not for the subgroup of persons who were born in Europe and the Americas (Fig 1).
Fig 1. Prevalence of H. pylori infection (%) (urea breath test>3.5) by age group (in years) and country of birth; Maccabi Health Services, Israel 2002–2012.
† Excluding countries that belonged to the Former Soviet Union.
The prevalences of H. pylori infection was similar between men and women. A significant gradual decrease (P<0.001) was observed in the prevalence of H. pylori infection according to socioeconomic status of place of residence, and a significant increase was found with increasing BMI (Table 1). H. pylori infection according to UBT was associated with an increased likelihood of having a diagnosis code of H. pylori-gastritis (age-adjusted Mantel-Haenszel OR 3.86 (95% CI 3.76–3.94) and gastric ulcer (age-adjusted Mantel-Haenszel OR 1.20 (95% CI 1.11–1.28)).
The prevalence of DM increased significantly (P<0.001) with increased age and BMI, and decreased according to socioeconomic rank of place of residence (P<0.001). It also differed according to country of birth (S1 Table). DM was slightly more common in men than in women (P<0.001), and markedly more prevalent in individuals with hypertension and/or dyslipidemia (S1 Table).
Associations of H. pylori infection, and of gastric and duodenal ulcer, with DM
Overall, in a crude analysis, prevalences of DM among H. pylori infected and uninfected patients were 7.9% vs 8.6%. Higher prevalence was found in persons with a diagnosis of gastric ulcer (14.8% vs 7.6%) and duodenal ulcer (14.1% vs 8.1%) than in those without such diagnoses.
Age-adjusted and age-stratified results
The age-stratified prevalences of DM according to each independent variable (H. pylori infection by UBT, gastric ulcer and duodenal ulcer), and BMI, are presented in Table 2.
Table 2. Prevalence of diabetes mellitus according to H. pylori infection, and gastric and duodenal ulcers, by age group and BMI.
| Age 25–29 years | Age 30–39 years | Age 40–49 years | Age 50–59 years | Age 60–69 years | Age 70–95 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: all BMI categories | Total | DM (%) | Total | DM (%) | Total | DM (%) | Total | DM (%) | Total | DM (%) | Total | DM (%) |
| H. pylori positive | 10,866 | 0.7 | 25,542 | 2.2 | 22,452 | 6.8 | 10,737 | 17.5 | 5274 | 26.8 | 2094 | 30.8 |
| H. pylori negative | 11,681 | 0.5 | 20,815 | 1.8 | 17,021 | 6.2 | 11,277 | 15.8 | 6842 | 25.9 | 3335 | 31.9 |
| Gastric ulcer | 295 | 0.3 | 678 | 2.9 | 783 | 8.7 | 680 | 21.3 | 448 | 32.1 | 269 | 33.1 |
| No gastric ulcer | 22,252 | 0.6 | 45,679 | 2.0 | 38,690 | 6.5 | 21,334 | 16.5 | 11,668 | 26.1 | 5160 | 31.4 |
| Duodenal ulcer | 775 | 1.4 | 2308 | 2.9 | 2637 | 8.3 | 2405 | 19.5 | 1458 | 29.6 | 783 | 31.5 |
| No duodenal ulcer | 21,772 | 0.6 | 44,049 | 2.0 | 36,836 | 6.4 | 19,609 | 16.3 | 10,658 | 25.8 | 4646 | 31.1 |
| B: BMI<25 kg/m2 | ||||||||||||
| H. pylori positive | 6524 | 0.2 | 12,417 | 0.7 | 8910 | 0.2 | 3256 | 6.6 | 1350 | 14.1 | 553 | 19.3 |
| H. pylori negative | 7756 | 0.2 | 11,226 | 0.6 | 7583 | 1.5 | 3804 | 5.1 | 1934 | 13.3 | 879 | 20.0 |
| Gastric ulcer | 177 | 0.0 | 330 | 0.6 | 335 | 2.1 | 197 | 5.6 | 126 | 17.5 | 71 | 18.3 |
| No gastric ulcer | 14,103 | 0.2 | 23,313 | 0.6 | 16,158 | 1.8 | 6863 | 5.8 | 3158 | 13.5 | 1361 | 19.8 |
| Duodenal ulcer | 427 | 0.7 | 1021 | 0.7 | 968 | 4.0 | 705 | 7.2 | 385 | 15.3 | 193 | 21.2 |
| No duodenal ulcer | 13,853 | 0.2 | 22,622 | 0.6 | 15,525 | 1.6 | 6355 | 5.6 | 2899 | 13.4 | 1239 | 19.5 |
| C: BMI≥25 kg/m2 | ||||||||||||
| H. pylori positive | 3559 | 1.8 | 11,645 | 4.2 | 12,787 | 10.5 | 7268 | 22.7 | 3839 | 31.6 | 1464 | 35.6 |
| H. pylori negative | 3146 | 1.3 | 8443 | 3.8 | 8941 | 10.3 | 7277 | 21.7 | 4840 | 31.1 | 2337 | 36.8 |
| Gastric ulcer | 103 | 1.0 | 312 | 5.8 | 432 | 14.1 | 473 | 27.9 | 315 | 38.4 | 185 | 38.9 |
| No gastric ulcer | 6602 | 1.6 | 19,776 | 4.0 | 21,296 | 10.4 | 14,072 | 22.0 | 8364 | 31.0 | 3616 | 36.2 |
| Duodenal ulcer | 271 | 2.6 | 1126 | 5.2 | 1577 | 11.4 | 1652 | 25.0 | 1050 | 35.0 | 557 | 38.4 |
| No duodenal ulcer | 6434 | 1.5 | 18,962 | 3.9 | 20,151 | 10.4 | 12,893 | 21.8 | 7629 | 30.8 | 3244 | 36.0 |
BMI: Body mass index; DM: diabetes mellitus
Adjustment for age showed increased likelihood for DM in relation to H. pylori infection [age-adjusted ORMH 1.10 (95% CI 1.05–1.14)], gastric ulcer [age-adjusted ORMH 1.31 (95% CI 1.18–1.46)] and duodenal ulcer [age-adjusted ORMH 1.25 (95% CI 1.17–1.33)] (Table 3A).
Table 3. Age adjusted and age specific odds ratios and 95% confidence intervals of the associations of H. pylori infection, and gastric and duodenal ulcers, with diabetes mellitus, by age (in years) and BMI (< and >25 kg/m2).
| Pooled age groups | Age 25–29 | Age 30–39 | Age 40–49 | Age 50–59 | Age 60–69 | Age 70–95 | P for age heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| ORMH (95% CI)a | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| A: all BMI categories | ||||||||
| H. pylori positive | 1.10 (1.05–1.14)b | 1.51 (1.08–2.13) | 1.21 (1.06–1.38) | 1.10 (1.02–1.20) | 1.13 (1.05–1.21) | 1.05 (0.97–1.14) | 0.95 (0.84–1.07) | 0.02 |
| Gastric ulcer | 1.31 (1.18–1.46)c | 0.50 (0.08–3.97) | 1.46 (0.93–2.29) | 1.38 (1.07–1.77) | 1.38 (1.14–1.66) | 1.34 (1.10–1.65) | 1.08 (0.83–1.40) | 0.5 |
| Duodenal ulcer | 1.25 (1.17–1.33)d | 2.47 (1.33–4.60) | 1.43 (1.11–1.85) | 1.34 (1.16–1.54) | 1.25 (1.12–1.39) | 1.21 (1.07–1.36) | 1.13 (0.96–1.32) | 0.13 |
| B: BMI<25 kg/m2 | ||||||||
| H. pylori positive | 1.16 (1.05–1.29) | 1.19 (0.58–2.43) | 1.16 (0.83–1.60) | 1.28 (1.01–1.62) | 1.32 (1.08–1.61) | 1.07 (0.87–1.31) | 0.96 (0.73–1.25) | 0.4 |
| Gastric ulcer | 1.10 (0.82–1.46) | 0.99 (0.99–1.00) | 0.97 (0.24–3.92) | 1.19 (0.56–2.54) | 0.97 (0.52–1.79) | 1.36 (0.85–2.18) | 0.91 (0.49–1.68) | 0.8 |
| Duodenal ulcer | 1.38 (1.17–1.61) | 3.62 (1.10–11.99) | 1.10 (0.51–2.36) | 2.53 (1.80–3.57) | 1.31 (0.97–1.78) | 1.17 (0.87–1.58) | 1.11 (0.77–1.61) | 0.004 |
| C: BMI≥25 kg/m2 | ||||||||
| H. pylori positive | 1.04 (1.00–1.09) | 1.49 (0.93–2.06) | 1.11 (0.96–1.28) | 1.02 (0.94–1.12) | 1.06 (0.98–1.15) | 1.03 (0.94–1.12) | 0.95 (0.83–1.09) | 0.29 |
| Gastric ulcer | 1.34 (1.19–1.51) | 0.61 (0.09–4.43) | 1.48 (0.92–2.40) | 1.42 (1.08–1.87) | 1.37 (1.12–1.68) | 1.39 (1.10–1.75) | 1.12 (0.83–1.52) | 0.7 |
| Duodenal ulcer | 1.18 (1.10–1.27) | 1.71 (0.79–3.73) | 1.35 (1.03–1.78) | 1.11 (0.94–1.30) | 1.19 (1.06–1.34) | 1.21 (1.06–1.39) | 1.11 (0.92–1.34) | 0.7 |
BMI: Body mass index; CI: confidence intervals; OR: Odds ratio
a Age-adjusted OR by Mantel-Haenzel. Each independent variable (H. pylori infection, gastric ulcer and duodenal ulcer) was analyzed separately
b P = 0.05,
c P = 0.2,
d P = 0.08 by chi square test for heterogeneity across BMI categories.
There was evidence (P = 0.02 for heterogeneity) for a positive association between H. pylori infection and DM prevalence, which differed by age: OR range of 1.51–1.10 for ages 25–59 years, and non-significant associations for ages 60–95 years (Table 3). The association between gastric ulcer and DM prevalence was statistically significant for ages 40–69, although the test for heterogeneity was not statistically significant (P = 0.5). The strength of the association of duodenal ulcer with DM prevalence differed slightly by age group (P = 0.13 by chi square test for heterogeneity) (Table 3A).
The heterogeneity test showed borderline statistical significance when pooling the age-adjusted ORMH of the associations of H. pylori infection and duodenal ulcer with DM across BMI categories (P = 0.05 and P = 0.08, respectively) (Table 3A–3C).
Multivariable analysis
In pooled multivariable analyses, the positive associations of H. pylori infection, gastric ulcer and duodenal ulcer, with DM, were slightly attenuated but remained significant: aOR 1.05 (95% CI 1.05–1.10), 1.20 (95% CI 1.06–1.34) and 1.20 (95% CI 1.12–1.28), respectively, after adjustment for age, socioeconomic status of place of residence, country of birth, smoking, BMI, dyslipidemia and hypertension. The results were similar when including into the same model the variables H. pylori positivity (by UBT) and gastric ulcer or duodenal ulcer: aOR 1.07 (95% CI 1.03–1.12), 1.20 (95% CI 1.06–1.33), 1.20 (95% CI 1.11–1.27) respectively.
Multivariable adjusted associations of H. pylori infection, gastric ulcer and duodenal ulcer, with DM, by age and BMI groups, differed slightly across the sub-groups. However, no significant heterogeneity by age group was found. The adjusted association of H. pylori infection with DM was stronger in people with BMI<25 kg/m2 than in those with BMI ≥25 kg/m2 (P = 0.04 by chi square test for heterogeneity); however, for the adjusted associations of gastric ulcer and duodenal ulcer with DM, no significant heterogeneity by BMI was found (P = 0.3) (Table 4A–4C).
Table 4. Adjusted odds ratio and 95% confidence intervals of the associations of H. pylori infection, and gastric and duodenal ulcers, with diabetes mellitus by age (in years) and BMI (< and >25 kg/m2).
| Pooled age groups | Age 25–29 | Age 30–39 | Age 40–49 | Age 50–59 | Age 60–69 | Age 70–95 | P for age heterogeneity | |
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | ||
| A: all BMI categories a | ||||||||
| H. pylori positive | 1.05 (1.01–1.10) c | 1.43 (1.00–2.05) | 1.11 (0.96–1.27) | 1.03 (0.94–1.12) | 1.09 (1.01–1.18) | 1.03 (0.95–1.13) | 0.94 (0.83–1.06) | 0.16 |
| Gastric ulcer | 1.20 (1.06–1.34) d | 0.51 (0.06–3.33) | 1.19 (0.91–1.55) | 1.19 (0.90–1.56) | 1.20 (0.98–1.48) | 1.28 (1.03–1.59) | 1.08 (0.81–1.43) | 0.9 |
| Duodenal ulcer | 1.20 (1.12–1.28) e | 1.96 (0.99–3.87) | 1.18 (0.73–1.89) | 1.18 (1.00–1.38) | 1.20 (1.06–1.34) | 1.22 (1.07–1.39) | 1.09 (0.92–1.30) | 0.6 |
| B: BMI<25 kg/m2 b | ||||||||
| H. pylori positive | 1.16 (1.04–1.29) | 1.18 (0.57–2.42) | 1.10 (0.79–1.53) | 1.21 (0.95–1.54) | 1.35 (1.10–1.66) | 1.05 (0.85–1.29) | 0.99 (0.75–1.30) | 0.4 |
| Gastric ulcer | 1.06 (0.79–1.42) | NA | 0.81 (0.20–3.35) | 0.96 (0.44–2.06) | 0.88 (0.47–1.66) | 1.30 (0.80–2.11) | 1.01 (0.54–1.90) | 0.8 |
| Duodenal ulcer | 1.27 (1.07–1.49) | 3.26 (0.98–10.90) | 0.93 (0.43–2.01) | 2.10 (1.47–3.00) | 1.20 (0.84–1.59) | 1.16 (0.86–1.58) | 1.06 (0.72–1.55) | 0.038 |
| C: BMI≥25 kg/m2 a | ||||||||
| H. pylori positive | 1.03 (0.98–1.08) | 1.57 (1.03–2.37) | 1.11 (0.95–1.29) | 1.00 (0.91–1.10) | 1.05 (0.97–1.14) | 1.03 (0.94–1.14) | 0.93 (0.81–1.07) | 0.18 |
| Gastric ulcer | 1.22 (1.08–1.39) | 0.51 (0.07–3.80) | 1.24 (0.75–2.06) | 1.23 (0.92–1.65) | 1.25 (1.01–1.56) | 1.23 (1.07–1.42) | 1.09 (0.79–1.49) | 0.9 |
| Duodenal ulcer | 1.17 (1.09–1.26) | 1.61 (0.71–3.63) | 1.22 (0.92–1.63) | 1.05 (0.88–1.24) | 1.20 (1.04–1.34) | 1.28 (1.01–1.64) | 1.10 (0.90–1.33) | 0.6 |
aOR: adjusted odds ratio; BMI: Body mass index; CI: confidence intervals; NA: not applicable, no cases of gastric ulcer in patients aged 25–29 years. Multivariable models presented in this table were fitted separately for each independent variable: H. pylori infection (by UBT), gastric ulcer and duodenal ulcer.
a Adjusted for age, socioeconomic status (SES) of place of residence, country of birth, BMI, smoking, dyslipidemia and hypertension.
b Adjusted for age, SES of place of residence, country of birth, smoking, dyslipidemia and hypertension. The age specific models did not include age.
c P = 0.049,
d P = 0.3,
e P = 0.3 by chi square test for heterogeneity across BMI categories.
An association between gastric ulcer and DM was found in both H. pylori negative persons aOR 1.24 (95% CI 1.06–1.44) and H. pylori positive persons: aOR 1.17 (95% CI 0.98–1.40); P = 0.6 by chi square test for heterogeneity. Similarly, an association between duodenal ulcer and DM was found in both H. pylori negative aOR 1.20 (95% CI 1.10–1.31) and positive persons: aOR 1.25 (95% CI 1.12–1.39); P = 0.5 by chi square test for heterogeneity.
Discussion
In this large population-based study, we assessed associations of H. pylori infection and its related gastroduodenal diseases with DM, among adults aged 25–95 years. To our knowledge, this is the largest study that addressed the role of H. pylori infection in DM. The overall association of H. pylori infection (by UBT) with DM was close to null (aOR 1.05 (95% CI 1.01–1.10)), although this association was stronger in patients with BMI<25 kg/m2 (aOR 1.17 (95% CI 1.04–1.29)), suggesting an effect modification by BMI (P for heterogeneity 0.049). We found significant positive associations of gastric and duodenal ulcers with DM, independent of known risk factors for DM such as age, socioeconomic status, BMI, dyslipidemia, hypertension and smoking. These associations were of small magnitude (aOR 1.20 (95% CI 1.06–1.34) and 1.20 (95% CI 1.12–1.28), respectively).
Previous studies that addressed the association between H. pylori infection and DM reported conflicting results. Some demonstrated a significant positive association between H. pylori infection and DM [8, 16, 26–28], while others reported null results [14, 17, 29] or positive associations that became non-significant following adjustment for potential confounders [9, 13, 30, 31]. These discrepancies might be explained, at least in part, by different methods employed across the studies in the detection of H. pylori infection, and in sample size and/or selection of the study population. Also, these studies employed various definitions of DM, some followed the American Diabetes Association criteria [14, 16, 28]. Others used one of several criteria such as reports on anti-diabetic medications, self-report physician diagnosis of DM or fasting blood glucose [8, 9, 15].
Our findings indicate complex patterns of associations between H. pylori infection and DM, and between peptic diseases and DM. First, the positive associations of gastric and duodenal ulcers with DM were attenuated when controlling for known risk factors for DM such as age, socioeconomic status, BMI, dyslipidemia, hypertension and smoking. The associations did not completely diminish; rather, adjustment had limited impact on the strength of the associations. This suggests that these factors may comprise partial confounders in the association between peptic ulcer disease and DM.
Second, we report a possible effect modification by BMI. The association between H. pylori infection and DM became more evident in people with BMI<25 kg/m2; although some variation in the association measure was observed across the BMI/age groups. Testing for heterogeneity by age was mostly not significant. Previous studies also proposed that H. pylori infection and DM might be modified by age and BMI [28, 32]. These observations highlight the importance of large population-based studies for examining an independent role of H. pylori infection, beyond well-known and established risk factors for DM.
The current evidence does not enable determination of causality in the observed associations of H. pylori infection, and gastric and duodenal ulcers, with DM. H. pylori infection might be a marker for unmeasured characteristics. Nonetheless, whether H. pylori is causally related to DM or is simply a risk marker, our observations have public health and clinical importance, and suggest that patients infected with H. pylori and those with peptic disease warrant special attention regarding the prevention of DM.
H. pylori infection is generally acquired in early childhood [33], several years before the usual onset of DM. H. pylori gastric colonization induces rigorous humoral and cell-mediated immune responses [10, 34], which do not clear the infection. The inflammation induced by H. pylori might provide a possible explanation for the positive associations observed of H. pylori infection and peptic disease with DM. The predominant human T cell response is the T-helper 1 mediated response, which is associated with releasing proinflammatory cytokines and activation of phagocytes [10, 34, 35]. H. pylori also induces Th2 and T-regulatory (Tregs) responses [10, 34, 35]. Imbalance between Th1 and Tregs responses might induce peptic disease [34, 36]. Our finding of a positive association between peptic ulcer disease and DM suggests that the long-term gastric inflammation and induced-damage to the gastric mucosa are likely involved in the relationship between H. pylori infection and DM.
H. pylori-induced inflammation affects gastric physiology. For example, H. pylori affects the levels of pepsinogen (PG) I and PGII; proenzymes of the digestive enzyme pepsin. PGI and PGII are secreted from cells in the corpus and PGII is also secreted from cells in the antrum and duodenum [37, 38]. Serum PGI and PGII are increased in H. pylori infected vs. uninfected individuals, and higher levels are found in those with more severe gastritis [39]. As the severity of gastritis progresses and corpus atrophic lesions appear, the PGI level decreases, while the PGII level remains stable; the result is a decrease in the PGI:PGII ratio [39, 40]. These markers predict various gastric pathologies [39]. A recent study [41] showed significant negative correlations between the PGI:PGII ratio and cardiovascular risk factors among individuals with type 2 DM [41]. H. pylori infection can also affect the regulation of ghrelin and leptin [42–44], two hormones that have important roles in energy homeostasis [45]; both hormones are secreted by the epithelial cells in the stomach [45, 46]. Ghrelin decreases energy expenditure and stimulates weight gain [47], while leptin reduces appetite and increases energy expenditure [45]. Altogether, these studies suggest that H. pylori can alter gastric physiology, which can in turn affect metabolic homeostasis and the risk of DM.
In the current study, H. pylori infections were detected in 52.0% of individuals who performed UBT. This is comparable with previous data on H. pylori positivity among Israeli adults, as well as the variation observed according to country of birth [21]. However, unexpectedly, the prevalence of the infection (by UBT) was lower in the subgroups of persons aged 50–95 years than in the younger age subgroups; this observation was not explained by country of birth, and suggests possible differences in referrals to UBT by age group. The prevalence of DM (8.3%) is also comparable to previous estimates (8.3–9.5%), despite the use of different criteria to determine DM [48, 49].
Our study has some limitations. We used data from a large HMO database, which were collected primarily for administrative purposes. The methods of collecting information on variables such as BMI and smoking may vary among medical personnel within the same HMO. Missing information was low for BMI, country of birth and socioeconomic status; yet relatively high (27%) for smoking. In persons with unknown smoking status, the prevalence of H. pylori infection was similar to that in never smokers, and DM prevalence was lower compared to those with documented smoking status. This might have resulted in partial adjustment for smoking.
Our study also has several strengths. First is the use of a large population-base sample. Second is the employment of standard criteria for the classification of DM, which combine results of laboratory tests, purchases of anti-diabetic medications and physicians' diagnoses. Lastly, H. pylori infection was determined based on UBT, which is among the most accurate non-invasive methods for the detection of H. pylori. Since only physicians can refer patients to UBT, our study population most likely represents symptomatic persons.
Conclusions
Significant positive associations of small magnitude were found between H. pylori infection and DM, and between peptic disease and DM, independent of known determinants of DM. The long-term gastric inflammation and associated-damage to the gastric mucosa, as reflected by peptic disease, might play a role in the association between H. pylori infection and DM. Further studies are needed to elucidate the underlying mechanisms of these associations.
Supporting information
(DOCX)
Acknowledgments
We thank Ms. Racheli Katz from Maccabi Health Services (Tel Aviv, Israel) for her help in retrieving the data from the computerized databases.
This work was performed in partial fulfillment of the requirements for a M.Sc. degree of Mrs. Rotem Refaeli, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Data Availability
In this study, we used deidentified patients' data from a computerized database of a health maintenance organization. These data are collected as part of patients' clinical care, and not for research purposes. Legal and ethical restrictions apply for secondary usage of these data in research. Our IRB approval is limited to using anonymous data, and accordingly we received exemption from informed consent. We are not allowed to provide datasets to be publicly available. Given IRB and legal restrictions, we cannot provide any access to individual level data. Readers may contact Dr. Khitam Muhsen (the corresponding author) for further information, at kmuhsen@post.tau.ac.il.
Funding Statement
The authors received no specific funding for this work.
References
- 1.van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17 Suppl 1:S3–8. doi: 10.1097/01.hjr.0000368191.86614.5a [DOI] [PubMed] [Google Scholar]
- 2.Powers AC. Harrison's Principles of Internal Medicine, 18e 18e ed Chapter 344. Diabetes Mellitus. The McGraw-Hill Companies, Inc.; 2013. [Google Scholar]
- 3.Medalie JH, Papier CM, Goldbourt U, Herman JB. Major factors in the development of diabetes mellitus in 10,000 men. Arch Intern Med. 1975;135(6):811–7. [PubMed] [Google Scholar]
- 4.Espelt A, Arriola L, Borrell C, Larranaga I, Sandin M, Escolar-Pujolar A. Socioeconomic position and type 2 diabetes mellitus in Europe 1999–2009: a panorama of inequalities. Curr Diabetes Rev. 2011;7(3):148–58. [DOI] [PubMed] [Google Scholar]
- 5.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):804–18. doi: 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- 6.Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J et al. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004;7(1A):147–65. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–7. doi: 10.1056/NEJMoa010492 [DOI] [PubMed] [Google Scholar]
- 8.Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35(3):520–5. doi: 10.2337/dc11-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutsey PL, Pankow JS, Bertoni AG, Szklo M, Folsom AR. Serological evidence of infections and Type 2 diabetes: the MultiEthnic Study of Atherosclerosis. Diabet Med. 2009;26(2):149–52. doi: 10.1111/j.1464-5491.2008.02632.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125 [DOI] [PubMed] [Google Scholar]
- 11.Baudron CR, Franceschi F, Salles N, Gasbarrini A. Extragastric diseases and Helicobacter pylori. Helicobacter. 2013;18:44–51. doi: 10.1111/hel.12077 [DOI] [PubMed] [Google Scholar]
- 12.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon ATR, Bazzoli F et al. Management of Helicobacter pylori infection-the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646–64. doi: 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 13.Dore MP, Bilotta M, Malaty HM, Pacifico A, Maioli M, Graham DY et al. Diabetes mellitus and Helicobacter pylori infection. Nutrition. 2000;16(6):407–10. [DOI] [PubMed] [Google Scholar]
- 14.Demir M, Gokturk HS, Ozturk NA, Kulaksizoglu M, Serin E, Yilmaz U. Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis Sci. 2008;53(10):2646–9. doi: 10.1007/s10620-007-0185-7 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205(8):1195–202. doi: 10.1093/infdis/jis106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bener A, Micallef R, Afifi M, Derbala M, Al-Mulla HM, Usmani MA. Association between type 2 diabetes mellitus and Helicobacter pylori infection. Turk J Gastroenterol. 2007;18(4):225–9. [PubMed] [Google Scholar]
- 17.Xia HHX, Talley NJ, Kam EPY, Young LJ, Hammer J, Horowitz M. Helicobacter pylori infection is not associated with diabetes mellitus, nor with upper gastrointestinal symptoms in diabetes mellitus. Am J Gastroenterol. 2001;96(4):1039–46. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Zhang C, Wu J, Zhang G. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract. 2013;99(2):200–8. doi: 10.1016/j.diabres.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Liu J, Lv ZS. Association of Helicobacter pylori infection with diabetes mellitus and diabetic nephropathy: A meta-analysis of 39 studies involving more than 20,000 participants. Scand J Infect Dis. 2013;45(12):930–8. doi: 10.3109/00365548.2013.844351 [DOI] [PubMed] [Google Scholar]
- 20.Harris MI. Epidemiologic studies on the pathogenesis of non-insulin-dependent diabetes mellitus (NIDDM). Clin Invest Med. 1995;18(4):231–9. [PubMed] [Google Scholar]
- 21.Muhsen K, Cohen D, Spungin-Bialik A, Shohat T. Seroprevalence, correlates and trends of Helicobacter pylori infection in the Israeli population. Epidemiol Infect. 2012;140(7):1207–14. doi: 10.1017/S0950268811002081 [DOI] [PubMed] [Google Scholar]
- 22.Heymann AD, Chodick G, Halkin H, Karasik A, Shalev V, Shemer J et al. The implementation of managed care for diabetes using medical informatics in a large Preferred Provider Organization. Diabetes Res Clin Pr. 2006;71(3):290–8. doi: 10.1016/j.diabres.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Weitzman D, Chodick G, Shalev V, Grossman C, Grossman E. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension. 2014;64(3):501–7. doi: 10.1161/HYPERTENSIONAHA.114.03718 [DOI] [PubMed] [Google Scholar]
- 24.Israel Centeral Bureau of Statistics. Characterization and classification of geagraphic units by the socio-economic level of the population 2008. 2013.
- 25.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8(1):1 doi: 10.1186/1742-5573-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devrajani BR, Shah SZ, Soomro AA, Devrajani T. Type 2 diabetes mellitus: A risk factor for Helicobacter pylori infection: A hospital based case-control study. Int J Diabetes Dev Ctries. 2010;30(1):22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj S, Rekwal L, Misra SP, Misra V, Yadav RK, Srivastava A. Association of helicobacter pylori infection with type 2 diabetes. Indian J Endocrinol Metab. 2014;18(5):694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh MC, Wang SS, Hsieh YT, Kuo FC, Soon MS, Wu DC. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur J Clin Invest. 2013;43(9):949–56. doi: 10.1111/eci.12124 [DOI] [PubMed] [Google Scholar]
- 29.Ko GT, Chan FK, Chan WB, Sung JJ, Tsoi CL, To KF et al. Helicobacter pylori infection in Chinese subjects with type 2 diabetes. Endocr Res. 2001;27(1–2):171–7. [DOI] [PubMed] [Google Scholar]
- 30.Gillum RF. Infection with Helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: the Third National Health and Nutrition Examination Survey. J Natl Med Assoc. 2004;96(11):1470–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura T, Morita E, Kawai S, Sasakabe T, Sugimoto Y, Fukuda N et al. No association between Helicobacter pylori infection and diabetes mellitus among a general Japanese population: a cross-sectional study. Springerplus. 2015;4 Artn 602 doi: 10.1186/S40064-015-1371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han X, Li Y, Wang J, Liu B, Hu H, Li X et al. Helicobacter pylori infection is associated with type 2 diabetes among a middle- and old-age Chinese population. Diabetes Metab Res Rev. 2016;32(1):95–101. doi: 10.1002/dmrr.2677 [DOI] [PubMed] [Google Scholar]
- 33.Muhsen K, Jurban M, Goren S, Cohen D. Incidence, age of acquisition and risk factors of Helicobacter pylori infection among Israeli Arab infants. J Trop Pediatr. 2012;58(3):208–13. doi: 10.1093/tropej/fmr068 [DOI] [PubMed] [Google Scholar]
- 34.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: ancient history, modern implications. J Clin Invest. 2009;119(9):2475–87. doi: 10.1172/JCI38605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goll R, Gruber F, Olsen T, Cui G, Raschpichler G, Buset M et al. Helicobacter pylori stimulates a mixed adaptive immune response with a strong T-regulatory component in human gastric mucosa. Helicobacter. 2007;12(3):185–92. doi: 10.1111/j.1523-5378.2007.00495.x [DOI] [PubMed] [Google Scholar]
- 36.Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57(10):1375–85. doi: 10.1136/gut.2007.137539 [DOI] [PubMed] [Google Scholar]
- 37.Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology. 1971;61(2):185–8. [PubMed] [Google Scholar]
- 38.Samloff IM, Liebman WM. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology. 1973;65(1):36–42. [PubMed] [Google Scholar]
- 39.Miki K, Urita Y. Using serum pepsinogens wisely in a clinical practice. J Dig Dis. 2007;8(1):8–14. doi: 10.1111/j.1443-9573.2007.00278.x [DOI] [PubMed] [Google Scholar]
- 40.Graham DY, Nurgalieva ZZ, El-Zimaity HM, Opekun AR, Campos A, Guerrero L et al. Noninvasive versus histologic detection of gastric atrophy in a Hispanic population in North America. Clin Gastroenterol Hepatol. 2006;4(3):306–14. doi: 10.1016/j.cgh.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 41.Bahadoran Z, Mirmiran P, Zarif-Yeaganeh M, Zojaji H, Azizi F. Helicobacter pylori Stool Antigen Levels and Serological Biomarkers of Gastric Inflammation are Associated with Cardio-Metabolic Risk Factors in Type 2 Diabetic Patients. Endocrinol Metab. 2015;30(3):280–7. doi: 10.3803/EnM.2015.30.3.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ et al. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. Clin Endocrinol Metab. 2008;93(6):2350–7. doi: 10.1210/jc.2007-2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francois F, Roper J, Joseph N, Pei Z, Chhada A, Shak JR et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterology. 2011;11:37 doi: 10.1186/1471-230X-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuang CH, Sheu BS, Yang HB, Lee SC, Kao AW, Cheng HC et al. Gender difference of circulating ghrelin and leptin concentrations in chronic Helicobacter pylori infection. Helicobacter. 2009;14(1):54–60. doi: 10.1111/j.1523-5378.2009.00653.x [DOI] [PubMed] [Google Scholar]
- 45.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117(1):13–23. doi: 10.1172/JCI30227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN et al. The stomach is a source of leptin. Nature. 1998;394(6695):790–3. doi: 10.1038/29547 [DOI] [PubMed] [Google Scholar]
- 47.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 48.Israel Minsitry of Health. National program for quality indicators in community health care. 2014.
- 49.Israel Minsitry of Health. Israel national health interview survey 2007–2010; selected findings. Israel Center for Disease Control. Tal Hashomer 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
In this study, we used deidentified patients' data from a computerized database of a health maintenance organization. These data are collected as part of patients' clinical care, and not for research purposes. Legal and ethical restrictions apply for secondary usage of these data in research. Our IRB approval is limited to using anonymous data, and accordingly we received exemption from informed consent. We are not allowed to provide datasets to be publicly available. Given IRB and legal restrictions, we cannot provide any access to individual level data. Readers may contact Dr. Khitam Muhsen (the corresponding author) for further information, at kmuhsen@post.tau.ac.il.