Abstract
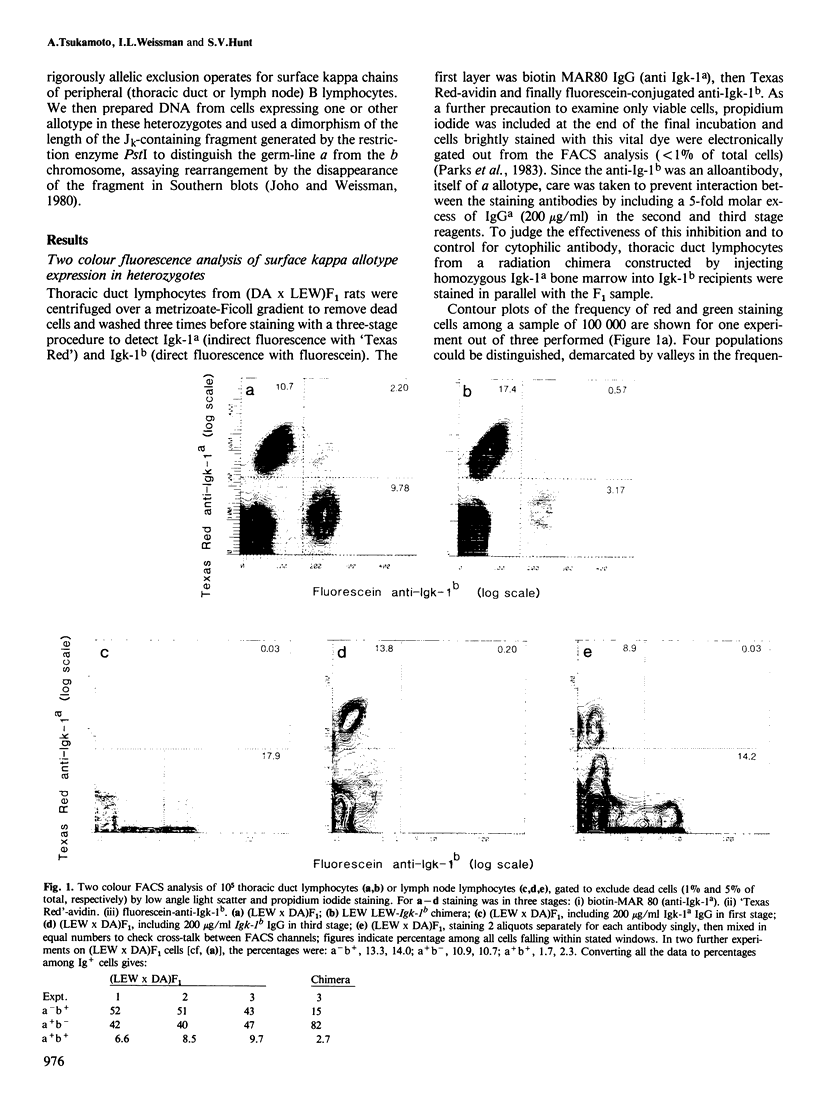
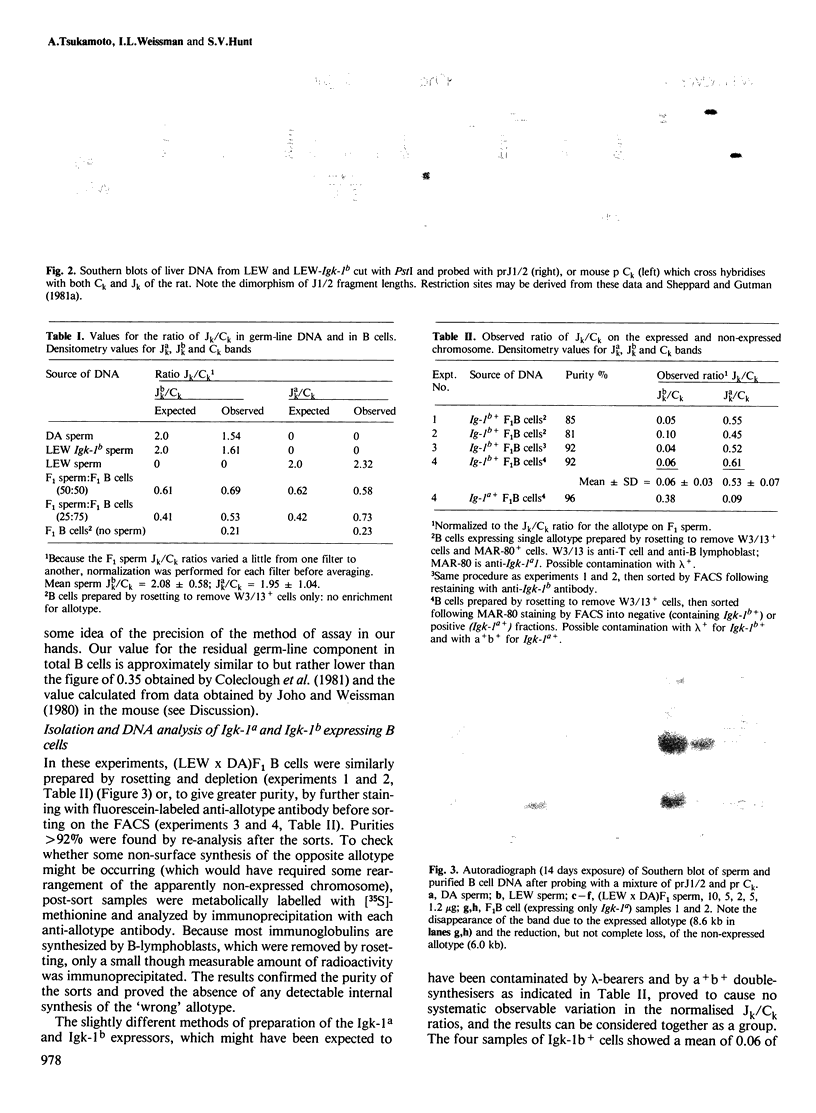
The frequency of normal rat peripheral B lymphocytes stained for surface immunoglobulin kappa allotypes a and b in (a X b) F1 heterozygotes was assessed by two-colour immunofluorescence on a fluorescence-activated cell sorter. The upper limit to the frequency of double- stainers was 8% among all kappa-positive cells, though it was not resolved how far cytophilic antibody accounted for these. Cells expressing each allotype singly were isolated and the extent of rearrangement of the genes encoding the joining-kappa segment on the expressed and non-expressed chromosome were independently assessed. The expressed allele was found to be virtually completely rearranged while the non-expressed allele showed approximately 45-60% rearrangement. The implications of this substantial non-productive rearrangement for models of allelic exclusion are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Mitchell J., Bernard O., Cory S. Transcriptionally active DNA region that rearranges frequently in murine lymphoid tumors. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6966–6970. doi: 10.1073/pnas.79.22.6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Altenburger W., Steinmetz M., Zachau H. G. Functional and non-functional joining in immunoglobulin light chain genes of a mouse myeloma. Nature. 1980 Oct 16;287(5783):603–607. doi: 10.1038/287603a0. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Breiner A. V., Brandt C. R., Milcarek C., Sweet R. W., Warszawski D., Ziv E., Schechter I. Recent duplication and germ-line diversification of rat immunoglobulin kappa chain gene joining segments. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5993–5997. doi: 10.1073/pnas.79.19.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement during pre-B cell differentiation. J Mol Cell Immunol. 1983;1(1):31–41. [PubMed] [Google Scholar]
- Coleclough C. Chance, necessity and antibody gene dynamics. Nature. 1983 May 5;303(5912):23–26. doi: 10.1038/303023a0. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Cooper D., Perry R. P. Rearrangement of immunoglobulin heavy chain genes during B-lymphocyte development as revealed by studies of mouse plasmacytoma cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1422–1426. doi: 10.1073/pnas.77.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Crews S., Barth R., Hood L., Prehn J., Calame K. Mouse c-myc oncogene is located on chromosome 15 and translocated to chromosome 12 in plasmacytomas. Science. 1982 Dec 24;218(4579):1319–1321. doi: 10.1126/science.7146913. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Martinotti S., Gallo R. C., Erikson J., Croce C. M. Translocation and rearrangements of the c-myc oncogene locus in human undifferentiated B-cell lymphomas. Science. 1983 Feb 25;219(4587):963–967. doi: 10.1126/science.6401867. [DOI] [PubMed] [Google Scholar]
- Early P., Hood L. Allelic exclusion and nonproductive immunoglobulin gene rearrangements. Cell. 1981 Apr;24(1):1–3. doi: 10.1016/0092-8674(81)90492-x. [DOI] [PubMed] [Google Scholar]
- Early P., Nottenburg C., Weissman I., Hood L. Immunoglobulin gene rearrangements in normal mouse B cells. Mol Cell Biol. 1982 Jul;2(7):829–836. doi: 10.1128/mcb.2.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Weissman I. L. Inheritance and strain distribution of a rat immunoglobulin allotype. J Immunol. 1971 Nov;107(5):1390–1393. [PubMed] [Google Scholar]
- Harris L. J., Lang R. B., Marcu K. B. Non-immunoglobulin-associated DNA rearrangements in mouse plasmacytomas. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4175–4179. doi: 10.1073/pnas.79.13.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Fowler M. H. A repopulation assay for B and T lymphocyte stem cells employing radiation chimaeras. Cell Tissue Kinet. 1981 Jul;14(4):445–464. doi: 10.1111/j.1365-2184.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Williams A. F. The origin of cell surface immunoglobulin of marrow-derived and thymus-derived lymphocytes of the rat. J Exp Med. 1974 Mar 1;139(3):479–496. doi: 10.1084/jem.139.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R., Nottenburg C., Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement and expression during lymphocyte development. Curr Top Dev Biol. 1983;18:15–58. doi: 10.1016/s0070-2153(08)60578-5. [DOI] [PubMed] [Google Scholar]
- Joho R., Weissman I. L. V-J joining of immunoglobulin kappa genes only occurs on one homologous chromosome. Nature. 1980 Mar 13;284(5752):179–181. doi: 10.1038/284179a0. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Cebra J. J., Herzenberg L. A. Immunoglobulin (Ig) allotype markers on rabbit lymphocytes: separation of cells bearing different allotypes and demonstration of the binding of Ig to lymphoid cell membranes. J Immunol. 1973 Nov;111(5):1334–1348. [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Loor F., Kelus A. S. Allelic exclusion in the B lineage cells of the rabbit. Eur J Immunol. 1978 May;8(5):315–324. doi: 10.1002/eji.1830080506. [DOI] [PubMed] [Google Scholar]
- Maki R., Kearney J., Paige C., Tonegawa S. Immunoglobulin gene rearrangement in immature B cells. Science. 1980 Sep 19;209(4463):1366–1369. doi: 10.1126/science.6774416. [DOI] [PubMed] [Google Scholar]
- Nezlin R., Rokhlin O. Allotypes of light chains of rat immunoglobulins and their application to the study of antibody biosynthesis. Contemp Top Mol Immunol. 1976;5:161–184. doi: 10.1007/978-1-4684-8142-6_6. [DOI] [PubMed] [Google Scholar]
- Nottenburg C., Weissman I. L. Cmu gene rearrangement of mouse immunoglobulin genes in normal B cells occurs on both the expressed and nonexpressed chromosomes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):484–488. doi: 10.1073/pnas.78.1.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Forni L., Dubiski S., Kelus A. S., Mandy W. J., Todd C. W. Heavy chain variable and constant region allotypes in single rabbit plasma cells. Immunochemistry. 1973 May;10(5):281–285. doi: 10.1016/0019-2791(73)90023-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Seidman J. G., Leder P., Tonegawa S., Matthyssens G., Weigert M. Transcription of mouse kappa chain genes: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1937–1941. doi: 10.1073/pnas.77.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Keath E. J., Piccoli S. P., Cole M. D. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell. 1982 Dec;31(2 Pt 1):443–452. doi: 10.1016/0092-8674(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Gutman G. A. Allelic forms of rat kappa chain genes: evidence for strong selection at the level of nucleotide sequence. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7064–7068. doi: 10.1073/pnas.78.11.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard H. W., Gutman G. A. Complex allotypes of rat kappa chains are encoded by structural alleles. Nature. 1981 Oct 22;293(5834):669–671. doi: 10.1038/293669a0. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Gutman G. A. Rat kappa-chain J-segment genes: two recent gene duplications separate rat and mouse. Cell. 1982 May;29(1):121–127. doi: 10.1016/0092-8674(82)90096-4. [DOI] [PubMed] [Google Scholar]
- Taub R., Kirsch I., Morton C., Lenoir G., Swan D., Tronick S., Aaronson S., Leder P. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S. L., Dubiski S., Mage R. G. Distribution of allotypic specificities A1, A2, A14, and A15 among immunoglobulin G molecules. J Immunol. 1970 Mar;104(3):641–647. [PubMed] [Google Scholar]
- Van Ness B. G., Coleclough C., Perry R. P., Weigert M. DNA between variable and joining gene segments of immunoglobulin kappa light chain is frequently retained in cells that rearrange the kappa locus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):262–266. doi: 10.1073/pnas.79.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanNess B. G., Shapiro M., Kelley D. E., Perry R. P., Weigert M., D'Eustachio P., Ruddle F. Aberrant rearrangement of the kappa light-chain locus involving the heavy-chain locus and chromosome 15 in a mouse plasmacytoma. Nature. 1983 Feb 3;301(5899):425–427. doi: 10.1038/301425a0. [DOI] [PubMed] [Google Scholar]
- Weiler E. Differential activity of allelic gamma-globulin genes in antibody-producing cells. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1765–1772. doi: 10.1073/pnas.54.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]




