Abstract
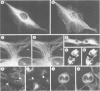
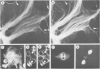
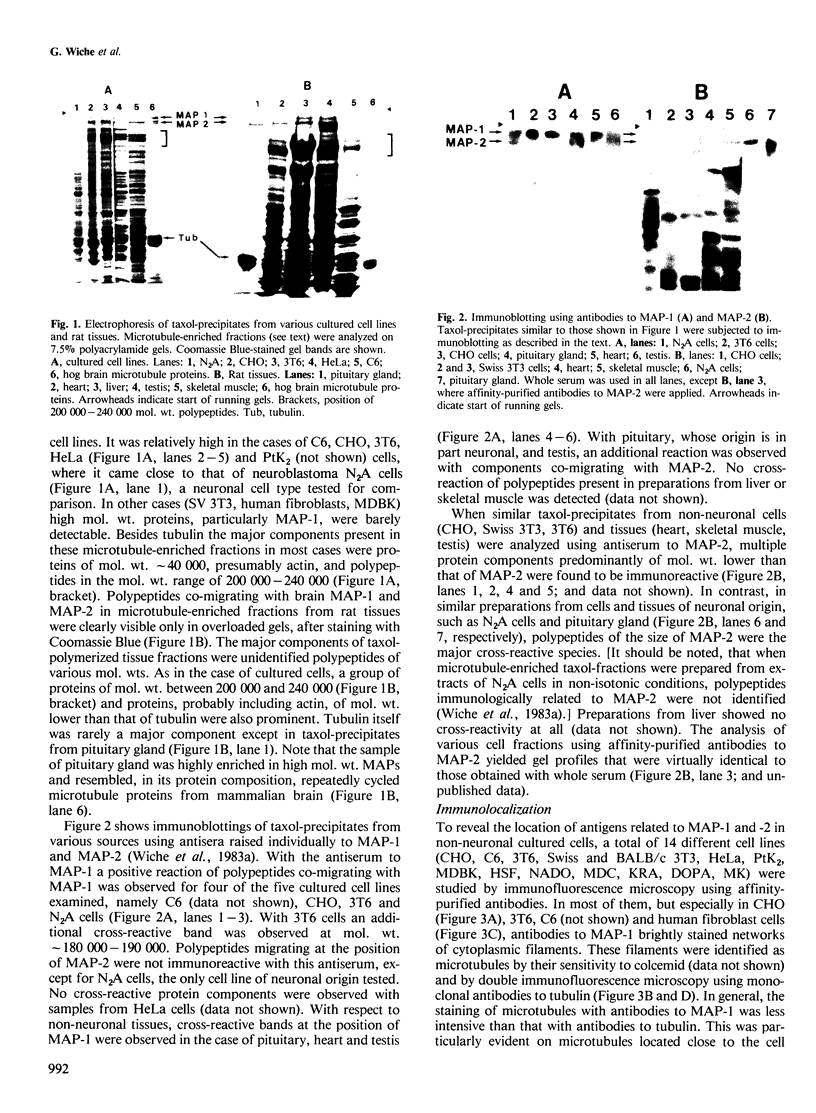
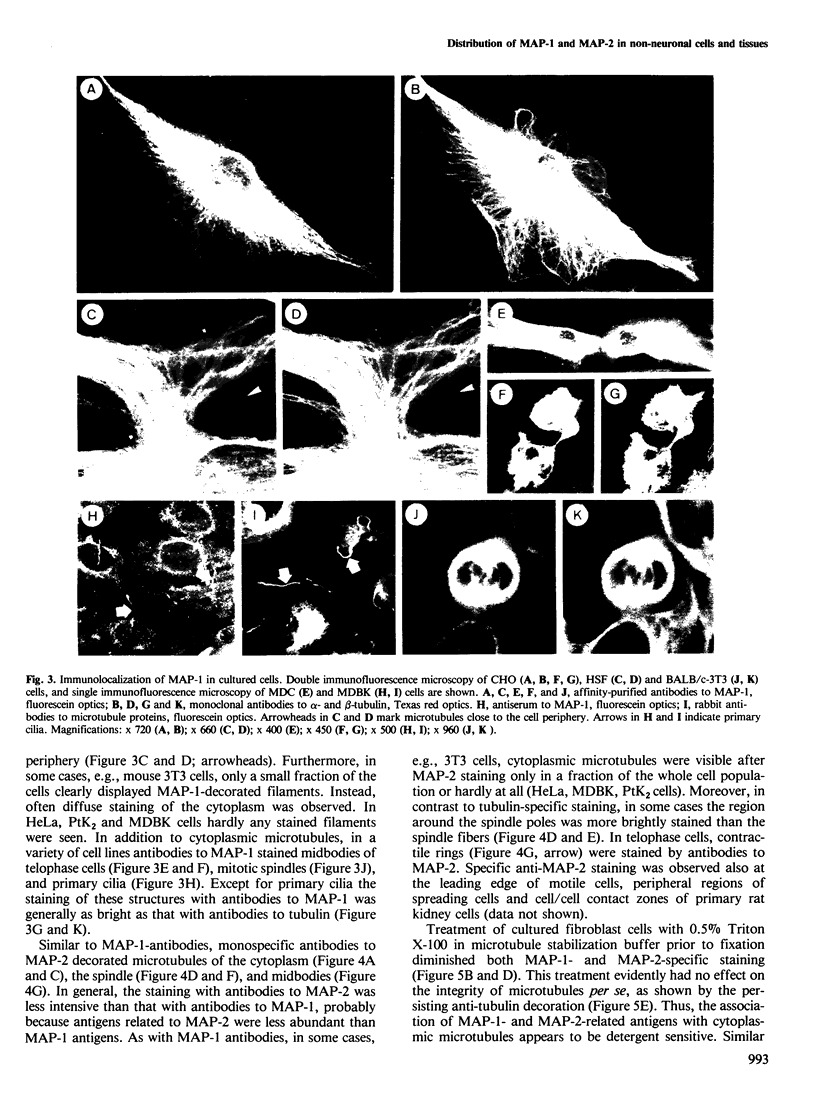
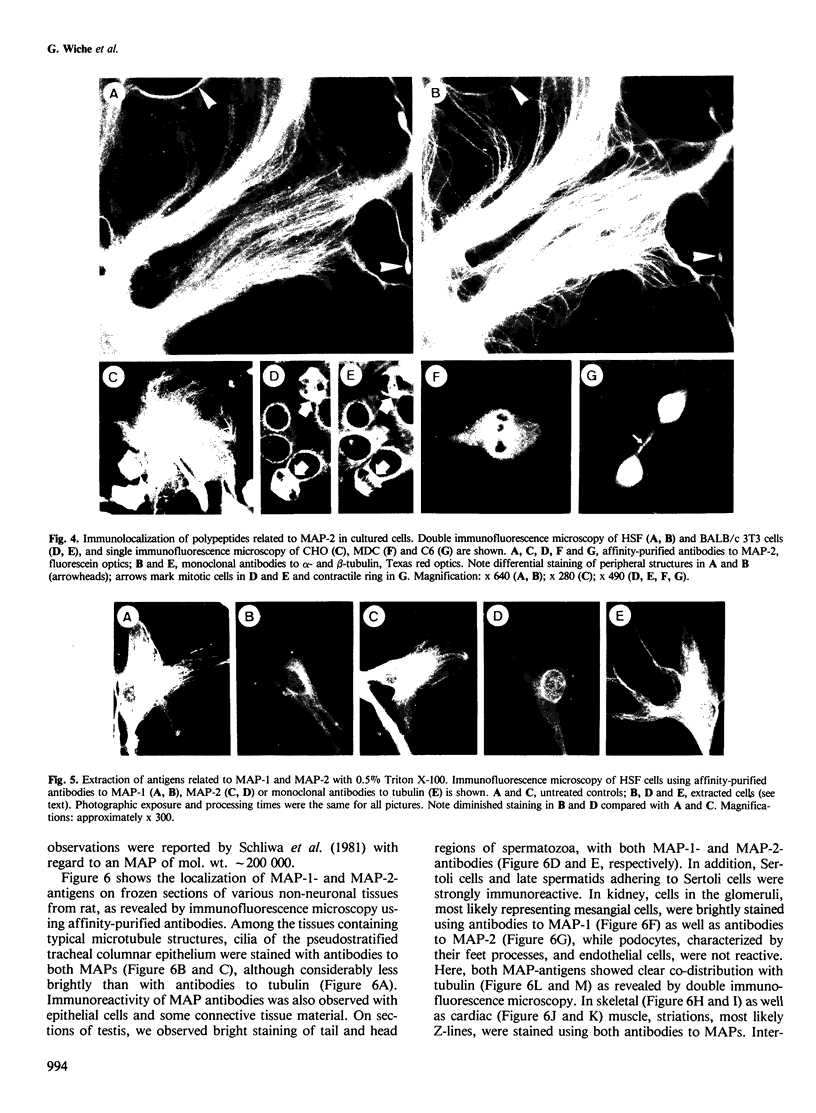
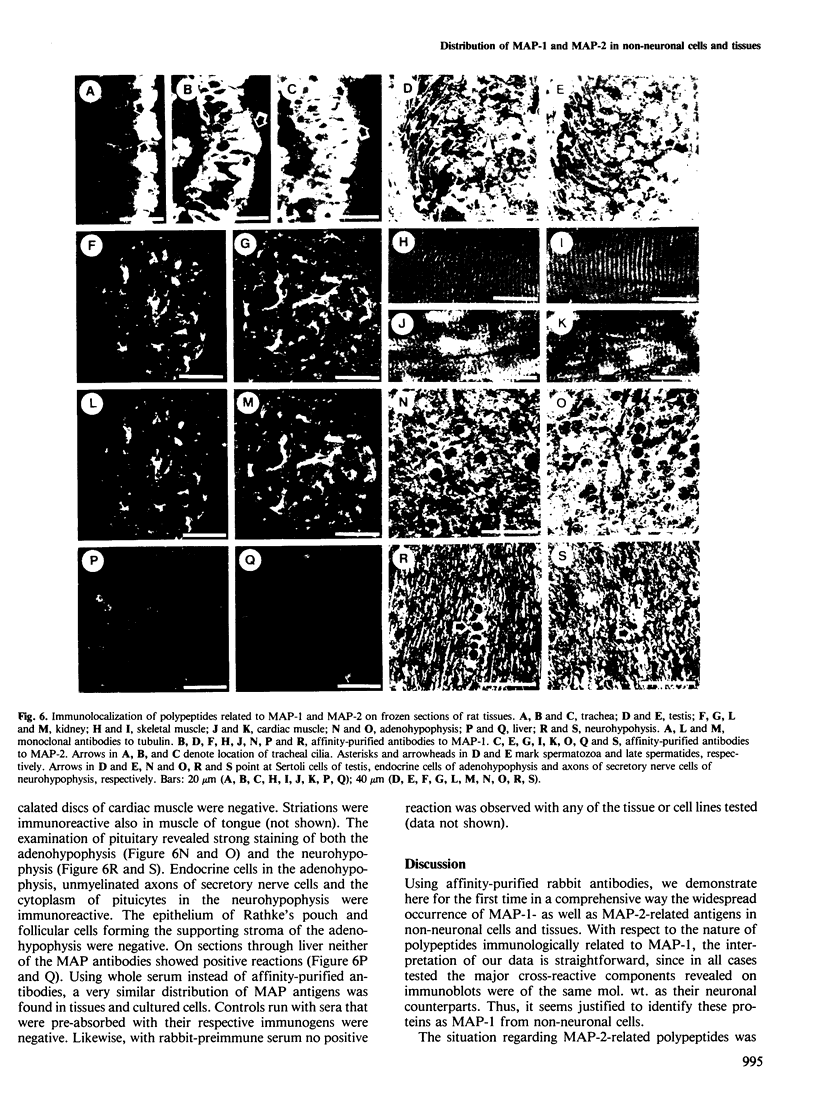
The occurrence and cellular localization of polypeptides related to hog brain microtubule-associated proteins 1 and 2 (MAP-1 and MAP-2) in non-neuronal cell lines of various species and types, and in several tissues from rat was studied. When insoluble cell fractions were prepared by incubation of isotonic cell extracts with 20 microM taxol, polypeptides co-migrating with MAP-1 and MAP-2 upon gel electrophoresis were observed in virtually all cases examined. Immunoblotting of preparations from 3T6, CHO, HeLa and N2A cells, as well as pituitary, heart, testis and liver revealed immuno-reactivity with antibodies to neuronal MAP-1 for polypeptides co-migrating with MAP-1 in all cases, except for HeLa cells and liver. With similar preparations, antibodies raised to neuronal MAP-2 were barely reactive with bands of the MAP-2 size except for N2A cells and pituitary gland. In all cases of non-neuronal cells and tissues, major cross-reactive bands, however, were of mol. wt. lower than that of MAP-2, indicating, most likely, proteolytic breakdown of MAP-2 during cell fractionation. As shown by double immunofluorescence microscopy of various cultured cell lines using affinity-purified antibodies to MAPs, and monoclonal antibodies to tubulin, MAP-1-as well as MAP-2-related antigens were generally, but not exclusively, associated with typical microtubule structures of the cytoplasm, spindle, midbody and primary cilia. Antigens related to both MAPs were also localized in frozen sections of rat trachea, testis, pituitary, kidney and cardiac and skeletal muscle.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A. Arrangement of high molecular weight associated proteins on purified mammalian brain microtubules. J Cell Biol. 1977 Mar;72(3):642–654. doi: 10.1083/jcb.72.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Initial phase of dendrite growth: evidence for the involvement of high molecular weight microtubule-associated proteins (HMWP) before the appearance of tubulin. J Cell Biol. 1982 Feb;92(2):589–593. doi: 10.1083/jcb.92.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Widespread cellular distribution of MAP-1A (microtubule-associated protein 1A) in the mitotic spindle and on interphase microtubules. J Cell Biol. 1984 Jan;98(1):331–340. doi: 10.1083/jcb.98.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Self-assembly of microtubules in extracts of cultured HeLa cells and the identification of HeLa microtubule-associated proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):293–297. doi: 10.1073/pnas.76.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Spiegelman B. M., Kirschner M. W. Conservation of microtubule associated proteins. Isolation and characterization of tau and the high molecular weight microtubule associated protein from chicken brain and from mouse fibroblasts and comparison to the corresponding mammalian brain proteins. J Biol Chem. 1979 Dec 25;254(24):12670–12678. [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Cleveland D. W., Kirschner M. W. Intracellular localization of the high molecular weight microtubule accessory protein by indirect immunofluorescence. J Cell Biol. 1978 Mar;76(3):781–786. doi: 10.1083/jcb.76.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Rosenbaum J. L. Ultrastructural localization of the high molecular weight proteins associated with in vitro-assembled brain microtubules. J Cell Biol. 1975 Apr;65(1):237–241. doi: 10.1083/jcb.65.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenges K. H., Nagle B. W., Uhlmann A., Bryan J. In vitro assembly of tubulin from nonneural cells (Ehrlich ascites tumor cells). Biochemistry. 1977 Jul 26;16(15):3455–3459. doi: 10.1021/bi00634a025. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell. 1983 Sep;34(2):503–512. doi: 10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Liem R. K., Shelanski M. L. Regulation of a high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J Cell Biol. 1983 Jan;96(1):76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982 Aug 10;257(15):9143–9151. [PubMed] [Google Scholar]
- Herrmann H., Pytela R., Dalton J. M., Wiche G. Structural homology of microtubule-associated proteins 1 and 2 demonstrated by peptide mapping and immunoreactivity. J Biol Chem. 1984 Jan 10;259(1):612–617. [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Izant J. G., McIntosh J. R. Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4741–4745. doi: 10.1073/pnas.77.8.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weatherbee J. A., McIntosh J. R. A microtubule-associated protein antigen unique to mitotic spindle microtubules in PtK1 cells. J Cell Biol. 1983 Feb;96(2):424–434. doi: 10.1083/jcb.96.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weatherbee J. A., McIntosh J. R. A microtubule-associated protein in the mitotic spindle and the interphase nucleus. Nature. 1982 Jan 21;295(5846):248–250. doi: 10.1038/295248a0. [DOI] [PubMed] [Google Scholar]
- Karr T. L., White H. D., Purich D. L. Characterization of brain microtubule proteins prepared by selective removal of mitochondrial and synaptosomal components. J Biol Chem. 1979 Jul 10;254(13):6107–6111. [PubMed] [Google Scholar]
- Klein I., Willingham M., Pastan I. A high molecular weight phosphoprotein in cultured fibroblasts that associates with polymerized tubulin. Exp Cell Res. 1978 Jun;114(1):229–238. doi: 10.1016/0014-4827(78)90056-3. [DOI] [PubMed] [Google Scholar]
- Krohne G., Stick R., Kleinschmidt J. A., Moll R., Franke W. W., Hausen P. Immunological localization of a major karyoskeletal protein in nucleoli of oocytes and somatic cells of Xenopus laevis. J Cell Biol. 1982 Sep;94(3):749–754. doi: 10.1083/jcb.94.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Bershadsky A. D., Gelfand V. I., Rosenblat V. A. High molecular weight protein MAP 2 promoting microtubule assembly in vitro is associated with microtubules in cells. Cell Biol Int Rep. 1980 Nov;4(11):1017–1024. doi: 10.1016/0309-1651(80)90174-5. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Gelfand V. I., Rosenblat V. A. Microtubule-associated protein MAP1 promotes microtubule assembly in vitro. FEBS Lett. 1981 Dec 7;135(2):241–244. doi: 10.1016/0014-5793(81)80791-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle B. W., Doenges K. H., Bryan J. Assembly of tubulin from cultured cells and comparison with the neurotubulin model. Cell. 1977 Nov;12(3):573–586. doi: 10.1016/0092-8674(77)90258-6. [DOI] [PubMed] [Google Scholar]
- Sattilaro R. F., Dentler W. L., LeCluyse E. L. Microtubule-associated proteins (MAPs) and the organization of actin filaments in vitro. J Cell Biol. 1981 Aug;90(2):467–473. doi: 10.1083/jcb.90.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Euteneuer U., Bulinski J. C., Izant J. G. Calcium lability of cytoplasmic microtubules and its modulation by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1037–1041. doi: 10.1073/pnas.78.2.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherline P., Schiavone K. Immunofluorescence localization of proteins of high molecular weight along intracellular microtubules. Science. 1977 Dec 9;198(4321):1038–1040. doi: 10.1126/science.337490. [DOI] [PubMed] [Google Scholar]
- Sheterline P. Localisation of the major high-molecular-weight protein on microtubules in vitro and in cultured cells. Exp Cell Res. 1978 Sep;115(2):460–464. doi: 10.1016/0014-4827(78)90310-5. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Dickersin K. Structure and composition of the cytoskeleton of nucleated erythrocytes I. The presence of microtubule-associated protein 2 in the marginal band. J Cell Biol. 1980 Oct;87(1):170–179. doi: 10.1083/jcb.87.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia M. M., Avila J., Coll J., Colaço C., Sandoval I. V. Quantitation and characterization of the microtubule associated MAP2 in porcine tissues and its isolation from porcine (PK15) and human (HeLa) cell lines. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1241–1249. doi: 10.1016/0006-291x(82)90920-2. [DOI] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Davis S. E. Low molecular weight microtubule-associated proteins are light chains of microtubule-associated protein 1 (MAP 1). Proc Natl Acad Sci U S A. 1983 Mar;80(5):1342–1346. doi: 10.1073/pnas.80.5.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee J. A., Luftig R. B., Weihing R. R. In vitro polymerization of microtubules from HeLa cells. J Cell Biol. 1978 Jul;78(1):47–57. doi: 10.1083/jcb.78.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee J. A., Sherline P., Mascardo R. N., Izant J. G., Luftig R. B., Weihing R. R. Microtubule-associated proteins of HeLa cells: heat stability of the 200,000 mol wt HeLa MAPs and detection of the presence of MAP-2 in HeLa cell extracts and cycled microtubules. J Cell Biol. 1982 Jan;92(1):155–163. doi: 10.1083/jcb.92.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Baker M. A. Cytoplasmic network arrays demonstrated by immunolocalization using antibodies to a high molecular weight protein present in cytoskeletal preparations from cultured cells. Exp Cell Res. 1982 Mar;138(1):15–29. doi: 10.1016/0014-4827(82)90086-6. [DOI] [PubMed] [Google Scholar]
- Wiche G., Briones E., Hirt H., Krepler R., Artlieb U., Denk H. Differential distribution of microtubule-associated proteins MAP-1 and MAP-2 in neurons of rat brain and association of MAP-1 with microtubules of neuroblastoma cells (clone N2A). EMBO J. 1983;2(11):1915–1920. doi: 10.1002/j.1460-2075.1983.tb01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Cole R. D. Reversible in vitro polymerization of tubulin from a cultured cell line (rat glial cell clone C6). Proc Natl Acad Sci U S A. 1976 Apr;73(4):1227–1231. doi: 10.1073/pnas.73.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Herrmann H., Leichtfried F., Pytela R. Plectin: a high-molecular-weight cytoskeletal polypeptide component that copurifies with intermediate filaments of the vimentin type. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):475–482. doi: 10.1101/sqb.1982.046.01.044. [DOI] [PubMed] [Google Scholar]
- Wiche G., Krepler R., Artlieb U., Pytela R., Denk H. Occurrence and immunolocalization of plectin in tissues. J Cell Biol. 1983 Sep;97(3):887–901. doi: 10.1083/jcb.97.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G., Solomon F. Proteins specifically associated with the microtubules of the mammalian mitotic spindle. Cell. 1982 Feb;28(2):233–242. doi: 10.1016/0092-8674(82)90341-5. [DOI] [PubMed] [Google Scholar]