ABSTRACT
Bacillary angiomatosis (BA) is an angioproliferative disease of immunocompromised patients that usually presents as vascular tumors in the skin and subcutaneous tissues. It is caused by chronic infections with either Bartonella henselae or B. quintana. Oral cavity BA is exceedingly rare and even rarer without simultaneous cutaneous disease. We report herein the case of a 51-year-old HIV-infected man who presented severe odynophagia and an eroded lesion on the hard palate that progressed to an oronasal fistula. No cutaneous lesions were recorded. Doxycycline led to complete resolution. To the best of our knowledge, only six previous cases of oral BA without tegumentary disease have been previously reported and none of them progressed to fistula.
Keywords: Bacillary angiomatosis, Bartonella spp, HIV infection, Oral cavity
INTRODUCTION
Bartonella are arthropod-borne pathogens that are highly adapted to their respective mammalian reservoir hosts. Their persistence in the bloodstream may occur as a result of intraerythrocytic infection. The genus is named after the Peruvian microbiologist Alberto Leonardo Barton, who described these organisms in 1909 while studying the agent of Oroya fever. More than 30 Bartonella spp. have been isolated from humans as well as from wild and domestic animals 1 . Two species are human-specific: Bartonella bacilliformis, the agent of Oroya fever, and B. quintana, the agent of trench fever. Incidental zoonotic human infection by other species will not lead to intraerythrocytic bacteremia, but may be associated with a wide spectrum of clinical disorders, such as cat-scratch disease, blood culture-negative endocarditis, peliosis hepatis, and Parinaud oculoglandular syndrome. Most human infections are caused by three species: B. henselae, B. quintana and B. bacilliformis 1 .
Bacillary angiomatosis (BA), also called epithelioid angiomatosis, is an angioproliferative disease of immunocompromised patients, mainly HIV-infected subjects with severe CD4 cell depletion. It was first described in 1983 by Stoler et al. 2 . BA is caused by chronic infection with either B. henselae or B. quintana. Cats are the primary reservoir for B. henselae and the infection is transmitted to humans via scratches or bites of infected animals. Among cats, B. henselae is transmitted by cat fleas (Ctenocephalides felis). By contrast, B. quintana is a human-specific pathogen with worldwide distribution and is transmitted by the human body louse (Pediculus humanus humanus).
BA usually presents with the gradual appearance of a few to hundreds of brown to violaceous vascular tumors of varying size in the skin and subcutaneous tissues. However, lesions may also be found in a variety of other tissues. Mucosal lesions may rarely occur in the oral cavity, pharynx, larynx, and endobronchial tissue. Therefore, visceral and mucosal disease should be sought in all patients diagnosed with cutaneous BA.
Only a few cases of BA in the oral cavity or other mucosal sites have been reported. These patients generally have concomitant cutaneous lesions 3 - 7 . Reports of oral BA in patients without concomitant tegumentary disease are exceedingly rare 8 - 13 . We wish to report on an unusual case of BA without simultaneous cutaneous disease in which an erosive lesion progressed to an oronasal fistula.
CASE REPORT
A 51-year-old HIV-infected male patient from Rio de Janeiro State, Brazil, sought medical care in December 2013 with a several week history of dysphagia and odynophagia. He had had a diagnosis of HIV infection in 1996 and was also infected with hepatitis C virus. Disseminated histoplasmosis was successfully treated 10 years before the current presentation. He was lost to follow-up several times between 1996 and 2013. When he first noticed the pain and difficulty in swallowing, the antiretroviral regimen had been paused for more than 8 months. At this point, the CD4 cell count was 316 cells/mm 3 . Laboratory evaluations, as well as chest X-ray, were unremarkable.
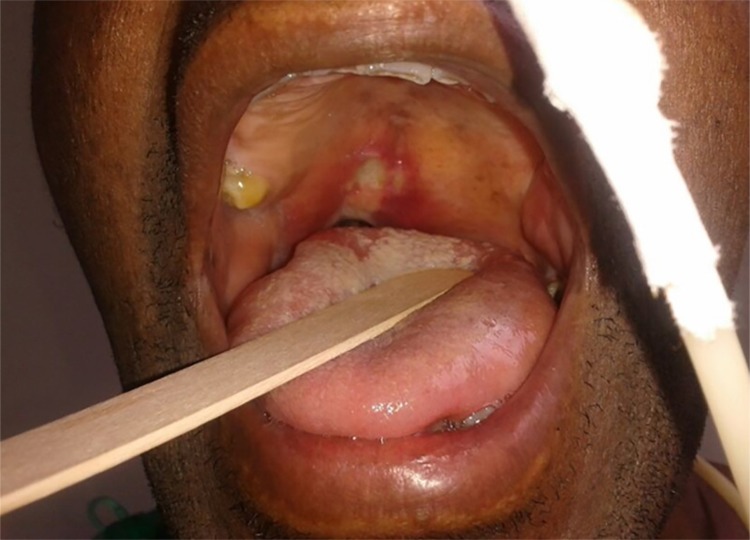
Clinical examination revealed a painful bluish-purple papular lesion over the right palatoglossal arch. There were no tegumentary lesions. Antiretroviral therapy was restarted with a combination of lamivudine, tenofovir, atazanavir and ritonavir. Acyclovir was empirically prescribed for presumed oral herpes, with no clinical response. One month later the lesion was larger, extended to the hard palate (Figure 1), had an erosive center and progressed to an oronasal fistula. The patient was febrile and complained of severe odynophagia. Freely movable submandibular and cervical lymph nodes were recorded. He was admitted to the inpatient unit and a biopsy of the lesion was then performed as a diagnostic procedure.
Figure 1. - Clinical image of a 51-year-old HIV-infected male patient who presented with a several week history of dysphagia and odynophagia. A painful bluish-purple papular lesion with an erosive center-on the right palatoglossal arch is seen. The lesion was very painful.
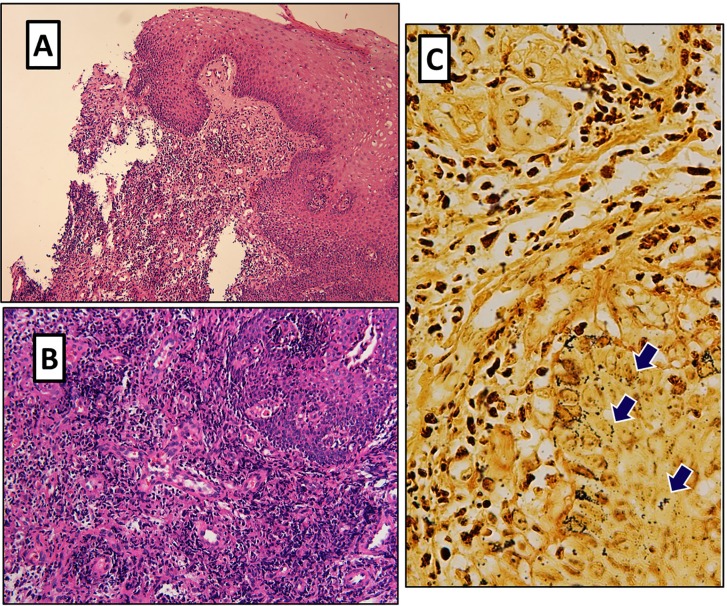
Histopathological studies showed lobular vascular proliferation with epithelioid endothelial cells and an intervening edematous stroma with an inflammatory infiltrate containing multiple neutrophils (Figures 2A and 2B). When stained with Warthin-Starry, nests of small, extracellular, dark-stained bacilli were revealed ( Figure 2C). A diagnosis of oral BA was then made. Antimicrobial therapy with doxycycline 100 mg bid was immediately instituted and continued for 3 months. The patient rapidly improved during the following days and experienced an uneventful recovery. The oronasal fistula left minimal sequelae of hyperrhinolalia. Two years later, an indirect fluorescence assay for Bartonella spp. IgG antibodies, performed according to the manufacturer instructions, yielded a titer of 1:256, indicating a previous exposure to Bartonella spp. Unfortunately, a serological assay could not be performed at the time of hospitalization. Therefore, comparison of paired samples is not possible in the present case. No evidence of relapse was recorded after more than three years of follow-up.
Figure 2. - A) Low power (original magnification 10x) hematoxylin and eosin stain shows neutrophilic inflammation and capillary proliferation; B) lobular vascular proliferation with epithelioid endothelial cells and an intervening edematous stroma with an inflammatory infiltrate of multiple neutrophils (original magnification 20x); C) Clumps of small extracellular, argyrophilic bacilli as unveiled by Warthin-Starry silver stain (original magnification 40x).
DISCUSSION
BA is classically associated with HIV-infection. However, it may also complicate the course of other immunosuppressive conditions 14 , 15 and may rarely occur in apparently immunocompetent subjects 16 . Most patients present with cutaneous disease. In 1987, Cockerell et al. 3 were the first to report a case of mucosal lesions resembling BA: a 32-year-old HIV-infected male who had numerous cutaneous lesions and died from asphyxiation due to laryngeal obstruction by multiple angiomata. However, no histopathological examination was available from the laryngeal lesions. Similar reports of mucosal lesions in patients with simultaneous cutaneous BA followed 4 - 6 . Speight et al. 8 were the first to report a case of histopathologically-proven mucosal BA. Their patient presented with BA confined to the oral cavity and, interestingly, had no associated cutaneous lesions. Few other reports of oral BA with 7 or without simultaneous cutaneous lesions 9 , 10 - 13 followed. Isolated reports of BA in other gastrointestinal mucosal sites, with 17 , 18 or without 19 , 20 simultaneous cutaneous disease, are also available.
The differential diagnosis of BA includes Kaposi`s sarcoma (KS), pyogenic granuloma, hemangioma, angiosarcoma, and cat scratch disease. It shares histopathological resemblance with KS, pyogenic granuloma, verruga peruana and angiosarcoma. The histopathological hallmark of BA is the presence of lobular proliferations of blood vessels, neutrophilic infiltration, and interstitial amorphous granular-like deposits that reveal small, extracellular, argyrophilic bacilli when stained with Warthin-Starry silver. In contrast, Kaposi’s sarcoma lesions show slit-like vascular spaces with lymphoplasmacytic inflammation and no bacilli can be stained. It should be stressed that even in patients who have a confirmed diagnosis of cutaneous KS, careful histopathological examination of mucosal lesions should be performed. López de Blanc et al. 11 described a patient in whom the cutaneous lesions were KS, but the oral lesions were BA. Therefore, the presence of confirmed cutaneous KS lesions should not be taken as evidence that the oral lesions are also KS.
Oral BA in HIV-infected patients is highly unusual but oral BA in patients without simultaneous cutaneous disease is even rarer. To the best of our knowledge, only six previous cases of oral BA without tegumentary disease have been reported to date 8 - 13 . All but one of these 12 were from the pre-highly active antiretroviral therapy era. Our patient presented with a painful bluish-purple papular lesion that eroded in the right palate and progressed to an oronasal fistula within days. A review of the clinical presentation of the few reported cases of oral BA shows that the lesions may be painful 9 , 10 , eroded 8 , 11 , associated with alveolar bone loss 9 , 11 and may relapse after inappropriate treatment 8 , 12 . They may present as sessile or lobulated swellings 11 , nodules 10 or simply as a raised lesion 8 . The lesions may bleed after oral hygiene 12 , have a red to bluish 9 or flat blue 8 aspect resembling KS. Gums and palate seem to be the preferred sites.
In summary, physicians caring for HIV-infected patients should be aware that BA may unusually present with lesions in the oral cavity or other mucosal sites, even in patients with no concomitant tegumentary disease. These lesions may be highly painful and eroded and will require biopsy and appropriate histopathological studies to be differentiated from other disorders such as KS. Recovery is usually straightforward when appropriate antimicrobial therapy is initiated. Timely diagnosis is of utmost importance since this treatable infectious disorder may rapidly progress.
REFERENCES
- 1.Angelakis E, Raoult D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents. 2014;44:16–25. doi: 10.1016/j.ijantimicag.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Stoler MH, Bonfiglio TA, Steigbigel RT, Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol. 1983;80:714–718. doi: 10.1093/ajcp/80.5.714. [DOI] [PubMed] [Google Scholar]
- 3.Cockerell CJ, Whitlow MA, Webster GF, Friedman-Kien AE. Epithelioid angiomatosis: a distinct vascular disorder in patients with the acquired immunodeficiency syndrome or AIDS-related complex. Lancet. 1987;2:654–656. doi: 10.1016/s0140-6736(87)92442-1. [DOI] [PubMed] [Google Scholar]
- 4.Axiotis CA, Schwartz R, Jennings TA, Glaser N. AIDS-related angiomatosis. Am J Dermatopathol. 1989;11:177–181. doi: 10.1097/00000372-198911020-00011. [DOI] [PubMed] [Google Scholar]
- 5.van der Wouw PA, Hadderingh RJ, Reiss P, Hulsebosch HJ, Walford N, Lange JM. Disseminated cat-scratch disease in a patient with AIDS. AIDS. 1989;3:751–753. [PubMed] [Google Scholar]
- 6.Szaniawski WK, Don PC, Bitterman SR, Schachner JR. Epithelioid angiomatosis in patients with AIDS. Report of seven cases and review of the literature. J Am Acad Dermatol. 1990;23:41–48. doi: 10.1016/0190-9622(90)70183-i. [DOI] [PubMed] [Google Scholar]
- 7.Levell NJ, Bewley AP, Chopra S, Churchill D, French P, Miller R, et al. Bacillary angiomatosis with cutaneous and oral lesions in an HIV-infected patient from the U.K. Br J Dermatol. 1995;132:113–115. doi: 10.1111/j.1365-2133.1995.tb08634.x. [DOI] [PubMed] [Google Scholar]
- 8.Speight PM, Zakrzewska J, Fletcher CD. Epithelioid angiomatosis affecting the oral cavity as a first sign of HIV infection. Br Dent J. 1991;171:367–370. doi: 10.1038/sj.bdj.4807726. [DOI] [PubMed] [Google Scholar]
- 9.Glick M, Cleveland DB. Oral mucosal bacillary epithelioid angiomatosis in a patient with AIDS associated with rapid alveolar bone loss: case report. J Oral Pathol Med. 1993;22:235–239. doi: 10.1111/j.1600-0714.1993.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 10.Hofman P, Raspaldo H, Michiels JF, Garnier G, Santini J. Angiomatose bacillaire de la cavité buccale au cours du SIDA - un diagnostic différentiel du sarcome de Kaposi muqueux. Rev Stomatol Chir Maxillofac. 1993;94:375–378. [PubMed] [Google Scholar]
- 11.López de Blanc S, Sambuelli R, Femopase F, Luna N, Gravotta M, David D, et al. Bacillary angiomatosis affecting the oral cavity. Report of two cases and review. J Oral Pathol Med. 2000;29:91–96. doi: 10.1034/j.1600-0714.2000.290207.x. [DOI] [PubMed] [Google Scholar]
- 12.Tucci E, Della Rocca C, Santilli F. Localized bacillary angiomatosis in the oral cavity: observations about a neoplasm with atypical behavior. Description of a case and review of the literature. Minerva Stomatol. 2006;55:67–75. [PubMed] [Google Scholar]
- 13.Gazineo JL, Trope BM, Maceira JP, May SB, Coelho JM, Lambert JS, et al. Bacillary angiomatosis: description of 13 cases reported in five reference centers for AIDS treatment in Rio de Janeiro, Brazil. Rev Inst Med Trop Sao Paulo. 2001;43:1–6. doi: 10.1590/s0036-46652001000100001. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz RA, Gallardo MA, Kapila R, Gascón P, Herscu J, Siegel I, et al. Bacillary angiomatosis in an HIV seronegative patient on systemic steroid therapy. Br J Dermatol. 1996;135:982–987. doi: 10.1046/j.1365-2133.1996.d01-1107.x. [DOI] [PubMed] [Google Scholar]
- 15.Psarros G, 4th Riddell J, Gandhi T, Kauffman CA, Cinti SK. Bartonella henselae infections in solid organ transplant recipients: report of 5 cases and review of the literature. Medicine (Baltimore) 2012;91:111–121. doi: 10.1097/MD.0b013e31824dc07a. [DOI] [PubMed] [Google Scholar]
- 16.Zarraga M, Rosen L, Herschthal D. Bacillary angiomatosis in an immunocompetent child: a case report and review of the literature. Am J Dermatopathol. 2011;33:513–515. doi: 10.1097/DAD.0b013e3181ec846a. [DOI] [PubMed] [Google Scholar]
- 17.Walford N, Van der Wouw PA, Das PK, Ten Velden JJ, Hulsebosch HJ. Epithelioid angiomatosis in the acquired immunodeficiency syndrome: morphology and differential diagnosis. Histopathology. 1990;16:83–88. doi: 10.1111/j.1365-2559.1990.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang AD, Drachenberg CI, James SP. Bacillary angiomatosis associated with extensive esophageal polyposis: a new mucocutaneous manifestation of acquired immunodeficiency disease (AIDS) Am J Gastroenterol. 1996;91:2220–2223. [PubMed] [Google Scholar]
- 19.Huh YB, Rose S, Schoen RB, Hunt S, Whitcomb DC, Finkelstein S. Colonic bacillary angiomatosis. Ann Intern Med. 1996;124:735–737. doi: 10.7326/0003-4819-124-8-199604150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Chetty R, Sabaratnam RM. Upper gastrointestinal bacillary angiomatosis causing hematemesis: a case report. Int J Surg Pathol. 2003;11:241–244. doi: 10.1177/106689690301100316. [DOI] [PubMed] [Google Scholar]