Abstract
Development of multifunctional nanomaterials, one of the most interesting and advanced research areas in the field of nanotechnology, is anticipated to revolutionize cancer diagnosis and treatment. Gold nanoparticles (AuNPs) are now being widely utilized in bio-imaging and phototherapy due to their tunable and highly sensitive optical and electronic properties (the surface plasmon resonance). As a new concept, termed “theranostics,” multifunctional AuNPs may contain diagnostic and therapeutic functions that can be integrated into one system, thereby simultaneously facilitating diagnosis and therapy and monitoring therapeutic responses. In this review, the important properties of AuNPs relevant to diagnostic and phototherapeutic applications such as structure, shape, optics, and surface chemistry are described. Barriers for translational development of theranostic AuNPs and recent advances in the application of AuNPs for cancer diagnosis, photothermal, and photodynamic therapy are discussed.
Keywords: multifunctional gold nanoparticles, cancer bioimaging, cancer photothermal and photodynamic therapy
Introduction
Cancer, one of the leading causes of mortality worldwide, has caused approximately 8.8 million deaths in 2015 (www.who.int). The number of people who are diagnosed with this malignancy is expected to rise to 22 million annually in the next 2 decades (www.who.int). Despite the increased knowledge about the causes of cancer and the improved interventions to prevent and manage the disease, survival rates are still low mainly due to the delay in diagnosis, lack of effective therapeutics, and high incidence of relapse.
As an emerging concept that facilitates simultaneous diagnosis and treatment, the implementation of theranostic nanomaterials (ie, metal and silica nanoparticles [NPs], liposomes, dendrimers, quantum dots, and carbon nanotubes) has great potential for improved cancer treatment and reduced side effects.1–6 Among these, gold NPs (AuNPs) exhibit favorable physical properties and tailored surface functionalization, providing a potential platform for developing cancer theranostics. This review provides a comprehensive overview of AuNPs as emerging nanomaterials for future cancer theranostics. In this regard, the key physicochemical properties of AuNPs such as structure, shape, optics, and surface chemistry are discussed. In addition, various AuNP-based diagnostic and phototherapeutic strategies under investigation are critically evaluated, with a particular emphasis on those developed to overcome delivery barriers.
Key properties of AuNPs for diagnosis and phototherapy
Types of AuNPs
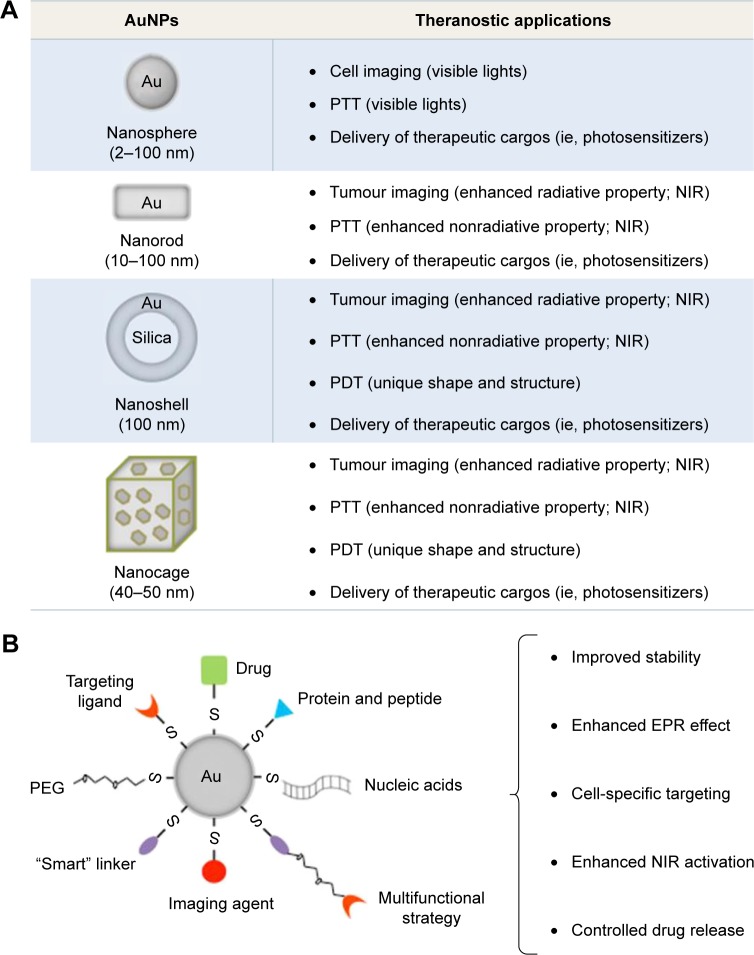
Colloidal AuNPs were first produced in 1857 by Faraday,7 where the “fine particles” were formed from the reduction of gold chloride by phosphorus and the stabilization of AuNPs by carbon disulfide. In 1951, Turkevich et al8 reported the formation of colloidal AuNPs using trisodium citrate (HOC(COONa)(CH2COONa)2) to reduce tetrachloroauric acid (gold [III] chloride [HAuCl4]) in water, and later Frens9 improved the formation using a slightly modified method. Recently, AuNPs have been produced with various sizes and shapes (ie, gold nanospheres, nanorods, nanocages, nanoshells, and nanostars), which are dependent upon the synthetic methods adopted for their preparation (Figure 1; a summary of synthetic approaches to obtain various gold nanostructures has been described previously).10–14
Figure 1.
Development of theranostic AuNPs in the treatment of cancer.
Notes: (A) Commonly used AuNPs can be categorized depending on the particle shape, including Au nanospheres, nanorods, nanoshells, and nanocages. These AuNPs with tunable optical and electronic properties and easy surface functionalizations have presented great potential for cancer bioimaging, PTT/PDT, and targeted drug delivery. (B) Functional components including stealth coating materials, bioresponsive moieties, bioactive targeting ligands, bioimaging agents, and therapeutic cargos can be integrated into one system, to achieve multifunctional AuNPs for future cancer treatment.
Abbreviations: AuNPs, gold nanoparticles; EPR, enhanced penetration and retention; NIR, near-infrared; PDT, photodynamic therapy; PEG, polyethylene glycol; PTT, photothermal therapy.
Gold nanospheres
Gold nanospheres (particle size 2–100 nm) are generally prepared after reducing HAuCl4 with the assistance of different reducing agents under various temperature and pressure. Trisodium citrate (sometimes referred to simply as sodium citrate), for example, is a commonly used reducing and stabilizing agent, which is capable of generating monodisperse gold nanospheres with different particle sizes by adjusting the concentration of citrate.11
The seeding growth strategy has been used to improve monodispersity and avoid the formation of large AuNPs with irregular shapes (diameter >50 nm), whereby large spherical AuNPs are produced by reducing HAuCl4 or Au (III)-surfactant complexes onto the surface of preexisting seeds using reducing agents such as hydroxylamine hydrochloride,15,16 ascorbic acid,17 2-mercaptosuccinic acid,18 hydroquinone,19 and hydrogen peroxide.20
Gold nanorods (GNRs)
GNRs were first synthesized by Foss et al,21 Martin,22 and Perez-Juste et al23 using the template method. They are generally synthesized with a size ranging from 10 nm to 100 nm and an aspect ratio between 1 (sphere) and 7 with a corresponding longitudinal plasmon of ~1,050 nm. However, the yield is low due to the fact that only one monolayer of nanorods is produced using the template approach.
Alternatively, the seed growth method is also used to synthesize GNRs.23 In this method, gold seeds are prepared by reducing the gold salt with a strong reducing agent (ie, sodium borohydride). These seeds that provide the nucleation sites are subsequently added to the aqueous surfactant media containing the gold salt and the reducing agents (ie, ascorbic acid and hexadecyltrimethylammonium bromide [CTAB]) for the growth steps.24,25 Additional nucleation during the growth stage can be inhibited by controlling the growth conditions such as 1) the rate of addition of reducing agents to the gold seed and gold salt solution and 2) the reduction potential of reducing agents. As a result, the aspect ratios of nanorods (the ratio of the longer side to the shorter side) can be controlled by using different amounts of gold seeds relative to the precursor.
Gold nanocages
Gold nanocages are hollow nanostructures (particle size ~40–50 nm) which can be prepared with controllable pores on the surface via the galvanic replacement reaction between truncated silver (Ag) nanocubes and HAuCl4.26 Silver nanostructures with controlled shapes can be generated via the polyol reduction, where AgNO3 is reduced by ethylene glycol to generate Ag atoms followed by nanocrystals or seeds. Silver nanostructures are utilized as the sacrificial template and can be transformed into AuNPs with hollow structures via the galvanic replacement.26 The molar ratio of Ag to HAuCl4 can be regulated to manipulate the diameter and wall thickness of resultant gold nanocages.26
Gold nanoshells
Gold nanoshells are normally composed of dielectric core materials (ie, silica and polystyrene) coated by a thin gold layer. Core materials such as silica and polystyrene have been widely used to provide high stability and monodispersity.27 These core materials can be tailored by varying the dimensions of the core and/or the shell; normally, the core has a diameter ~100 nm and a thin shell of gold about several nanometers (~1–20 nm).28,29
Modification of the core surface with a bifunctional ligand that enhances the shell coverage is a common method for the preparation of gold nanoshells. In the case of silica, the core surface is modified by 3-aminopropyltriethoxysilane (APS) with both ethoxy and amine groups. The ethoxy group binds covalently to silica surface through the hydroxyl group, while the amine group attaches to gold seeds. In addition, bifunctional linkers such as 3-aminopropyltrimethoxysilane (APTMS) and 3-mercaptopropyltrimethoxysilane (MPTMS) have also been used to modify the silica, leading to amino-and thiol-functionalized surfaces that can efficiently attach to gold seeds. As a result, the complete shell is formed by aging the gold attached onto the silica core.30
Optical characteristics of AuNPs
It is known that the oscillating electromagnetic field of light induces a collective coherent oscillation of the free electrons (also known as conduction band electrons) of the metal.31 The amplitude of the oscillation that reaches a maximum at a specific frequency is termed the surface plasmon resonance (SPR).31 A strong absorption of the incident light is induced by the SPR and can be measured using an ultraviolet (UV)–visible absorption spectrometer.32 The SPR band of noble metals (ie, gold and silver) is known to be much stronger than other metals.32 The SPR wavelength of AuNPs can be tuned from the visible to the near-infrared (NIR) region by changing the size, shape, and structure of AuNPs, as theoretically described by the Mie theory.32
Two main processes, namely, absorption and scattering, occur when the light passes through matter resulting in the energy loss of electromagnetic wave.33 The scattered light has the same frequency as the incident light (it is termed as Rayleigh scattering) or a shifted frequency (it is termed as Raman scattering).33 AuNPs can significantly enhance the light scattering, ie, five- to six-fold stronger than most strongly absorbing organic dyes and higher than the emission of most strongly fluoresceins,34 which makes AuNPs very promising imaging and detection platforms for cancer, as described in the “AuNPs for cancer diagnosis” section.
The scattering properties are highly dependent on the size and shape of AuNPs, which are given in the following sections.
Particle size
AuNPs with particle sizes of ~40 nm may be easily detected down to a particle concentration of 10−14 M.35,36 Moreover, the scattering of ~60 nm AuNPs is ~100-fold stronger than the emission of a fluorescein.36 Likewise, ~70 nm AuNPs can scatter orders of magnitude stronger than that of a polystyrene sphere of the same size.37 It has been reported that ~30–100 nm AuNPs can be detected under a microscope using dark-field illumination conditions (only the light scattered from indirect illumination of the sample is detected).38 Recently, Jain et al34 and Lee and El-Sayed39 have substantially studied the relationship between the optical absorption/scattering and the size of AuNPs using the Mie theory. They reported that the total extinction of ~20 nm AuNPs was mostly contributed by absorption; when the particle size of AuNPs was ~40 nm, they initiated scattering, and when the particle size was ~80 nm, the extinction of AuNPs was contributed by absorption and scattering in a very similar degree.34,39 This fact that the ratio of scattering to absorption increases significantly for larger size of particles can guide the development of AuNPs for bioimaging.
Particle shape
The optical properties of AuNPs can also be tuned by shape.40–42 When the shape of AuNPs is changed from sphere to rod, the SPR will be split into two bands, namely, a strong band in the NIR region and a weak band in the visible region, as predicted by the Gans theory. The strong band, also referred to as the longitudinal band, results from electron oscillations along the long axis. The weak band, also known as the transverse band, is similar to the wavelength of gold nanospheres. Tong et al43 reported that the longitudinal band of GNRs was red shifted largely by increasing the aspect ratios (length/width), which causes the color change from blue to red. In addition, Lee and El-Sayed44 have shown that when the aspect ratio of GNRs was increased, light scattering was significantly enhanced.
It is known that the SPR of gold nanocages may be tuned to the NIR region with specified wavelengths.45 For instance, the particle size of gold nanocages is generally ~50 nm edge width with several nanometer walls and holes for an SPR wavelength of ~800 nm.46
Particle composition
It is known that the composition of NPs (particularly semiconductor NPs such as quantum dots) is also able to affect the scattering properties;47 recently, several studies have focused on the scattering properties dependent on the composition of Au nanocomplexes.48–50 Jain et al34 have investigated the scattering properties of the core–shell composition in silica–Au nanoshells using Mie theory and discrete dipole approximation method. Results show that the thicknesses of the core and the shell and the radius ratio of core/shell significantly influenced the optical characteristics such as the resonance wavelength, the extinction cross-section, and the ratio of scattering to absorption.34 In addition, it was reported that the SPR wavelength of silica–Au nanoshells may be changed by controlling the Au shell thickness; for example, when the shell thickness was decreased from 20 to 5 nm, the SPR was red shifted ~300 nm, which most likely results from the increased coupling between the inner and outer shell surface plasmons for thinner shell particles.30
Surface functionalization of AuNPs
The surface chemistry of gold makes AuNPs a promising platform for biomedical applications (Figure 1).11 Brust et al51 exploited the high affinity of thiol for gold to produce AuNP-thiolates (Au-S); this was achieved using HAuCl4, thiol, tetraoctylammonium bromide, and NaBH4 in water–toluene and was stabilized via Au-S-stabilizer bonds. These NPs were dispersed in organic solvent and required further phase transfer or ligand exchange to transfer them into water. An essential purification step to remove impurities for use in biological application was also necessary.
In addition, ligands containing amine and phosphine groups, which also have high affinity with the surface of gold, have been used as efficient stabilizing agents.52 For example, cationic surfactant-free AuNPs (~2–200 nm) have been developed that were synthesized in water using a seed growth method, in the presence of L-cysteine methyl ester hydrochloride (HSCH2CH(NH2)COOCH3⋅HCl) as a capping agent.53 Furthermore, Chhour et al54 have functionalized AuNPs using a group of thiol and amine ligands, including 11-mercaptoundecanoic acid (11-MUDA), 16-mercaptohexadecanoic acid (16-MHDA), polyethylenimine (PEI), 4-mercapto-1-butanol (4-MB), and 11-mercaptoundecyl-tetra (ethylene glycol) (MTEG). These ligands enhanced the AuNP stability and provided different surface functionalities which can influence cell uptake and cytotoxicity.54 Among them, the 11-MUDA AuNPs were used to monitor the recruitment of monocytes into atherosclerotic plaques in a disease mouse model using X-ray computed tomography (CT), potentially allowing detection of monocyte recruitment in the presence of emerging atherosclerosis therapies.54 In addition, Liu et al55 have recently stabilized AuNPs using thiol-polyethylene glycol (SH-PEG) at two different ratios of thiol to PEG (1:1 and 1:2), significantly improving the AuNP stability in biological media. When AuNPs with higher PEG content were intravenously injected, they were found to accumulate in the liver at a lower level relative to counterparts with lower PEG content.55 These results suggest that when designing AuNPs, a rational ratio between the anchoring group (ie, thiol) and the hydrophilic group (ie, PEG) should be carefully considered, this is important for integrating the properties of NPs in certain bio-related application.
Targeting ligands56–60 may be modified onto the AuNP surface to specifically deliver therapeutic cargos into tissues and organs. Recently, tumor-targeted mesoporous silica-encapsulated GNRs (GNRs@mSiO2) for chemotherapy and photothermal therapy (PTT) have been developed by Shen et al.61 In this study, RGD peptides, a targeting ligand for αvβ3 integrin receptors that are known to overexpress on several cancer cells, were conjugated to the terminal groups of PEG on GNRs@mSiO2 (namely, pGNRs@mSiO2-RGD). The pGNRs@mSiO2-RGD presented significant stability in bio-environments and efficient loading of doxorubicin (DOX, an antitumor chemotherapeutics). Following the NIR irradiation, the combination of photothermal ablation and DOX-mediated cytotoxicity using pGNRs@mSiO2-RGD resulted in significant tumor reduction in subcutaneous xenografted mice.61 In addition, it has been reported that PEI-capped AuNPs (Au-PEI) were conjugated with anisamide (AA, which is known to bind to the sigma receptor overexpressed on prostate cancer cells)62,63 to produce the AA-targeted AuNPs (Au-PEI-AA).59 As a result, Au-PEI-AA facilitated siRNA uptake into prostate cancer PC-3 cells via binding to the sigma receptor and achieved efficient downregulation of the targeted oncogene.59
In addition to the aforementioned stabilizing and targeting ligands, the gold surface can also be modified using several suitable imaging agents, photosensitive molecules, and bioactive/bioresponsive moieties (ie, pH-sensitive linkers, matrix metalloproteinase [MMP]-sensitive linkers, temperature-sensitive linkers, fusogenic/synthetic peptides, and endosomal membrane-disruptive materials) to achieve multifunctional AuNPs for cancer diagnosis and phototherapy, which are discussed in the following sections.
AuNPs for cancer diagnosis
Spectroscopic cancer imaging
For wavelengths >650 and <2,000 nm, the tissue absorption is weak, so the NIR light (wavelength from 700 to 2,500 nm) is normally chosen to image tumor deeply within the body. It is worth noting that the penetration depth of NIR light into tissues is highly dependent on the tissue type, the wavelength, and the condition of the incident beam (ie, the laser power, irradiation time, and time interval).64–66 For example, it was shown that no penetration was found in the skin, skull, or brain for NIR light with low-power laser; however, 0.45%–2.90% of 810 nm NIR light at high power (10–15 W) was delivered into 3 cm of the aforementioned tissues.66
AuNPs on their own may act as an NIR-active imaging probe for cancer detection facilitating whole-body scans due to the unique optical properties. The use of targeted AuNPs as the contrast agent was demonstrated by Sokolov et al,37 where AuNPs were conjugated with an antibody against the epidermal growth factor receptor (EGFR, it is known to overexpress on many cancers). These AuNP conjugates were used for detecting cancer cells using a scanning confocal microscope in the reflectance mode with a 647 nm laser to excite the SPR of AuNPs; as a result, cells with AuNP conjugates were clearly imaged on a dark background.37 El-Sayed et al67 improved the use of AuNPs (~35 nm) as the contrast agent via dark-field microscopy. Following excitation by white light, only the wavelength of light corresponding to the maximum of the SPR of AuNPs was displayed intensely, where a bright image of cells with AuNPs could be seen on a dark background.67 Moreover, cetuximab (CET, a chimeric monoclonal antibody against the EGFR)-conjugated PEGylated GNRs (CET-pGNRs) was developed for cancer imaging in xenografted mice.68 The results of in vivo NIR absorption imaging show that specific targeting of CET-pGNRs to the tumor region was evident by a significant increase in the absorption signal. The biodistribution data also show that the amount of AuNPs in tumor tissues from mice injected with CET-pGNRs was eightfold greater than that recorded by nontargeted pGNRs, confirming the results from the NIR absorption imaging. These indicate that CET-pGNRs can specifically target tumor tissues with high specificity and provide a potential tool for NIR-based cancer diagnosis.
Recently, the photoacoustic imaging has taken advantage of plasmonic systems, such as AuNPs with various sizes and shapes.69 Plasmon resonances of AuNPs can be tuned to enhance the optical response,70,71 which can give rise to heat conversion with high efficiency and to the subsequent pressure wave generating the photoacoustic signal. Indeed, these properties have been utilized to develop AuNPs as contrast agents for the photoacoustic imaging.72 Recently, an amphiphilic GNR coated with PEG and poly(lactic-co-glycolic acid) (PLGA; AuNR⋅PEG⋅PLGA) was developed for the photoacoustic imaging in xenografted mice.73 The AuNR⋅PEG⋅PLGA could self-assemble into vesicles with the AuNRs embedded in the shell formed by the PLGA and PEG extending into the aqueous environments to stabilize the structure. Furthermore, the in vivo two-dimensional (2D) and three-dimensional (3D) photoacoustic images show that the strong plasmonic coupling of GNRs in the vesicles induced a high photothermal effect and a photoacoustic signal, which may potentially be used for image-guided phototherapy in the future.73
In addition, AuNPs have also been utilized as high-quality CT imaging agents due to better X-ray attenuation properties (atomic number Z =79; k-edge value =80.7 keV) than that of iodinated CT contrast agents.74,75 In addition, the conventional small-molecular CT contrast agents are rapidly cleared by the kidney resulting in short imaging times, whereas the gold surface can be modified with biological stabilizing groups (ie, PEG) to improve the pharmacokinetic properties,76 which is beneficial for cancer imaging. Recently, a folic acid (FA)-targeted gold nanosphere (FA-PEG-PEI-AuNPs) was developed using PEI and PEG as stabilizing ligands.77 The intravenous injection of FA-PEG-PEI-AuNPs into an overexpressed folate receptor tumor model resulted in significantly higher CT values in the tumor region compared with nontargeted PEG-PEI-AuNPs. In addition to the “enhanced penetration and retention” (EPR)-based passive tumor targeting, the FA-mediated active targeting was also able to significantly enhance the AuNP accumulation in tumor tissues, resulting in enhanced cancer CT imaging.
In addition, AuNPs are also known to enhance the Raman scattering signal of adjacent molecules, and therefore, surface-enhanced Raman spectroscopy (SERS) imaging aided by gold nanomaterials (spheres, rods, cubes, etc.) has also widely been used in the detection of viruses and cancer cells.78,79
Functionalized imaging agents for cancer detection
Hybrid dual imaging technologies, including positron emission tomography (PET)/CT, PET/magnetic resonance imaging (MRI), and ultrasound/CT, have recently become available.80 Cancer diagnosis clearly benefits from these techniques due to multimodality, as a single agent may avoid the administration of multiple doses. However, the choice of imaging modality must be carefully considered since each one has its own advantages and limitations (ie, modalities with high sensitivity may have poor resolution).
AuNPs can be easily functionalized with additional imaging agents, and improvement in AuNP-based imaging systems may allow the observation of tissues not only on its basic anatomic configuration but also on the molecular level.42,81,82 Moreover, the real-time noninvasive monitoring potentially enables a rapid decision on whether the treatment regimen is effective in a given patient.40,83
Recently, Zhao et al84 have synthesized gold nanospheres doped with 199Au atoms using a one-step procedure for single-photon emission CT (SPECT)/CT imaging in an orthotopic mouse xenograft of triple-negative breast cancer (TNBC). The high-stable radiolabeling ability resulted from the incorporation of 199Au atoms into the crystal lattice of AuNPs. In addition, the 199Au-doped AuNPs were further modified with 1) PEGylation for favorable pharmacokinetics and 2) D-Ala1-peptide T-amide (DAPTA) for targeting C–C chemokine receptor 5 (CCR5, a prognostic biomarker for breast cancer progression).84 Results demonstrate the suitability of 199Au for SPECT/CT imaging and the potential of 199Au-AuNP-PEG-DAPTA for accurately detecting CCR5 in vivo.
Moreover, He et al85 have recently synthesized novel AuNPs for magnetic and CT dual-mode imaging in a mouse xenograft of colorectal cancer. Fe2O3 was first coated with Au nanoshell (Fe2O3/AuNPs), and subsequently the surface of the Fe2O3/AuNPs was modified with lectins (sugar-binding proteins specifically bind to the carbohydrate moieties of the glycans on colorectal cancer cells) through bifunctional PEG-N-hydroxysuccinimide ester disulfide linkers (lectin–PEG–Fe2O3/AuNPs). The lectin–PEG–Fe2O3/AuNPs demonstrated long circulation time, site-specific tumor distribution, and high-quality MRI and CT contrast enhancement effects in tumor tissues, suggesting that the resultant AuNPs are a promising contrast agent for dual-mode MRI/CT colorectal cancer imaging.
Furthermore, selected examples of AuNP-based systemic cancer imaging are provided in Table 1, including the types of AuNPs, functional ligands, cancer types, in vivo animal models, imaging techniques, and end point comments.
Table 1.
A summary of studies on the in vivo use of gold nanocomplexes in systemic cancer imaging
| Functional ligand | Cancer type | In vivo model | Imaging technique | Comment | Reference |
|---|---|---|---|---|---|
| Gold nanospheres | |||||
| Stabilizing ligand: PEG and PEI Targeting ligand: FA |
Papilloma (KB cells) | S.C. xenograft mouse | CT | The AuNP-PEI was modified with FA-linked PEG, forming FA-targeted PEGylated AuNPs. The resultant targeted AuNPs presented potential role as a nanoprobe for CT imaging of FA receptor-overexpressing xenografted tumor | 77 |
| Stabilizing ligand: PEG fluorescent dye | Colon carcinoma (CT26 cells) | S.C. allograft mouse | CT | The signal intensity and nanoprobe accumulation of Au-NPAPF-PEG in the tumor were up to 24 h post i.v. injection, suggesting the role as a promising nanoprobe for in vivo tumor-targeted CT imaging | 87 |
| Stabilizing ligands: PEG and glucose Targeting ligand: glucose |
Melanoma (SKMEL23 cells) | S.C. xenograft mouse | CT | The AuNP-labeled T cells were injected intravenously to mice-bearing human melanoma xenografts, and whole-body CT imaging allowed examination of the distribution, migration, and kinetics of T cells | 88 |
| Stabilizing ligand: PEG | Lung cancer (SPC- A1 cells) | S.C. xenograft mouse | CT | Results suggest that PEGylated AuNPs can be used as a promising contrast agent with enhanced biocompatibility for CT imaging in cancer diagnosis | 89 |
| Hybrid formulation: mesoporous silica NPs emitter: 64Cu |
Lung cancer | Urethane- induced lung cancer mouse | PET | 64Cu-labeled gold/mesoporous silica hybrid NPs can successfully detect the existence of clinically relevant spontaneous lung tumors in a urethane-induced lung cancer mouse model through PET imaging | 90 |
| Stabilizing ligand: PEG Targeting ligand: TAT Emitter: Gd3+ |
Glioblastoma (U87 cells) | Orthotopic xenograft mouse | MRI | Compared with the Gd3+ chelate, TAT-Au NP-Gd conjugates showed a 2.2-fold higher relaxivity and 82-fold enhancement in Gd3+ cellular uptake, which allowed for sensitive detection of the cancer cells via MRI | 91 |
| Stabilizing ligand: PEG Targeting ligand: RGD Emitter: 125I |
Glioblastoma (U87 MG cells) | S.C. xenograft mouse | SPECT/CT | In vivo SPECT/CT imaging results showed that the 125I-labeled RGD-PEG-AuNP probes can target the tumor site as soon as 10 min after injection | 92 |
| Stabilizing ligand: PEG Targeting ligand: DAPTA |
TNBC (4T1 cells) | Orthotopic allograft mouse | SPECT/CT | The synthesis of AuNPs was doped with 199Au atoms into the crystal lattice of each AuNP, which ensured the highest possible stability for the radiolabel. When conjugated with DAPTA for the CCR5 receptor, the targeted AuNPs resulted in the in vivo sensitive and specific detection | 84 |
| Stabilizing ligand: GC MMP sensitive linker: MMP peptide NIR dye: Cy5.5 |
Colorectal cancer (HT-29 cells) | S.C. xenograft mouse | CT NIR fluorescence imaging |
The quenched Cy5.5 was recovered by cleavage of the peptide substrates upon exposure to the active MMPs, which is overexpressed in tumor tissue. As a result, the AuNPs simultaneously provided CT images with high spatial resolution and optical images with high sensitivity | 93 |
| Stabilizing ligand: PEG Targeting ligand: FA Emitter: Gd3+ |
Papilloma (KB cells) | S.C. xenograft mouse | CT MRI |
With the modification of PEG and the FA- targeting ligand, the multifunctional AuNPs were able to be used for dual-mode CT/MRI of xenograft tumor models overexpressing FA receptors | 94 |
| Photostability enhancer: PB | Colon adenocarcinoma (HT-29 cells) | S.C. xenograft mouse | PAI CT |
The AuNPs were coated with PB to form the core/shell Au@PB NPs, which were found to be an excellent photoabsorbing agent for both PTT and PAI. The gold core ensured a remarkable contrast enhancement for CT imaging | 95 |
| Stabilizing ligand: PEG NIR dye: Cy5.5 | Squamous carcinoma (SCC7 cells) | S.C. allograft mouse | PAI NIR fluorescence imaging |
The resultant AuNPs showed high fluorescence and PAI signals in the tumor over time, which peaked at the 6 h time point (tumor-to-normal tissue ratio of 3.64±0.5I for optical imaging and 2.5±0.27 for PAI) | 96 |
| GNRs | |||||
| Stabilizing ligand: PEG Targeting ligand: biotin |
Squamous carcinoma (SCC7 cells) | S.C. allograft mouse | PAI | Under the photothermal/photoacoustic imaging, the in vivo pharmacodynamic effect of resultant GNRs could be monitored by precisely controlling the irradiation time and intensity of the NIR light | 97 |
| Amphiphilic ligands: PEG and PLGA | Glioblastoma (U87 MG cells) | S.C. xenograft mouse | PAI | Amphiphilic AuNRs were prepared by grafting with PEG and PLGA forming vesicles. Enhanced PA signals were due to the strong plasmonic coupling of the gold in the vesicular shell | 73 |
| Stabilizing ligand: PEG Targeting ligand: CET |
Epithelial carcinoma (A431 cells) | S.C. xenograft mouse | NIR fluorescence imaging | The NIR absorption images showed that the relative total photon counts from targeted Au nanorods in tumor tissue at 6 h were 10-fold higher than those from nontargeted counterparts | 68 |
| Stabilizing ligand: PEG NIR dye and photosensitizer: AlPcS4 |
Squamous carcinoma (SCC7 cells) | S.C. xenograft mouse | NIR fluorescence imaging | After i.v. injection of the AuNP-AlPcS4 complex, tumor sites were clearly identified on NIR fluorescence imaging as early as 1 h after injection | 98 |
| Stabilizing ligand: PNIPAAmMA MRI contrast agents: Fe3O4 NPs |
Glioma (C6 cells) | S.C. xenograft mouse | PET PAI |
GNRs were coated with PNIPAAmMA and Fe3O4 NPs using a simple LbL method, demonstrating the accurate tumor location using dual MRI and PAI | 99 |
| Stabilizing ligand: PEG SERS reporters | Ovarian cancer (MDA-435S, HEY, SKOv3 cells) | S.C. xenograft mouse | PAI SERS imaging |
PEGylated Au nanorods allowed presurgical PAI visualization of a tumor for locoregional staging as well as intraoperative SERS imaging for complete resection of tumor margins | 100 |
| Stabilizing ligand: liposome | Liver cancer (HepG2, Huh-7) | Orthotopic xenograft mouse | PAI NIR fluorescence imaging |
ICG-loaded liposome-Au nanorods exhibit favorable biocompatibility, high stability, and enhanced dual-model imaging signal | 101 |
| Gold nanoshells | |||||
| Stabilizing ligand: PPAA shell Targeting ligand: CET Emitter:89Zr |
Epithelial carcinoma (A431 cells) | S.C. xenograft mouse | PET | PET studies showed that the resultant AuNPs-PPAA-CET-89Zr provided high tumor- to-background ratio, suggesting a valuable tool for theranostic purposes | 102 |
| Stabilizing ligand: PEG Emitter: 64Cu |
Head and neck squamous cell carcinoma (SCC4 cells) | S.C. xenograft rat | PET/CT | The in vivo distribution of 64Cu-Au nanoshells was monitored using PET/CT imaging at various time points after i.v. injection | 103 |
| Targeting ligand: lectin MRI contrast agents: Fe3O4NPs |
Colorectal cancer (Sw620 cells) | S.C. xenograft mouse | MRI CT |
The lectin-Fe2O3@Au nanoshells showed great potential for dual-mode MRI and CT imaging of colorectal cancer in vivo | 85 |
| Targeting ligand: antibody (anti-NGAL) MRI contrast agents: Fe3O4NPs |
Pancreatic cancer (AsPC-1 cells) | S.C. xenograft mouse | MRI NIR fluorescence imaging |
Antibody-conjugated Au nanoshells specifically targeted pancreatic cancer cells in vivo providing contrast for both NIR fluorescence and T2-weighted MRI with high tumor contrast | 104 |
| Stabilizing ligand: PEG Emitter: Gd3+ |
Melanoma (B16- F10 cells) | S.C. xenograft mouse | MRI X-ray imaging Optical imaging |
The Gd3+-conjugated Au-silica nanoshells showed great potential for multimode MRI, X-ray imaging, and optical imaging of melanoma in vivo | 105 |
| MMP-triggering ligand: gelatin MRI contrast agent:Fe3O4 |
Hepatoma (H22 cells) | S.C. allograft mouse | CT and PAT imaging and MRI | A bio-eliminable MPNA, assembled from Fe3O4 nanocluster and gold nanoshell, could respond to the local microenvironment with acidic pH and enzymes in tumors, collapse into small molecules and discrete NPs, and finally be cleared from the body | 106 |
| Gold nanoclusters | |||||
| Stabilizing ligand: BSA Targeting ligand: methionine NIR dye: hydrophilic ICG |
Breast cancer (MDA-MB-231 cells) | S.C. xenograft mouse | NIR fluorescence imaging | The fluorescence signal in receptor-positive tumor was distinguishable from the normal tissues at 2 h post injection, reached peak intensity at 10 h post injection, and was still detectable at 96 h | 107 |
| Stabilizing ligand: BSA Targeting ligand: FA and HA |
Liver cancer (HepG2 cells) Adenocarcinoma (A549 cells) | S.C. xenograft mouse | NIR fluorescence imaging | The strong fluorescence was observed at the tumor sites derived from the selectively accumulated targeted AuNPs, demonstrating a promising probe for the cancer diagnosis | 108 |
| Stabilizing ligand: BSA Nuclear imaging moiety: Hoechst |
Pancreatic tumor (MiaPaca-2 cells) | S.C. xenograft mouse | Maestro™ 2 in vivo imaging system | The in vivo imaging was performed via blue and red channels which displayed the accumulation of Hoechst-AuNCs mainly in the tumor and partly in the liver and kidneys | 109 |
| Stabilizing ligand: PEG Emitter: 64Cu |
Prostate cancer (PC3 cells) | S.C. xenograft mouse | PET/CT | PET/CT results demonstrated the heterogeneous intratumoral distribution of 64CuAuNCs-PEG350 and 64CuAuNCs-PEG1000 | 110 |
| Emitter: 64Cu | Glioblastoma (U87 MG cells) | S.C. xenograft mouse | PET IvIS® imaging system |
64Cu-dopped AuNCs showed satisfactory synergistic dual-modality PET and self- illuminating NIR tumor imaging | 111 |
| Stabilizing ligand: BSA Emitter: Gd3+ |
Breast cancer (MCF-7 cells) | S.C. xenograft mouse | CT NIR fluorescence imaging MRI |
The hybrid gold-gadolinium nanoclusters provided a promising nanoprobe for cancer- targeted imaging and diagnosis in vivo | 112 |
| Stabilizing ligand: hairpin-DNA | Melanoma (M14 cells) | S.C. xenograft mouse | NIR fluorescence imaging | The hairpin-DNA-modified NaYF4@SiO4-Au promoted simultaneous deep tissue imaging and drug molecule release when combining single-band anti-stokes NIR emission and the photothermal effect | 113 |
| pH-sensitive ligand: azide and alkyne functionalities | Glioma (U87MG cells) | Orthotopic xenograft mouse | MRI and SERS imaging | Multifunctional AuNPs could not only preoperatively define orthotopic glioblastoma xenografts by MRI with high sensitivity and durability in vivo but also intraoperatively guide tumor excision with the assistance of a handheld Raman scanner | 114 |
| Hollow AuNPs | |||||
| Stabilizing ligand: PEG | Adenocarcinoma (A549 cells) | S.C. xenograft mouse | CT | The attenuation coefficient of hollow AuNPs is 5.3 times higher than that of the iodine-based contrast agent at the same concentration, demonstrating the potential of hollow AuNPs for CT imaging | 115 |
| Stabilizing ligand: PEG Targeting ligand: RGD Emitter: 64Cu |
Liver carcinoma vX2 tumor) | Orthotopic allograft rabbit | PET/CT | PET/CT images showed that the 64Cu-RGD- PEG-HAuNS showed higher tumor uptake than control groups at 24 h post injection | 116 |
| Stabilizing ligand: PEG Targeting ligand: RGD Emitter: 64Cu |
Glioblastoma (U87 cells) | Orthotopic xenograft mouse |
PET/CT PAI |
The dual-modality PAI and PET/CT imaging provided a promising targeted AuNP-mediated glioma therapy | 117 |
| Gold nanostars | |||||
| Stabilizing ligand: PEG Raman reporter: p-mercaptobenzoic acid |
Primary soft-tissue sarcomas | Transgenic mouse | CT Two-photon luminescence imaging |
The CT and optical results showed that 30 nm nanostars have higher tumor uptake, as well as deeper penetration into tumor interstitial space compared with 60 nm counterparts | 118 |
| Stabilizing ligand: PEG | Breast cancer (4T1 cells) | S.C. allograft mouse | PAI | Novel Fe2O3@Au core/shell magnetic gold nanoflowers were synthesized through interactive growth of Au on Fe2O3 NPs. The nanoflowers exhibited remarkable SERS enhancement | 119 |
| Emitter: Gd3+ | Adenocarcinoma (A549 cells) | S.C. xenograft mouse | MRI, CT, and NIR fluorescence imaging | The existence of Gd3+ ions on GNCNs exhibits significant luminescence intensity enhancement for NIR fluorescence imaging, high X-ray attenuation for CT imaging, and reasonable r1 relaxivity for MRI | 120 |
| SERS labeling ligand: DTNB | Ovarian cancer (SKOv3) | S.C. xenograft mouse | Raman spectroscopy | The SERS Au nanostars were developed as a highly sensitive contrast agent for tumor detection in xenografted mice | 121 |
| Gold nanocages | |||||
| Stabilizing ligand: PEG Emitter: 64Cu |
Breast cancer (EMT-6 cells) | S.C. xenograft mouse | PET/CT | PET/CT images clearly showed rapid localization of the 64Cu -PEG-AU nanocages in tumor at 1 h post injection with the administration of a trace amount | 122 |
| Gold nanoprisms | |||||
| Stabilizing ligand: PEG | Colorectal cancer (HT-29 cells) | S.C. xenograft mouse | PAI | PEGylated Au nanoprisms showed the capacity to penetrate tumors and provided a high- resolution signal amplifier for optoacoustic imaging | 123 |
| Gold nanotripods | |||||
| Stabilizing ligand: PEG Targeting ligand: RGD |
Glioblastoma (U87 MG cells) | S.C. xenograft mouse | PAI | i.v. injection of RGD-conjugated Au-tripods showed PAI contrasts in tumors up to threefold higher than for the blocking group (coinjection with RGD) | 124 |
Abbreviations: AIE, aggregation-induced emission; AuNPs, gold NPs; BSA, bovine serum albumin; CET, cetuximab; CT, computed tomography; DAPTA, D-Ala1-peptide T-amide; DTNB, 5,5-dithio-bis-(2-nitrobenzoic acid); FA, folic acid; GC, glycol chitosan; GNRs, gold nanorods; HA, hyaluronic acid; ICG, indocyanine green; i.v., intravenous; LbL, layer-by-layer; MMP, matrix metalloproteinase; MPNA, magnetoplasmonic nanoassembly; MRI, magnetic resonance imaging; NGAL, neutrophil gelatinase-associated lipocalin; NIR, near-infrared; NPs, nanoparticles; PAI, photoacoustic imaging; PB, Prussian blue; PEG, polyethylene glycol; PEI, polyethylenimine; PET, positron emission tomography; PLGA, poly(lactic-co-glycolic acid); PPAA, plasma-polymerized allylamine shell; PTT, photothermal therapy; SPECT, single-photon emission CT; S.C., subcutaneous; SERS, surface-enhanced Raman spectroscopy; TAT, transactivator of transcription; TNBC, triple-negative breast cancer.
AuNPs for cancer phototherapy
PTT
In addition to the aforementioned enhanced light scattering (referred as a radiative property) useful for optical imaging, AuNPs can also convert the absorbed light into heat via a series of nonradiative processes.86,125–129 Two main processes occur based on the heat energy contents: 1) the heat from the energy transformation is passed to the surrounding medium via the phonon–phonon relaxation within ~100 ps and 2) a competitive process takes place between the heating by the electrons and the cooling by the surrounding medium, and when the heating rate is much faster than the cooling rate, AuNPs are melted in hundreds of femtoseconds.125–128 To facilitate cancer PTT, the first process has to dominate, and therefore, continuous-wave (CW) lasers that overlap maximally with the AuNP SPR absorption band need to be utilized.130,131
PTT can be achieved using gold nanospheres under the stimulation of pulsed or CW visible lasers due to the SPR absorption in the visible region, whereby such treatment is suitable for shallow tumors (ie, skin cancer).132,133 Recently, antibody-targeted gold nanospheres were developed to specifically target the EGFR on squalors carcinoma cells, and following stimulation by single 10 ns laser pulses at visible wavelengths, the resultant AuNPs generated intracellular photothermal micro bubbles and induced PTT for tumor inhibition in a subcutaneous cancer model.134
To treat tumors under the skin, NIR-active PTT is favorable as the light can penetrate more deeply due to the minimal absorption of the hemoglobin and water in tissues in this spectral region. Therefore, it is important to tune the SPR absorption of AuNPs to the NIR region by means of altering the shape, morphology, and structure, as described in the “Optical characteristics of AuNPs” section.
Recently, RGD-conjugated dendrite-modified GNRs (RGD-diners) were developed by Li et al135 for selective tumor targeting and PTT in xenografted mice. At 6 h after intravenous injection, the resultant AuNPs accumulated inside tumor tissues via targeting the αVβ3 overexpressed on cancer cells, and when it was irradiated with a NIR laser with a wavelength of 808 nm at a power density of 24 W/cm2 (~0.5 cm diameter illuminated region), tumor growth was significantly reduced by RGD-diners in comparison with the control groups.
In addition, polymeric per fluorocarbon Nan capsules were prepared using the oil-in-water emulsion method, followed by the addition of PEGylated gold nanoshells on the surface.136 These resultant Nan capsules could not only enhance the contrast for ultrasound/CT imaging but also function as photo-absorbers for NIR-active PTT in xenografted mice.136
Recently, Ayala-Orozco et al137 have developed novel gold nanomatryoshkas composed of concentric gold–silica–gold layers and PEG-stabilizing ligands, for PTT of TNBC. In comparison with ~150 nm conventional silica–Au nanoshells, the resultant gold nanomatryoshkas (<100 nm) facilitated higher accumulation in tumors due to better penetration of smaller AuNPs into tissue via the EPR effect. Under irradiation with a CW laser emitting 3 W/cm2 at a wavelength of 808 nm (~1.2 cm diameter illuminated region), the survival rate of an advanced TNBC model with >1,000 mm3 tumors was significantly improved by gold nanomatryoshkas relative to conventional silica–Au nanoshells.137
Photodynamic therapy (PDT)
When photosensitizers are stimulated under the light of specific wavelengths, they convert the surrounding oxygen into toxic reactive oxygen species (ie, singlet oxygen) that may destroy malignant cells in surrounding proximity, which is now known as cancer PDT.138 However, most of the organic photosensitizers are only activated by UV and visible lights, which have poor tissue penetration and therefore are limited to the treatment of surface tumors.139 In addition, organic photosensitizers possess low molar extinction coefficients and therefore can undergo photobleaching and enzymatic degradation.139 In contrast, metal nanostructures (ie, gold, silver, and platinum) can overcome these limitations, as they possess 5–6 orders of molar extinction coefficients, better photostability, and enhanced resistant to enzymatic degradation.140
To tackle the treatment of deeply buried tumors, AuNPs that are able to exert PDT upon NIR light activation have recently been developed. For example, lipid-coated gold nanocages were produced to activate PTT and PDT simultaneously in melanoma-xenografted mice.141 Following direct injection of lipid-coated gold nanocages into the tumor site, a 980 nm CW laser (150 mW/cm2; 10 min) was used to stimulate AuNPs, which raised temperature (~10°C) and generated the singlet oxygen. As a result, this treatment significantly inhibited tumor growth in comparison with an irradiation of the 808 nm diode laser (150 mW/cm2; 12 min; only PTT was induced in this wavelength).
In addition, a variety of organic photosensitizers with NIR-active property can also be incorporated into AuNPs for PDT with a low dose of organic photosensitizers and short exposure of laser irradiation. For example, indocyanine green (ICG, a medical diagnostic agent) is used to produce singlet oxygen for PDT.142 Recently, GNR/ICG-loaded chitosan nanospheres (CS-AuNR-ICG NSs) were prepared by the non-solvent counterion complexation method and electrostatic interaction.142 The CS-AuNR-ICG NSs could effectively load ICG and protect it from rapid hydrolysis. Results of in vivo NIR fluorescence imaging and biodistribution showed that these Au-chitosan NPs could be specifically delivered to the tumor site. Under an 808 nm laser, CS-AuNR-ICG NSs simultaneously produced hyperthermia and reactive oxygen species, which achieved complete inhibition of tumor growth in xenografted mice. Compared with PTT (GNRs) or PDT (ICG) alone, the combined therapy showed a significantly improved therapeutic efficacy.142
In addition, selected examples of AuNP-based cancer phototherapy are provided in Table 2, including the types of AuNPs, functional ligands, cancer types, in vivo animal models, laser types, and end point comments.
Table 2.
A summary of studies on the in vivo use of gold nanocomplexes in systemic cancer PTT and PDT
| AuNP type | Functional ligand | Cancer type | In vivo model | Laser | Comment | Reference |
|---|---|---|---|---|---|---|
| PTT | ||||||
| Au nanorods | Stabilizing ligand: PEG | Melanoma (MDA- MB-435 cells) | S.C. xenograft mouse | 810 nm laser 2 w/cm2 5 min |
A single i.v. injection of PEG-Au nanorods enabled destruction of the irradiated human xenograft tumors in mice | 143 |
| Au nanorods | Stabilizing ligand: PEG Targeting ligand: RGD |
Glioblastoma (U87 MG cells) | S.C. xenograft mouse | 808 nm laser 1 w/cm2 10 min |
Au nanorods showed high tumor-targeting ability via receptor-mediated pathway and were successfully used for PTT | 144 |
| Au nanorods | Coating material: silica | Breast cancer (4T1 cells) | S.C. allograft mouse | 808 nm laser 4 w/cm2 10 min |
When Au nanorods were stimulated with the NIR laser, DOX was released for synergistic therapeutic effect in combination with PTT | 145 |
| Au nanorods | Stabilizing ligand: PEG and dendrimers | Colon carcinoma (26 cells) | S.C. allograft mouse | 808 nm laser 0.24 w/cm2 10 min |
The combined photothermal-chemo treatment using AuNPs containing DOX for synergistic PPT and chemotherapy exhibited higher therapeutic efficacy than either single treatment alone | 146 |
| Au nanorods | Coating materials: PvP and AgNO3 Targeting ligand: aptamer |
Adenocarcinoma (A549 cells) | S.C. xenograft mouse | 980 nm laser 0.84 w/cm2 5 min |
The resultant AuNPs specifically accumulated into tumor tissues and induced PTT for dramatically stronger antitumor effect upon NIR laser irradiation | 147 |
| Au nanorods | Au nanorods encapsulated in CHI/sodium ALG microcapsules | Breast cancer (4T1 cells) | S.C. allograft mouse | 808 nm laser 3.83 J/cm2 5 min |
Self-assembled Au nanorods in bilayer- modified microcapsules localized at tumor sites, generated vapor bubbles under NIR exposure, and subsequently damaged tumor tissues | 148 |
| Au nanorods | Stabilizing ligand: PEG Targeting ligand: antibody for anaerobic bacteria (C difficile) |
Adenocarcinoma (A549 cells) | S.C. xenograft mouse | 808 nm laser 0.5 w/cm2 10 min |
The C. difficile spores was i.v. injected for 2 days, followed by the injection of antibody-Au nanorods to specifically target the germination of the C. difficile spores in tumor tissues (low level of oxygen microenvironment). Under the NIR laser, antibody-Au nanorods completely inhibited tumor growth | 149 |
| Au nanorods | Stabilizing ligand: dendrimer | Non-small-cell lung cancer (PC-9 cells) | S.C. xenograft mouse | 808 nm laser 3.6 w/cm2 8 min |
Dendrimer-stabilized Au nanorods (DSAuNRs, sub-10 nm in length) showed significantly enhanced absorption in the NIR region compared with dendrimer- stabilized Au nanospheres. The tumor growth was significantly retarded by the photothermal efficiency of DSAuNRs | 150 |
| Au nanorods | Coating material: silica Targeting ligand: antibody for CXCR4 |
Gastric cancer (MGC803 cells) | S.C. xenograft mouse | 808 nm laser 1.5 w/cm2 3 min |
iPS cells were transfected with the resulted AuNRs@SiO2@CXCR4 via receptor-mediated pathway. The transfected iPS cells were homing to tumor tissues, and the tumor growth was significantly slowed down by the photothermal efficiency of AuNRs@ SiO2@CXCR4 | 151 |
| Au nanoshells | Multilayered AuNPs with silica and gold, also termed Au nanomatryoshkas | Breast cancer (MDA-MB-231 cells) | Orthotopic xenograft mouse | 810 nm laser 2 w/cm2 5 min |
Au nanomatryoshkas exhibited improved PTT efficacy when compared with conventional gold nanoshells | 152 |
| Au nanoshells | Stabilizing ligand: PEG | Breast cancer (4T1 cells) | S.C. allograft mouse | 808 nm laser 1 w/cm2 10 min |
In combination with chemotherapeutics, the resultant Au nanoshells achieved complete destruction of the tumors at a low laser irradiation without weight loss or recurrence of tumors | 153 |
| Au nanoshells | Stabilizing ligand: PEG Inner core: PLGA NPs |
Glioblastoma (U87 MG cells) | S.C. xenograft mouse | 808 nm laser 1.5 w/cm2 1.5 min |
The temperature of tumor treated with the resultant Au nanoshells was rapidly increased to 46.6 °C, which released DOX for synergistic therapeutic effect in combination with PTT | 154 |
| Au nanoshells | Stabilizing ligand: PEG Inner core: PEI-PASP (DIP/MEA) NPs |
Liver cancer Bel-7402 cells | S.C. xenograft mouse | 808 nm laser 1.5 w/cm2 2 min |
A polymeric vesicle encapsulating DOX was prepared and then decorated with a gold layer using a modified method of in situ gold seed growth. The NIR light energy was converted into heat, which killed cancer cells in the vicinity and induced the rupture of nanoshell to release DOX inside tumor | 155 |
| Au nanoshells | Stabilizing coating: MPCMs Inner core: silica |
Breast cancer (4T1 cells) | S.C. allograft mouse | 808 nm laser 1 w/cm2 5 min |
MPCM-coated Au nanoshells presented longer blood circulation and tumor accumulation in a xenograft mouse model of breast cancer. Tumor growth was significantly slowed down by irradiation of NIR laser | 156 |
| Au nanostars | Stabilizing ligand: PEG Targeting ligand: RGD |
Glioblastoma (U87 MG cells) | S.C. xenograft mouse | 790 nm laser 1 w/cm2 10 min |
RGD-Au nanostars were designed to specifically target overexpressed integrin αvβ3 on tumor neovasculature, enabling highly sensitive PTT | 157 |
| Au nanostars | Surface coating: organosilica | Breast cancer (MDA-MB-231 cells) | S.C. xenograft mouse | 808 nm laser 0.5 w/cm2 5 min |
In 5 min of irradiation, the temperature at the tumor region of mice treated with Au nanostars increased remarkably to about 57°C | 158 |
| Au nanocages | Targeting ligand: HA | Breast cancer (MDA-MB-231 cells) | S.C. xenograft mouse | 808 nm laser 1 w/cm2 5 min |
HA-coated Au nanocages accumulated inside tumor tissues via HA-CD44 interaction. Under the NIR stimulation, HA-coated Au nanocages significantly slowed down the tumor growth. In addition, a complete tumor inhibition was achieved when combined with chemotherapy | 159 |
| Au nanocages | Gold surface was coated with PvP and RBC membranes | Breast cancer (4T1 cells) | S.C. allograft mouse | 850 nm laser 1 w/cm2 10 min |
RBC-AuNCs exhibited significantly enhanced in vivo blood retention and circulation lifetime. with NIR laser, RBC-AuNCs achieved 100% survival of tumor-bearing mice over a span of 45 days | 160 |
| Hollow Au nanospheres | Stabilizing ligand: PEG Targeting ligand: a peptide (TNYL) |
Ovarian carcinoma (SKOv3 cells) | S.C. xenograft mouse | 808 nm laser 1.5 w/cm2 3 min |
Under NIR laser irradiation, the resultant hollow Au nanospheres induced PTT for dramatically stronger antitumor effect against EphB4-positive tumors than EphB4- negative tumors | 161 |
| Hollow Au nanospheres | Stabilizing ligand: PvP and citrate | Ovarian carcinoma (SKOv3 cells) | S.C. xenograft mouse | 808 nm 3.0 w/cm2 10 min |
The resultant AuNPs exhibited a significantly enhanced surface plasmon absorption in the NIR region, inducing an efficient photothermal conversion and stronger anticancer ability under NIR laser irradiation | 162 |
| Au nanoclusters | A pH-sensitive ligand inducing Au nanoclusters in mild acidic environments | Fibrosarcoma (HT-1080 cells) | S.C. xenograft mouse | 660 nm laser 0.5 w/cm2 1 min |
MSCs were first transfected with the resultant AuNPs. The MSC-AuNPs showed a 37-fold higher tumor-targeting efficiency and resulted in a significantly enhanced anticancer effect upon irradiation | 163 |
| Au nanoplates | Stabilizing ligand: PEG | Breast cancer (4T1 cells) | S.C. allograft mouse | 808 nm laser 0.5 w/cm2 10 min |
PEGylated AuNPs presented good biocompatibility, prolonged blood circulation, and relatively high tumor accumulation. The NIR laser irradiation induced PTT and retarded tumor growth | 164 |
| PDT | ||||||
| Au nanospheres | Coating materials: heparin Photosensitizer: PhA |
Adenocarcinoma (A549 cells) | S.C. xenograft mouse | 670 nm laser 3 mw/cm2 30 min |
The PDT effects of PhA-H/AuNP significantly retarded tumor growth in comparison with PhA alone | 165 |
| Au nanorods | Coating materials: silica and PEG Photosensitizer: PPIX | Adenocarcinoma (HeLa cells) | S.C. xenograft mouse | 532 nm laser 25 mw/cm2 15 min |
A real-time and specific in vivo SERS and fluorescence detection method using the resultant AuNPs was applied for tumor detection and subsequent PDT | 166 |
| Au nanorods | AuNPs encapsulated in Pluronic nanogel Photosensitizer: Ce6 | Squamous carcinoma (SCC7 cells) | S.C. allograft mouse | 655 nm laser 20 J/cm2 30 min |
A remarkable synergy for anticancer treatment was observed when PDT was applied before PTT, both in vitro and in vivo | 167 |
| Au nanorods | Stabilizing ligand: PEG Targeting ligand: FA |
Melanoma (B16F0 cells) | S.C. allograft mouse | 915 nm 130 mw/cm2 15 min |
GNRs alone can sensitize the formation of singlet oxygen and exert dramatic PDT effects on complete destruction of tumors in mice under light excitation | 168 |
| Au nanorods | Stabilizing ligand: CHI Photosensitizer: ICG |
Liver cancer (H22 cells) | S.C. allograft mouse | 808 nm laser 2 w/cm2 10 min |
The resultant NPs have been successfully prepared to facilitate in vivo PDT resulting in abundant ROS produced by ICG under NIR irradiation | 142 |
| Au nanorods | Stabilizing ligand: poly(allylamine hydrochloride) Photosensitizer: RB |
Oral squamous carcinoma | A carcinogen was topically injected into the left cheek pouch mucosa | 532 nm green light 1.79 w/cm2 10 min |
The PDT-only treatment achieved a 46.5% tumor inhibition rate; when combined with PTT effects under NIR laser stimulation, 95.5% tumor inhibition rate was achieved | 169 |
| Au nanorods | Endosome disruptive ligand: Tat/HA2 Photosensitizer: AlPcS4 |
Adenocarcinoma (HeLa cells) | S.C. xenograft mouse | 808 nm laser 400 mw/cm2 15 min and 680 nm LED light 10 mw/cm2 40 min |
AuNRs absorbed an SPR wavelength (808 nm) and converted it into heat, causing the release of AlPcS4. Subsequently, upon illumination at 680 nm, the released AlPcS4 transferred the photon energy to oxygen molecules, stimulating ROS generation to slow down the tumor growth | 170 |
| Au nanocages | Stabilizing ligand: PEG Photosensitizer: HPPH |
Colon cancer (Colon-26 cells) | S.C. allograft mouse | 665 nm laser 75 mw/cm2 30 min |
The tumor growth was suppressed due to the enhanced phototoxicity of the HPPH- Au nanocages under the laser stimulation | 171 |
| Au nanoclusters | AuNPs encapsulated in silica Photosensitizer: Ce6 |
Melanoma (MDA- MB-435 cells) | S.C. xenograft mouse | 671 nm laser 100 mw/cm2 10 min |
The resultant AuNCs@SiO2-Ce6 completely inhibited tumor growth in mice due to PDT effects when compared with Ce6 alone and AuNCs@SiO2 alone | 172 |
| Au quantum clusters | Stabilizing ligand: lipoic acid Targeting ligand: FA Photosensitizer: PPIX |
Glioma (C6 cells) | S.C. xenograft mouse | 532 nm laser 1.5 w/cm2 15 min |
Under the laser stimulation, singlet oxygen efficiency of the resultant NPs was significantly higher when compared with that of the PPIX alone | 173 |
Abbreviations: ALG, alginate; AuNPs, gold NPs; C. difficile, Clostridium difficile; Ce6, chlorin e6; CHI, chitosan; DOX, doxorubicin; FA, folic acid; GNRs, gold nanorods; HA, hyaluronic acid; HPPH, 3-devinyl-3-(1′-hexyloxyethyl)pyropheophorbide; ICG, indocyanine green; iPS, Induced pluripotent stem; i.v., intravenous; LED, light-emitting diode; MPCMs, macrophage cell membranes; MSCs, mesenchymal stem cells; NIR, near-infrared; NPs, nanoparticles; PDT, photodynamic therapy; PEG, polyethylene glycol; PEI, polyethylenimine; PEI-PAsp (DIP/MEA), polyethylenimine-b-poly(2-diisopropylamino/2-mercaptoethylamine) ethyl aspartate; PhA, pheophorbide a; PLGA, poly(lactic-co-glycolic acid); PPIX, protoporphyrin IX; PTT, photothermal therapy; PVP, polyvinylpyrrolidone; RB, Rose Bengal; RBC, red blood cell; ROS, reactive oxygen species; S.C., subcutaneous; SERS, surface-enhanced Raman spectroscopy; SPR, surface plasmon resonance.
Barriers to the translation of AuNPs for cancer theranostics
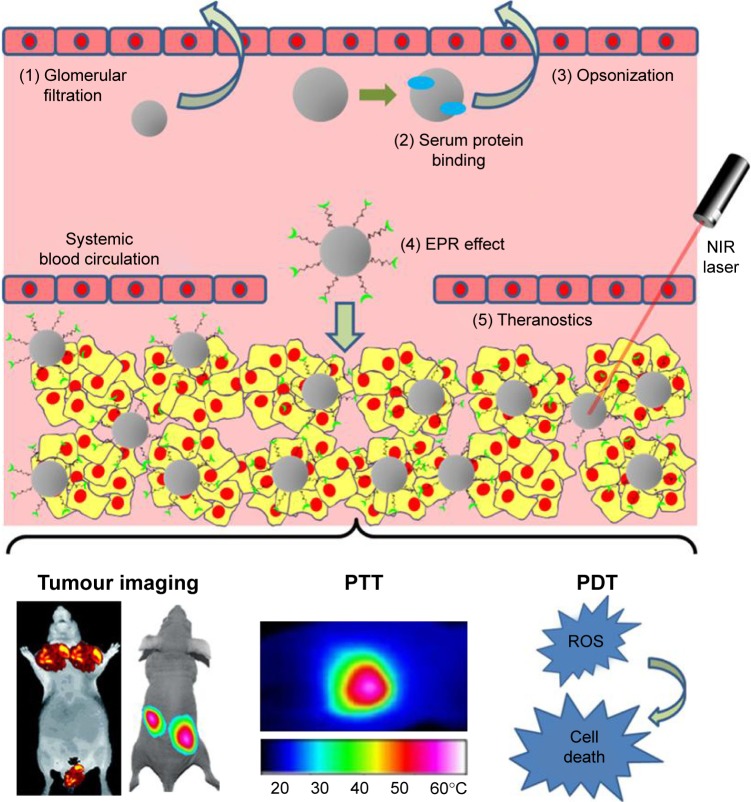
Although local administration of therapeutics has demonstrated significant potential for the treatment of shallow cancers (ie, skin cancer and head and neck cancer) due to the ease of access, many diseases (eg, metastatic cancers) still require systemic administration of therapeutic agents into the bloodstream. In this section, major challenges, namely, 1) instability in the blood circulation, 2) targeting to specific cells of interest, and 3) activation in response to the tumor microenvironment (TME),174 for systemic AuNP delivery in terms of cancer diagnosis and phototherapeutics are discussed (Figure 2).
Figure 2.
Systemic delivery of multifunctional AuNPs for cancer bioimaging and phototherapeutics.
Notes: (1) Following intravenous injection into the blood, AuNPs with a particle size of <6 nm are prone to glomerular filtration. (2 and 3) When blood proteins bind to AuNPs nonspecifically, the resultant complexes tend to be taken up by MPS for opsonization (a means of identifying the invading particle to the phagocyte). (4) Multifunctional AuNPs (~100 nm) with stealth coating materials, bioresponsive moieties, bioactive targeting ligands, and/or bioimaging agents can efficiently accumulate inside tumor tissues via the “EPR” effect and specifically target cancer cells via ligand–receptor pathway. (5) As a result, theranostic AuNPs can sensitively image the tumors and effectively induce PTT and/or PDT under the irradiation of the NIR light.
Abbreviations: AuNPs, gold nanoparticles; EPR, enhanced penetration and retention; MPS, mononuclear phagocyte system; NIR, near-infrared; PDT, photodynamic therapy; PTT, photothermal therapy; ROS, reactive oxygen species.
Blood circulation
It has been reported, following intravenous administration, that rapid glomerular filtration occurs in the case of NPs with a hydrodynamic size of <6 nm, while those with particle sizes of >8 nm are able to avoid kidney removal.175 While NPs with >10 nm diameter may reduce renal filtration, larger particles (ie, >100 nm) can evoke the reticuloendothelial system (RES).176 The RES, also known as the mononuclear phagocyte system (MPS), is part of the immune system, which primarily involves the liver, spleen, and lymph nodes.176,177 Foreign materials will be removed out of the blood circulation by the immune cells in MPS, thus dramatically reducing the circulatory half-time (t1/2) of NPs.177 In addition, nonspecific adsorption of blood proteins onto the surface of NPs (ie, hydrophobic interactions between hydrophobic patches on proteins and hydrophobic ligands on NPs, and electrostatic interactions between anionic albumin and cationic NPs) may inhibit NPs’ targeting capabilities or increase the overall particle size of NPs, and eventually the resultant large complexes are either trapped in the capillary endothelium of the lung or taken up by MPS.176
The modification of stabilizing ligands (ie, PEG, block copolymers, and hyaluronic acid [HA]) onto the gold surface, forming the so-called “stealth” particles capable of improving pharmacokinetic profiles, has been substantially reviewed previously.76,178,179 In addition to these stabilizing components, long-circulating NPs have also been developed where the NP surface is coated with the cellular membrane of red blood cells (RBCs) and leukocytes.150,154,174,175 As an alternative stealth coating material, the membrane of these cells completely covers the NP surface with their “self-markers” (ie, proteins, glycans, and acidic sialyl moieties), and the resultant NPs, recognized as the host’s own cells, can actively evade the immune system. For example, Piao et al160 reported that the RBC-coated gold nanocages exhibited longer blood retention compared with the PEGylated counterparts. In addition, the fusion of RBC membranes over gold surface did not alter the unique porous and hollow structures of gold nanocages. Following systemic administration, the RBC-coated gold nanocages demonstrated enhanced tumor accumulation, induced NIR-active PTT, and eventually achieved 100% survival of tumor-bearing mice over a span of 45 days.160
Targeting to specific cells of interest
It is known that tumor grows rapidly via a process termed angiogenesis (new blood vessels are developed from the preexisting vasculature).177,180 The porous vasculature of a tumor is known to provide access to blood-circulating particles with sizes <500 nm, usually <150 nm.176 In addition, the lymphatic drainage system of tumor tissues is normally underdeveloped, and thus, NPs may not be removed. This EPR effect will facilitate “passive” accumulation of NPs in tumor tissues. In addition, targeting ligands (ie, antibodies and peptides) can be functionalized on NPs for “active” cancer targeting. Taking advantage of the “passive” and “active” targeting, recent advances in the design of AuNPs have offered great potential for accurate diagnosis and targeted therapy. For example, targeted gold nanocages were developed by coating with HA (a targeting ligand for CD44) on the gold surface to specifically recognize cancer cells over-expressing CD44.159 The HA–Au nanocages containing DOX could be efficiently uptaken by a receptor-mediated process, and subsequently, the coated HA molecules were degraded in lysosomes, resulting in the release of DOX. Biodistribution results demonstrate that targeted gold nanocages achieved significantly higher tumor accumulation relative to nontargeted counterparts in tumor-bearing mice. As a result, HA-coated gold nanocages containing DOX significantly slowed down the tumor growth, and more importantly, complete tumor inhibition was achieved when combined with PTT under the NIR stimulation.159
Activation in response to TME
TME is specifically adapted to support the growth, invasion, and metastasis of primary tumor tissues; this has been substantially reviewed previously.181 “Smart” bioresponsive NPs have been developed for targeted delivery and controlled drug release by exploiting the TME, where a slightly acidic environment, a low oxygen (hypoxia) niche, and overexpression of extracellular matrix (ECM) components (ie, MMPs) are evident.181
It is now known that the hypoxia region in solid tumors is a complex microenvironment with a low oxygen concentration and deficient nutrients.182 The hypoxic environment can not only lower the susceptibility of cancer cells to anticancer drugs but also reduce the response of cancer cells to free radicals.182 Although the hypoxia of solid tumors causes a hurdle in therapy, the low level of oxygen microenvironment serves as an ideal habitat for a number of anaerobic bacteria. Recently, Luo et al149 reported a tumor-targeted AuNP delivery using two anaerobic bacterial strains, namely, Bifidobacterium breve UCC2003 and Clostridium difficile CCUG 37780. In this study, two approaches were designed for the active NP delivery: 1) a direct conjugation of GNRs on the surface of the vegetative B. breve for intravenous delivery into hypoxic region (a cargo-carrying approach) and 2) the injection of the C. difficile spores first, followed by the intravenous administration of the antibody–GNR conjugates to specifically target the germination of the C. difficile spores (an antibody-guiding approach). Under NIR excitation, the antibody-directed strategy showed stronger imaging and achieved effective PTT to completely inhibit tumor growth in a subcutaneous mouse cancer model.149
In addition, Sun et al93 developed novel AuNPs for dual CT/optical imaging of cancer. First, AuNPs were modified with glycol chitosan (GC) polymers (GC-AuNPs) for excellent stability and enhanced EPR effect. Second, fluorescent probes were chemically modified to GC-AuNPs via MMP-active peptides (MMP-GC-AuNPs). The NIR fluorescent probes were strongly quenched by the combinational quenching effects between the organic black hole quencher and the gold surface, but the quenched probes were reactivated upon exposure to MPPs in the TME. As a result, CT images with high spatial resolution and optical images with high sensitivity were simultaneously achieved using these AuNP-based CT/optical dual imaging probes.93
Biodistribution, metabolism, and nanotoxicity
In addition to tumor accumulation via the EPR effect, a majority of intravenously administrated NPs are nonspecifically distributed into healthy tissues (ie, phagocytic cell-rich organs such as the liver and spleen) before the renal clearance.183 Although the gold core is generally inert, nontoxic, and biocompatible, significant toxicity of AuNPs can be induced by the synthesis method of gold nanostructures, physicochemical properties (ie, size, morphology, and surface charge), surface conjugates and ligands, the doses, and the administration routes.173 Recently, numerous studies have described AuNPs designed to investigate whether their in vivo behaviors such as accumulation in healthy tissues and renal clearance cause unwanted effects.184–187
For example, the glomerular filtration, biodistribution, and toxicity of glutathione (GSH)- and bovine serum albumin (BSA)-capped AuNPs were evaluated using mice.188 As a result, smaller AuNPs (GSH-coated, ~2 nm) caused highly efficient kidney removal and were more readily metabolized relative to the larger AuNPs (BSA-coated, ~8 nm), suggesting that the toxicity was related to the size of AuNPs. In addition, although both AuNPs caused acute infection, inflammation, and kidney function damage after 24 h, these effects were eliminated for ~2 nm AuNPs after 28 days; in contrast, ~8 nm AuNPs further accumulated in the liver and spleen causing irreparable toxicity.188
It has been difficult to substantially evaluate the biodistribution, clearance, and toxicity of AuNPs, due to the fact that experimental designs are diverse, including the particle size, charge and shape, functionalization methods, types of animal models, delivery doses, and administration routes. Therefore, standard approaches are urgently needed in the future to facilitate development and approval of AuNPs for cancer theranostics.189
Conclusion and future perspectives
The newly emerging concept termed theranostics is well established as the development of novel strategies to combine diagnostic and therapeutic capabilities into a single agent facilitating specific and personalized therapies for diseases.190 The rationale behind the concept is that diseases, such as cancers, are extremely heterogeneous, and all existing treatments are effective for only certain patients and at selective stages of disease development.191 Therefore, a combined approach of diagnostics and therapeutics may provide promising treatment protocols that are more specific to individuals, ie, personalized medicines, and therefore more likely to offer improved prognoses.
Recent advances in understanding the optical and chemical properties of gold have enabled the design of novel AuNPs containing diagnostic and therapeutic functions that can be integrated into one system.40,42,192 Au-nanocomplex-based cancer imaging and phototherapy are reviewed in the “AuNPs for cancer diagnosis” and “AuNPs for cancer phototherapy” sections, respectively (Tables 1 and 2); many of these Au nanocomplexes, by tuning the size, shape, and structure, and by using different functional ligands, are capable of improving sensitive cancer diagnosis and targeted phototherapy simultaneously, under the stimulation of the appropriate light resources (NIR light is preferred in most cases). In addition, a number of AuNP-based theranostics have advanced into clinical trials (NCT03020017, NCT01270139 NCT02755870, NCT01420588, and NCT02782026). For example, the safety evaluation of a drug named NU-0129 is now undertaking an early Phase I trial (NCT03020017). NU-0129 is composed of siRNA arranged on the surface of Au nanospheres, and when infused in patients with recurrent glioblastoma multiforme or gliosarcoma, the Au nanocomplexes can penetrate the blood–brain barrier and deliver siRNA into tumor cells knocking down the expression of oncoprotein Bcl2Like12, which may result in significant inhibition of tumor growth (NCT03020017). Furthermore, CNM-Au8 (a drug candidate now being tested in clinical trial), an Au nanocrystal suspension drug, has recently been developed by Clene Nanomedicine (Salt Lake City, UT, USA) using Clean-Surface Nanosuspension™ (CSN) technology (it produces atomically clean-surface elemental nanocrystals, free of any residual surface chemicals, or surface-capping agents) (www.clene.com). The oral administration of CNM-Au8 has demonstrated efficient immunomodulation in established demyelination animal models (www.clene.com). The safety, tolerability, and pharmacokinetics of CNM-Au8 are now under evaluation in healthy volunteers, with a hope of facilitating the application of the drug for the demyelinating disorder neuromyelitis optica (NMO) in the future (NCT02755870).
To overcome in vivo delivery barriers, Au nanocomplexes are normally modified with functional moieties such as stabilizing materials, targeting ligands, and bioresponsive linkers. However, it should be borne in mind that extensive functional modifications may cause unwanted toxic side effects, and therefore, further investigation is required to quantify the benefit of a treatment versus the risk of toxicity before theranostic Au nanocomplexes can be translated into clinically accepted strategies for cancer therapy.
Acknowledgments
We acknowledge the funding provided by the Outstanding Youth Foundation from the Department of Science and Technology, Jilin Province (Project Number: 20170520046JH); the Start-Up Research Grant Program from Jilin University (Project Number: 451160102052); the HAI-Novartis Fellowship Award from the Haematology Association of Ireland and Novartis Oncology; and the CNRS Lebanon GRP2015.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41(7):2656–2672. doi: 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 2.Ryu JH, Koo H, Sun IC, et al. Tumor-targeting multi-functional nanoparticles for theragnosis: new paradigm for cancer therapy. Adv Drug Deliv Rev. 2012;64(13):1447–1458. doi: 10.1016/j.addr.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed N, Fessi H, Elaissari A. Theranostic applications of nanoparticles in cancer. Drug Discov Today. 2012;17(17–18):928–934. doi: 10.1016/j.drudis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Lim EK, Kim T, Paik S, Haam S, Huh YM, Lee K. Nanomaterials for theranostics: recent advances and future challenges. Chem Rev. 2015;115(1):327–394. doi: 10.1021/cr300213b. [DOI] [PubMed] [Google Scholar]
- 5.Namiki Y, Fuchigami T, Tada N, et al. Nanomedicine for cancer: lipid-based nanostructures for drug delivery and monitoring. Acc Chem Res. 2011;44(10):1080–1093. doi: 10.1021/ar200011r. [DOI] [PubMed] [Google Scholar]
- 6.Doane TL, Burda C. The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem Soc Rev. 2012;41(7):2885–2911. doi: 10.1039/c2cs15260f. [DOI] [PubMed] [Google Scholar]
- 7.Faraday M. The Bakerian Lecture: experimental relations of gold (and other metals) to light. Philos T R Soc A. 1857;147:145–181. [Google Scholar]
- 8.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. [Google Scholar]
- 9.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature. 1973;241(105):20–22. [Google Scholar]
- 10.Khoury CG, Vo-Dinh T. Gold nanostars for surface-enhanced Raman scattering: synthesis, characterization and optimization. J Phys Chem C Nanomater Interfaces. 2008;2008(112):18849–18859. doi: 10.1021/jp8054747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41(7):2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigderman L, Zubarev ER. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv Drug Deliv Rev. 2013;65(5):663–676. doi: 10.1016/j.addr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Singhana B, Slattery P, Chen A, Wallace M, Melancon MP. Light-activatable gold nanoshells for drug delivery applications. AAPS PharmSciTech. 2014;15(3):741–752. doi: 10.1208/s12249-014-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, Zhang S, Yu Y, Zhang H, He Q. Structural-engineering rationales of gold nanoparticles for cancer theranostics. Adv Mater Deerfield. 2016;28(39):8567–8585. doi: 10.1002/adma.201602080. [DOI] [PubMed] [Google Scholar]
- 15.Brown KR, Lyon LA, Fox AP, Reiss BD, Natan MJ. Hydroxylamine seeding of colloidal Au nanoparticles. 3. Controlled formation of conductive Au films. Chem Mater. 2000;12(2):314–323. [Google Scholar]
- 16.Rahme K, Nolan MT, Doody T, et al. Highly stable PEGylated gold nanoparticles in water: applications in biology and catalysis. RSC Adv. 2013;3(43):21016–21024. [Google Scholar]
- 17.Sau TK, Pal A, Jana NR, Wang ZL, Pal T. Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles. J Nanopart Res. 2001;3(4):257–261. [Google Scholar]
- 18.Niu JL, Zhu T, Liu ZF. One-step seed-mediated growth of 30–150 nm quasispherical gold nanoparticles with 2-mercaptosuccinic acid as a new reducing agent. Nanotechnology. 2007;18(32):7. [Google Scholar]
- 19.Perrault SD, Chan WCW. Synthesis and surface modification of highly monodispersed, spherical gold nanoparticles of 50–200 nm. J Am Chem Soc. 2009;131(47):17042. doi: 10.1021/ja907069u. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Xu H, Xia H, Wang D. Rapid seeded growth of monodisperse, quasi-spherical, citrate-stabilized gold nanoparticles via H2O2 reduction. Langmuir. 2012;28(38):13720–13726. doi: 10.1021/la3027804. [DOI] [PubMed] [Google Scholar]
- 21.Foss CA, Hornyak GL, Stockert JA, Martin CR. Optical-properties of composite membranes containing arrays of nanoscopic gold cylinders. J Phys Chem. 1992;96(19):7497–7499. [Google Scholar]
- 22.Martin CR. Nanomaterials: a membrane-based synthetic approach. Science. 1994;266(5193):1961–1966. doi: 10.1126/science.266.5193.1961. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, Mulvaney P. Gold nanorods: synthesis, characterization and applications. Coordin Chem Rev. 2005;249(17–18):1870–1901. [Google Scholar]
- 24.Jana NR, Gearheart L, Murphy CJ. Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chem Mater. 2001;13(7):2313–2322. [Google Scholar]
- 25.Nikoobakht B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater. 2003;15(10):1957–1962. [Google Scholar]
- 26.Skrabalak SE, Chen JY, Sun YG, et al. Gold nanocages: synthesis, properties, and applications. Acc Chem Res. 2008;41(12):1587–1595. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yong KT, Sahoo Y, Swihart MT, Prasad PN. Synthesis and plasmonic properties of silver and gold nanoshells on polystyrene cores of different size and of gold-silver core-shell nanostructures. Colloid Surf A. 2006;290(1–3):89–105. [Google Scholar]
- 28.Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Nanoengineering of optical resonances. Chem Phys Lett. 1998;288(2–4):243–247. [Google Scholar]
- 29.Pham T, Jackson JB, Halas NJ, Lee TR. Preparation and characterization of gold nanoshells coated with self-assembled monolayers. Langmuir. 2002;18(12):4915–4920. [Google Scholar]
- 30.Prodan E, Radloff C, Halas NJ, Nordlander P. A hybridization model for the plasmon response of complex nanostructures. Science. 2003;302(5644):419–422. doi: 10.1126/science.1089171. [DOI] [PubMed] [Google Scholar]
- 31.Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Berlin Heidelberg: Springer-Verlag; 1995. [Google Scholar]
- 32.Cobley CM, Chen JY, Cho EC, Wang LV, Xia YN. Gold nanostructures: a class of multifunctional materials for biomedical applications. Chem Soc Rev. 2011;40(1):44–56. doi: 10.1039/b821763g. [DOI] [PubMed] [Google Scholar]
- 33.Huang X, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28. [Google Scholar]
- 34.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B. 2006;110(14):7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 35.Yguerabide J, Yguerabide EE. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications – I. Theory. Anal Biochem. 1998;262(2):137–156. doi: 10.1006/abio.1998.2759. [DOI] [PubMed] [Google Scholar]
- 36.Yguerabide J, Yguerabide EE. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications – II. Experimental characterization. Anal Biochem. 1998;262(2):157–176. doi: 10.1006/abio.1998.2760. [DOI] [PubMed] [Google Scholar]
- 37.Sokolov K, Follen M, Aaron J, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63(9):1999–2004. [PubMed] [Google Scholar]
- 38.Orendorff CJ, Sau TK, Murphy CJ. Shape-dependent plasmon-resonant gold nanoparticles. Small. 2006;2(5):636–639. doi: 10.1002/smll.200500299. [DOI] [PubMed] [Google Scholar]
- 39.Lee KS, El-Sayed MA. Dependence of the enhanced optical scattering efficiency relative to that of absorption for gold metal nanorods on aspect ratio, size, end-cap shape, and medium refractive index. J Phys Chem B. 2005;109(43):20331–20338. doi: 10.1021/jp054385p. [DOI] [PubMed] [Google Scholar]
- 40.Hauck TS, Chan WC. Gold nanoshells in cancer imaging and therapy: towards clinical application. Nanomedicine (Lond) 2007;2(5):735–738. doi: 10.2217/17435889.2.5.735. [DOI] [PubMed] [Google Scholar]
- 41.Skrabalak SE, Au L, Lu X, Li X, Xia Y. Gold nanocages for cancer detection and treatment. Nanomedicine (Lond) 2007;2(5):657–668. doi: 10.2217/17435889.2.5.657. [DOI] [PubMed] [Google Scholar]
- 42.Huang X, Neretina S, El-Sayed MA. Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv Mater. 2009;21(48):4880–4910. doi: 10.1002/adma.200802789. [DOI] [PubMed] [Google Scholar]
- 43.Tong L, Wei QS, Wei A, Cheng JX. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol. 2009;85(1):21–32. doi: 10.1111/j.1751-1097.2008.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee KS, El-Sayed MA. Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J Phys Chem B. 2006;110(39):19220–19225. doi: 10.1021/jp062536y. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Saeki F, Wiley BJ, et al. Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005;5(3):473–477. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 46.Sun YG, Mayers BT, Xia YN. Template-engaged replacement reaction: a one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors. Nano Lett. 2002;2(5):481–485. [Google Scholar]
- 47.Agasti SS, Rana S, Park MH, Kim CK, You CC, Rotello VM. Nanoparticles for detection and diagnosis. Adv Drug Deliv Rev. 2010;62(3):316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portales H, Saviot L, Duval E, et al. Resonance and composition effects on the Raman scattering from silver-gold alloy clusters. Eur Phys J D. 2001;16(1–3):197–200. [Google Scholar]
- 49.Amendola V, Scaramuzza S, Agnoli S, Polizzi S, Meneghetti M. Strong dependence of surface plasmon resonance and surface enhanced Raman scattering on the composition of Au-Fe nanoalloys. Nanoscale. 2014;6(3):1423–1433. doi: 10.1039/c3nr04995g. [DOI] [PubMed] [Google Scholar]
- 50.Juluri BK, Zheng YB, Ahmed D, Jensen L, Huang TJ. Effects of geometry and composition on charge-induced plasmonic shifts in gold nanoparticles. J Phys Chem C. 2008;112(19):7309–7317. [Google Scholar]
- 51.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J Chem Soc Chem Comm. 1994;(7):801–802. [Google Scholar]
- 52.Sardar R, Funston AM, Mulvaney P, Murray RW. Gold nanoparticles: past, present, and future. Langmuir. 2009;25(24):13840–13851. doi: 10.1021/la9019475. [DOI] [PubMed] [Google Scholar]
- 53.Guo J, Armstrong MJ, O’Driscoll CM, Holmes JD, Rahme K. Positively charged, surfactant-free gold nanoparticles for nucleic acid delivery. RSC Adv. 2015;5:17862–17871. [Google Scholar]
- 54.Chhour P, Naha PC, O’Neill SM, et al. Labeling monocytes with gold nanoparticles to track their recruitment in atherosclerosis with computed tomography. Biomaterials. 2016;87:93–103. doi: 10.1016/j.biomaterials.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu XS, Huang N, Wang HB, Li H, Jin Q, Ji J. The effect of ligand composition on the in vivo fate of multidentate poly(ethylene glycol) modified gold nanoparticles. Biomaterials. 2013;34(33):8370–8381. doi: 10.1016/j.biomaterials.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 56.Conde J, Tian F, Hernandez Y, et al. In vivo tumor targeting via nanoparticle-mediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials. 2013;34(31):7744–7753. doi: 10.1016/j.biomaterials.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 57.Shiao YS, Chiu HH, Wu PH, Huang YF. Aptamer-functionalized gold nanoparticles as photoresponsive nanoplatform for co-drug delivery. ACS Appl Mater Interfaces. 2014;6(24):21832–21841. doi: 10.1021/am5026243. [DOI] [PubMed] [Google Scholar]
- 58.Arosio D, Chiodo F, Reina JJ, et al. Effective targeting of DC-SIGN by alpha-fucosylamide functionalized gold nanoparticles. Bioconjugate Chem. 2014;25(12):2244–2251. doi: 10.1021/bc500467u. [DOI] [PubMed] [Google Scholar]
- 59.Fitzgerald KA, Rahme K, Guo JF, Holmes JD, O’Driscoll CM. Anisamide-targeted gold nanoparticles for siRNA delivery in prostate cancer – synthesis, physicochemical characterisation and in vitro evaluation. J Mater Chem B. 2016;4(13):2242–2252. doi: 10.1039/c6tb00082g. [DOI] [PubMed] [Google Scholar]
- 60.Guo J, O’Driscoll CM, Holmes JD, Rahme K. Bioconjugated gold nanoparticles enhance cellular uptake: a proof of concept study for siRNA delivery in prostate cancer cells. Int J Pharm. 2016;509(1–2):16–27. doi: 10.1016/j.ijpharm.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 61.Shen S, Tang HY, Zhang XT, et al. Targeting mesoporous silica-encapsulated gold nanorods for chemo-photothermal therapy with near-infrared radiation. Biomaterials. 2013;34(12):3150–3158. doi: 10.1016/j.biomaterials.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 62.Guo JF, Ogier JR, Desgranges S, Darcy R, O’Driscoll C. Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials. 2012;33(31):7775–7784. doi: 10.1016/j.biomaterials.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Fitzgerald KA, Malhotra M, Gooding M, et al. A novel, anisamide-targeted cyclodextrin nanoformulation for siRNA delivery to prostate cancer cells expressing the sigma-1 receptor. Int J Pharm. 2016;499(1–2):131–145. doi: 10.1016/j.ijpharm.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 64.Stolik S, Delgado JA, Perez A, Anasagasti L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J Photochem Photobiol B. 2000;57(2–3):90–93. doi: 10.1016/s1011-1344(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 65.Sakudo A. Near-infrared spectroscopy for medical applications: current status and future perspectives. Clin Chim Acta. 2016;455:181–188. doi: 10.1016/j.cca.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsych Dis Treat. 2015;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5(5):829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 68.Choi J, Yang J, Bang D, et al. Targetable gold nanorods for epithelial cancer therapy guided by near-IR absorption imaging. Small. 2012;8(5):746–753. doi: 10.1002/smll.201101789. [DOI] [PubMed] [Google Scholar]
- 69.Yang XM, Stein EW, Ashkenazi S, Wang LHV. Nanoparticles for photoacoustic imaging. Wires Nanomed Nanobi. 2009;1(4):360–368. doi: 10.1002/wnan.42. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Brandl DW, Nordlander P, Halas NJ. Plasmonic nanostructures: artificial molecules. Acc Chem Res. 2007;40(1):53–62. doi: 10.1021/ar0401045. [DOI] [PubMed] [Google Scholar]
- 71.Halas NJ, Lal S, Chang WS, Link S, Nordlander P. Plasmons in strongly coupled metallic nanostructures. Chem Rev. 2011;111(6):3913–3961. doi: 10.1021/cr200061k. [DOI] [PubMed] [Google Scholar]
- 72.Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond) 2015;10(2):299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song JB, Yang XY, Jacobson O, et al. Ultrasmall gold nanorod vesicles with enhanced tumor accumulation and fast excretion from the body for cancer therapy. Adv Mater Deerfield. 2015;27(33):4910–4917. doi: 10.1002/adma.201502486. [DOI] [PubMed] [Google Scholar]
- 74.Popovtzer R, Agrawal A, Kotov NA, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8(12):4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu H, Shen M, Zhao J, et al. Tunable synthesis and acetylation of dendrimer-entrapped or dendrimer-stabilized gold-silver alloy nanoparticles. Colloids Surf B Biointerfaces. 2012;94:58–67. doi: 10.1016/j.colsurfb.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 76.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 2011;6(4):715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou B, Yang J, Peng C, et al. PEGylated polyethylenimine-entrapped gold nanoparticles modified with folic acid for targeted tumor CT imaging. Colloids Surf B Biointerfaces. 2016;140:489–496. doi: 10.1016/j.colsurfb.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 78.Paul AM, Fan Z, Sinha SS, et al. Bioconjugated gold nanoparticle based SERS probe for ultrasensitive identification of mosquito-borne viruses using Raman fingerprinting. J Phys Chem C. 2015;119(41):23669–23675. doi: 10.1021/acs.jpcc.5b07387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: a potential cancer diagnostic marker. Nano Lett. 2007;7(6):1591–1597. doi: 10.1021/nl070472c. [DOI] [PubMed] [Google Scholar]
- 80.Hoejgaard L, Hesse B. Hybrid imaging: conclusions and perspectives. Curr Med Imaging Rev. 2011;7(3):252–253. [Google Scholar]
- 81.Meir R, Motiei M, Popovtzer R. Gold nanoparticles for in vivo cell tracking. Nanomedicine (Lond) 2014;9(13):2059–2069. doi: 10.2217/nnm.14.129. [DOI] [PubMed] [Google Scholar]
- 82.Jin Y. Multifunctional compact hybrid Au nanoshells: a new generation of nanoplasmonic probes for biosensing, imaging, and controlled release. Acc Chem Res. 2014;47(1):138–148. doi: 10.1021/ar400086e. [DOI] [PubMed] [Google Scholar]
- 83.Zhao J, Wallace M, Melancon MP. Cancer theranostics with gold nanoshells. Nanomedicine. 2014;9(13):2041–2057. doi: 10.2217/nnm.14.136. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Y, Pang B, Luehmann H, et al. Gold nanoparticles doped with (199) Au atoms and their use for targeted cancer imaging by SPECT. Adv Healthc Mater. 2016;5(8):928–935. doi: 10.1002/adhm.201500992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He XX, Liu FY, Liu L, Duan TC, Zhang HM, Wanq ZX. Lectin-conjugated Fe2O3@Au core@shell nanoparticles as dual mode contrast agents for in vivo detection of tumor. Mol Pharm. 2014;11(3):738–745. doi: 10.1021/mp400456j. [DOI] [PubMed] [Google Scholar]
- 86.Huang S, Li C, Wang WP, et al. A54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. Int J Nanomedicine. 2017;12:5163–5176. doi: 10.2147/IJN.S131089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang JM, Li C, Zhang X, et al. In vivo tumor-targeted dual-modal fluorescence/CT imaging using a nanoprobe co-loaded with an aggregation-induced emission dye and gold nanoparticles. Biomaterials. 2015;42:103–111. doi: 10.1016/j.biomaterials.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meir R, Shamalov K, Betzer O, et al. Nanomedicine for cancer immunotherapy: tracking cancer-specific T-cells in vivo with gold nanoparticles and CT imaging. ACS Nano. 2015;9(6):6363–6372. doi: 10.1021/acsnano.5b01939. [DOI] [PubMed] [Google Scholar]
- 89.Peng C, Zheng L, Chen Q, et al. PEGylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomography. Biomaterials. 2012;33(4):1107–1119. doi: 10.1016/j.biomaterials.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 90.Cheng B, He HC, Huang T, et al. Gold nanosphere gated mesoporous silica nanoparticle responsive to near-infrared light and redox potential as a theranostic platform for cancer therapy. J Biomed Nanotechnol. 2016;12(3):435–449. doi: 10.1166/jbn.2016.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng Y, Dai Q, Morshed RA, et al. Blood-brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10(24):5137–5150. doi: 10.1002/smll.201400654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim YH, Jeon J, Hong SH, et al. Tumor targeting and imaging using cyclic RGD-PEGylated gold nanoparticle probes with directly conjugated iodine-125. Small. 2011;7(14):2052–2060. doi: 10.1002/smll.201100927. [DOI] [PubMed] [Google Scholar]
- 93.Sun IC, Eun DK, Koo H, et al. Tumor-targeting gold particles for dual computed tomography/optical cancer imaging. Angew Chem Int Ed Engl. 2011;50(40):9348–9351. doi: 10.1002/anie.201102892. [DOI] [PubMed] [Google Scholar]
- 94.Chen Q, Li KA, Wen SH, et al. Targeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials. 2013;34(21):5200–5209. doi: 10.1016/j.biomaterials.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 95.Jing LJ, Liang XL, Deng ZJ, et al. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials. 2014;35(22):5814–5821. doi: 10.1016/j.biomaterials.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Gao S, Zhang LW, Wang GH, et al. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials. 2016;79:36–45. doi: 10.1016/j.biomaterials.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 97.Chen WH, Yang CX, Qiu WX, et al. Multifunctional theranostic nanoplatform for cancer combined therapy based on gold nanorods. Adv Healthc Mater. 2015;4(15):2247–2259. doi: 10.1002/adhm.201500453. [DOI] [PubMed] [Google Scholar]
- 98.Jang B, Park JY, Tung CH, Kim IH, Choi Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano. 2011;5(2):1086–1094. doi: 10.1021/nn102722z. [DOI] [PubMed] [Google Scholar]
- 99.Yang HW, Liu HL, Li ML, et al. Magnetic gold-nanorod/PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials. 2013;34(22):5651–5660. doi: 10.1016/j.biomaterials.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 100.Jokerst JV, Cole AJ, Van de Sompel D, Gambhir SS. Gold nanorods for ovarian cancer detection with photoacoustic imaging and resection guidance via Raman imaging in living mice. ACS Nano. 2012;6(11):10366–10377. doi: 10.1021/nn304347g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guan T, Shang W, Li H, et al. From detection to resection: photoacoustic tomography and surgery guidance with indocyanine green loaded gold nanorod@liposome core-shell nanoparticles in liver cancer. Bioconjug Chem. 2017;28(4):1221–1228. doi: 10.1021/acs.bioconjchem.7b00065. [DOI] [PubMed] [Google Scholar]
- 102.Karmani L, Labar D, Valembois V, et al. Antibody-functionalized nanoparticles for imaging cancer: influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media Mol Imaging. 2013;8(5):402–408. doi: 10.1002/cmmi.1539. [DOI] [PubMed] [Google Scholar]
- 103.Xie H, Wang ZJ, Bao A, Goins B, Phillips WT. In vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenografts. Int J Pharm. 2010;395(1–2):324–330. doi: 10.1016/j.ijpharm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 104.Chen W, Ayala-Orozco C, Biswal NC, et al. Targeting pancreatic cancer with magneto-fluorescent theranostic gold nanoshells. Nanomedicine (Lond) 2014;9(8):1209–1222. doi: 10.2217/nnm.13.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coughlin AJ, Ananta JS, Deng NF, Larina IV, Decuzzi P, West JL. Gadolinium-conjugated gold nanoshells for multimodal diagnostic imaging and photothermal cancer therapy. Small. 2014;10(3):556–565. doi: 10.1002/smll.201302217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L, Fu S, Chen C, et al. Microenvironment-driven bioelimination of magnetoplasmonic nanoassemblies and their multimodal imaging-guided tumor photothermal therapy. ACS Nano. 2016;10(7):7094–7105. doi: 10.1021/acsnano.6b03238. [DOI] [PubMed] [Google Scholar]
- 107.Chen HY, Li BW, Ren XY, et al. Multifunctional near-infrared-emitting nano-conjugates based on gold clusters for tumor imaging and therapy. Biomaterials. 2012;33(33):8461–8476. doi: 10.1016/j.biomaterials.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 108.Zhang P, Yang XX, Wang Y, Zhao NW, Xiong ZH, Huang CZ. Rapid synthesis of highly luminescent and stable Au-20 nanoclusters for active tumor-targeted imaging in vitro and in vivo. Nanoscale. 2014;6(4):2261–2269. doi: 10.1039/c3nr05269a. [DOI] [PubMed] [Google Scholar]
- 109.Croissant JG, Zhang D, Alsaiari S, et al. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J Control Release. 2016;229:183–191. doi: 10.1016/j.jconrel.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 110.Zhao YF, Sultan D, Detering L, Luehmann H, Liu YJ. Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of Cu-64-Au alloy nanoclusters. Nanoscale. 2014;6(22):13501–13509. doi: 10.1039/c4nr04569f. [DOI] [PubMed] [Google Scholar]
- 111.Hu H, Huang P, Weiss OJ, et al. PET and NIR optical imaging using self-illuminating Cu-64-doped chelator-free gold nanoclusters. Biomaterials. 2014;35(37):9868–9876. doi: 10.1016/j.biomaterials.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu DH, Sheng ZH, Zhang PF, et al. Hybrid gold-gadolinium nanoclusters for tumor-targeted NIRF/CT/MRI triple-modal imaging in vivo. Nanoscale. 2013;5(4):1624–1628. doi: 10.1039/c2nr33543c. [DOI] [PubMed] [Google Scholar]
- 113.Han S, Samanta A, Xie X, et al. Gold and hairpin DNA functionalization of upconversion nanocrystals for imaging and in vivo drug delivery. Adv Mater Deerfield. 2017 Mar 10;29(18) doi: 10.1002/adma.201700244. Epub. [DOI] [PubMed] [Google Scholar]
- 114.Gao X, Yue Q, Liu Z, et al. Guiding brain-tumor surgery via blood-brain-barrier-permeable gold nanoprobes with acid-triggered MRI/SERRS signals. Adv Mater. 2017 Mar 15;29(21) doi: 10.1002/adma.201603917. Epub. [DOI] [PubMed] [Google Scholar]
- 115.Park J, Park J, Ju EJ, et al. Multifunctional hollow gold nanoparticles designed for triple combination therapy and CT imaging. J Control Release. 2015;207:77–85. doi: 10.1016/j.jconrel.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Tian M, Lu W, Zhang R, et al. Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model. Mol Imaging Biol. 2013;15(5):614–624. doi: 10.1007/s11307-013-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu W, Melancon MP, Xiong C, et al. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. 2011;71(19):6116–6121. doi: 10.1158/0008-5472.CAN-10-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y, Ashton JR, Moding EJ, et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics. 2015;5(9):946–960. doi: 10.7150/thno.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang J, Guo M, Ke HT, et al. Rational design and synthesis gamma Fe2O3@Au magnetic gold nanoflowers for efficient cancer theranostics. Adv Mater. 2015;27(34):5049. doi: 10.1002/adma.201501942. [DOI] [PubMed] [Google Scholar]
- 120.Hou W, Xia F, Alfranca G, et al. Nanoparticles for multi-modality cancer diagnosis: simple protocol for self-assembly of gold nanoclusters mediated by gadolinium ions. Biomaterials. 2017;120:103–114. doi: 10.1016/j.biomaterials.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 121.D’hollander A, Mathieu E, Jans H, et al. Development of nanostars as a biocompatible tumor contrast agent: toward in vivo SERS imaging. Int J Nanomedicine. 2016;11:3703–3714. doi: 10.2147/IJN.S91340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Liu Y, Luehmann H, et al. Evaluating the pharmacokinetics and in vivo cancer targeting capability of Au nanocages by positron emission tomography imaging. ACS Nano. 2012;6(7):5880–5888. doi: 10.1021/nn300464r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bao CC, Beziere N, del Pino P, et al. Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers. Small. 2013;9(1):68–74. doi: 10.1002/smll.201201779. [DOI] [PubMed] [Google Scholar]
- 124.Cheng K, Kothapalli SR, Liu HG, et al. Construction and validation of nano gold tripods for molecular imaging of living subjects. J Am Chem Soc. 2014;136(9):3560–3571. doi: 10.1021/ja412001e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Link S, El-Sayed MA. Shape and size dependence of radiative, nonradiative and photothermal properties of gold nanocrystals. Int Rev Phys Chem. 2000;19(3):409–453. [Google Scholar]
- 126.Link S, Furube A, Mohamed MB, Asahi T, Masuhara H, El-Sayed MA. Hot electron relaxation dynamics of gold nanoparticles embedded in MgSO4 powder compared to solution: the effect of the surrounding medium. J Phys Chem B. 2002;106(5):945–955. [Google Scholar]
- 127.Link S, Ei-Sayed MA. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu Rev Phys Chem. 2003;54:331–366. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- 128.Link S, Hathcock DJ, Nikoobakht B, El-Sayed MA. Medium effect on the electron cooling dynamics in gold nanorods and truncated tetrahedra. Adv Mater. 2003;15(5):393. [Google Scholar]
- 129.Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley interdisciplinary reviews. Nanomed Nanobiotechnol. 2017;9(4):e1449. doi: 10.1002/wnan.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang XH, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostic and therapy. Nanomedicine. 2007;2(5):681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 131.Hwang S, Nam J, Jung S, Song J, Doh H, Kim S. Gold nanoparticle-mediated photothermal therapy: current status and future perspective. Nanomedicine (Lond) 2014;9(13):2003–2022. doi: 10.2217/nnm.14.147. [DOI] [PubMed] [Google Scholar]
- 132.Huang X, Qian W, El-Sayed IH, El-Sayed MA. The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy. Lasers Surg Med. 2007;39(9):747–753. doi: 10.1002/lsm.20577. [DOI] [PubMed] [Google Scholar]
- 133.El-Sayed IH, Huang XH, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 134.Hleb EY, Hafner JH, Myers JN, et al. LANTCET: elimination of solid tumor cells with photothermal bubbles generated around clusters of gold nanoparticles. Nanomedicine. 2008;3(5):647–667. doi: 10.2217/17435889.3.5.647. [DOI] [PubMed] [Google Scholar]
- 135.Li Z, Huang P, Zhang X, et al. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol Pharm. 2010;7(1):94–104. doi: 10.1021/mp9001415. [DOI] [PubMed] [Google Scholar]
- 136.Ke HT, Wang JR, Tong S, et al. Gold nanoshelled liquid perfluorocarbon magnetic nanocapsules: a nanotheranostic platform for bimodal ultrasound/magnetic resonance imaging guided photothermal tumor ablation. Theranostics. 2014;4(1):12–23. doi: 10.7150/thno.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ayala-Orozco C, Urban C, Bishnoi S, et al. Sub-100nm gold nanomatryoshkas improve photo-thermal therapy efficacy in large and highly aggressive triple negative breast tumors. J Control Release. 2014;191:90–97. doi: 10.1016/j.jconrel.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Allison RR, Moghissi K. Photodynamic therapy (PDT): PDT mechanisms. Clin Endosc. 2013;46(1):24–29. doi: 10.5946/ce.2013.46.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schweitzer VG, Somers ML. PHOTOFRIN-mediated photodynamic therapy for treatment of early stage (Tis-T2N0M0) SqCCa of oral cavity and oropharynx. Laser Surg Med. 2010;42(1):1–8. doi: 10.1002/lsm.20881. [DOI] [PubMed] [Google Scholar]
- 140.Vankayala R, Sagadevan A, Vijayaraghavan P, Kuo CL, Hwang KC. Metal nanoparticles sensitize the formation of singlet oxygen. Angew Chem Int Ed Engl. 2011;50(45):10640–10644. doi: 10.1002/anie.201105236. [DOI] [PubMed] [Google Scholar]
- 141.Vankayala R, Lin CC, Kalluru P, Chiang CS, Hwang KC. Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light. Biomaterials. 2014;35(21):5527–5538. doi: 10.1016/j.biomaterials.2014.03.065. [DOI] [PubMed] [Google Scholar]
- 142.Chen R, Wang X, Yao XK, Zheng XC, Wang J, Jiang XQ. Near-IR-triggered photothermal/photodynamic dual-modality therapy system via chitosan hybrid nanospheres. Biomaterials. 2013;34(33):8314–8322. doi: 10.1016/j.biomaterials.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 143.von Maltzahn G, Park JH, Agrawal A, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69(9):3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun XL, Huang XL, Yan XF, et al. Chelator-free Cu-64-integrated gold nanomaterials for positron emission tomography imaging guided photothermal cancer therapy. ACS Nano. 2014;8(8):8438–8446. doi: 10.1021/nn502950t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Z, Wang J, Nie X, et al. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J Am Chem Soc. 2014;136(20):7317–7326. doi: 10.1021/ja412735p. [DOI] [PubMed] [Google Scholar]
- 146.Li XJ, Takashima M, Yuba E, Harada A, Kono K. PEGylated PAMAM dendrimer-doxorubicin conjugate-hybridized gold nanorod for combined photothermal-chemotherapy. Biomaterials. 2014;35(24):6576–6584. doi: 10.1016/j.biomaterials.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 147.Shi H, Ye XS, He XX, et al. Au@Ag/Au nanoparticles assembled with activatable aptamer probes as smart “nano-doctors” for image-guided cancer thermotherapy. Nanoscale. 2014;6(15):8754–8761. doi: 10.1039/c4nr01927j. [DOI] [PubMed] [Google Scholar]
- 148.Shao JX, Xuan MJ, Dai LR, Si TY, Li JB, He Q. Near-infrared-activated nanocalorifiers in microcapsules: vapor bubble generation for in vivo enhanced cancer therapy. Angew Chem Int Edit. 2015;54(43):12782–12787. doi: 10.1002/anie.201506115. [DOI] [PubMed] [Google Scholar]
- 149.Luo CH, Huang CT, Su CH, Yeh CS. Bacteria-mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett. 2016;16(6):3493–3499. doi: 10.1021/acs.nanolett.6b00262. [DOI] [PubMed] [Google Scholar]
- 150.Wang X, Wang H, Wang Y, et al. A facile strategy to prepare dendrimer-stabilized gold nanorods with sub-10-nm size for efficient photothermal cancer therapy. Sci Rep. 2016;6:22764. doi: 10.1038/srep22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu Y, Yang M, Zhang J, et al. Human induced pluripotent stem cells for tumor targeted delivery of gold nanorods and enhanced photothermal therapy. ACS Nano. 2016;10(2):2375–2385. doi: 10.1021/acsnano.5b07172. [DOI] [PubMed] [Google Scholar]
- 152.Ayala-Orozco C, Urban C, Knight MW, et al. Au nanomatryoshkas as efficient near-infrared photothermal transducers for cancer treatment: benchmarking against nanoshells. ACS Nano. 2014;8(6):6372–6381. doi: 10.1021/nn501871d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li W, Zhang XJ, Zhou MJ, et al. Functional core/shell drug nanoparticles for highly effective synergistic cancer therapy. Adv Healthc Mater. 2014;3(9):1475–1485. doi: 10.1002/adhm.201300577. [DOI] [PubMed] [Google Scholar]
- 154.Hao YW, Zhang BX, Zheng CX, et al. The tumor-targeting core-shell structured DTX-loaded PLGA@Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J Control Release. 2015;220(pt A):545–555. doi: 10.1016/j.jconrel.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 155.Wang L, Yuan YY, Lin SD, et al. Photothermo-chemotherapy of cancer employing drug leakage-free gold nanoshells. Biomaterials. 2016;78:40–49. doi: 10.1016/j.biomaterials.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 156.Xuan M, Shao J, Dai L, Li J, He Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 157.Nie LM, Wang SJ, Wang XY, et al. In vivo volumetric photoacoustic molecular angiography and therapeutic monitoring with targeted plasmonic nanostars. Small. 2014;10(8):1585–1593. doi: 10.1002/smll.201302924. [DOI] [PubMed] [Google Scholar]
- 158.Gao YP, Li YS, Chen JZ, et al. Multifunctional gold nanostar-based nanocomposite: synthesis and application for noninvasive MR-SERS imaging-guided photothermal ablation. Biomaterials. 2015;60:31–41. doi: 10.1016/j.biomaterials.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 159.Wang ZZ, Chen ZW, Liu Z, et al. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials. 2014;35(36):9678–9688. doi: 10.1016/j.biomaterials.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 160.Piao JG, Wang LM, Gao F, You YZ, Xiong YJ, Yang LH. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8(10):10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 161.Wang ZH, Sun JH, Qiu YQ, et al. Specific photothermal therapy to the tumors with high EphB4 receptor expression. Biomaterials. 2015;68:32–41. doi: 10.1016/j.biomaterials.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 162.Zhou JL, Wang ZH, Li QP, et al. Hybridized doxorubicin-Au nanospheres exhibit enhanced near-infrared surface plasmon absorption for photothermal therapy applications. Nanoscale. 2015;7(13):5869–5883. doi: 10.1039/c4nr07279k. [DOI] [PubMed] [Google Scholar]
- 163.Kang S, Bhang SH, Hwang S, et al. Mesenchymal stem cells aggregate and deliver gold nanoparticles to tumors for photothermal therapy. ACS Nano. 2015;9(10):9678–9690. doi: 10.1021/acsnano.5b02207. [DOI] [PubMed] [Google Scholar]
- 164.Chen M, Tang SH, Guo ZD, et al. Core-shell Pd@Au nanoplates as theranostic agents for in-vivo photoacoustic imaging, CT imaging, and photothermal therapy. Adv Mater. 2014;26(48):8210–8216. doi: 10.1002/adma.201404013. [DOI] [PubMed] [Google Scholar]
- 165.Li L, Nurunnabi M, Nafiujjaman M, Lee YK, Huh KM. GSH-mediated photoactivity of pheophorbide a-conjugated heparin/gold nanoparticle for photodynamic therapy. J Control Release. 2013;171(2):241–250. doi: 10.1016/j.jconrel.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Y, Qian J, Wang D, Wang YL, He SL. Multifunctional gold nanorods with ultrahigh stability and tunability for in vivo fluorescence imaging, SERS detection, and photodynamic therapy. Angew Chem Int Edit. 2013;52(4):1148–1151. doi: 10.1002/anie.201207909. [DOI] [PubMed] [Google Scholar]
- 167.Kim JY, Choi WI, Kim M, Tae G. Tumor-targeting nanogel that can function independently for both photodynamic and photothermal therapy and its synergy from the procedure of PDT followed by PTT. J Control Release. 2013;171(2):113–121. doi: 10.1016/j.jconrel.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 168.Vankayala R, Huang YK, Kalluru P, Chiang CS, Hwang KC. First demonstration of gold nanorods-mediated photodynamic therapeutic destruction of tumors via near infra-red light activation. Small. 2014;10(8):1612–1622. doi: 10.1002/smll.201302719. [DOI] [PubMed] [Google Scholar]
- 169.Wang BK, Wang JH, Liu Q, et al. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials. 2014;35(6):1954–1966. doi: 10.1016/j.biomaterials.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 170.Ye SF, Kang N, Chen M, et al. Tat/HA2 peptides conjugated AuNR@pNIPAAm as a photosensitizer carrier for near infrared triggered photodynamic therapy. Mol Pharm. 2015;12(7):2444–2458. doi: 10.1021/acs.molpharmaceut.5b00161. [DOI] [PubMed] [Google Scholar]
- 171.Srivatsan A, Jenkins SV, Jeon M, et al. Gold nanocage-photosensitizer conjugates for dual-modal image-guided enhanced photodynamic therapy. Theranostics. 2014;4(2):163–174. doi: 10.7150/thno.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang P, Lin J, Wang SJ, et al. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials. 2013;34(19):4643–4654. doi: 10.1016/j.biomaterials.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nair LV, Nazeer SS, Jayasree RS, Ajayaghosh A. Fluorescence imaging assisted photodynamic therapy using photosensitizer-linked gold quantum clusters. ACS Nano. 2015;9(6):5825–5832. doi: 10.1021/acsnano.5b00406. [DOI] [PubMed] [Google Scholar]
- 174.Broering R, Real CI, John MJ, et al. Chemical modifications on siRNAs avoid Toll-like-receptor-mediated activation of the hepatic immune system in vivo and in vitro. Int Immunol. 2014;26(1):35–46. doi: 10.1093/intimm/dxt040. [DOI] [PubMed] [Google Scholar]
- 175.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nanosized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24(3):438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 177.Guo JF, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29(4):402–417. doi: 10.1016/j.biotechadv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 178.Kumar A, Zhang X, Liang XJ. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol Adv. 2013;31(5):593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 179.Guo JF, Rahme K, Fitzgerald KA, Holmes JD, O’Driscoll CM. Biomimetic gold nanocomplexes for gene knockdown: will gold deliver dividends for small interfering RNA nanomedicines? Nano Res. 2015;8(10):3111–3140. [Google Scholar]
- 180.Chwalek K, Bray LJ, Werner C. Tissue-engineered 3D tumor angiogenesis models: potential technologies for anti-cancer drug discovery. Adv Drug Deliver Rev. 2014;79–80:30–39. doi: 10.1016/j.addr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 181.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13(10):727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 183.Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Annu Rev Pharmacol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 184.Zhou C, Long M, Qin YP, Sun XK, Zheng J. Luminescent gold nanoparticles with efficient renal clearance. Angew Chem Int Edit. 2011;50(14):3168–3172. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hirn S, Semmler-Behnke M, Schleh C, et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm. 2011;77(3):407–416. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alric C, Miladi I, Kryza D, et al. The biodistribution of gold nanoparticles designed for renal clearance. Nanoscale. 2013;5(13):5930–5939. doi: 10.1039/c3nr00012e. [DOI] [PubMed] [Google Scholar]
- 187.Simpson CA, Salleng KJ, Cliffel DE, Feldheim DL. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomedicine. 2013;9(2):257–263. doi: 10.1016/j.nano.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 188.Zhang XD, Wu D, Shen X, Liu PX, Fan FY, Fan SJ. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials. 2012;33(18):4628–4638. doi: 10.1016/j.biomaterials.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 189.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40(3):1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 190.Xie J, Lee S, Chen XY. Nanoparticle-based theranostic agents. Adv Drug Deliver Rev. 2010;62(11):1064–1079. doi: 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 192.Xia Y, Li W, Cobley CM, et al. Gold nanocages: from synthesis to theranostic applications. Acc Chem Res. 2011;44(10):914–924. doi: 10.1021/ar200061q. [DOI] [PMC free article] [PubMed] [Google Scholar]