Abstract
Objective:
To identify the shared neuroimaging signature of gait slowing and cognitive impairment.
Methods:
We assessed a cohort of older adults (n = 175, mean age 73 years, 57% female, 65% white) with repeated measures of gait speed over 14 years, MRI for gray matter volume (GMV) at year 10 or 11, and adjudicated cognitive status at year 14. Gait slowing was calculated by bayesian slopes corrected for intercepts, with higher values indicating faster decline. GMV was normalized to intracranial volume, with lower values indicating greater atrophy for 10 regions of interest (hippocampus, anterior and posterior cingulate, primary and supplementary motor cortices, posterior parietal lobe, middle frontal lobe, caudate, putamen, pallidum). Nonparametric correlations adjusted for demographics, comorbidities, muscle strength, and knee pain assessed associations of time to walk with GMV. Logistic regression models calculated odds ratios (ORs) of gait slowing with dementia or mild cognitive impairment with and without adjustment for GMV.
Results:
Gait slowing was associated with cognitive impairment at year 14 (OR per 0.1 s/y slowing 1.47; 95% confidence interval 1.04–2.07). The right hippocampus was the only region that was related to both gait slowing (ρ = −0.16, p = 0.03) and cognitive impairment (OR 0.17, p = 0.009). Adjustment for right hippocampal volume attenuated the association of gait slowing with cognitive impairment by 23%.
Conclusions:
The association between gait slowing and cognitive impairment is supported by a shared neural substrate that includes a smaller right hippocampus. This finding underscores the value of long-term gait slowing as an early indicator of dementia risk.
Prevention and early treatment may hold the key to reducing the global burden of dementia.1 Early detection of those most at risk will be necessary to appropriately target early treatments, but the current screening approaches are too invasive and costly to be used at a population level. Therefore, early clinical indicators of risk are needed.
Slow gait speed predicts future onset of dementia and mild cognitive impairment (MCI),2–6 and changes in gait may appear earlier than cognitive changes.7,8 Gait speed decline may occur as early as 12 years before the onset of MCI.9 Therefore, repeated measures of gait speed could provide an early screening tool for increased dementia risk. Gait speed declines are multifactorial in nature,10 and risk factors include age-related changes in cerebral integrity.11 Slow gait speed is related to specific age-related MRI abnormalities in those without neurologic disease, including smaller volume of motor regions, prefrontal cortex, basal ganglia, and medial temporal lobe.11,12 Changes in gray matter regions, including the hippocampus, have been proposed as a shared underlying mechanism for declines in both cognitive and physical functioning with age.11 We propose that gait slowing is indicative of dementia risk due to reduced cerebral integrity, possibly specific to hippocampal atrophy.
We used a well-characterized cohort of older adults with annual follow-up over 14 years to assess the associations of long-term changes in gait speed with later cognitive impairment. We then assessed how regional gray matter volumes (GMVs) were associated with both gait slowing and cognitive impairment and whether GMV attenuated the association between gait decline and cognitive impairment.
METHODS
Study population.
The Health Aging and Body Composition (Health ABC) study is a prospective cohort study of black and white older adults. At baseline in 1997, participants were 70 to 79 years of age and lived in Memphis, TN, or Pittsburgh, PA. Participants were recruited from designated ZIP codes by a random sample of Medicare-eligible adults. Eligibility was based on reporting no difficulties in performing activities of daily living, walking a quarter mile, or climbing 10 steps without resting. Men and black participants were oversampled.
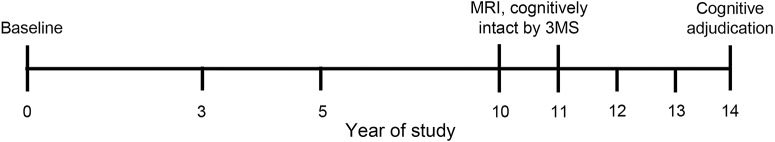
Participants at the Pittsburgh study site were asked to participate in a neuroimaging substudy between 2006 and 2008 (year 10 or 11 of the study; figure 1). Cognitive adjudication occurred at the Pittsburgh site during the 2011 to 2012 visit (year 14 of the study). Because we wanted to look at preclinical neuroimaging markers, participants were excluded if they had a Modified Mini-Mental State (3 MS) score <85 at the time of MRI, indicating likely major cognitive impairment. The mean time between MRI and cognitive adjudication was 3.3 years (SD 0.5 years).
Figure 1. Timeline of measures from the Health ABC.
Participants in Health ABC were seen annually with gait speed measured at study years 0, 3, and 5 and annually after year 10. MRI was conducted in year 10 or 11, and participants were included in these analyses if they had a 3 MS score ≥85 at time of MRI. Cognitive adjudication for dementia or mild cognitive impairment occurred in year 14. Health ABC = Health Aging and Body Composition; 3 MS = Modified Mini Mental State.
Of the 1,527 participants enrolled in the study in 1997 to 1998 at the Pittsburgh site, 819 were alive and were contacted to participate in the neuroimaging substudy; 315 completed it. Participants with cognitive adjudication (n = 246) were excluded from our analytic sample if they did not have at least 2 gait measures (n = 1), had no MRI data (n = 5), were missing ≥1 covariates (n = 22), or had a 3 MS score <85 at the time of MRI (n = 27). Our final analytic sample included 193 participants. There were no differences between included and excluded participants in age, sex, heart disease, diabetes mellitus, hypertension, knee pain, muscle strength, baseline gait speed, gait slope, or total GMV (all p > 0.1). The included individuals were more likely to be white (p = 0.01) and to have at least a high school education (p = 0.0006) compared to the excluded individuals.
Standard protocol approvals, registrations, and patient consents.
This study was approved by institutional review boards of all participating institutions. All participants signed written informed consent.
Gait speed.
Repeated measures of time to walk 6 m at the usual pace were collected between 2 and 9 times between 1997 to 1998 and 2011 to 2012. Changes in time to walk 6 m were computed with bayesian slopes from longitudinal mixed models with random slopes and intercepts corrected for intercepts and are reported as increases in seconds to walk per year. Slopes were calculated separately by sex. Higher values indicate a greater increase in time to walk (i.e., faster slowing of gait speed).
Cognitive impairment.
Cognitive status was clinically adjudicated on Health ABC participants who were seen at the year 14 site visit in 2011 to 2012 from data collected at the year 14 and prior visits, as described elsewhere.13 Because of the restricted sample size, participants were categorized as cognitively normal or cognitively impaired (MCI or dementia) for these analyses.
Neuroimaging.
A 3T Siemens Tim Trio magnetic resonance scanner with a Siemens 12-channel head coil was used (Siemens, Munich, Germany). Image acquisition and analysis for this study have been previously described.14 Magnetization-prepared rapid gradient echo images were acquired to obtain GMV of regions of interest (ROIs).
GMV was normalized to intracranial volume, with lower values indicating greater atrophy, and was assessed bilaterally for 10 prespecified ROIs on the basis of previous literature11,12,15 (hippocampus, anterior and posterior cingulate, primary and supplementary motor cortices, posterior parietal lobe, middle frontal lobe, caudate, putamen, and pallidum).
White matter hyperintensity (WMH) volumes were obtained from T2-weighted fluid-attenuated inversion recovery images with a semiautomated method.16 Total brain WMH volume was normalized to brain volume.
APOE genotype.
APOE genotype was quantified by standard single-nucleotide polymorphism genotyping techniques and categorized as having any APOE ε4 alleles or no allele.
Covariates.
At baseline, demographic data, including age, race, sex, and education, were self-reported. Prevalent and incident disease algorithms based both on self-report and on physician diagnoses, recorded medications, and laboratory data were used to create time-dependent comorbidity variables indicating the development of diabetes mellitus, coronary heart disease, and hypertension during the entire study period. Fall history was assessed for the past year at each annual visit. History of recurrent falls over the entire study was defined as reporting multiple falls at least once or reporting a single fall at multiple visits. Muscle strength, measured as peak torque of quadriceps strength measured on a dynamometer (125 AP, Kin-Com, Chattanooga, TN), and self-reported joint pain at the knee were collected at the time of MRI.
Statistical analysis.
Descriptive statistics comparing those who were cognitively normal and those who were cognitively impaired at the end of the study were calculated by t tests for continuous variables and by χ2 statistics for categorical variables.
Associations of 5 different gait speed measures (3 single time points and 2 slopes) with cognitive impairment were assessed by logistic regression to determine which had the strongest association using odds ratios (ORs) and 95% confidence intervals (CIs). The measures included time to walk 6 m at study baseline, at the time of MRI, and at the time of cognitive adjudication, as well as the slope of time to walk during the full 14-year study period and during the last 4 years of the study between the MRI and cognitive adjudication. Of those gait measures, the one most strongly associated with cognitive impairment was then assessed in relation to GMV of the prespecified ROIs, with an emphasis on hippocampal volume. Left and right ROIs were assessed separately. Because of nonnormal distributions of some ROI GMVs, partial Spearman correlations were used to obtain correlation coefficients (ρ) and p values. Associations of total GMV and ROI GMV with cognitive impairment were assessed by logistic regression. Sensitivity analyses assessed the associations separately for MCI and dementia. Analyses were repeated stratified by APOE ε4 allele status.
To determine whether differences in ROI GMV might explain the association between gait slope and cognitive impairment, GMV variables from regions associated with both gait slowing and cognitive impairment were included in logistic regression models to assess how much each GMV variable attenuated the association between gait slope and cognitive impairment. All analyses were adjusted for age, sex, race, education, coronary heart disease, diabetes mellitus, hypertension, falls, knee pain, muscle strength, and WMH. All analyses used SAS, version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
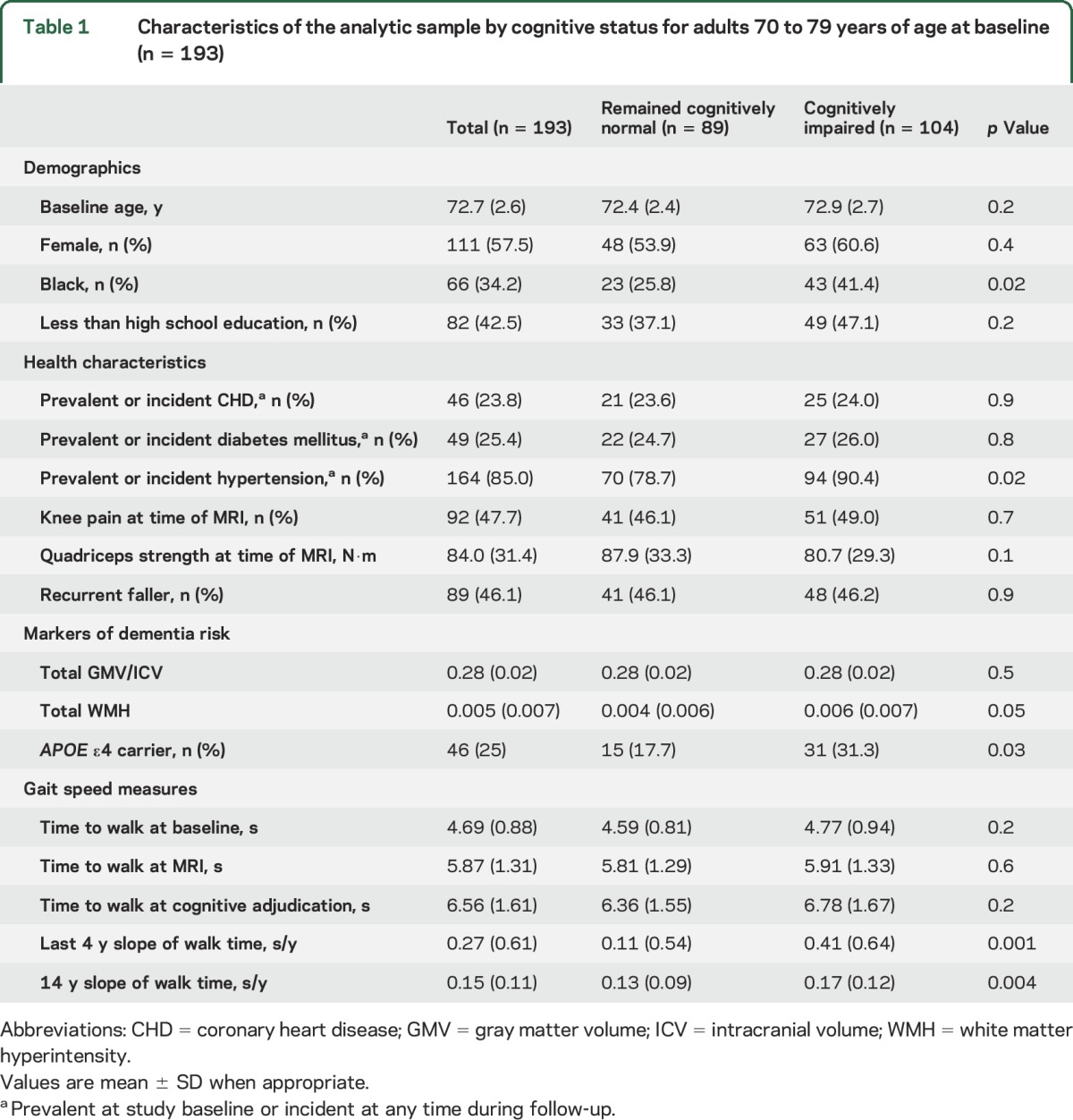
At baseline, the sample was on average 73 years old. A slight majority (57.5%) were female, and 34.2% were black (table 1). At adjudication, 104 (53.9%) had cognitive impairment. Of these, 69 (35.8%) had MCI and 35 (18.1%) had dementia. Participants with cognitive impairment were more likely to be black (41.4% vs 25.8%, p = 0.02) and to be hypertensive (90.4% vs 78.7%, p = 0.02) than those who were cognitively normal (table 1). Those with cognitive impairment also had higher WMH volumes than those who remained cognitively normal (p = 0.05; table 1). Faster gait slowing was associated with older age, presence of knee pain, lower muscle strength, lower total GMV, greater WMH volume, and being an APOE ε4 carrier (table e-1 at Neurology.org).
Table 1.
Characteristics of the analytic sample by cognitive status for adults 70 to 79 years of age at baseline (n = 193)
The mean time to walk 6 m was consistently longer (i.e., slower gait) for the cognitively impaired group during all 14 years of follow-up (figure 2), but no single time point gait measure was significantly associated with later cognitive impairment (table 1). The greatest mean differences between the 2 groups appeared during the last 4 years before cognitive adjudication (figure 2). However, the slope of gait change during the last 4 years was only weakly associated with cognitive impairment in unadjusted models (OR per 0.1-s/y slowing 1.10, 95% CI 1.04–1.17) and after adjustment for demographic and health variables (OR per 0.1-s/y slowing 1.13, 95% CI 1.05–1.22). In contrast, the slope of gait change over the full 14 years before cognitive adjudication was more strongly associated with later cognitive impairment before (OR per 0.1-s/y slowing 1.52, 95% CI 1.13–2.04) and after adjustment for demographic and health variables (OR per 0.1-s/y slowing 1.47, 95% CI 1.04–2.07). Sensitivity analyses assessed the association of gait slowing with MCI and dementia separately and found similar results for each (MCI: OR 1.45, 95% CI 0.99–2.15; dementia: OR 1.69, 95% CI 1.05–2.71).
Figure 2. Mean gait speed by cognitive status over 14 years.
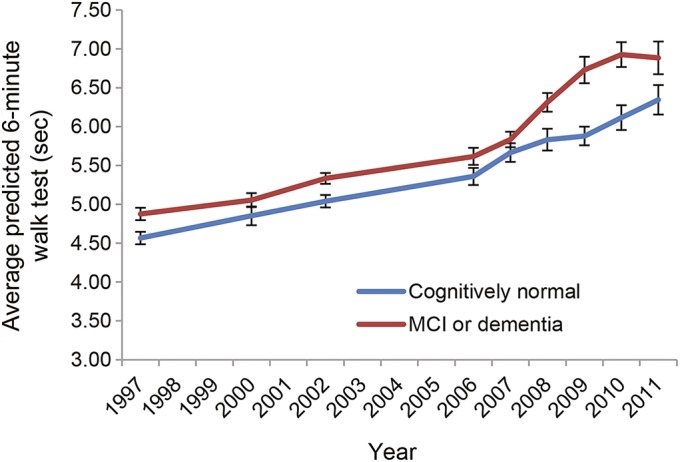
Predicted mean time and standard errors in seconds to walk 6 m over the full 14-year follow-up of the Health ABC study for those who were cognitively normal or cognitively impaired (MCI or dementia) at adjudication in year 14. Predicted estimates come from longitudinal mixed models. Health ABC = Health Aging and Body Composition; MCI = mild cognitive impairment.
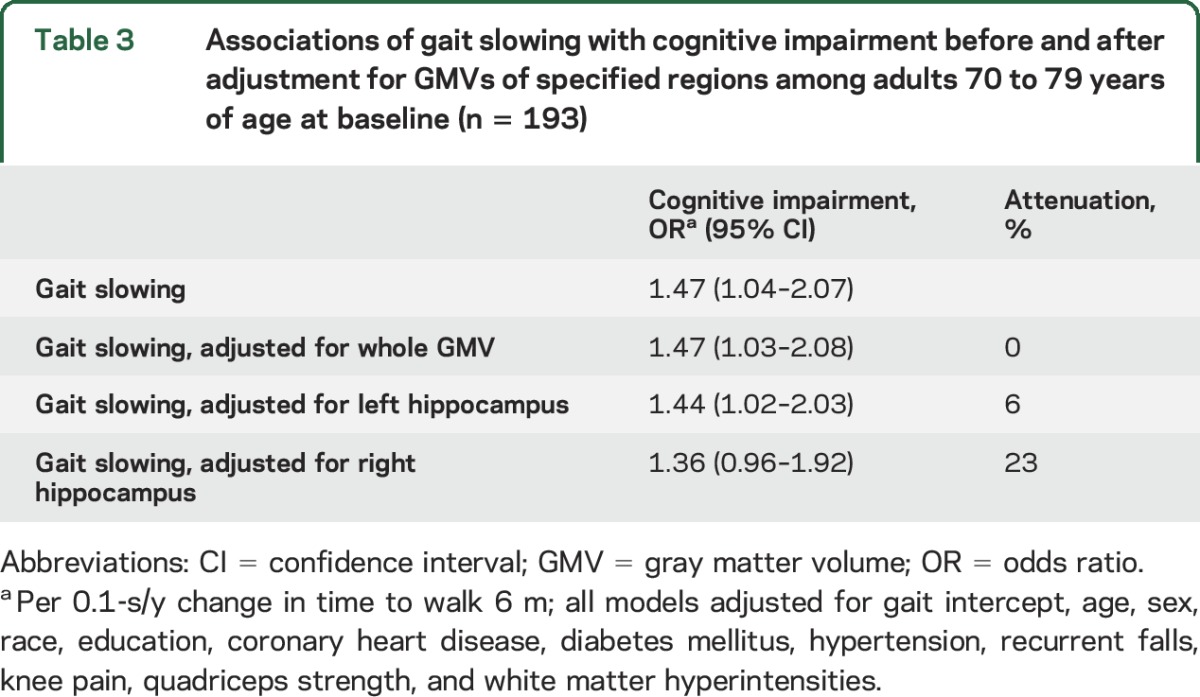
Lower total GMV was significantly associated with greater gait slowing over 14 years (ρ = −0.18, p = 0.02). Smaller right hippocampus (ρ = −0.16, p = 0.03), but not left hippocampus (ρ = −0.03, p = 0.6), was associated with faster gait slowing (table 2). A number of additional ROIs were associated with faster gait slowing in fully adjusted models (table 2), including the right anterior cingulate gyrus, bilateral caudate, and bilateral putamen (ρ range −0.17 to −0.25, p range 0.001–0.02). Of the ROIs associated with gait slowing, only smaller volume of the right hippocampus was significantly associated with greater odds of being cognitively impaired (OR = 0.17, 95% CI 0.05–0.55; table 3) and attenuated the association between gait slowing and cognitive impairment (23% attenuation; table 3). In contrast, left hippocampal volume was also associated with cognitive impairment (OR = 0.30, 95% CI 0.11–0.83) but was not associated with gait slowing (table 2) and did not appreciably attenuate the association between gait slowing and cognitive impairment (table 3).
Table 2.
Spearman correlations of GMVa with gait slowingb among adults 70 to 79 years of age at baseline (n = 193)
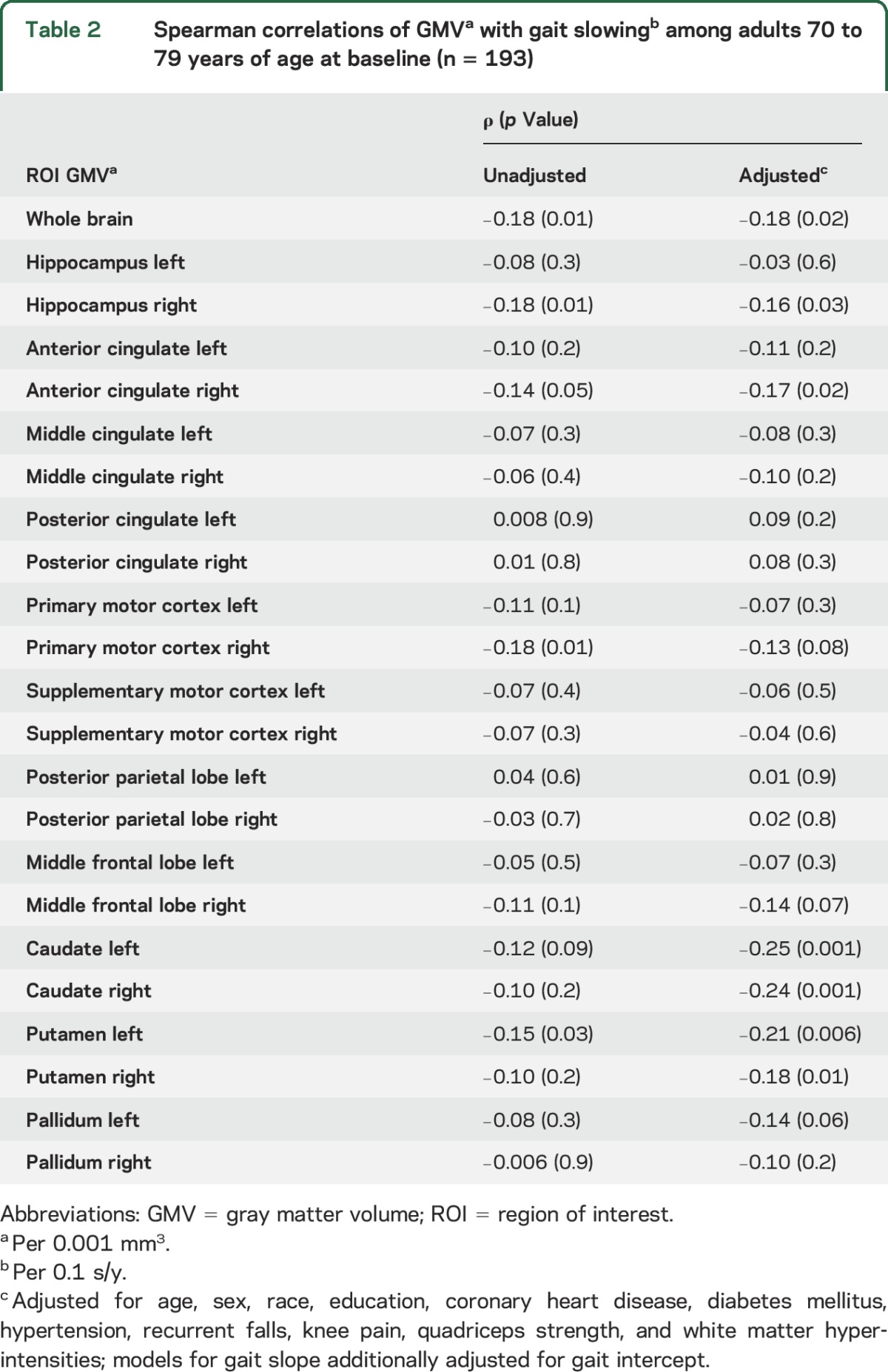
Table 3.
Associations of gait slowing with cognitive impairment before and after adjustment for GMVs of specified regions among adults 70 to 79 years of age at baseline (n = 193)
To assess the possible modifying role of APOE ε4 allele status, we reran analyses stratified by APOE. Associations between right hippocampal volume and both gait slowing and cognitive impairment were stronger in APOE ε4 allele carriers (data not shown). In models testing the association of gait slowing with cognitive impairment, there was no significant interaction between gait slowing and APOE status (p for interaction = 0.8).
DISCUSSION
We found that a longitudinal measure of gait speed decline over 14 years predicted later cognitive impairment (adjudicated MCI or dementia) in an initially healthy sample of older adults. This association was independent of a number of demographic and health characteristics, including WMH volume. Gait speed change predicted cognitive impairment in the absence of associations for single time point gait speed measures. This association was attributable, at least in part, to differences in right hippocampal volume.
These findings support the theory that the relation between gait speed and cognitive impairment is due to a shared underlying neuropathology.11 While multiple brain regions are likely implicated in both gait slowing and cognitive decline, smaller right hippocampal volume appears to be shared by both. This provides a mechanistic link between gait slowing and cognitive decline, indicating that the observed association is not just due to parallel, independent declines in the 2 domains during aging. Loss of hippocampal integrity is a well-recognized contributor to cognitive impairment. Volume of the hippocampus is implicated in cognitive decline and dementia onset,17 and hippocampal atrophy is observed in both Alzheimer disease17 and vascular dementia.18 Of the gray matter regions assessed previously, the hippocampus has been most consistently associated with measures indicting slower and less stable gait patterns.19–27 Cross-sectional studies have found positive associations between gait measures and hippocampal integrity,19–25 but 2 other studies found no association.15,28 The only study to assess longitudinal, concurrent changes in gait and regional GMV found that only hippocampal atrophy was associated with declines in gait speed over a mean of 30 months.26 Studies that have assessed associations of gait and hippocampal integrity cross-sectionally in cognitively healthy individuals and those with MCI found more consistent associations in the healthy individuals,22,23 indicating that the association may be present only before the onset of cognitive impairment.
In our analyses, hypertension was significantly associated with cognitive impairment and marginally significantly associated with gait slowing. Hypertension is a known risk factor for both gait slowing and cognitive impairment and has been shown to be related to smaller hippocampal volumes.29 It is possible that hypertension is a key risk factor for hippocampal volume loss leading to both gait and cognitive declines.
The majority of studies have not assessed laterality of the hippocampal association with gait. One study using an ROI approach and assessing right and left hippocampal volume separately found that only right hippocampal volume was associated with the Timed Up and Go Test in cognitively healthy individuals.22 A second study found that smaller volumes of the right, but not left, medial temporal lobe were associated with worse Timed Up and Go Test performance.27 The hippocampus is involved in sensorimotor integration,30 and the right side, in particular, is involved in spatial memory.31,32 Patients with multiple cognitive domain MCI have greater atrophy of the temporal gyrus on the right side compared to greater atrophy on the left in those with amnestic MCI.33 This is consistent with gait speed having stronger associations with nonamnestic MCI34 and vascular dementia35 as opposed to amnestic MCI and Alzheimer disease. It should be noted, however, that atrophy of the right hippocampus has also been associated with Alzheimer disease.36,37
Our findings underscore the importance of assessing gait speed decline over a number of years as opposed to at a single time point, consistent with previous findings.5 While single time point measures of gait speed can be informative,38 we found that only the slope of gait speed change was associated with later cognitive impairment. We also found that gait slowing measured over 14 years was more strongly associated with cognitive impairment than gait slowing over the last 4 years of follow-up. This was despite the fact that the largest differences in gait speed seemed to appear during the last 4 years. The variability in gait speed change was also much larger during this time period, which may have reduced the strength of the association. This larger variability between individuals may be due to the onset of declines in multiple other systems that contribute to declines in gait speed10 as participants entered their 80s.
Our study has several important limitations. Our sample was likely healthier than the average population of older adults. They had to be free of mobility disability at baseline when they were on average 73 years of age. In addition, we excluded those individuals with likely cognitive impairment based on 3 MS scores at the time of MRI when participants were in their early 80s, and they had to survive until cognitive adjudication when they were in their mid-80s. We did not distinguish between types of cognitive impairment. By mixing amnestic/Alzheimer-type impairments and nonamnestic/vascular-type impairments, we could have underestimated associations if they were specific to a particular type of cognitive impairment.39 However, we did not have power to assess subtypes of cognitive impairment. Finally, we did not assess nonspeed aspects of gait. Aspects of rhythm and variability of gait may also be predictive of dementia onset2 and should be further explored as early markers of dementia risk. Together, these limitations likely led to a conservative estimate of the true association between gait changes and cognitive outcomes.
The association between gait slowing and cognitive impairment is supported by a shared neural substrate that includes a smaller right hippocampus. This finding underscores the value of long-term gait slowing as an early indicator of dementia risk. Gait speed has several advantages over other early indicators of risk for cognitive impairment. It is easily measured in clinical settings and may precede detectable changes on many cognitive tests. Assessment of gait speed should be included in regular geriatric evaluations40 to flag those to be further evaluated for dementia risk, possibly with MRI to determine hippocampal integrity. Gait speed declines could be a key component of early detection for cognitive impairment and dementia that will allow better planning, prevention, and early treatment options.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge coauthor Dr. Suzanne Satterfield, who passed away before the publication of this article.
GLOSSARY
- CI
confidence interval
- Health ABC
Health Aging and Body Composition
- MCI
mild cognitive impairment
- OR
odds ratio
- ROI
region of interest
- 3 MS
Modified Mini-Mental State
- WMH
white matter hyperintensity
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Andrea L. Rosso: study concept and design, analysis and interpretation of data, statistical analysis, critical revision of manuscript for intellectual content. Joe Verghese: study concept and design, interpretation of data, critical revision of manuscript for intellectual content. Andrea L. Metti and Robert M. Boudreau: analysis and interpretation of data, statistical analysis, critical revision of manuscript for intellectual content. Howard J. Aizenstein: critical revision of manuscript for intellectual content. Stephen B. Kritchevsky, Tamara B. Harris, Kristine Yaffe, and Suzanne Satterfield: study supervision, critical revision of manuscript for intellectual content. Stephanie A. Studenski: study concept and design, interpretation of data, critical revision of manuscript for intellectual content. Caterina Rosano: study concept and design, interpretation of data, critical revision of manuscript for intellectual content, study supervision.
STUDY FUNDING
Health ABC was supported by National Institute on Aging contracts (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106; National Institute on Aging grant R01-AG-028050; and National Institute of Nursing Research grant R01-NR-012459). Healthy Brain Project was supported by the National Institute on Aging (K23-AG-028966, R01-AG-029232). This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging; the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827-07; the NIH through grant KL2-TR000146; and the National Institute on Aging through grant K01-AG053431.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 2.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 2007;78:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clouston SA, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev 2013;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumurgier J, Artaud F, Touraine C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci 2017;72:655–661. [DOI] [PubMed] [Google Scholar]
- 5.Welmer AK, Rizzuto D, Qiu C, Caracciolo B, Laukka EJ. Walking speed, processing speed, and dementia: a population-based longitudinal study. J Gerontol A Biol Sci Med Sci 2014;69:1503–1510. [DOI] [PubMed] [Google Scholar]
- 6.Montero-Odasso MM, Barnes B, Speechley M, et al. Disentangling cognitive-frailty: results from the Gait and Brain Study. J Gerontol A Biol Sci Med Sci 2016;71:1476–1482. [DOI] [PubMed] [Google Scholar]
- 7.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci 2013;68:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best JR, Liu-Ambrose T, Boudreau RM, et al. An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J Gerontol A Biol Sci Med Sci 2016;71:1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 2010;67:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci 2015;70:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci 2013;68:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci 2014;69:1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metti AL, Aizenstein H, Yaffe K, et al. Trajectories of peripheral interleukin-6, structure of the hippocampus, and cognitive impairment over 14 years in older adults. Neurobiol Aging 2015;36:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosano C, Aizenstein HJ, Newman AB, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage 2012;62:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2007;62:1048–1055. [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006;148:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.den Heijer T, van der Lijn F, Koudstaal PJ, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain 2010;133:1163–1172. [DOI] [PubMed] [Google Scholar]
- 18.Kril JJ, Patel S, Harding AJ, Halliday GM. Patients with vascular dementia due to microvascular pathology have significant hippocampal neuronal loss. J Neurol Neurosurg Psychiatry 2002;72:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezzati A, Katz MJ, Lipton ML, Lipton RB, Verghese J. The association of brain structure with gait velocity in older adults: a quantitative volumetric analysis of brain MRI. Neuroradiology 2015;57:851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makizako H, Shimada H, Doi T, Park H, Yoshida D, Suzuki T. Six-minute walking distance correlated with memory and brain volume in older adults with mild cognitive impairment: a voxel-based morphometry study. Demen Geriatr Cogn Disord Extra 2013;3:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callisaya ML, Beare R, Phan TG, Chen J, Srikanth VK. Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PLoS One 2014;9:e84909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allali G, Annweiler C, Predovan D, Bherer L, Beauchet O. Brain volume changes in gait control in patients with mild cognitive impairment compared to cognitively healthy individuals: GAIT study results. Exp Gerontol 2016;76:72–79. [DOI] [PubMed] [Google Scholar]
- 23.Beauchet O, Launay CP, Annweiler C, Allali G. Hippocampal volume, early cognitive decline and gait variability: which association? Exp Gerontol 2015;61:98–104. [DOI] [PubMed] [Google Scholar]
- 24.Rosso AL, Olson Hunt MJ, Yang M, et al. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture 2014;40:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res 2009;1291:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callisaya ML, Beare R, Phan TG, et al. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc 2013;61:1074–1079. [DOI] [PubMed] [Google Scholar]
- 27.Kose Y, Ikenaga M, Yamada Y, et al. Timed Up and Go Test, atrophy of medial temporal areas and cognitive functions in community-dwelling older adults with normal cognition and mild cognitive impairment. Exp Gerontol 2016;85:81–87. [DOI] [PubMed] [Google Scholar]
- 28.Dumurgier J, Crivello F, Mazoyer B, et al. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage 2012;60:871–878. [DOI] [PubMed] [Google Scholar]
- 29.Beauchet O, Celle S, Roche F, et al. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens 2013;31:1502–1516. [DOI] [PubMed] [Google Scholar]
- 30.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res 2001;127:119–136. [DOI] [PubMed] [Google Scholar]
- 31.Piekema C, Kessels RP, Mars RB, Petersson KM, Fernandez G. The right hippocampus participates in short-term memory maintenance of object-location associations. Neuroimage 2006;33:374–382. [DOI] [PubMed] [Google Scholar]
- 32.de Toledo-Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus 2000;10:136–142. [DOI] [PubMed] [Google Scholar]
- 33.Bell-McGinty S, Lopez OL, Meltzer CC, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol 2005;62:1393–1397. [DOI] [PubMed] [Google Scholar]
- 34.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc 2008;56:1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med 2002;347:1761–1768. [DOI] [PubMed] [Google Scholar]
- 36.Morra JH, Tu Z, Apostolova LG, et al. Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Hum Brain Mapp 2009;30:2766–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soldan A, Pettigrew C, Lu Y, et al. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer's disease. Hum Brain Mapp 2015;36:2826–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauchet O, Annweiler C, Callisaya ML, et al. Poor gait performance and prediction of dementia: results from a meta-analysis. J Am Med Dir Assoc 2016;17:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility: giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA 2014;311:2061–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.