Abstract
Objective:
To study factors associated with permanent CSF diversion and the relationship between shunting and functional outcomes in spontaneous intraventricular hemorrhage (IVH).
Methods:
Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III), a randomized, multicenter, double-blind, placebo-controlled trial, was conducted to determine if pragmatically employed external ventricular drainage (EVD) plus intraventricular alteplase improved outcome, in comparison to EVD plus saline. Outcome measures were predictors of shunting and blinded assessment of mortality and modified Rankin Scale at 180 days.
Results:
Among the 500 patients with IVH, CSF shunting was performed in 90 (18%) patients at a median of 18 (interquartile range [IQR] 13–30) days. Patient demographics and IVH characteristics were similar among patients with and without shunts. In the multivariate analysis, black race (odds ratio [OR] 1.98; 95% confidence interval [CI] 1.18–3.34), duration of EVD (OR 1.10; CI 1.05–1.15), placement of more than one EVD (OR 1.93; CI 1.13–3.31), daily drainage CSF per 10 mL (OR 1.07; CI 1.04–1.10), and intracranial pressure >30 mm Hg (OR 1.70; CI 1.09–2.88) were associated with higher odds of permanent CSF shunting. Patients who had CSF shunts had similar odds of 180-day mortality, while survivors with shunts had increased odds of poor functional outcome, compared to survivors without shunts.
Conclusions:
Among patients with spontaneous IVH requiring emergency CSF diversion, those with early elevated intracranial pressure, high CSF output, and placement of more than one EVD are at increased odds of permanent ventricular shunting. Administration of intraventricular alteplase, early radiographic findings, and CSF measures were not useful predictors of permanent CSF diversion.
Spontaneous intracerebral hemorrhage (ICH) has a high mortality, and only a fifth of survivors achieve functional independence at 6 months.1 Intraventricular hemorrhage (IVH) and the ensuing hydrocephalus, which occur in up to 45% of ICH patients, independently contribute to poor outcomes,2–4 and these patients represent a critically ill subset of the ICH population. Hence, presence of IVH or hydrocephalus often warrants emergent temporary CSF drainage using an external ventricular drain (EVD).5 The standard practice is to subsequently wean or clamp the EVD when resolution of intracranial pressure elevation and hydrocephalus has occurred. In the event of an unsuccessful EVD challenge, permanent CSF shunt is indicated.6 Rates of permanent shunting are estimated to be 18%–30% among ICH patients requiring an EVD.7,8
Two factors consistently associated with shunt dependence after ICH are thalamic location of ICH and elevations in intracranial pressure (ICP) beyond 25 mm Hg.7,8 Given the paucity of data in ICH, physicians often extrapolate results from subarachnoid hemorrhage studies, where age,9,10 low Glasgow Coma Scale score,11 elevated CSF protein,12 presence of intraventricular hemorrhage,9,10 CSF erythrocyte count,12,13 ventriculitis,14 and duration of EVD13 are independent predictors of permanent shunting. Our current understanding of shunt dependence in patients with ICH and subarachnoid hemorrhage is based on studies from small single-center patient cohorts studied retrospectively, and subject to biases resulting from institutional practices and neurosurgical protocols. Moreover, prior studies in ICH patients have included mostly intraparenchymal hemorrhages with variable extent of IVH. We hence aimed to study factors associated with permanent CSF diversion in a large, multi-institution cohort of spontaneous IVH patients with obstructive hydrocephalus requiring EVD, and also assess the relationship between shunting and ICH outcomes.
METHODS
Study design and patients.
We performed a prospective observational cohort study using patients enrolled in the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR) III trial. CLEAR III was a multicenter, randomized, double-blind, placebo-controlled trial conducted to determine if pragmatically employed EVD plus intraventricular alteplase improved outcome by removing IVH and controlling ICP, in comparison to EVD plus saline. The main inclusion criteria were (1) adult patients aged 18–80 years, (2) spontaneous (hypertensive) ICH with hematoma volume <30 mL or primary IVH, (3) obstruction of the third or fourth ventricles, (4) presentation within 24 hours of symptom onset, and (5) stability of ICH, IVH, and any EVD tract hemorrhage prior to 72 hours from diagnostic noncontrast CT scan. The trial randomized patients to receive up to 12 doses of alteplase or 0.9% saline every 8 hours via the EVD until third and fourth ventricles were radiographically open.15
Measurements.
Patient demographics and comorbidities were recorded at the time of enrollment. The following baseline characteristics were collected: age; sex; stroke comorbidities; medication history, particularly antithrombotics; and admission severity variables such as Glasgow Coma Scale and NIH Stroke Scale. Noncontrast CT scans were obtained on admission, at 12-hour intervals until stability, and postrandomization once daily until after last dose of study agent. CT scans were assessed daily for radiographic opening of the third or fourth ventricles. Hematoma volumes were calculated using semiautomated planimetry and read centrally by a single neuroradiologist blinded to treatments and outcomes. IVH and ICH volumes were calculated at diagnosis, stability (no further evidence of new bleeding from any site), and at end of treatment defined as 24 hours after last dose of study agent. We assessed third ventricular obstruction at 24 hours after last dose of study agent, defined as third ventricular obstruction either by IVH or mechanical compression by thalamic hematoma. CSF output was recorded daily while EVD was in place. Since our study population had distal ventricular obstruction, the majority of the patients had hydrocephalus. We hence did not assess hydrocephalus as a categorical variable. Degree of hydrocephalus was instead evaluated on diagnostic CT using the Evans index,16 which was defined as maximal frontal horn ventricular width divided by the maximal transverse inner diameter of the skull, and the bicaudate index,17 defined as the ratio between the bicaudate distance and the inner table of the skull at the same level. These indices were assessed at baseline and at 30 days.
Our predictor variable was permanent CSF shunt procedure. Decisions regarding EVD weaning, clamping, and shunt insertion were at the discretion of the enrolling centers. ICP was recorded every 4 hours from EVD insertion until day 7 postrandomization in all patients, or until removal of the EVD. Any ICP greater than 20 mm Hg had to be sustained for at least 15 minutes. CSF sampling was performed daily for 7 days postrandomization and when clinically indicated. CSF parameters measured were cell count, protein, glucose, and Gram stain. To account for practice variability in the management of patients by enrolling centers, we identified centers that enrolled more than 10 patients. In addition, we divided the centers into 5 categories based on geographic location: northeast, south, midwest, and west for hospitals in the United States, and international for hospitals abroad.
Outcomes.
Our primary outcome measures were factors associated with permanent CSF diversion and blinded assessment of poor functional outcome defined as a modified Rankin Scale (mRS) score of 4–6 at 6 months. Secondary outcomes were mortality and disability (mRS 4–5) assessed separately.
Statistical analysis.
We used the Wilcoxon rank sum test or Student t test for continuous variables depending on the normality of distribution. Categorical variables were analyzed using the Pearson χ2 square test (Fisher exact test when appropriate). Binary logistic regression was used to assess factors associated with shunting, and the relationship between permanent shunting and ICH outcomes. Covariates for the regression models were chosen based on bivariate logistic regression with a significance of p < 0.05. Based on these criteria, in the regression model that assessed predictors of permanent CSF diversion, we adjusted for race, duration of EVD, more than one EVD, any occurrence of ICP over 30 mm Hg, and daily CSF output. Similarly, the regression models that studied the relationship between permanent shunting and ICH outcomes were adjusted for age, race, stability IVH and ICH volume, thalamic obstruction, Glasgow Coma Scale score at screening, ICP over 30 mm Hg, and withdrawal of care. Statistical analyses were performed using Stata (version 14.0, College Station, TX). All analyses were 2-tailed, and significance level was determined by p < 0.05.
Standard protocol approvals, registrations, and patient consents.
The CLEAR III trial was performed at 73 sites in Brazil, Canada, Germany, Hungary, Israel, Spain, the United Kingdom, and the United States, following local institutional review board and country ethics approval.18 This study is registered with ClinicalTrials.gov, NCT00784134. Written informed consent for research was obtained from all participants (or legal representatives or surrogates when applicable) in the study.
RESULTS
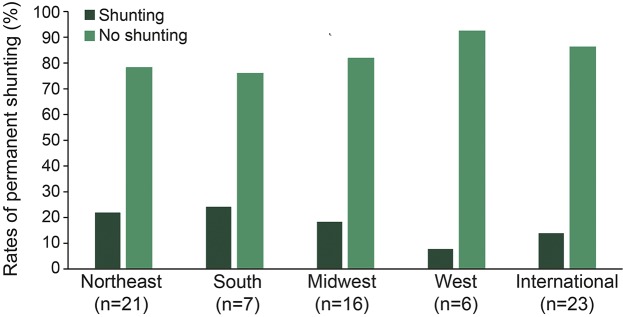
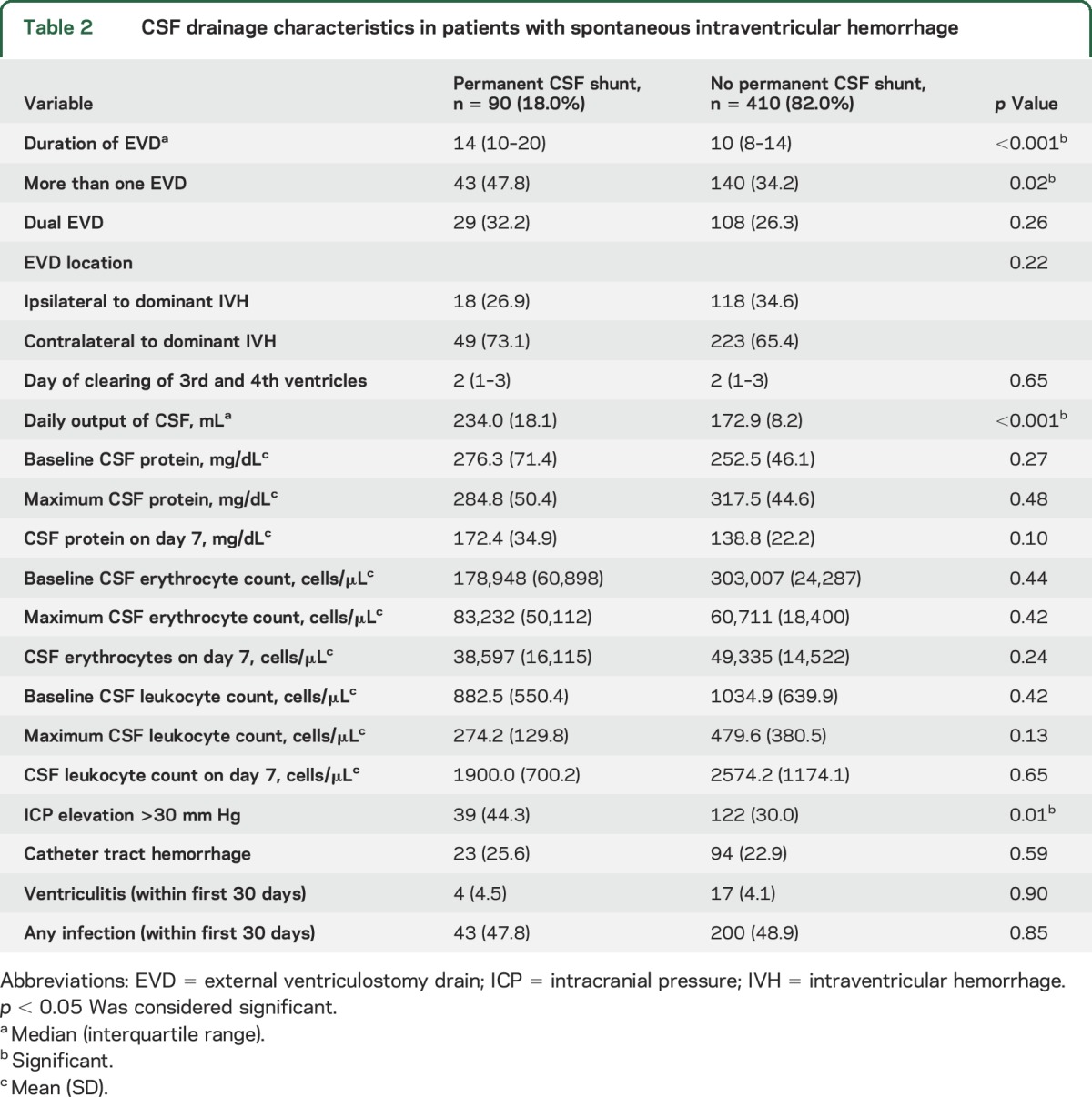
Of the 500 patients with IVH included in the study, shunting was performed in 90 patients (18%; 95% confidence interval [CI] 14.9%–21.6%) and did not differ by use of intraventricular alteplase (p = 0.78). Among the 90 patients with permanent CSF diversion, the median time to shunting was 18.0 days (interquartile range [IQR] 13.0–30.0). While black patients were more likely to have permanent shunts compared to other race groups (p < 0.001), other patient demographics and comorbidities were similar among patients with and without shunts (table 1). Patients with shunts had higher rates of third ventricular obstruction at 72 hours post last dose of study agent compared to patients who did not require permanent shunting (29.6% vs 18.3%, p = 0.02); however, other markers of hematoma severity, hydrocephalus indices, and day of radiographic opening of the third and fourth ventricles were similar between the 2 groups. Rates of permanent CSF shunting did not differ by geographic location of the enrollment site (figure). Patients with shunts had longer duration of EVD (p < 0.001), had higher daily CSF output (p < 0.001), and were more likely to have any ICP >30 mm Hg (p = 0.01), and placement of more than one EVD (p = 0.02), compared to patients who did not require permanent CSF diversion (table 2).
Table 1.
Baseline demographics, comorbidities, and clinical severity factors in patients with spontaneous intraventricular hemorrhage
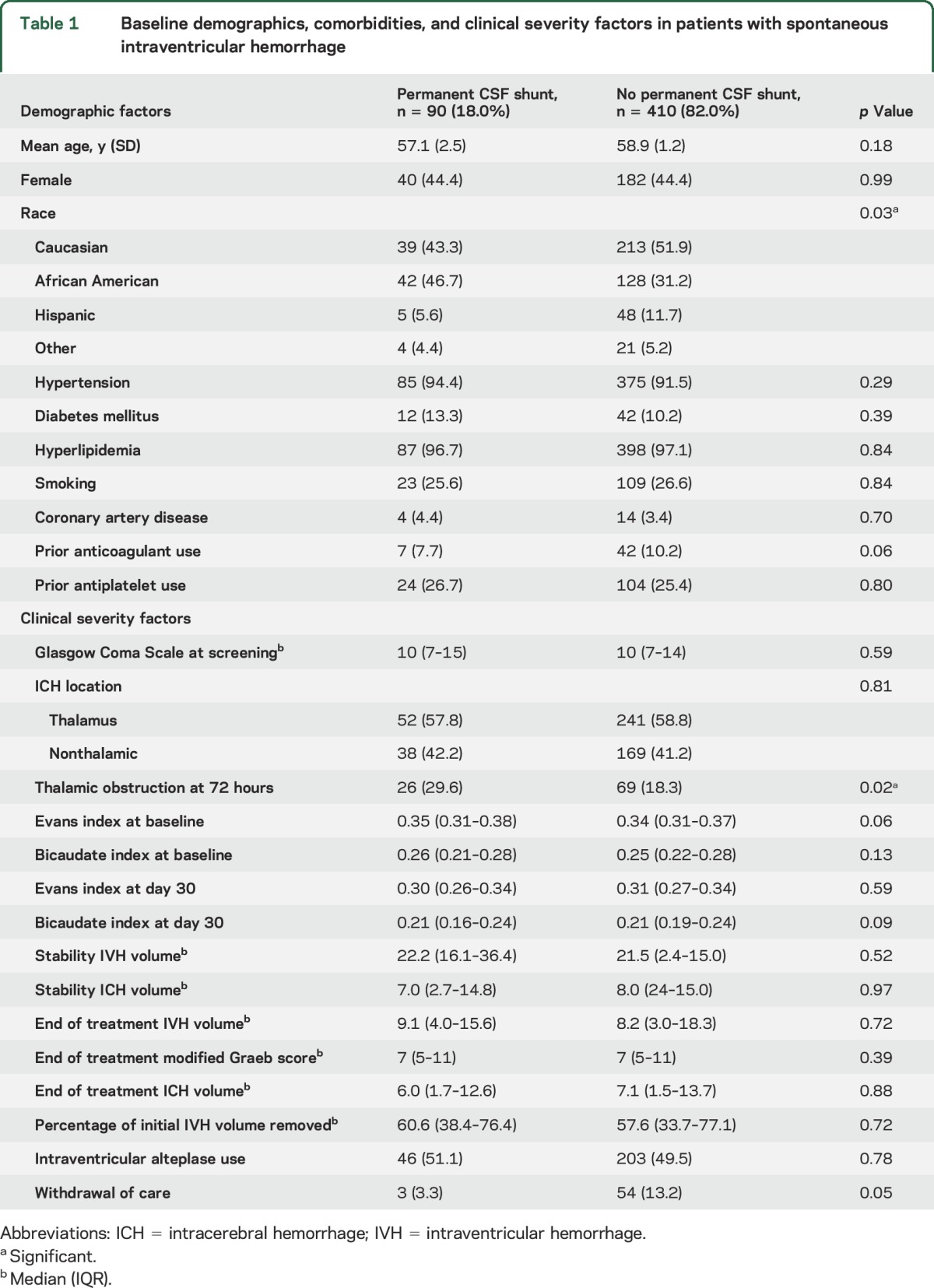
Figure. Rates of permanent CSF shunting by geographic location of the enrollment site.
Table 2.
CSF drainage characteristics in patients with spontaneous intraventricular hemorrhage
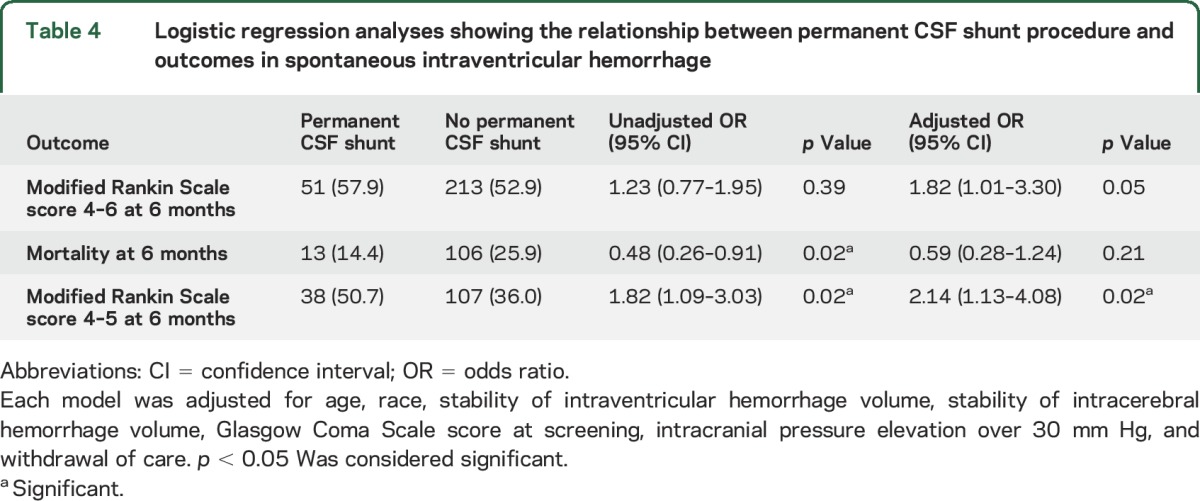
In logistic regression models adjusted for the aforementioned confounders (table 3), factors that were associated with a higher odds of shunting included black race (odds ratio [OR] 1.98; 95% CI 1.18–3.34), duration of EVD (OR 1.10; CI 1.05–1.15), placement of more than one EVD (OR 1.93; CI 1.13–3.31), daily drainage of CSF per 10 mL (OR 1.07; CI 1.04–1.10), and any ICP elevation >30 mm Hg (OR 1.70; CI 1.09–2.88). Further, patients with permanent shunts had similar odds of mortality and functional outcome at 6 months compared to patients without shunts after adjusting for known markers of mortality such as age, Glasgow Coma Scale, ICH and IVH volumes at screening, third ventricle obstruction at 72 hours, and ICP elevations over 30 mm Hg (table 4). However, after exclusion of IVH patients who died (mRS 6), patients with permanent CSF diversion had higher odds of disability (mRS 4–5) at 6 months (OR 2.14; 95% CI 1.13–4.08).
Table 3.
Factors associated with permanent CSF shunting in patients with spontaneous intraventricular hemorrhage (IVH)
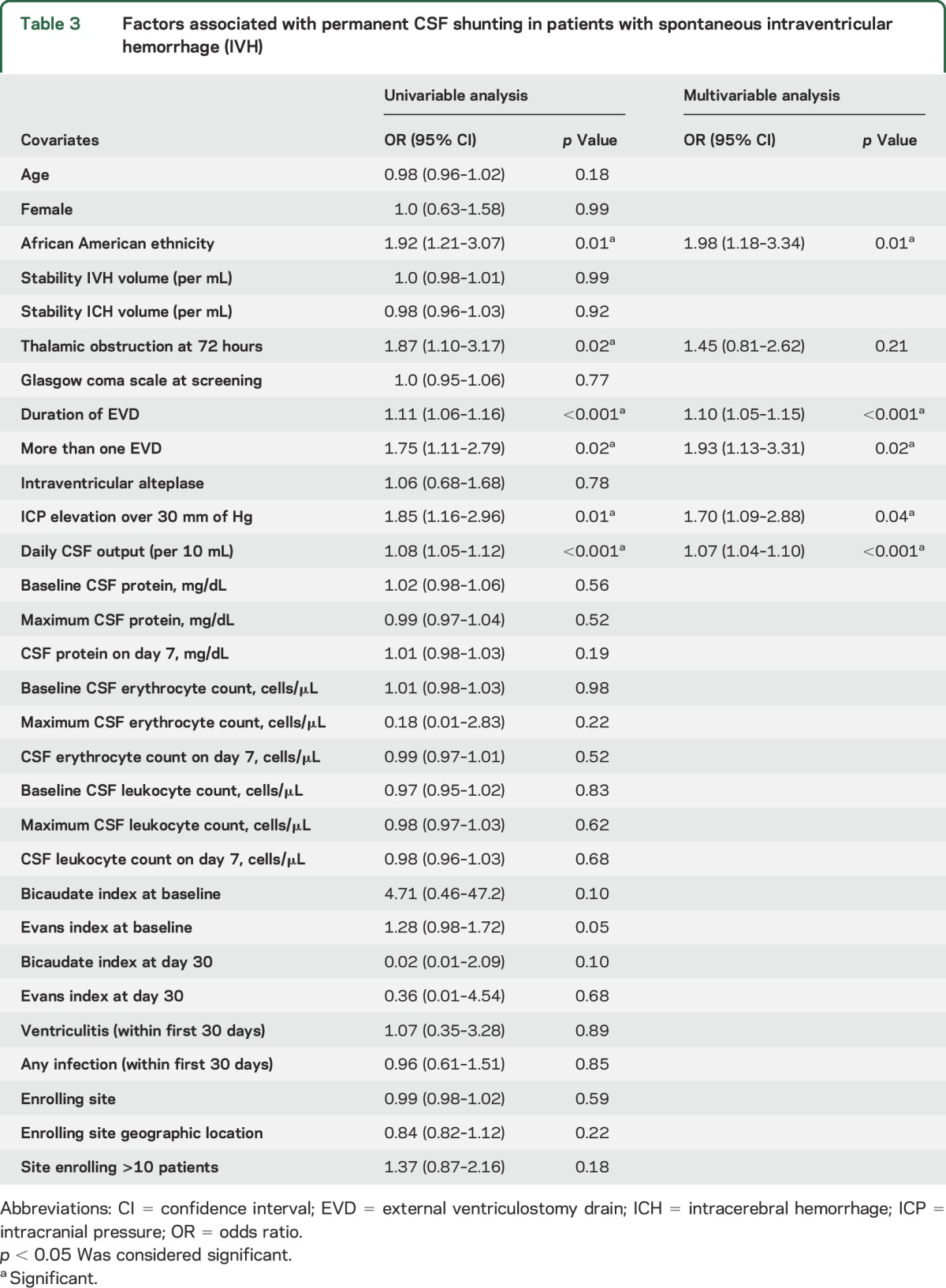
Table 4.
Logistic regression analyses showing the relationship between permanent CSF shunt procedure and outcomes in spontaneous intraventricular hemorrhage
DISCUSSION
In a large prospective observational cohort of primary IVH patients, we found that race, duration of EVD, requirement of more than one EVD, daily CSF drainage, and ICP elevation >30 mm Hg were independently associated with permanent CSF diversion. The incidence of shunt dependency was 18% and did not differ by treatment group (intraventricular alteplase or saline). Shunting was not associated with mortality, but was correlated with poor functional outcome (mRS 4 and 5) among survivors in this group of patients with severe IVH.
In our study, elevations in ICP were independently associated with shunt dependency, which corroborates results of 2 previous studies.7,8 In addition, we found higher daily CSF output through the EVD also correlated with permanent shunting in IVH patients, which agrees with studies in subarachnoid hemorrhage.19–21 CSF drainage volume may be a surrogate for persistent ventricular outflow obstruction or may reflect EVD management. We did not find any relationship between stability or end-of-treatment IVH volumes and shunting. Furthermore, use of intraventricular thrombolysis also did not influence shunting. This is in contrast to the findings of a small prospective study of 22 IVH patients where a combination of EVD, intraventricular thrombolysis, and lumbar spinal fluid drainage obviated the need for shunt surgery.22 Our study protocol, however, did not involve lumbar spinal fluid drainage. Moreover, the median percentage of IVH volume removed in the CLEAR III trial was about 60%, which meant the remaining IVH volume (40%) may have contributed to shunt dependency irrespective of alteplase use. These results suggest that a complex interplay of factors such as poor ventricular compliance, altered CSF flow dynamics, impaired CSF reabsorption, and hematoma-driven inflammation may influence shunt dependency in the posthemorrhagic hydrocephalus patient.23,24
Thalamic location of ICH is a known predictor of shunt dependency,7,8 although there was no such association in our study. A possible explanation for this finding is that prior studies had small sample sizes with fewer than 15 patients who had permanent shunts, which limited the number of covariates that could be controlled for in the regression models. This study benefited from a large number of patients with well-matched baseline hematoma and clinical characteristics, the majority of whom had thalamic ICH with complete obstruction of the distal ventricular system, allowing us to adequately control for potential confounders. An intriguing finding was the influence of black race on permanent CSF diversion. A recent population-based study also reported higher rates of IVH requiring EVD among black patients, although permanent shunting was not evaluated.25 In light of evidence suggesting that younger patients are more likely to have hydrocephalus,4 the authors attributed their findings to the younger age of black patients.25 Given the possibility of residual confounding by age, we did an additional age-adjusted regression analysis and found black race to be an independent predictor of shunting. Further study is needed to elucidate any underlying genetic polymorphisms or altered CSF flow dynamics in black patients compared to the general population that predisposes them to shunt dependency.
We also found that CSF protein, leukocyte, and erythrocyte concentrations at various time points (baseline, maximum, and day 7) had no bearing on shunting. This challenges the traditional approach of using CSF protein and erythrocytes to guide optimal timing for performing CSF diversion although our CSF analysis only included the first week postrandomization and not CSF analysis immediately prior to shunting. Mechanistically reduced flow due to high CSF viscosity, sticking of the valve components, peritoneal malabsorption, and protein deposition obstructing the shunt lumen have been proposed to incriminate high CSF protein and erythrocyte counts with shunting.26–29 However, emerging evidence has quelled these theories.27–29 Although CSF protein and erythrocyte counts did not influence shunting in our study, this may have contributed to the variation in the timing of CSF shunting (13–30 days). The wide range of shunt insertion times also suggests differences in EVD weaning protocols, which could not be monitored in the study. Furthermore, inclusion of patients with early (<2 weeks) and delayed shunting (>2 weeks) collectively in our analysis likely contributed to the wide IQR of 13–30 days. The standard practice in most centers in the United States, however, is to consider permanent CSF diversion within the first 2 weeks.7,8
Permanent CSF diversion was independently associated with morbidity but not mortality in our study. Our findings mirror those of Adams et al.,30 where shunt dependency correlated with higher morbidity in a population-based study of 1,533 patients with aneurysmal subarachnoid hemorrhage. Withdrawal of care was not significantly higher in patients who did not receive shunts in our study. This suggests that prior advanced directives, family/surrogate wishes, and hesitancy in initiation of comfort care measures may have influenced decisions on permanent CSF diversion. Implementation of aggressive measures in IVH patients with a high likelihood for poor outcomes may have accounted for our findings.
The strengths of our study include the largest cohort of IVH patients studied, well-defined inclusion criteria, preestablished time points for neuroimaging, multiple daily assessments of EVD parameters, and blinded assessment of outcome. Several limitations are important. First, inclusion of a selective cohort of IVH patients such as those with small hematoma volumes and complete obstruction of the third and fourth ventricles and rigid screening and monitoring performed in the setting of a trial limit the generalizability of the results. Second, all decisions regarding EVD management, challenge, and permanent CSF shunting were made at the discretion of the treating physicians at the different participating institutions. Hence, it is likely that some centers were more aggressive in placing shunts compared to others. Although we found no difference in permanent CSF diversion stratified by enrollment site and geographic location, residual confounding is still a possibility given that there were 73 different participating centers.
In a large, prospectively evaluated cohort of IVH patients, we found black race, duration of EVD, placement of more than one EVD, daily CSF drainage, and ICP elevation >30 mm Hg to be independently associated with permanent CSF diversion. We found no relationship with use of intraventricular thrombolysis, volume of IVH removed, or CSF parameters within the first week, suggesting that early clinical indicators of CSF flow obstruction (ICP and CSF drainage) are better predictors of future need for permanent shunting than radiographic and laboratory parameters in the spontaneous IVH population.
Supplementary Material
GLOSSARY
- CI
confidence interval
- CLEAR
Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage
- EVD
external ventricular drain
- ICH
intracerebral hemorrhage
- ICP
intracranial pressure
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- mRS
modified Rankin Scale
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. Murthy and Ziai had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Murthy, Hanley, and Ziai. Acquisition, analysis, or interpretation of data: Drs. Murthy, Eslami, and Ziai. Drafting of the manuscript: Drs. Murthy, Awad, Hanley, and Ziai. Critical revision of the manuscript for important intellectual content: Drs. Murthy, Awad, Harnof, Aldrich, Harrigan, Jallo, Caron, Huang, Camarata, and Rivera Lara, R. Dlugash, N. McBee, and Drs. Eslami, Hanley, and Ziai. Statistical analysis: Drs. Murthy and Ziai. Administrative, technical, or material support: Dr. Ziai. Study supervision: Dr. Ziai.
STUDY FUNDING
Dr. Murthy is supported by the American Academy of Neurology, American Brain Foundation, and the Leon Levy Neuroscience Foundation. Dr. Awad and Dr. Hanley were awarded substantial research support through grants 5U01NS062851 for Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage III and for Minimally Invasive Surgery Plus r-tPA for Intracerebral Hemorrhage Evacuation (MISTIE) III 1U01NS08082. Dr. Harnof, Dr. Aldrich, Dr. Caron, Dr. Huang, Dr. Camrata, Dr. Lara, R. Dlugash, and N. McBee report no disclosures. Dr. Ziai is supported by grants 5U01NS062851 and 1U01NS08082.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 2.Ziai WC, Nyquist PA, Hanley DF. Ventriculostomy and lytic therapy for intracerebral hemorrhage. Front Neurol Neurosci 2015;37:130–147. [DOI] [PubMed] [Google Scholar]
- 3.Phan TG, Koh M, Vierkant RA, Wijdicks EF. Hydrocephalus is a determinant of early mortality in putaminal hemorrhage. Stroke 2000;31:2157–2162. [DOI] [PubMed] [Google Scholar]
- 4.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke 1998;29:1352–1357. [DOI] [PubMed] [Google Scholar]
- 5.Engelhard HH, Andrews CO, Slavin KV, Charbel FT. Current management of intraventricular hemorrhage. Surg Neurol 2003;60:15–21; discussion 21–12. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol 2000;247:117–121. [DOI] [PubMed] [Google Scholar]
- 7.Miller C, Tsivgoulis G, Nakaji P. Predictors of ventriculoperitoneal shunting after spontaneous intraparenchymal hemorrhage. Neurocrit Care 2008;8:235–240. [DOI] [PubMed] [Google Scholar]
- 8.Zacharia BE, Vaughan KA, Hickman ZL, et al. Predictors of long-term shunt-dependent hydrocephalus in patients with intracerebral hemorrhage requiring emergency cerebrospinal fluid diversion. Neurosurg Focus 2012;32:E5. [DOI] [PubMed] [Google Scholar]
- 9.Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 2003;52:763–769; discussion 769–771. [DOI] [PubMed] [Google Scholar]
- 10.Little AS, Zabramski JM, Peterson M, et al. Ventriculoperitoneal shunting after aneurysmal subarachnoid hemorrhage: analysis of the indications, complications, and outcome with a focus on patients with borderline ventriculomegaly. Neurosurgery 2008;62:618–627; discussion 618–627. [DOI] [PubMed] [Google Scholar]
- 11.O'Kelly CJ, Kulkarni AV, Austin PC, Urbach D, Wallace MC. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: incidence, predictors, and revision rates: clinical article. J Neurosurg 2009;111:1029–1035. [DOI] [PubMed] [Google Scholar]
- 12.Chan M, Alaraj A, Calderon M, et al. Prediction of ventriculoperitoneal shunt dependency in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 2009;110:44–49. [DOI] [PubMed] [Google Scholar]
- 13.Esposito DP, Goldenberg FD, Frank JI, Ardelt AA, Roitberg BZ. Permanent cerebrospinal fluid diversion in subarachnoid hemorrhage: influence of physician practice style. Surg Neurol Int 2011;2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rincon F, Gordon E, Starke RM, et al. Predictors of long-term shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: clinical article. J Neurosurg 2010;113:774–780. [DOI] [PubMed] [Google Scholar]
- 15.Ziai WC, Tuhrim S, Lane K, et al. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Int J Stroke 2014;9:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng SE, Low AM, Tang KK, Chan YH, Kwok RK. Value of quantitative MRI biomarkers (Evans' index, aqueductal flow rate, and apparent diffusion coefficient) in idiopathic normal pressure hydrocephalus. Journal of magnetic resonance imaging. JMRI 2009;30:708–715. [DOI] [PubMed] [Google Scholar]
- 17.Barr AN, Heinze WJ, Dobben GD, Valvassori GE, Sugar O. Bicaudate index in computerized tomography of Huntington disease and cerebral atrophy. Neurology 1978;28:1196–1200. [DOI] [PubMed] [Google Scholar]
- 18.Hanley DF, Lane K, McBee N, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet 2017;389:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zolal A, Juratli T, Dengl M, Ficici KH, Schackert G, Sobottka SB. Daily drained CSF volume is a predictor for shunt dependence: a retrospective study. Clin Neurol Neurosurg 2015;138:147–150. [DOI] [PubMed] [Google Scholar]
- 20.Lewis A, Kimberly TW. A retrospective analysis of cerebrospinal fluid drainage volume in subarachnoid hemorrhage and the need for early or late ventriculoperitoneal shunt placement. J Neurosurg Sci 2016;60:289–295. [PubMed] [Google Scholar]
- 21.Hayek MA, Roth C, Kaestner S, Deinsberger W. Impact of external ventricular drainage volumes on shunt dependency after subarachnoid hemorrhage. J Neurol Surg A Cent Eur Neurosurg 2017;78:227–230. [DOI] [PubMed] [Google Scholar]
- 22.Staykov D, Huttner HB, Struffert T, et al. Intraventricular fibrinolysis and lumbar drainage for ventricular hemorrhage. Stroke 2009;40:3275–3280. [DOI] [PubMed] [Google Scholar]
- 23.Simard PF, Tosun C, Melnichenko L, Ivanova S, Gerzanich V, Simard JM. Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Transl Stroke Res 2011;2:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czosnyka M, Czosnyka ZH, Whitfield PC, Donovan T, Pickard JD. Age dependence of cerebrospinal pressure-volume compensation in patients with hydrocephalus. J Neurosurg 2001;94:482–486. [DOI] [PubMed] [Google Scholar]
- 25.Faigle R, Marsh EB, Llinas RH, Urrutia VC, Gottesman RF. Race-specific predictors of mortality in intracerebral hemorrhage: differential impacts of intraventricular hemorrhage and age among blacks and whites. J Am Heart Assoc Epub 2016 Aug 16. [DOI] [PMC free article] [PubMed]
- 26.Brydon HL, Bayston R, Hayward R, Harkness W. The effect of protein and blood cells on the flow-pressure characteristics of shunts. Neurosurgery 1996;38:498–504; discussion 505. [DOI] [PubMed] [Google Scholar]
- 27.Brydon HL, Hayward R, Harkness W, Bayston R. Physical properties of cerebrospinal fluid of relevance to shunt function: 1: the effect of protein upon CSF viscosity. Br J Neurosurg 1995;9:639–644. [DOI] [PubMed] [Google Scholar]
- 28.Brydon HL, Hayward R, Harkness W, Bayston R. Physical properties of cerebrospinal fluid of relevance to shunt function: 2: the effect of protein upon CSF surface tension and contact angle. Br J Neurosurg 1995;9:645–651. [DOI] [PubMed] [Google Scholar]
- 29.Brydon HL, Keir G, Thompson EJ, Bayston R, Hayward R, Harkness W. Protein adsorption to hydrocephalus shunt catheters: CSF protein adsorption. J Neurol Neurosurg Psychiatry 1998;64:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams H, Ban VS, Leinonen V, et al. Risk of shunting after aneurysmal subarachnoid hemorrhage: a collaborative study and initiation of a consortium. Stroke 2016;47:2488–2496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.