Abstract
Objective:
To assess odor identification (OI) as an indicator of presymptomatic Alzheimer disease (AD) pathogenesis in cognitively normal aging individuals at increased risk of AD dementia.
Methods:
In 274 members of the PREVENT-AD cohort of healthy aging persons with a parental or multiple-sibling history of AD dementia, we assessed the cross-sectional association of OI with potential indicators of presymptomatic AD. Some 101 participants donated CSF, thus enabling assessment of AD pathology with the biomarkers total tau (t-tau), phospho-tau (P181-tau), and their ratios with β-amyloid (Aβ1-42). Adjusted analyses considered age, cognition, APOE ε4 status, education, and sex as covariates. We measured OI using the University of Pennsylvania Smell Identification Test and cognitive performance using the Repeatable Battery for Assessment of Neuropsychological Status. Standard kits provided assays of the AD biomarkers. Analyses used robust-fit linear regression models.
Results:
Reduced OI was associated with lower cognitive score and older age, as well as increased ratios of CSF t-tau and P181-tau to Aβ1-42 (all p < 0.02). However, the observed associations of OI with age and cognition were unapparent in adjusted models that restricted observations to CSF donors and included AD biomarkers. OI showed little association with CSF Aβ1-42 alone except in APOE ε4 carriers having lowest-quartile Aβ1-42 levels.
Conclusions:
These findings from healthy high-risk older individuals suggest that OI reflects degree of preclinical AD pathology, while its relationships with age and cognition result from the association of these latter variables with such pathology. Diminished OI may be a practical and affordable biomarker of AD pathology.
Prevention of Alzheimer disease (AD) dementia can be accomplished by retarding the progression of the disease in its presymptomatic stages, thus postponing the onset of clinical symptoms. Hence, research on the identification and development of AD preventives can be aided by quantitative measures of disease progression before symptom onset.1 Such presymptomatic disease progress may be revealed by subtle cognitive changes, various MRI or PET imaging techniques, or AD biomarkers in the CSF. These measures are generally inconvenient, and more accessible markers of preclinical AD pathology are needed.
Because rhinencephalic brain regions are especially vulnerable to AD pathology,2 a candidate marker for this purpose may be odor identification (OI), i.e., the ability to identify and name specific odorants.3–10 Like cognition, OI is known to be impaired in both aging11,12 and dementia.7,13,14 In longitudinal studies, reduced OI performance predicts faster cognitive decline in elderly controls and persons with mild cognitive impairment or AD dementia.5,8–10,15–17 Finally, an important study of cognitively healthy persons showed that reduced OI ability predicted postmortem AD pathology.5
We therefore sought to evaluate OI as a measure of presymptomatic AD pathogenesis. In a study of aging asymptomatic individuals at risk of AD dementia, we investigated the association between OI and recognized in vivo AD biomarkers such as CSF total tau (t-tau) and phospho-tau (P181-tau) and their ratio with Alzheimer β-amyloid (Aβ1-42). We hypothesized that degree of impairment in OI would predict biomarker evidence of AD neuropathology.
METHODS
The PREVENT-AD cohort.
We investigated cross-sectional baseline measures from 274 participants in a cohort of cognitively unimpaired individuals assembled for Presymptomatic Evaluation of Experimental or Novel Treatments for Alzheimer's Disease (PREVENT-AD).1 PREVENT-AD enrollees had a parent or multiple siblings with a history of AD-like dementia. They were ≥60 years of age, except that individuals 55 to 59 years old were eligible if their current age was within 15 years of dementia onset in their youngest-affected relative. In general, persons with a first-degree family history have an elevated risk of AD.18 We first screened their cognitive state using the Montreal Cognitive Assessment19 and the Clinical Dementia Rating scale.20 Individuals with questionable cognitive difficulties underwent a formal neuropsychological assessment. We analyzed data collected between September 2011 and August 2015 and archived in PREVENT-AD data release 2.0.
Nested within PREVENT-AD was a randomized trial of Impact of Naproxen Treatment on Presymptomatic Alzheimer's Disease (INTREPAD), a placebo-controlled, biomarker-endpoint prevention trial of the nonsteroidal anti-inflammatory drug naproxen in 184 participants. We obtained CSF data from a subset of 101 volunteers from that trial.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained at each stage of the research from all participants and their collateral partners. Protocols and French and English consent forms were approved by the Institutional Review Board of the McGill University Faculty of Medicine. INTREPAD is registered with clinicatrials.gov as NCT02702817. The research was conducted in compliance with the ethics principles of the Declaration of Helsinki. For additional information, see www.prevent-alzheimer.ca.
Methods of assessment.
Review of health and neurocognitive status.
Participants were evaluated while accompanied by an informant. Assessments included a health history and review of systems, a standardized neurologic examination, and a cognitive examination with version A of the Repeatable Battery for Assessment of Neuropsychological Status (RBANS).21 The RBANS is available in both English and Canadian French. It measures 5 domains of cognitive performance. Participants also underwent phlebotomy for routine laboratory tests and banking of plasma and a multimodal MRI/fMRI scan session.
CSF collection.
INTREPAD volunteers' lumbar punctures were performed in the morning after an overnight fast. CSF was collected with the Sprotte 24-gauge atraumatic needle. The time of collection was recorded. All procedures followed recommendations of the BiomarkAPD project in the EU Joint Programme in Neurodegeneration.22 Briefly, CSF was centrifuged at 3,000 RPM (2,000g) at room temperature to precipitate cells and other insoluble material. Within 4 hours of collection, CSF samples were frozen and stored in 0.4-mL aliquots at −80°C in 500 μL polypropylene cryotubes. The samples went through only one freeze-thaw cycle. We assayed CSF t-tau, P181-tau, and Aβ1-42 levels using the Innotest/Fujirebio (previously Innogenetics, Ghent, Belgium) ELISA kit, again following Joint Programme in Neurodegeneration–specified procedures. This technology is based on specific fluorescent antibody labeling. We used the biomarker ratios CSF t-tau/Aβ1-42 and P181-tau/Aβ1-42 to indicate disease state.
APOE genotyping.
DNA was extracted automatically from buffy coat samples with the QiaSymphony DNA mini kit (Qiagen, Toronto, ON, Canada). APOE genotype was determined with the PyroMark Q96 pyrosequencer (Qiagen). The DNA was amplified using reverse transcriptase–PCR, forward primers 5′-ACGGCTGTCCAAGGAGCTG-3′ (rs429358) and 5′-CTCCGCGATGCCGATGAC-3′ (rs7412), and reverse biotinylated primers 5′-CACCTCGCCGCGGTACTG-3′ (rs429358) and 5′-CCCCGGCCTGGTACACTG-3′ (rs7412). The DNA was sequenced with these primers: 5′-CGGACATGGAGGACG-3′ (rs429358) and 5′-CGATGACCTGCAGAAG-3′ (rs7412).
Odor identification.
We assessed OI abilities using the 40-item University of Pennsylvania Smell Identification Test (UPSIT).23 This test includes a simple scratch-and-sniff booklet along with multiple-choice response forms for OI. The UPSIT has been validated in people 5 to 85 years of age and shows a test-retest reliability of r = 0.92 to 0.95.24,25 Its score is computed as the sum of the correct responses of a maximum possible 40. Both francophone and anglophone participants were presented with odors from the US version of the UPSIT. The francophone test used an in-house French translation. In a leave-one-out analysis, we assessed the reliability of the UPSIT in the PREVENT-AD cohort among an initial sample of 159 participants, obtaining a Cronbach α of 0.821, which suggests high internal consistency.
Data analyses.
To avoid left skewness and a leptokurtic distribution, we transformed the raw OI scores to an UPSIT error score of log10 (41 − raw UPSIT score), as described by Moberg et al.26 Higher transformed scores thus represented greater deficit in OI. We used a Kruskal-Wallis test to compare scores in APOE ε4 carriers and noncarriers. To assess the main effects on OI of various indicators of interest, we first examined bivariate relationships using simple linear regression. To examine the effect of each predictor variable in full perspective, we then constructed multivariable models, iteratively adding measures individually or in combination. Both the bivariate and multivariable analyses used robust-fit linear regression with a tuning constant of 1.205 to down-weight outliers. The latter general linear model analyses considered, in various combinations, age, sex, years of education, APOE ε4 carrier status, RBANS total score (global cognition), and the CSF t-tau/Aβ1-42 ratio (sometimes substituted as specified below by other CSF indicators of AD pathogenesis). Adjusting for age, sex, years of education, and APOE ε4 carrier status enabled us to compare our work to other highly cited findings.5,6 We verified the absence of collinearity in the multivariable models by investigating the variance inflation factor and tolerance. To test our primary hypothesis, we explored the relationships between OI and CSF AD biomarkers, seeking identifiable subgroups and examining interaction terms of interest.
Two sensitivity analyses assessed the effects of potential confounders on olfactory function. For both analyses, we grouped available data on brain injury, TIA, and stroke into a binary categorical variable called brain health. A second binary variable grouped nasal polyps, nasal surgeries, deviated septum, and history of a broken nose. A third such variable identified participants with a history of asthma, and a fourth identified current smokers of any substance. A final potential confounder characterized participants with any of the foregoing conditions (past or present) mentioned only at the time of olfactory testing. The first sensitivity analysis added all 5 of these potential confounders as covariates in the analytic framework of model 7. The other was a version of model 7 that excluded data from all participants with a positive rating on any of the 5 potential confounding variables.
RESULTS
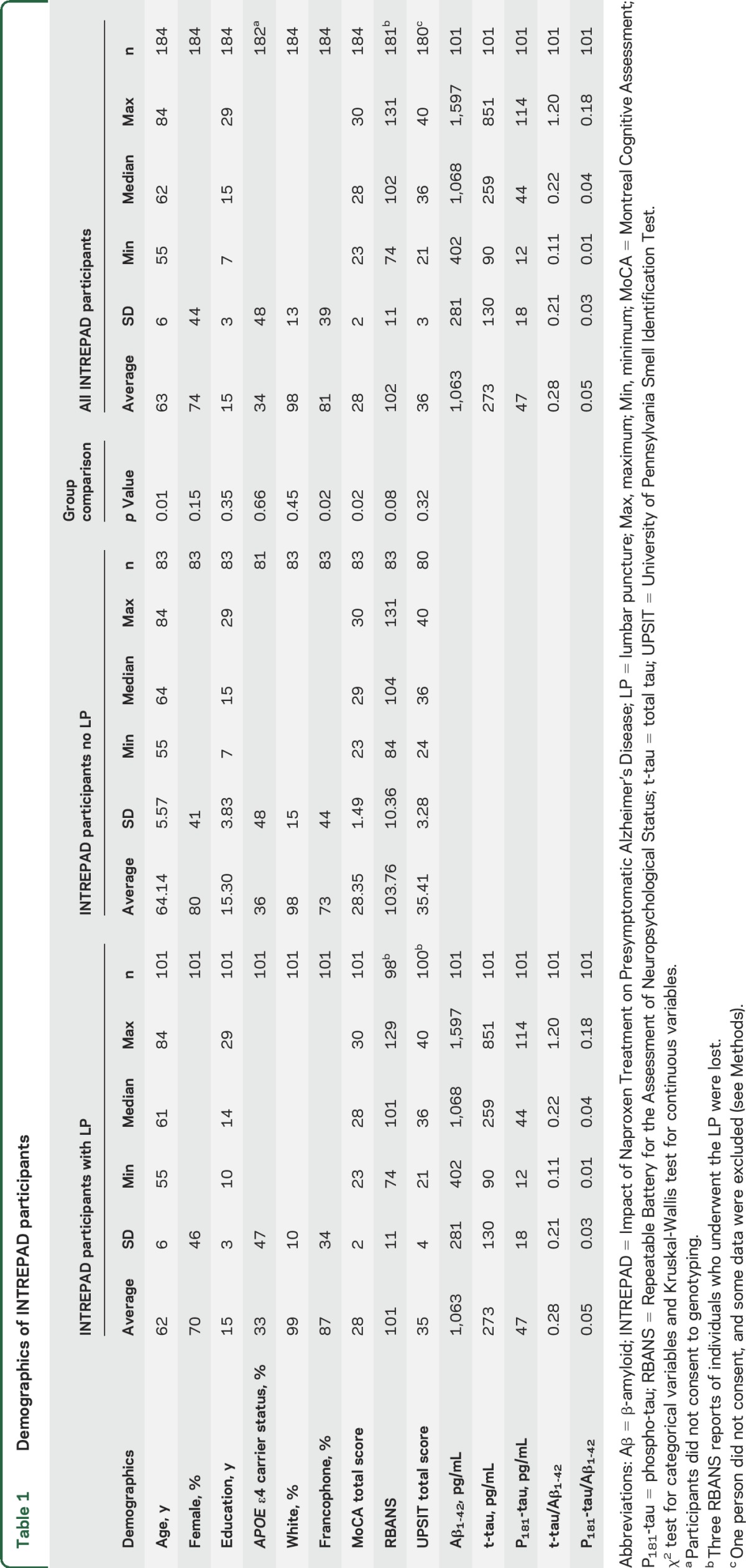
Of the 274 PREVENT-AD participants who met inclusion and exclusion criteria, 1 individual who consented for a lumbar puncture did not consent to the OI test. RBANS test results were lost for 4 participants. We excluded data from 8 participants who had incomplete test scores or nasal congestion on the day of testing. The analytic sample then comprised 265 PREVENT-AD participants, including 100 INTREPAD lumbar puncture volunteers who had complete CSF biomarker and OI data. The participant pool included predominantly francophone and female individuals who were well educated. As expected, their proportion of APOE ε4 carriers was higher than population norms (table 1). The INTREPAD participants from whom we collected CSF were slightly younger than other INTREPAD participants and had a higher proportion of francophone individuals and slightly lower Montreal Cognitive Assessment scores. The CSF donors appeared demographically similar to the entire PREVENT-AD group of 274 PREVENT-AD enrollees (table 1 and table e-1 at Neurology.org).
Table 1.
Demographics of INTREPAD participants
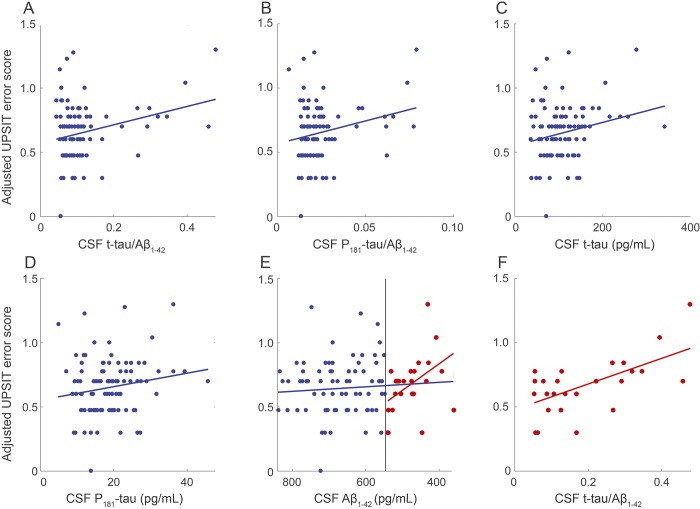
In bivariate modeling, the UPSIT error score was higher in older participants (n = 265, β = 0.0134, p = 2.24 ×10−6; figure e-1) and in participants with lower RBANS total score (n = 261, β = −0.00666, p = 1.28 ×10−6; figure e-1). However, because we were interested especially in the CSF AD biomarker data, we also evaluated models restricted to the 100 participants who had both OI and CSF data. In the reduced sample, the statistical association with older age was seen only at a trend level (β = 6.79 ×10−3, p = 0.095; figure e-2), but the association of reduced OI with decreased cognition remained robust (β = −4.76 ×10−3, p = 0.011; figure e-2). Similar unadjusted analyses showed strong association between higher UPSIT error score and increased values of CSF t-tau/Aβ1-42 (β = 0.286, p = 4.94 ×10−3), P181-tau/Aβ1-42 (β = 1.77, p = 0.0165), and CSF t-tau levels (β = 3.61 ×10−4, p = 0.0257; figure 1). There was a weaker but still suggestive relationship between increased UPSIT error score and elevated CSF P181-tau (β = 2.10 ×10−3, p = 0.0724) but no relationship with Aβ1-42 alone (β = −7.01 × 10−5, p = 0.359).
Figure 1. Robust-fit linear regression models of UPSIT error score vs CSF biomarkers of AD.
(A) CSF t-tau/Aβ1-42 (β = 0.287, p = 0.00494, n = 100). (B) CSF P181-tau/Aβ1-42 (β = 1.77, p = 0.0165, n = 100). (C) CSF t-tau (β = 3.61 ×10−4, p = 0.0257, n = 100). (D) CSF P181-tau (β = 2.10 ×10−3, p = 0.0724, n = 100). (E) CSF Aβ1-42 (β = −7.01 ×10−5, p = 0.359, n = 100) and in individuals with CSF Aβ1-42 levels below the 25th percentile (β = −0.000827, p = 0.0135, n = 25). (F) CSF t-tau/Aβ1-42 in individuals with CSF Aβ1-42 levels below the 25th percentile (β = 0.399, p = 0.00260, n = 25). In panel (E), blue circles represent the top 3 quartiles for Aβ concentrations, while red circles in panels (E) and (F) are data from the lowest quartile of Aβ concentrations (suggesting that they have more advanced preclinical AD). Aβ = β-amyloid; AD = Alzheimer disease; P181-tau = phospho-tau; t-tau = total tau; UPSIT = University of Pennsylvania Smell Identification Test.
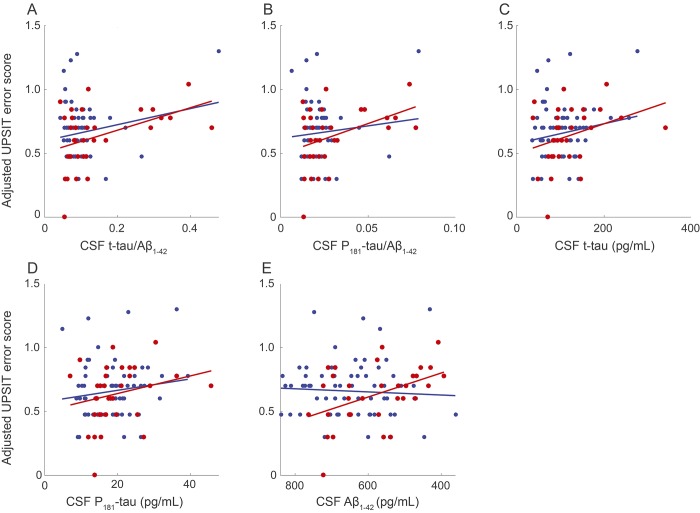
We observed no group difference in OI between APOE ε4 carriers and noncarriers (n = 262, p = 0.271). A similar result was observed in the reduced sample of 100 participants with CSF (p = 0.7129). However, previously observed correlations between UPSIT error score and several CSF markers of AD pathology were now apparent only in ε4 carriers. In contrast to unstratified samples, the carriers showed an association between higher UPSIT error scores and reduced levels of Aβ1-42 (n = 33, β = −3.76 ×10−4, p = 0.00841). In keeping with previously noted findings, UPSIT error score was associated in the ε4 carriers with higher t-tau/Aβ1-42 (β = 0.352, p = 0.0158) and P181-tau/Aβ1-42 (β = 2.416, p = 0.0270), but a comparable association appeared only at a trend level for t-tau (β = 4.68 ×10−4, p = 0.0914) and not at all for P181-tau (β = 2.76 ×10−3, p = 0.204; figure 2). In addition, we saw that individuals with lowest CSF Aβ1-42 levels appeared to have a higher proportion of APOE ε4 carriers (supplemental materials).
Figure 2. Robust-fit linear regression models of UPSIT error score vs CSF biomarkers, stratified by APOE ε4 status.
Red circles represent APOE ε4 carriers. Blue circles represent APOE ε4 noncarriers. (A) CSF t-tau/Aβ1-42 in carriers (β = 0.352, p = 0.0158, n = 33) and in noncarriers (β = 0.252, p = 0.131, n = 67). (B) CSF P181-tau/Aβ1-42 in carriers (β = 2.416, p = 0.0270, n = 33) and in noncarriers (β = 0.980, p = 0.408, n = 67). (C) CSF t-tau in carriers (β = 4.68 ×10−4, p = 0.0914, n = 33) and in noncarriers (β = 0.000312, p = 0.144, n = 67). (D) CSF P181-tau in carriers (β = 2.76 ×10−3, p = 0.204, n = 33) and in noncarriers (β = 0.00178, p = 0.218, n = 67). (E) CSF Aβ1-42 in carriers (β = −3.76 ×10−4, p = 0.00841, n = 33) and in noncarriers (β = 4.890e−05, p = 0.598, n = 67). Aβ = β-amyloid; AD = Alzheimer disease; P181-tau = phospho-tau; t-tau = total tau; UPSIT = University of Pennsylvania Smell Identification Test.
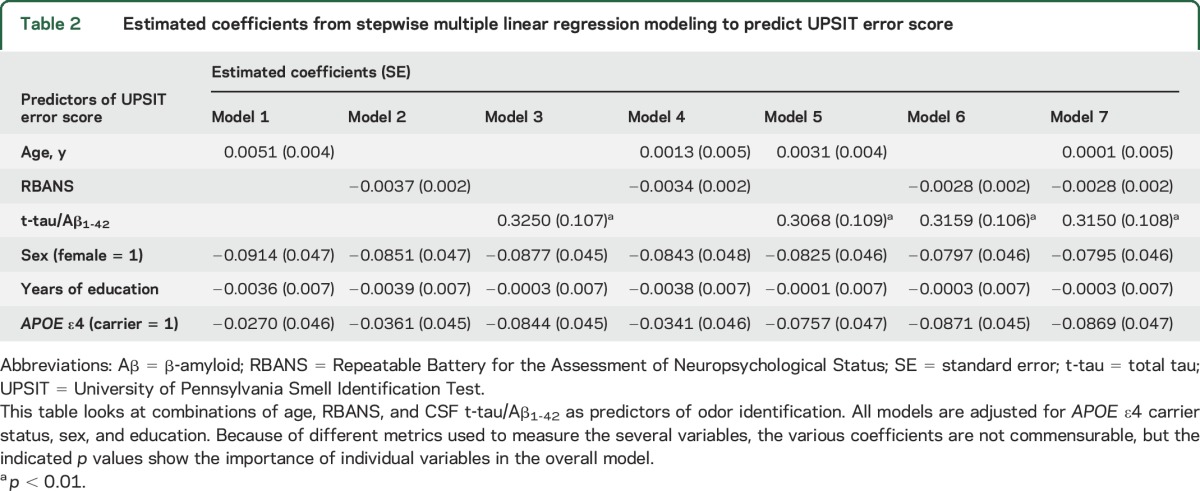
The multiple linear regression models assessed the relationships between OI and age, cognition, and CSF biomarkers added sequentially in models adjusted for sex, education, and APOE ε4 carrier status. Multivariable models from the full PREVENT-AD dataset (without CSF variables) showed strong associations of UPSIT error score with age and diminished cognition, either alone or in combination (table e-2). Table 2 shows comparable analyses in the reduced sample, now including CSF data. Models 1, 2, and 3 in table 2 (supplemental materials) indicate no association of UPSIT error score with age, a trend with cognitive score (p = 0.06), but a strong association with CSF t-tau/Aβ1-42 (p = 0.003). Model 4 suggests that the trend association with cognition was unapparent after adjustment for age (p = 0.145). Model 5 confirms the absence (in adjusted models) of any association between OI and age, and it shows the absence of a material effect of age adjustment on the association of OI with CSF t-tau/Aβ1-42. Model 6 indicates that any association of OI with cognition became unapparent after the inclusion of CSF t-tau/Aβ1-42 (RBANS, p = 0.151; CSF t-tau/Aβ1-42, p = 0.004). Model 7, which includes all the described variables, made these findings clearer by suggesting that OI was predicted by its relationship with CSF t-tau/Aβ1-42 (p = 0.005) regardless of age, cognition, APOE ε4 status, sex, or education (figure e-3). This last model explained 19.7% of the variance in OI score (F7,90 = 3.68, p < 0.00258). Both sensitivity analysis variants of model 7 produced nearly identical results (in the first analysis, F12,85 = 2.47, p < 0.00993; in the second, F7,83 = 2.59, p < 0.0236). The latter reduced model still explained 15.9% of the variance in OI score. Other similar multiple linear regression analyses (not shown) essentially reproduced the findings of model 7, substituting t-tau or P181-tau alone or the ratio of P181-tau/Aβ1-42 as independent CSF biomarker predictors.
Table 2.
Estimated coefficients from stepwise multiple linear regression modeling to predict UPSIT error score
DISCUSSION
We investigated relationships of performance in OI with global cognitive scores, established AD risk factors, and several CSF biomarkers known to predict subsequent dementia. Our main finding was that a decrease in OI was associated with increasing CSF biomarker evidence of AD pathology. This association survived adjustment not only for sex, educational attainment, APOE ε4 carrier status, and potential brain and olfactory health history confounders but also for age or cognitive score. The relationships of OI performance with age and cognitive ability recapitulate earlier findings.11,12 In the present sample, however, the relationship of OI with cognitive performance appears to be spurious because it represents the conjoint (confounded) relationship of both variables with the CSF biomarkers. We suggest that the relationship between OI and age (observed in the full dataset) may similarly represent a confounded association of these 2 measures with AD pathology (hence with related impairment in OI). To the best of our knowledge, there has been no previous direct demonstration that the association of OI with cognition is driven by a confounded relationship of both variables with AD pathology.
Overall, we observed no correlation of OI with CSF Aβ1-42 levels alone. Such a relationship was observed, however, among individuals whose CSF Aβ1-42 levels were in the lowest quartile. This subgroup had a greater proportion of APOE ε4 carriers (48%), which was close to the proportion typically seen in patients with AD. Thus, impairment in OI may in fact reflect cerebral accumulation of Aβ in these sicker participants, consistent with observations with amyloid PET.6 This notion was reinforced in our work by a statistically significant interaction between APOE ε4 carrier status and CSF Aβ1-42 levels as predictors of OI performance. The same was not so for global cognition, suggesting that OI may be an earlier indicator of accumulated pathology before symptom onset. Related recent work in transgenic mice expressing human APOE ε4 vs ε3 demonstrated genotype-specific structural differences in midlife. These modifications appear to precede later functional differences and increasing structural abnormality in brain regions related to olfaction.27
More generally, OI deficit is associated with presymptomatic AD pathology.5 Reduced structural integrity of brain regions that subserve olfaction appears especially vulnerable to such pathology. These changes include reduced entorhinal cortical thickness and hippocampus and amygdala volumes,6,16,28 as well as fibrillar amyloid accumulation in the posterior cingulate, temporoparietal, and lingual cortical regions.6,29 Data from 15O-H2O-PET experiments on olfactory-evoked regional cerebral blood flow also demonstrate that patients with AD have a pattern of functional activation different from healthy controls. Specifically, regional cerebral blood flow in the right frontotemporal area shows a correlation with OI.30 CSF clearance is reduced in AD, relating to increased amyloid accumulation. Finally, dynamic monitoring of CSF has confirmed that the fluid reaches the nasal turbinates and clears through the cribriform plate.31
We note that our findings appear to contradict one recent report in which OI appeared not to be related to brain accumulation of amyloid as revealed by PET.32 As we did, the authors of that study attempted to control for known detrimental olfactory health issues. They chose to exclude participants with such issues. In addition, the earlier research was characterized by intervals ranging up to 5 years between tests of OI and PET scans, whereas our work consistently tested OI within 3 months of CSF collection. A recent study demonstrated that OI deficits are more readily detected in patients with acute brain trauma when olfactory testing is performed within days of injury.33 Recent PET studies of olfactory sensory neurons in lesioned, aging, or AD-like animal models further support this point.34
Limitations of this study include its high proportion of women and participants' level of educational attainment, attributes that are common in aging volunteer cohorts. Although we attempted to control for these factors in multivariable models, participants who volunteered for lumbar punctures may be an even more select population, an important concern because these 100 participants produced our most informative findings. Because our results come from cross-sectional observations only, it remains unknown whether altered biomarker levels represent a process of ongoing change and, if so, the rate at which such change is accumulating. Longitudinal studies for this purpose are now in progress, as are studies assessing the physical accumulation of amyloid and tau with specific PET tracers. Lastly, we acknowledge that our failure to observe a relationship between OI and age in the restricted sample may result from the limited sample size of the CSF donor participant pool. Generally, age is reported to be the strongest known predictor of impaired OI35,36 and the best known predictor of AD dementia. However, at least some of this age-related loss in olfactory function may relate to factors unrelated to AD pathogenesis (e.g., deterioration of nasal epithelium or calcification of the cribriform plate36).
While impaired OI may in fact help identify persons who could for various reasons eventually have cognitive impairment, we strongly urge that our present cross-sectional results not be regarded as rationale for clinical use of olfactory testing as an AD diagnostic. We suggest, however, that OI may serve for research purposes as a simple and inexpensive indicator of evolving AD pathology. Indeed, we are exploring its use as a biomarker in clinical trials among asymptomatic persons at risk of later dementia symptoms. In this and other ways, OI may add valuably to the measures available for AD prevention research.
Supplementary Material
ACKNOWLEDGMENT
The authors thank David Fontaine, PhD, who oversaw cognitive testing at enrollment, scored the RBANS cognitive evaluations, and administered additional cognitive batteries when indicated. Anne Labonté, BSc, conducted the CSF biomarker analyses. Cécile Madjar, MSc, directed data management, and Doris Dea and Louise Théroux did the genotyping. Cynthia Picard, PhD, helped write the descriptions of laboratory methods. Tanya Lee, Melissa Appleby, Laura Mahar, Galina Pogossova, Renuka Giles, and Karen Wan collected cognitive and olfaction data. All are with the Centre for Studies on Prevention of AD, Douglas Mental Health University Institute. Thomas Beaudry, BSc (McGill Centre for Studies in Aging, Douglas Mental Health University Institute; Perform Centre, Concordia University), helped edit the manuscript. Tharick Ali Pascoal, MD, Marina Tedeshi Dauar, MD, and Laksanun Cheewakriengkrai, MD (McGill Centre for Studies in Aging and Centre for Studies on Prevention of AD, Douglas Mental Health University Institute), collected the CSF. Katarina Dedovic, PhD (Canadian Institutes for Health Research, Ottawa), provided valuable advice, as did Miranda Tuwaig, MSc, Angela Tam, MSc, Christina Kazazian, Pierre-Francois Meyer, MSc, and Melissa Savard, MSc (all at the Centre for Studies on Prevention of AD, Douglas Mental Health University Institute). Special thanks go to Marianne Dufour, administrative assistant, and to Ginette Mayrand, Joanne Frenette, MSc, Isabelle Vallée, Rana El-Khoury, and Fabiola Ferdinand, all nurses who met with participants, as well as the entire PREVENT-AD Research Group (https://preventad.loris.ca/acknowledgements/acknowledgements.php?date=2017-1-26). The authors acknowledge the generosity and commitment of all research participant volunteers for this work.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- INTREPAD
Impact of Naproxen Treatment on Presymptomatic Alzheimer's Disease
- OI
odor identification
- P181-tau
phospho-tau
- PREVENT-AD
Presymptomatic Evaluation of Experimental or Novel Treatments for Alzheimer's Disease
- RBANS
Repeatable Battery for Assessment of Neuropsychological Status
- t-tau
total tau
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Marie-Elyse Lafaille-Magnan: study concept and design, data collection (RBANS and UPSIT), statistical analysis, data interpretation, drafting/revising the manuscript, accepts responsibility for conduct of research. John Breitner: study concept and design, data interpretation, drafting/revising the manuscript. Judes Poirier: study concept and design, data interpretation, revising the manuscript. Pierre Etienne and Pedro Rosa-Neto: study concept and design, revising the manuscript. Jennifer Tremblay-Mercier and Joanne Frenette: study concept and design.
STUDY FUNDING
This study was funded by an unrestricted gift from Pfizer Canada, by generous support from McGill University, and by infrastructure support from the Canada Fund for Innovation. Support for genetic and laboratory work was provided by the Fonds de Récherche du Québec–Santé and by the Levesque Foundation. The Douglas Mental Health University Institute Foundation provided additional support.
DISCLOSURE
M.-E. Lafaille-Magnan has received research support from the Lazlo & Etelka Kollar fellowship fund. M.-E. Lafaille-Magnan is the Brain Reach High School program coordinator, for which she has received an award from the McGill Integrated Program in Neuroscience. M.-E. Lafaille-Magnan receives honoraria from serving as a board member of the clinical excellence committee on social services with a focus on youth, mental health, and dependence for the Institut National d'excellence en santé et en services sociaux. J. Poirier receives royalties from a publication he has cowritten titled Alzheimer's Disease: A Guide, published in 2011 in French, English, German, Portuguese, and Chinese. P. Etienne, J. Tremblay-Mercier, and J. Frenette report no disclosures relevant to the manuscript. P. Rosa-Neto receives a salary award from le Fonds de recherche du Québec–Santé (Chercheurs-boursiers–Junior 2). He receives research support from the Canadian Institutes of Health Research, Canadian Consortium on Neurodegeneration in Aging, Weston Brain Institute, and Enigma radiopharmaceuticals. Dr. Rosa-Neto is a principal investigator in industry-sponsored clinical trials for TauRx, Jensen, and Eli Lilly. He received honoraria from serving on Eli Lilly's scientific advisory board. He serves as a consultant for Kalgene and Enigma radiopharmaceuticals. He does not hold stock in or receive royalties from any pharma or biotech company. J. Breitner has received research support from Pfizer Canada. He also received research support from McGill University, the Douglas Hospital Foundation, le Fonds de recherche du Québec–Santé, Canadian Consortium on Neurodegeneration in Aging, Weston Brain Institute, and the government of Canada (Canadian Institutes for Health Research, Tier 1 Canada Research Chair, and the Canadian Fund for Innovation). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Breitner JCS, Poirier J, Etienne PE, Leoutsakos JM; PREVENT-AD Research Group. Rationale and structure for a new center for Studies on Prevention of Alzheimer's Disease (StoP-AD). J Prev Alzheimers Dis 2016;3:236–242. [DOI] [PubMed] [Google Scholar]
- 2.Daulatzai MA. Olfactory dysfunction: its early temporal relationship and neural correlates in the pathogenesis of Alzheimer's disease. J Neural Transm (Vienna) 2015;122:1475–1497. [DOI] [PubMed] [Google Scholar]
- 3.Tabert MH, Liu X, Doty RL, et al. A 10-item smell identification scale related to risk for Alzheimer's disease. Ann Neurol 2005;58:155–160. [DOI] [PubMed] [Google Scholar]
- 4.Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 2008;29:693–706. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann NY Acad Sci 2009;1170:730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Growdon ME, Schultz AP, Dagley AS, et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 2015;84:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velayudhan L, Pritchard M, Powell JF, Proitsi P, Lovestone S. Smell identification function as a severity and progression marker in Alzheimer's disease. Int Psychogeriatr 2013;25:1157–1166. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology 2017;88:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts RO, Christianson TJH, Kremers WK, et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 2016;73:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 2015;84:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ship J, Pearson J, Cruise L, Brant L, Metter EJ. Longitudinal changes in smell identification. J Gerontol A Biol Med Sci 1996;51:86–91. [DOI] [PubMed] [Google Scholar]
- 12.Wehling EI, Lundervold AJ, Nordin S, Wollschlaeger D. Longitudinal changes in familiarity, free and cued odor identification, and edibility judgments for odors in aging individuals. Chem Senses 2016;41:155–161. [DOI] [PubMed] [Google Scholar]
- 13.Sun GH, Raji CA, MacEachern MP, Burke JF. Olfactory identification testing as a predictor of the development of Alzheimer's dementia: a systematic review. Laryngoscope 2012;122:1455–1462. [DOI] [PubMed] [Google Scholar]
- 14.Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer's disease and Parkinson's disease on olfaction: a meta-analysis. Behav Brain Res 2012;231:60–74. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 2007;78:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devanand DP, Liu X, Tabert MH, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer's disease. Biol Psychiatry 2008;64:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc 2014;20:209–217. [DOI] [PubMed] [Google Scholar]
- 18.Lampert EJ, Roy Choudhury K, Hostage CA, Petrella JR, Doraiswamy PM. Prevalence of Alzheimer's pathologic endophenotypes in asymptomatic and mildly impaired first-degree relatives. PLoS One 2013;8:e60747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasreddine Z, Phillips N, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 20.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical state for the staging of dementia. Br J Psychiatry 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 21.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 22.Lelental N, Brandner S, Kofanova O, et al. Comparison of different matrices as potential quality control samples for neurochemical dementia diagnostics. J Alzheimers Dis 2016;52:51–64. [DOI] [PubMed] [Google Scholar]
- 23.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502. [DOI] [PubMed] [Google Scholar]
- 24.Doty RL, Newhouse MG. Azzalina JD. Internal consistency and short-term test-retest reliability of the University of Pennsylvania Smell Identification Test. Chem Senses 1985;10:297–300. [Google Scholar]
- 25.Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys 1989;45:381–384. [DOI] [PubMed] [Google Scholar]
- 26.Moberg PJ, Doty RL, Turetsky BI, et al. Olfactory identification in elderly schizophrenia and Alzheimer's disease. Neurobiol Aging 1997;18:163–167. [DOI] [PubMed] [Google Scholar]
- 27.Peng KY, Mathews PM, Levy E, Wilson DA. Apolipoprotein E4 causes early olfactory network abnormalities and short-term olfactory memory impairments. Neuroscience 2017;343:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagemeier J, Woodward MR, Rafique UA, et al. Odor identification deficit in mild cognitive impairment and Alzheimer's disease is associated with hippocampal and deep gray matter atrophy. Psychiatry Res 2016;255:87–93. [DOI] [PubMed] [Google Scholar]
- 29.Bahar-Fuchs A, Chételat G, Villemagne VL, et al. Olfactory deficits and amyloid-β burden in Alzheimer's disease, mild cognitive impairment, and healthy aging: a PiB PET Study. J Alzheimers Dis 2010;22:1081–1087. [DOI] [PubMed] [Google Scholar]
- 30.Kareken DA, Mosnik D, Doty RL, et al. Olfactory-evoked regional cerebral blood flow in Alzheimer's disease. Neuropsychology 2001;15:18–29. [DOI] [PubMed] [Google Scholar]
- 31.Leon De MJ, Li Y, Okamura N, et al. CSF clearance in Alzheimer disease measured with dynamic PET. J Nucl Med Epub 2017 Mar 16. [DOI] [PMC free article] [PubMed]
- 32.Albers AD, Asafu-Adjei J, Delaney MK, et al. Episodic memory of odors stratifies Alzheimer biomarkers in normal elderly. Ann Neurol 2016;80:846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xydakis MS, Mulligan LP, Smith AB, Olsen CH, Lyon DM, Belluscio L. Olfactory impairment and traumatic brain injury in blast-injured combat troops: a cohort study. Neurology 2015;84:1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van de Bittner GC, Riley MM, Cao L, et al. Nasal neuron PET imaging quantifies neuron generation and degeneration. J Clin Invest 2017;127:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doty RL. The olfactory system and its disorders. Semin Neurol 2009;29:74–81. [DOI] [PubMed] [Google Scholar]
- 36.Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol 2014;5:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.