Abstract
The multigene family which codes for the mouse major urinary proteins (MUPs) consists of approximately 35 genes. Most of these are members of two different groups, Group 1 and Group 2, which can be distinguished by nucleic acid hybridisation. Here we describe the structure of a Group 1 gene and show that two size classes of MUP mRNA which are found in mouse liver result from different splicing events in the 3'-non-coding region and contain different polyadenylation sites. Short mRNA is approximately 750 nucleotides long, contains six exons, and is the main product of the Group 2 genes. Long mRNA is approximately 880 nucleotides long, contains seven exons and is the main product of the Group 1 genes. Five exons and part of the sixth are common to long and short mRNA and contain the coding region. This codes for an acidic protein of 180 amino acids containing an 18 residue signal peptide. A comparison of the mouse sequence with a homologous rat alpha 2u-globulin sequence shows that the rate of evolutionary divergence of the two proteins has been high. Silent sites have diverged four times more rapidly than replacement sites, showing that there has been selection against change in the protein sequence.
Full text
PDF


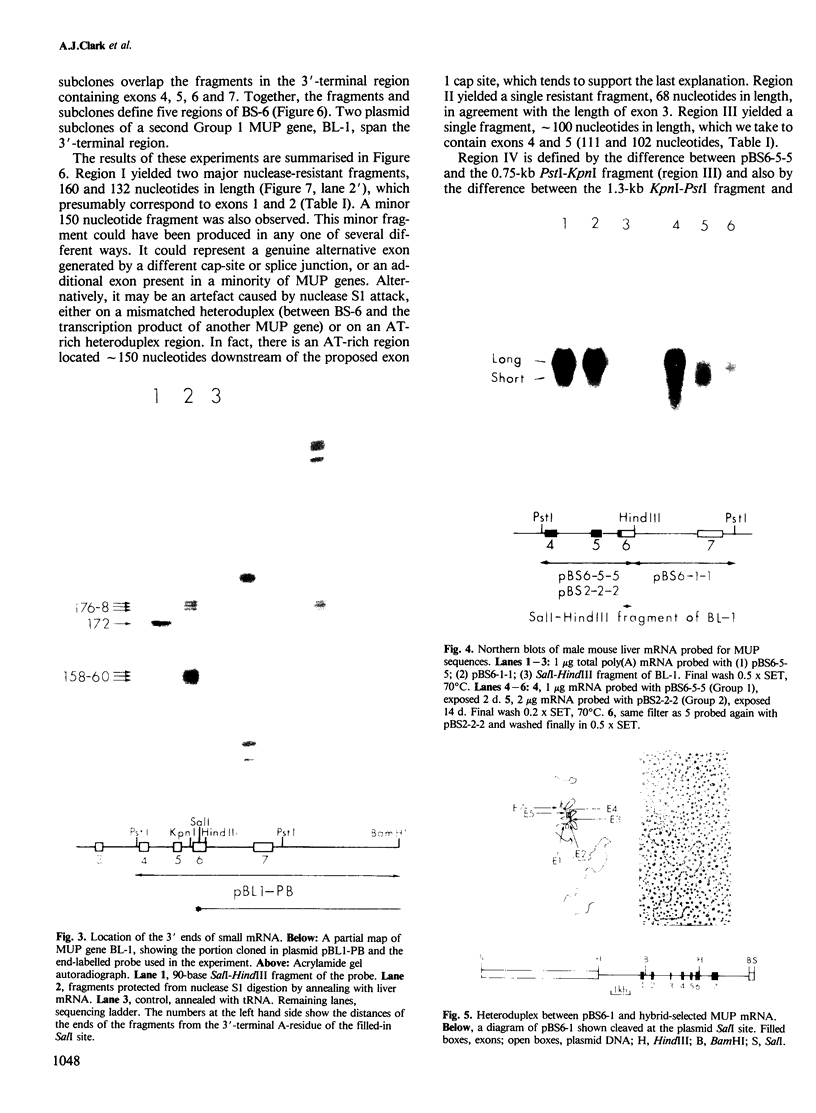
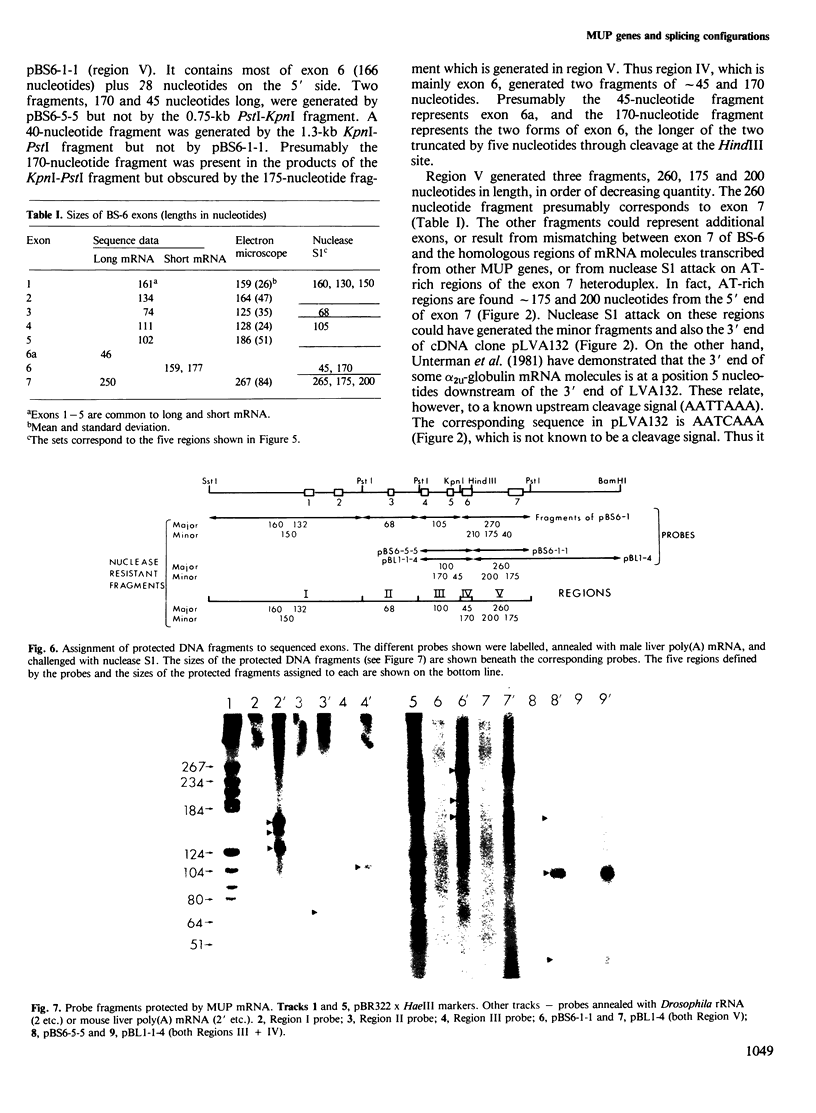



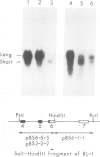
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Rosenberg N., Casanova R. J., Thomas E., Baltimore D. Immunoglobulin heavy-chain expression and class switching in a murine leukaemia cell line. Nature. 1982 Mar 25;296(5855):325–331. doi: 10.1038/296325a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., Gait M. J., Mayol L., Young I. G. A short primer for sequencing DNA cloned in the single-stranded phage vector M13mp2. Nucleic Acids Res. 1980 Apr 25;8(8):1731–1743. doi: 10.1093/nar/8.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K. L., Lalley P. A., Barth R. K., Hastie N. D. Mapping the structural genes coding for the major urinary proteins in the mouse: combined use of recombinant inbred strains and somatic cell hybrids. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1220–1224. doi: 10.1073/pnas.79.4.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Clark A. J., Clissold P. M., Hainey S., Francke U. Two main groups of mouse major urinary protein genes, both largely located on chromosome 4. EMBO J. 1982;1(5):615–620. doi: 10.1002/j.1460-2075.1982.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Davies J. A. Plasmid cloning vectors that can be nicked at a unique site. Mol Gen Genet. 1980;179(3):573–580. doi: 10.1007/BF00271747. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Clissold P. M., Bishop J. O. Variation between mouse major urinary protein genes isolated from a single inbred line. Gene. 1982 Jun;18(3):221–230. doi: 10.1016/0378-1119(82)90159-7. [DOI] [PubMed] [Google Scholar]
- Clissold P. M., Bishop J. O. Molecular cloning of cDNA sequences transcribed from mouse liver endoplasmic reticulum poly(A)mRNA. Gene. 1981 Nov;15(2-3):225–235. doi: 10.1016/0378-1119(81)90132-3. [DOI] [PubMed] [Google Scholar]
- Clissold P. M., Bishop J. O. Variation in mouse major urinary protein (MUP) genes and the MUP gene products within and between inbred lines. Gene. 1982 Jun;18(3):211–220. doi: 10.1016/0378-1119(82)90158-5. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Organization of the rat gamma-fibrinogen gene: alternative mRNA splice patterns produce the gamma A and gamma B (gamma ') chains of fibrinogen. Cell. 1982 Nov;31(1):159–166. doi: 10.1016/0092-8674(82)90415-9. [DOI] [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E. Isolation of a cDNA clone for mouse urinary proteins: age- and sex-related expression of mouse urinary protein genes is transcriptionally controlled. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5425–5429. doi: 10.1073/pnas.78.9.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K. P., Unterman R., McLaughlin M., Nakhasi H. L., Lynch K. R., Feigelson P. The structure and expression of very closely related members of the alpha 2u globulin gene family. J Biol Chem. 1982 Nov 25;257(22):13527–13534. [PubMed] [Google Scholar]
- Drickamer K., Kwoh T. J., Kurtz D. T. Amino acid sequence of the precursor of rat liver alpha 2 micro-globulin. J Biol Chem. 1981 Apr 25;256(8):3634–3636. [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Finlayson J. S., Potter M., Shinnick C. S., Smithies O. Components of the major urinary protein complex of inbred mice: determination of NH2-terminal sequences and comparison with homologous components from wild mice. Biochem Genet. 1974 Apr;11(4):325–335. doi: 10.1007/BF00486000. [DOI] [PubMed] [Google Scholar]
- Hainey S., Bishop J. O. Allelic variation at several different genetic loci determines the major urinary protein phenotype of inbred mouse strains. Genet Res. 1982 Feb;39(1):31–39. doi: 10.1017/s0016672300020723. [DOI] [PubMed] [Google Scholar]
- Hardison R. C., Butler E. T., 3rd, Lacy E., Maniatis T., Rosenthal N., Efstratiadis A. The structure and transcription of four linked rabbit beta-like globin genes. Cell. 1979 Dec;18(4):1285–1297. doi: 10.1016/0092-8674(79)90239-3. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A. Analysis of mRNA populations by cDNA.mRNA hybrid-mediated inhibition of cell-free protein synthesis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1217–1221. doi: 10.1073/pnas.75.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A., Toole J. J. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979 Jun;17(2):449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Heilig R., Perrin F., Gannon F., Mandel J. L., Chambon P. The ovalbumin gene family: structure of the X gene and evolution of duplicated split genes. Cell. 1980 Jul;20(3):625–637. doi: 10.1016/0092-8674(80)90309-8. [DOI] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Jung A., Sippel A. E., Grez M., Schütz G. Exons encode functional and structural units of chicken lysozyme. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5759–5763. doi: 10.1073/pnas.77.10.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Krauter K., Leinwand L., D'Eustachio P., Ruddle F., Darnell J. E., Jr Structural genes of the mouse major urinary protein are on chromosome 4. J Cell Biol. 1982 Aug;94(2):414–417. doi: 10.1083/jcb.94.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laperche Y., Lynch K. R., Dolan K. P., Feigelson P. Tissue-specific control of alpha 2u globulin gene expression: constitutive synthesis in the submaxillary gland. Cell. 1983 Feb;32(2):453–460. doi: 10.1016/0092-8674(83)90465-8. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Crerar M. M., Swain W. F., Pictet R. L., Thomas G., Rutter W. J. Structure of a family of rat amylase genes. Nature. 1980 Sep 11;287(5778):117–122. doi: 10.1038/287117a0. [DOI] [PubMed] [Google Scholar]
- Maki R., Roeder W., Traunecker A., Sidman C., Wabl M., Raschke W., Tonegawa S. The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes. Cell. 1981 May;24(2):353–365. doi: 10.1016/0092-8674(81)90325-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Inhibition of RNA cleavage but not polyadenylation by a point mutation in mRNA 3' consensus sequence AAUAAA. Nature. 1983 Oct 13;305(5935):600–605. doi: 10.1038/305600a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Lin C. R., Amara S. G., Stolarsky L., Roos B. A., Ong E. S., Evans R. M. Calcitonin mRNA polymorphism: peptide switching associated with alternative RNA splicing events. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1717–1721. doi: 10.1073/pnas.79.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. K., Chatterjee B., Demyan W. F., Nath T. S., Motwani N. M. Pretranslational regulation of alpha 2u-globulin in rat liver by growth hormone. J Biol Chem. 1982 Jul 10;257(13):7834–7838. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Shaw P. H., Held W. A., Hastie N. D. The gene family for major urinary proteins: expression in several secretory tissues of the mouse. Cell. 1983 Mar;32(3):755–761. doi: 10.1016/0092-8674(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Williams J. G. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. doi: 10.1093/nar/6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka P. R., Gallagher J. F., Held W. A. In vitro synthesis and characterization of precursors to the mouse major urinary proteins. J Biol Chem. 1980 Feb 25;255(4):1367–1373. [PubMed] [Google Scholar]
- Szoka P. R., Paigen K. Regulation of mouse major urinary protein production by the Mup-A gene. Genetics. 1978 Nov;90(3):597–612. doi: 10.1093/genetics/90.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi M., Young R. A., Hagenbüchle O., Schibler U. Multiple polyadenylation sites in a mouse alpha-amylase gene. Nucleic Acids Res. 1981 May 25;9(10):2313–2323. doi: 10.1093/nar/9.10.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Unterman R. D., Lynch K. R., Nakhasi H. L., Dolan K. P., Hamilton J. W., Cohn D. V., Feigelson P. Cloning and sequence of several alpha 2u-globulin cDNAs. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3478–3482. doi: 10.1073/pnas.78.6.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandoren G., Mertens B., Heyns W., Van Baelen H., Rombauts W., Verhoeven G. Different forms of alpha 2u-globulin in male and female rat urine. Eur J Biochem. 1983 Jul 15;134(1):175–181. doi: 10.1111/j.1432-1033.1983.tb07548.x. [DOI] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]