Abstract
Background
Among persons with obstructive airway disease, the relative contributions of chronic obstructive pulmonary disease (COPD), asthma, and common comorbid conditions to health care utilization and patient-centered outcomes (PCOs) have not been previously reported.
Methods
We followed a total of 3,486 persons aged ≥40 years with COPD, asthma, or both at baseline, from the Medical Expenditure Panel Survey (MEPS) cohorts enrolled annually from 2008 through 2012 for 1 year. MEPS is a prospective observational study of US households recording self-reported COPD, asthma, and ten medical conditions: angina, arthritis, cancer, coronary heart disease, cognitive impairment, diabetes, hypertension, lung cancer, myocardial infarction, and stroke/transient ischemic attack. We studied the separate contributions of these conditions to health care utilization (all-cause and respiratory disease hospitalization, any emergency department [ED] visit, and six or more outpatient visits) and PCOs (seven or more days spent in bed due to illness, incident loss of mobility, and incident decline in self-perceived health).
Results
COPD made the largest contributions to all-cause and respiratory disease hospitalization and ED visits, while arthritis made the largest contribution to outpatient health care. Arthritis and COPD, respectively, made the greatest contributions to the PCOs.
Conclusion
COPD made the largest and second largest contributions to health care utilization and PCOs among US adults with obstructive airway disease. The twelve medical conditions collectively accounted for between 52% and 61% of the health care utilization outcomes and between 53% and 68% of the PCOs. Cognitive impairment, diabetes, hypertension, and stroke also made significant contributions.
Keywords: average attributable fraction, AAF, chronic obstructive pulmonary disease, COPD, patient-centered outcomes, health care utilization
Introduction
Chronic obstructive pulmonary disease (COPD) is an important cause of morbidity and mortality and remains a primary driver of health care utilization in the USA.1 Recent studies have reported that patients with COPD demonstrate high prevalence of cardiovascular disease, diabetes, and depression and that the concurrence of these medical conditions is related to disproportionately higher utilization of health care resources.2–6 There is also growing recognition of the need to better understand how health-related quality of life can be improved for patients with COPD,7–10 as evidenced by a recent joint declaration by the American Thoracic Society and the European Respiratory Society calling for research that focuses on the most effective ways of improving care for these patients.11
We note two gaps in the research conducted to date in this area. First, there has been little effort to discern the relative contributions of COPD to health care utilization and patient-centered outcomes (PCOs) from those of its most common comorbid conditions. The second gap is the need to study cohorts that include patients both with and without COPD to enable the calculation of its contribution to outcomes relative to those from other conditions. In this study we address both gaps using a nationally representative sample of US families with adult persons who have either asthma, COPD, or both at baseline. This cohort permits the simultaneous estimation of the relative contributions of COPD and the other conditions, on health care utilization and PCOs with a technique called the average attributable fraction (AAF). The resultant AAFs represent the proportional contributions to an outcome attributable to individual conditions whether they occur alone or in combination with others.
To date, the AAF has been largely applied to studies of mortality in both national12 and regional13 cohorts. However, its application has been steadily growing and includes one study where the relative proportion of cases of pneumonia requiring hospitalization among community dwelling older adults were attributed to both modifiable and non-modifiable risk factors.14 To our knowledge, it has not been previously employed to assess the relative contribution of COPD to any outcome.
In this report, we apply the AAF15 to persons aged ≥40 years with obstructive airway disease in the Medical Expenditure Panel Survey (MEPS) of US households. Our goal was to estimate the proportions of select outcomes that can be attributed to the COPD and asthma, as well as ten important comorbid conditions.16
Methods
Study population
The study population included participants from MEPS, a resource sponsored by the US Agency for Health care Research and Quality. Households were chosen to constitute a nationally representative subsample of the US civilian population from within the National Health Interview Survey.16 We followed five cohorts respectively initiated in the years from 2008 to 2012 for 1 year in order to track changes in health status and utilization of medical services. Construction of the cohort has been previously described.6
Figure 1 shows that our analytic sample of 3,486 adults consisted of individuals who had information on smoking history, were ≥40 years old, and who reported asthma, COPD, or both at the baseline interview. COPD was defined as self-reporting of a physician diagnosis of chronic bronchitis (present in the 12 months prior to baseline) or emphysema (ever diagnosed). Asthma was identified as self-reporting of a corresponding physician diagnosis that was still present (ie, current asthma). The study was approved by the Human Investigation Committee of the Yale School of Medicine per Protocol Number 1510016585; it was granted exemption from participant consent.
Figure 1.
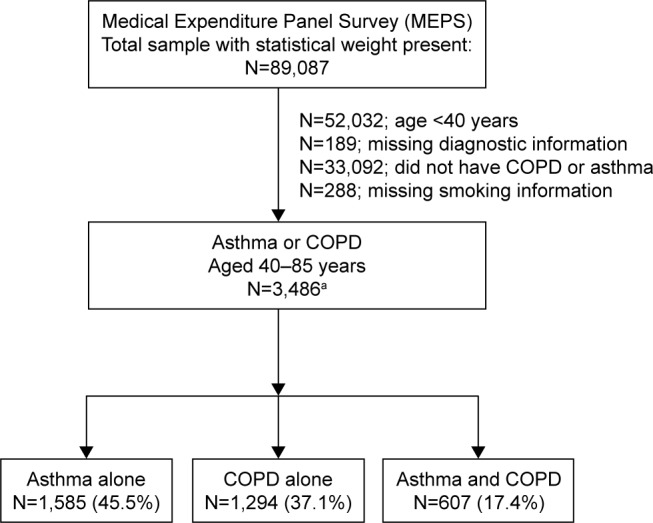
Derivation of samples from the 2008–2012 cohorts of the Medical Expenditures Panel Survey.
Notes: aThe following number of deaths occurred over the follow-up period: none at round 2 of the survey; 43 at round 3 of the survey; 28 at round 4 of the survey; and 27 at round 5 of the survey. The participants who died were not excluded from our analytical sample, since they had data at rounds 1 (baseline) and 2 of the survey.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Baseline sociodemographic, medical conditions, and clinical characteristics
Sociodemographic characteristics included age (≥65 versus 40–64 years), gender, race (African–American versus other), Hispanic ethnicity, and education (≥12 versus ≤11). History of medical conditions was assessed by self-report of a physician diagnosis of these conditions at baseline: angina, arthritis, asthma, cancer, coronary heart disease, COPD, diabetes, hypertension, lung cancer, myocardial infarction, stroke, or transient ischemic attack (TIA). We also included cognitive impairment, defined as self-reporting of confusion, memory loss, difficulty making decisions, or requiring supervision for safety, as a medical condition in our models.
Utilization of health care services
Health care services utilized over the first year of the study included all-cause hospitalization, respiratory hospitalization (identified by ICD9 codes: 416, 464, 466, 471–473, 477, 478, 480, 482, 483, 485–488, 490–493, 496, 505, 511, 514, 515, 518), any emergency department (ED) visit, and six or more outpatient (office or outpatient clinic) visits, the latter representing the median number of outpatient visits in this cohort.
Patient-centered outcomes
Three PCO measures were considered. A mobility measure assessed difficulty in walking, climbing stairs, grasping objects, reaching overhead, lifting, bending, and stooping or standing for long periods of time. Incident loss of mobility was calculated for those who did not report any difficulty with those aspects of mobility at baseline (n=2,040) and subsequently reported difficulty with one or more aspects at the 1-year follow-up interview. The second measure assessed the respondents’ overall perception of their health, coded as 1= poor or fair versus 0= excellent, very good, or good. Incident decline in self-perceived health was calculated for the subset of participants that reported good to excellent health at baseline (n=1,847) and subsequently reported fair or poor health at the 1-year follow-up interview. The third PCO was an indicator of the number of days the respondent spent in bed for a half day or more due to a physical illness or emotional problem during the first year of the study. Because our intent was to capture influence of COPD on highly bothersome disability, we subsequently coded this as one for ≥7 or more half days (the 75th percentile) versus zero for ≤6 days.
Statistical analysis
We first tabulated the prevalence of the medical conditions and sociodemographic characteristics at baseline, as well as the incidence of each outcome during the yearlong observation period. We subsequently fit multivariable logistic models of each of the seven dichotomous outcomes by regressing on the twelve medical conditions and five covariates: age, education, ethnicity, race, and sex. Model performance was evaluated using the C-statistic for discrimination and the Hosmer–Lemeshow statistic for calibration. For each given outcome, all possible two-factor interactions of the significant medical conditions were considered. Those interactions among the twelve conditions that demonstrated an individual association after adjustment were jointly tested in a separate model. Only those interactions that retained a significant association in the presence of all other individually associated interactions were retained in each multivariable model. The resultant estimates from these multivariable logistic models and the prevalence of the medical conditions drive the calculation of the corresponding AAFs.
The AAF is a descendant of the attributable fraction (AF) introduced by Levin in 1953.17 The AF is the degree to which the occurrence of a disease can be hypothetically reduced, assuming the entire population remains free from exposure. Calculation of the AF for a specific medical condition is based on the probability of the outcome (conditional on the presence of that condition) as well as the prevalence of that same condition. In the case where multiple conditions contribute to a specific health outcome, for example, death, the AF has a few notable mathematical limitations. The most important is that the AFs for multiply concurrent conditions can add up to >100%.18 This is because each AF represents the maximum possible reduction in the outcome obtained by removing all exposure to a given condition. Because the AF is deliberately accounting for the maximum possible contribution, it does not adjust for the overlapping contributions of multiple conditions.
For this reason, we prefer the AAF proposed by Eide and Gefeller,19 also referred to as partial attributable risk,20 which has the property of additivity. Additivity means that for any group of conditions, the sum of the individual AAFs will not exceed 100%, providing greater face validity. The AAF has subsequently been extended by Lin et al for longitudinal estimation,13 by Murphy et al for a large number of conditions21 and by Allore et al for rigorous accounting of covariates.15
Calculation of the AAF requires the initial generation of a design matrix consisting of all unique combinations of the medical conditions and covariates, and the subsequent calculation is based on the multivariable model of each outcome. The computational procedure consists of stepping through each row of the design matrix and calculating the reduction in the predicted probability of the outcome corresponding to the removal of a given medical condition from each possible subset of the model coefficients corresponding to the terms in that particular row of the design matrix. It has been shown previously that the overall average of the reductions in probability from all possible orderings within these subsets yields an estimate of AAF that is both additive and symmetric.19 As the calculation of the AAF is computationally intense, we chose to calculate it from 1,000 bootstrapped samples and taking the 2.5 and 97.5 percentiles as the limits of the 95% confidence intervals for the point estimate of each AAF.
Results
While our study included persons of age 40 through 85, Table 1 indicates that one third (33.3%) were aged ≥65 years with a majority of females (65.0%). Racially, 21.0% were African–American with only 14.1% identified as having Hispanic ethnicity, and strong majority (72.7%) had an education of high school or greater. The twelve conditions exhibited prevalence ranging from 1.4% (lung cancer) to 62.9% (asthma) and the outcomes exhibited incidence ranging from 5.5% for respiratory-specific hospitalization to 50.1% for six or more outpatient visits.
Table 1.
Baseline characteristics and study outcomes (N=3,486)
| Characteristics | n | % |
|---|---|---|
| Age: ≥65 vs 40–64 years | 1,161 | 33.30 |
| Female | 2,267 | 65.03 |
| African-American | 733 | 21.03 |
| Hispanic ethnicity | 491 | 14.08 |
| Education: ≥12 years | 2,522 | 72.66 |
| Medical conditions | ||
| Angina | 350 | 10.04 |
| Arthritis | 2,029 | 58.22 |
| Asthma | 2,192 | 62.88 |
| Cancer | 695 | 19.94 |
| Coronary heart disease | 595 | 17.08 |
| Cognitive impairment | 538 | 15.47 |
| Chronic obstructive pulmonary disease | 1,901 | 54.53 |
| Diabetes | 806 | 23.12 |
| Hypertension | 2,169 | 62.27 |
| Lung cancer | 50 | 1.44 |
| Myocardial infarction | 402 | 11.53 |
| Stroke/transient ischemic attack | 382 | 10.96 |
| Patient-centered outcomes | ||
| Incident mobility difficulties | 292 | 14.31 |
| Incident decline in perceived health | 312 | 16.89 |
| ≥7 days in bed due to physical illness or emotional problem | 930 | 26.75 |
| Utilization of health care services over 1 year | ||
| Any hospitalization | 719 | 20.63 |
| Hospitalization for respiratory condition | 190 | 5.45 |
| Any emergency department visit | 1,034 | 29.66 |
| ≥6 outpatient visits | 1,746 | 50.09 |
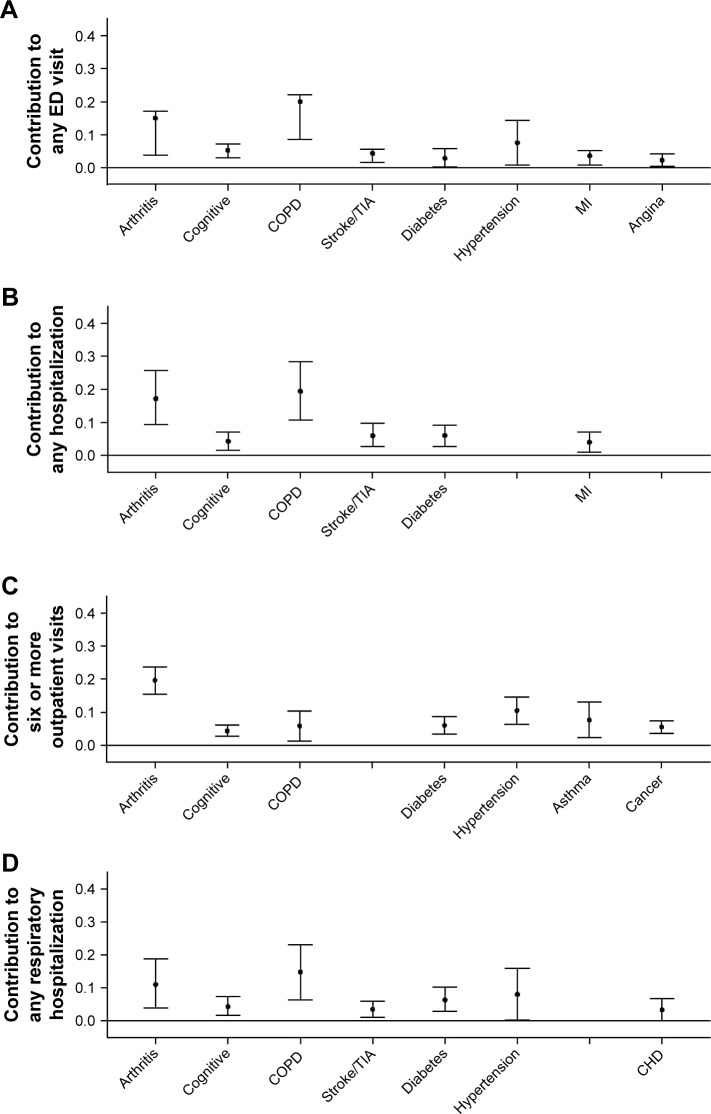
Regarding the four utilization outcomes, Figure 2 shows that COPD made the largest contribution to all except for six or more outpatient visits, for which arthritis dominated. Arthritis was also the second biggest contributor to the other three utilization outcomes. The only other conditions contributing to all four utilization outcomes were cognitive impairment and diabetes, although stroke and hypertension each contributed to three separate outcomes. Collectively, between six and eight individual conditions explained the following total percentages of the four outcomes as follows: any ED visit 61.3%, any hospitalization 57.0%, six or more outpatient visits 60.0%, and respiratory hospitalization 51.9%.
Figure 2.
Contribution of medical conditions to health care utilization outcomes over 1 year of follow-up in US adults with obstructive airway disease.
Notes: Conditions with 0 contribution not shown. Cognitive = cognitive impairment. (A) All-cause ED visit. The sum of the mean contribution of each condition was 0.61. (B) All-cause hospitalization. The sum of the mean contribution of each condition was 0.57. (C) Six or more outpatient (office or outpatient clinic) visits. The sum of the mean contribution of each condition was 0.60. (D) Hospitalization for respiratory illness. The sum of the mean contribution of each condition was 0.52.
Abbreviations: CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; ED, emergency department; MI, myocardial infarction; TIA, transient ischemic attack.
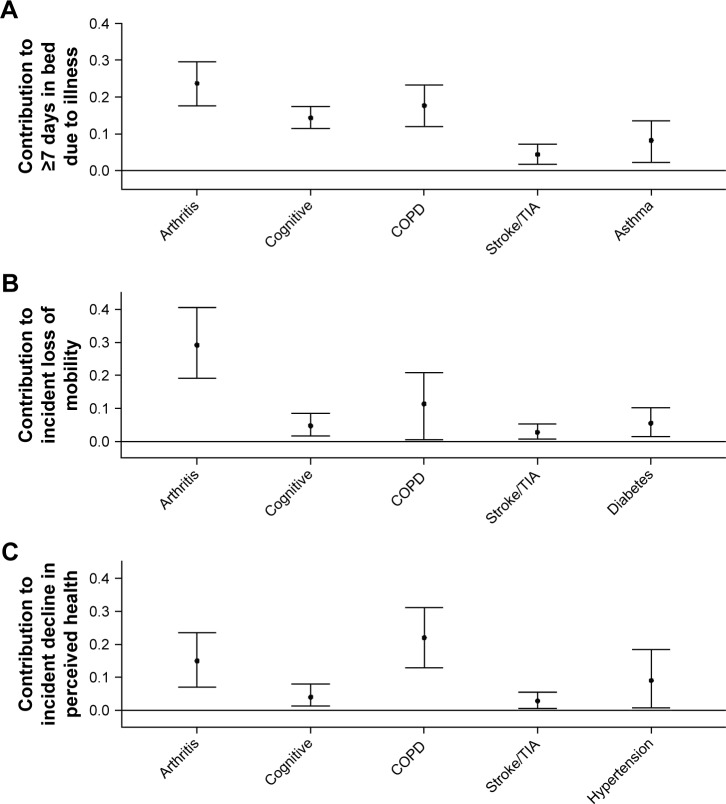
With respect to the PCOs, Figure 3 shows that arthritis made the largest contribution to ≥7 days in bed and to incident loss of mobility. COPD made the largest contribution to incident decline in self-perceived health and the second largest contribution to the other two PCOs. Cognitive impairment and stroke each made significant contributions to all of the PCOs. For each PCO, five individual conditions explained the following total percentages of the specific outcomes: ≥7 days in bed, 68.3%; incident loss of mobility, 53.7%; and incident loss of perceived health, 53.1%.
Figure 3.
Contribution of medical conditions to patient-centered outcomes over 1 year of follow-up in US adults with obstructive airway disease.
Notes: Conditions with zero contribution not shown. Cognitive = cognitive impairment. (A) Spent ≥7 days in bed due to physical or emotional illness. The sum of the mean contribution of each condition was 0.68. (B) Incident loss of mobility reflects the onset of mobility difficulty in those without any difficulty at baseline. The sum of the mean contribution of each condition was 0.54. (C) Incident decline in health reflects the onset of fair or poor perceived health in those with good to excellent perceived health at baseline. The sum of the mean contribution of each condition was 0.53.
Abbreviations: COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack.
Discussion
To our knowledge, this investigation is the first to apply the AAF methodology to patients with obstructive lung disease from a large representative sample of US adults. We show that out of 12 conditions COPD and arthritis collectively explain the largest proportions of four health care utilization outcomes and three PCOs. In particular, COPD explains the largest proportion of three of the four health care utilization measures, as well as patient-perceived incident decline in health. Conversely, arthritis explains the largest proportions of high frequency outpatient visits, instances of ≥7 days in bed, and incident loss of mobility. A recent report showed that patients with both asthma and COPD account for a larger share of the health care utilization and negative patient outcomes than either condition on its own.6 Regarding the additive effects of asthma and COPD, the current study shows that asthma complements the contributions of COPD to higher rates of outpatient visits and episodes of ≥7 days in bed. In addition to demonstrating that the influence of COPD on the utilization of health care is greater than that of asthma, the current study confirms and extends previous results by showing that contributions from COPD also eclipse those of the ten other medical conditions examined.
There are two noteworthy strengths of this study. First is its use of a large, nationally representative sample of US adults with obstructive airway disease. This permitted the inclusion of patients with and without COPD, thereby enabling the calculation of the relative contributions of COPD, asthma, and ten comorbid medical conditions. The second strength is its application of a sophisticated form of the AAF, which can accommodate a large number of concurrent conditions21 even while adjusting for sociodemographic covariates.15
Prior studies have demonstrated that COPD patients with comorbidities have higher rates of health care utilization than those without comorbid medical conditions.22–24 What has been lacking prior to the current study is a clear delineation of the explicit contributions of COPD in driving the use of health care relative to its concurring medical conditions. For example, Mannino et al showed that relative to normal function with no comorbidity, severe impairment of lung function (modified Global Institute of Obstructive Lung Disease stages 3 or 4) was significantly associated with higher hazards of all-cause hospitalization over 5 years.24 Specifically, they demonstrated hazard ratios (HRs) ranging from above 2.5 (modified by hypertension) to over 4.0 (modified by cardiovascular disease). While highly significant, these HRs on their own do not permit a direct estimation of how many hospitalizations can be accounted for by COPD with those comorbid conditions. In contrast, the AAF reported in this study indicates that COPD accounts for 19% of hospitalizations within 1 year, where this contribution represents an average over all existing combinations between COPD and the other eleven medical conditions.
Our study further illustrates that COPD itself, rather than its companion medical conditions, is the single largest contributor to emergency room use and inpatient admission. Our findings contrast with those of Gershon et al who report that COPD-associated comorbidities, rather than COPD itself, are the principal cause of the increased utilization of health care utilization characteristic of patients with COPD.5 However, any direct comparison with their findings is precluded by the vastly different methodology used by Gershon et al in their study.5
Our finding that PCOs among patients with COPD are influenced as much by comorbidity as by the lung disease itself is consistent with prior research.25–27 In particular, we found that among the 12 medical conditions considered, arthritis had the greatest impact on ≥7 days in bed and incident mobility disability, while COPD had the greatest impact on patient-perceived incident decline in health. Further supporting our results is the study by Wijnhoven et al, who report that among patients with COPD, musculoskeletal disease plays a major role in PCOs.27
The primary limitation of this study, shared by other studies based on data from large national surveys, is its reliance on the self-reporting of the physician diagnoses of chronic bronchitis, emphysema, asthma, and the ten comorbid conditions. However, it is encouraging that the results of this study corroborate some of the results from previous studies based on both self-reported data28 and studies of state-based23 and national22 registries of utilization. A second limitation of our study is its lamentable lack of diagnoses for mental health disorders, as prior studies have shown that depression and substance abuse in patients with COPD contribute tangibly to health care utilization.23,29 Last, because our study is observational in nature, we make no claims of causality.
Conclusion
This study supports those prior findings stating that COPD alone accounts for the greatest proportion of health care utilization4 and, to a lesser degree, the adverse PCOs suffered by patients with obstructive airway disease.7 By virtue of its rigorous assessment of the relative contributions of COPD to important outcomes, that is, in the context of asthma and ten important comorbid medical conditions, this study substantively extends the literature. These findings imply that future studies aimed at reducing health care utilization among patients with COPD should prioritize treatment of the underlying lung disease. They also suggest that interventions aimed at improving PCOs should consider both lung function and comorbidity, particularly with respect to the musculoskeletal system.
Acknowledgments
This study was supported by grants from the US National Institute on Aging (R24 AG045050 and R01 AG047891) and conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342, PI-Gill).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dhamane AD, Moretz C, Zhou Y, et al. COPD exacerbation frequency and its association with health care resource utilization and costs. Int J Chron Obstruct Pulmon Dis. 2015;10:2609–2618. doi: 10.2147/COPD.S90148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anecchino C, Rossi E, Fanizza C, et al. Prevalence of chronic obstructive pulmonary disease and pattern of comorbidities in a general population. Int J Chron Obstruct Pulmon Dis. 2007;2(4):567–574. [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001–2012 and Nationwide Emergency Department Sample 2006–2011. Chest. 2015;147(4):989–998. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon AS, Guan J, Victor JC, Goldstein R, To T. Quantifying health services use for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(6):596–601. doi: 10.1164/rccm.201211-2044OC. [DOI] [PubMed] [Google Scholar]
- 6.Vaz Fragoso CA, Murphy TE, Agogo GO, Allore HG, McAvay GJ. Asthma-COPD overlap syndrome in the US: a prospective population-based analysis of patient-reported outcomes and health care utilization. Int J Chron Obstruct Pulmon Dis. 2017;12:517–527. doi: 10.2147/COPD.S121223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer JP, Yusen RD. Defining patient-reported outcomes in chronic obstructive pulmonary disease: the patient-centered experience. Med Clin North Am. 2012;96(4):767–787. doi: 10.1016/j.mcna.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Dulohery MM, Schroeder DR, Benzo RP. Cognitive function and living situation in COPD: is there a relationship with self-management and quality of life? Int J Chron Obstruct Pulmon Dis. 2015;10:1883–1889. doi: 10.2147/COPD.S88035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones PW, Rennard S, Tabberer M, Riley JH, Vahdati-Bolouri M, Barnes NC. Interpreting patient-reported outcomes from clinical trials in COPD: a discussion. Int J Chron Obstruct Pulmon Dis. 2016;11:3069–3078. doi: 10.2147/COPD.S117378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon HY, Kim E. Factors contributing to quality of life in COPD patients in South Korea. Int J Chron Obstruct Pulmon Dis. 2016;11:103–109. doi: 10.2147/COPD.S90566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celli BR, Decramer M, Wedzicha JA, et al. An Official American Thoracic Society/European Respiratory Society Statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):e4–e27. doi: 10.1164/rccm.201501-0044ST. [DOI] [PubMed] [Google Scholar]
- 12.Tinetti ME, McAvay GJ, Murphy TE, Gross CP, Lin H, Allore HG. Contribution of individual diseases to death in older adults with multiple diseases. J Am Geriatr Soc. 2012;60(8):1448–1456. doi: 10.1111/j.1532-5415.2012.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin H, Allore HG, McAvay G, et al. A method for partitioning the attributable fraction of multiple time-dependent coexisting risk factors for an adverse health outcome. Am J Public Health. 2013;103(1):177–182. doi: 10.2105/AJPH.2011.300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juthani-Mehta M, De Rekeneire N, Allore H, et al. Modifiable risk factors for pneumonia requiring hospitalization of community-dwelling older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2013;61(7):1111–1118. doi: 10.1111/jgs.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allore HG, Zhan Y, Cohen AB, Tinetti ME, Trentalange M, McAvay G. Methodology to estimate the longitudinal average attributable fraction of guideline-recommended medications for death in older adults with multiple chronic conditions. J Gerontol A Biol Sci Med Sci. 2016;71(8):1113–1116. doi: 10.1093/gerona/glv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Department of Health & Human Services Medical Expenditure Panel Survey; survey background [revised August 2009] [Accessed December 8, 2016]. Available at: https://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp.
- 17.Levin ML. The occurrence of lung cancer in man. Acta Unio Internationalis Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- 18.Rowe AK, Powell KE, Flanders WD. Why population attributable fractions can sum to more than one. Am J Prev Med. 2004;26(3):243–248. doi: 10.1016/j.amepre.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Eide GE, Gefeller O. Sequential and average attributable fractions as aids in the selection of preventive strategies. J Clin Epidemiol. 1995;48(5):645–655. doi: 10.1016/0895-4356(94)00161-i. [DOI] [PubMed] [Google Scholar]
- 20.Ramsch C, Pfahlberg AB, Gefeller O. Point and interval estimation of partial attributable risks from case-control data using the R-package ‘pARccs’. Computer Methods Programs Biomed. 2009;94:88–95. doi: 10.1016/j.cmpb.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Murphy TE, McAvay G, Carriero NJ, et al. Deaths observed in Medicare beneficiaries: average attributable fraction and its longitudinal extension for many diseases. Stat Med. 2012;31(27):3313–3319. doi: 10.1002/sim.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 23.Yeatts KB, Lippmann SJ, Waller AE, et al. Population-based burden of COPD-related visits in the ED: return ED visits, hospital admissions, and comorbidity risks. Chest. 2013;144(3):784–793. doi: 10.1378/chest.12-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 25.van Manen JG, Bindels PJ, Dekker EW, et al. Added value of comorbidity in predicting health-related quality of life in COPD patients. Respir Med. 2001;95(6):496–504. doi: 10.1053/rmed.2001.1077. [DOI] [PubMed] [Google Scholar]
- 26.van Manen JG, Bindels PJ, Dekker FW, et al. The influence of COPD on health-related quality of life independent of the influence of comorbidity. J Clin Epidemiol. 2003;56(12):1177–1184. doi: 10.1016/s0895-4356(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 27.Wijnhoven HA, Kriegsman DM, Hesselink AE, de Haan M, Schellevis FG. The influence of co-morbidity on health-related quality of life in asthma and COPD patients. Respir Med. 2003;97(5):468–475. doi: 10.1053/rmed.2002.1463. [DOI] [PubMed] [Google Scholar]
- 28.Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003–2006) Heart Lung. 2013;42(3):163–170. doi: 10.1016/j.hrtlng.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer AS, Bhatt SP, Garner JJ, et al. Depression is associated with readmission for acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):197–203. doi: 10.1513/AnnalsATS.201507-439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]