In this study, we evaluated the yield of blood cultures among >7500 children hospitalized with CAP at 6 US children’s hospitals.
Abstract
BACKGROUND AND OBJECTIVES:
National guidelines recommend blood cultures for children hospitalized with presumed bacterial community-acquired pneumonia (CAP) that is moderate or severe. We sought to determine the prevalence of bacteremia and characterize the microbiology and penicillin-susceptibility patterns of positive blood culture results among children hospitalized with CAP.
METHODS:
We conducted a cross-sectional study of children hospitalized with CAP in 6 children’s hospitals from 2007 to 2011. We included children 3 months to 18 years of age with discharge diagnosis codes for CAP using a previously validated algorithm. We excluded children with complex chronic conditions. We reviewed microbiologic data and classified positive blood culture detections as pathogens or contaminants. Antibiotic-susceptibility patterns were assessed for all pathogens.
RESULTS:
A total of 7509 children hospitalized with CAP were included over the 5-year study period. Overall, 34% of the children hospitalized with CAP had a blood culture performed; 65 (2.5% of patients with blood cultures; 95% confidence interval [CI]: 2.0%–3.2%) grew a pathogen. Streptococcus pneumoniae accounted for 78% of all detected pathogens. Among detected pathogens, 50 (82%) were susceptible to penicillin. Eleven children demonstrated growth of an organism nonsusceptible to penicillin, representing 0.43% (95% CI: 0.23%–0.77%) of children with blood cultures obtained and 0.15% (95% CI: 0.08%–0.26%) of all children hospitalized with CAP.
CONCLUSIONS:
Among children without comorbidities hospitalized with CAP in a non-ICU setting, the rate of bacteremia was low, and isolated pathogens were usually susceptible to penicillin. Blood cultures may not be needed for most children hospitalized with CAP.
What’s Known on This Subject:
The Pediatric Infectious Diseases Society and the Infectious Diseases Society of America national guideline recommends that blood cultures should be obtained in children requiring hospitalization for presumed bacterial community-acquired pneumonia.
What This Study Adds:
Among children hospitalized with community-acquired pneumonia, the rate of bacteremia is low, and isolated pathogens are usually susceptible to penicillin.
The Pediatric Infectious Diseases Society and the Infectious Diseases Society of America national guideline recommends that blood cultures should be obtained in children requiring hospitalization for presumed bacterial community-acquired pneumonia (CAP) that is moderate or severe, particularly in those with complicated pneumonia.1 This recommendation was intended to facilitate targeted antimicrobial therapy when an organism was isolated because culture-directed therapy may be associated with improved outcomes while offering opportunities to reduce unnecessary broad-spectrum antimicrobial use.2–4 Furthermore, epidemiologic data derived from blood culture results may inform empirical treatment approaches and the national vaccination policy.1
However, moderate to severe pneumonia is not well defined in the CAP guideline, and there is no overall recommendation regarding whether to obtain blood cultures for hospitalized children with CAP. Thus, debate persists over the utility of blood cultures in children hospitalized with CAP. Studies of pneumonia after licensure of the pneumococcal conjugate vaccine have demonstrated low rates of bacteremia, ranging from 1% to 7%, among children hospitalized with CAP.5–7 Consequently, the impact of blood cultures on clinical management may be limited, whereas the costs (including the costs of additional health care use prompted by false-positive or contaminated culture results) may be substantial.5,8 However, these previous studies were limited by small sample sizes, limited periods of data collection, and/or single-center designs.5,6,8–10 Furthermore, the recent shift toward greater use of narrow-spectrum antibiotics (especially ampicillin instead of ceftriaxone) for CAP in the context of evolving antimicrobial resistance may lead to delays in appropriate antibiotic therapy for children with CAP because of penicillin-nonsusceptible organisms, including Streptococcus pneumoniae and Staphylococcus aureus.11 Delays in appropriate antibiotic therapy have been associated with worse clinical outcomes in adults with pneumonia.12,13
Based on the limited data in this area, we sought to determine the prevalence of bacteremia and characterize the microbiology and penicillin-susceptibility patterns of positive blood culture results in a large, multicenter cohort of children hospitalized with CAP. This information may help inform clinical practice around the judicious use of blood cultures as well as future guideline recommendations for the management of children with CAP.
Methods
Study Design
This retrospective cohort study used data from the Pediatric Health Information System Plus (PHIS+) database. The PHIS+ augments administrative and billing information contained within the PHIS database (Children’s Hospital Association, Lenexa, KS) with detailed laboratory and radiographic information from the following 6 tertiary children’s hospitals: the Children’s Hospital of Philadelphia (Philadelphia, PA), Boston Children’s Hospital (Boston, MA), the Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center (Pittsburgh, PA), Seattle Children’s Hospital (Seattle, WA), Primary Children’s Hospital (Salt Lake City, UT), and Cincinnati Children’s Hospital Medical Center (Cincinnati, OH). It has been standardized and harmonized to biomedical terminologies and data models and assessed for its quality.14 This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Study Population
We included children 3 months to 18 years of age hospitalized with CAP between January 1, 2007, and December 31, 2011. The diagnosis of CAP was ascertained on the basis of International Classification of Diseases, Ninth Revision, Clinical Modification discharge diagnosis codes and required either a primary diagnosis of pneumonia (480–483 and 485–487) or a primary diagnosis of pleural effusion (510.0, 510.9, 511.0, 511.1, 511.9) and a secondary diagnosis of pneumonia.15,16
We excluded children who were transferred into a hospital that was participating in the PHIS+ because these children may have had a blood culture performed or received antibiotic treatment before their arrival. In addition, we excluded children with a complex chronic condition recorded among their discharge diagnoses because these children may warrant a different diagnostic evaluation than children without comorbidities.17 We also excluded children with a secondary discharge diagnosis consistent with an underlying chronic condition not identified by the complex chronic condition algorithm, suggesting hospitalization for reasons other than pneumonia.16
Bacteremia
We identified blood cultures performed on the first or second hospital day for each child included in the cohort. Blood culture results were classified as positive or negative for bacterial growth. Children with bacterial growth in their cultures were further classified as harboring a pathogen or a contaminant by using a previously defined classification scheme for children hospitalized with pneumonia.7 We then calculated the prevalence of bacteremia among children hospitalized with CAP. For each pathogen, we also assessed whether the organism was susceptible versus nonsusceptible (including intermediate susceptibility as “nonsusceptible”) to penicillin. For S pneumoniae, we classified the isolate as susceptible to penicillin if the minimum inhibitory concentration was <2 μg/mL.1
We performed a subanalysis restricting the cohort to children who received an antibiotic on the first or second hospital day to best represent the population of children with suspected bacterial pneumonia. Additionally, we attempted to identify patients who were hospitalized with severe or complicated pneumonia. Patients were considered to have severe or complicated pneumonia if they were either admitted to an ICU or underwent a pleural drainage procedure on the first or second day of their hospitalization, respectively. Pleural drainage was defined by International Classification of Diseases, Ninth Revision, Clinical Modification procedure codes for thoracentesis (34.91), chest tube placement (34.04), video-assisted thoracoscopic surgery (34.21), and thoracotomy (34.02 and 34.09).18
Data Analysis
Demographic characteristics were summarized by using frequencies and percentages. We compared the characteristics of children receiving a blood culture with those not receiving a blood culture by using χ2 statistics. The prevalence of bacteremia detections with exact binomial 95% confidence intervals (CIs) were calculated among all children in whom a blood culture was obtained and in the subpopulations of interest. Additional analyses were conducted by stratifying the cohort on the basis of the presence or absence of severe or complicated pneumonia. Calculations were performed by site and in aggregate. All statistical analyses were performed by using SAS v.9.4 (SAS Institute, Cary, NC), and P values <.05 were considered statistically significant.
Results
During the study period, a total of 14 166 CAP hospitalizations were identified at the 6 study sites. After excluding children who were transferred in (n = 1281), those with a complex chronic condition (n = 5196), and those with other chronic conditions (n = 180), the final cohort consisted of 7509 CAP hospitalizations, ranging from 816 to 1675 encounters per site (Supplemental Tables 5 and 6).
Demographics of the study population stratified by the performance of a blood culture are shown in Table 1. Overall, 34.2% of the children hospitalized with CAP had a blood culture obtained. Compared with children without blood cultures obtained, the children with blood cultures performed were more likely to have private insurance, present during the summer season, and have longer hospital length of stay.
TABLE 1.
Characteristics of Children Stratified by Performance of Blood Culture
| Overall N = 7509 | No Blood Culture N = 4941 (65.8%) | Blood Culture N = 2568 (34.2%) | P | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age, y | ||||
| 1–5 | 5443 (72.5) | 3586 (72.6) | 1857 (72.3) | .108 |
| 6–12 | 1623 (21.6) | 1083 (21.9) | 540 (21.0) | |
| 13–18 | 443 (5.9) | 272 (5.5) | 171 (6.7) | |
| Girls | 3659 (48.7) | 2405 (48.7) | 1254 (48.8) | .897 |
| Race/ethnicity | ||||
| Non-Hispanic white | 4113 (59.5) | 2739 (59.9) | 1374 (58.7) | <.001 |
| Non-Hispanic black | 1576 (22.8) | 1020 (22.3) | 556 (23.8) | |
| Hispanic | 783 (11.3) | 496 (10.8) | 287 (12.3) | |
| Asian | 294 (4.3) | 191 (4.2) | 103 (4.4) | |
| Other | 149 (2.2) | 128 (2.8) | 21 (0.9) | |
| Insurance | ||||
| Government | 2894 (38.5) | 1999 (40.5) | 895 (34.9) | <.001 |
| Private | 3927 (52.3) | 2442 (49.4) | 1485 (57.8) | |
| Other | 688 (9.2) | 500 (10.1) | 188 (7.3) | |
| Disposition | ||||
| Home health service | 278 (3.7) | 132 (2.7) | 146 (5.7) | <.001 |
| Home | 6899 (91.9) | 4576 (92.6) | 2323 (90.5) | |
| Other | 326 (4.3) | 227 (4.6) | 99 (3.9) | |
| Skilled | 6 (0.1) | 6 (0.1) | 0 (0.0) | |
| Length of stay, d | ||||
| 0–1 | 2197 (29.3) | 1650 (33.4) | 547 (21.3) | <.001 |
| 2–3 | 3489 (46.5) | 2266 (45.9) | 1223 (47.6) | |
| 4–6 | 1142 (15.2) | 678 (13.7) | 464 (18.1) | |
| 7+ | 681 (9.1) | 347 (7.0) | 334 (13.0) | |
| Season | ||||
| Spring | 1983 (26.4) | 1299 (26.3) | 684 (26.6) | <.001 |
| Summer | 1033 (13.8) | 622 (12.6) | 411 (16.0) | |
| Fall | 1810 (24.1) | 1190 (24.1) | 620 (24.1) | |
| Winter | 2683 (35.7) | 1830 (37.0) | 853 (33.2) |
Performance of blood culture varied by site (range 13.6%–49.1%) for children hospitalized with CAP (Table 2).
TABLE 2.
Performance and Results of Blood Culture by Site
| Site | N of Hospitalized Children | N of Blood Cultures Performed (%) | N of Blood Culture Results (% of Patients in Whom Blood Culture Was Performed) | |
|---|---|---|---|---|
| Pathogen | Contaminant | |||
| A | 1236 | 328 (26.5) | 7 (2.1) | 3 (0.9) |
| B | 1334 | 287 (21.5) | 6 (2.1) | 0 (0.0) |
| C | 816 | 111 (13.6) | 5 (4.5) | 1 (0.9) |
| D | 1675 | 651 (38.9) | 18 (2.8) | 6 (0.9) |
| E | 1173 | 576 (49.1) | 6 (1.0) | 4 (0.7) |
| F | 1275 | 615 (48.2) | 23 (3.7) | 11 (1.8) |
| Total | 7509 | 2568 (34.2) | 65 (2.5) | 25 (1.0) |
Overall, bacteremia was identified in 0.9% (95% CI: 0.7%–1.1%) of the 7509 children hospitalized with CAP. Among the 2568 children in whom a blood culture was obtained, 65 (2.5% of all blood cultures; 95% CI: 2.0%–3.2%) demonstrated growth of a pathogen. S pneumoniae (n = 51) was the most common organism identified, accounting for 78% of all pathogens (Table 3), but it was detected in only 2.0% of all children with blood cultures obtained. Contaminants were identified in 25 (1.0%; 95% CI: 0.6%–1.4%) patients with CAP in whom a blood culture was obtained (Table 2); coagulase-negative staphylococci accounted for the most contaminants.
TABLE 3.
Specific Pathogens Identified in Blood Cultures and Susceptibility to Penicillins
| Organism | N | % of All Pathogens | % of All Blood Cultures Performed (n = 2568) | % of All Children Hospitalized With CAP (n = 7509) | Susceptibility to Penicillinsa | ||
|---|---|---|---|---|---|---|---|
| N Tested | N Susceptible (%) | N Nonsusceptible (%) | |||||
| S pneumoniae | 51 | 78.5 | 1.99 | 0.68 | 50 | 46 (92)b | 4 (8) |
| S aureus | 5 | 7.7 | 0.19 | 0.07 | 5 | — | 5 (100) |
| Haemophilus influenzae | 3 | 4.6 | 0.12 | 0.04 | 2 | 1 (50) | 1 (50) |
| β hemolytic streptococci | 2 | 3.1 | 0.08 | 0.03 | 1 | 1 (100) | — |
| Streptococcus pyogenes | 2 | 3.1 | 0.08 | 0.03 | 2 | 2 (100) | — |
| Enterobacter | 1 | 1.5 | 0.04 | 0.01 | 1 | — | 1 (100) |
| Moraxella sp. | 1 | 1.5 | 0.04 | 0.01 | 0 | — | — |
| Total | 65 | 100 | 2.53 | 0.87 | 61 | 50 (82) | 11 (18) |
Growth of contaminants include the following: coagulase-negative staphylococci (n = 20), Viridans group streptococci (n = 5), diphtheriods (n = 2), micrococci (n = 1), Actinomyces (n = 1), Corynebacterium (n = 1), Streptococci species (n = 1), and other Gram-positive cocci (n = 1). —, not applicable.
Percent susceptible and nonsusceptible reflect the percentages among those tested (4 patients had missing susceptibility data).
S pneumoniae isolates with minimum inhibitory concentration <2 were considered susceptible to penicillin when not otherwise stated by a local microbiology laboratory.
Data regarding susceptibility to penicillins were available for 61 of the 65 detected pathogens. Fifty (82%) pathogens were susceptible to penicillin (Table 3), including 92% of S pneumoniae isolates. Eleven children demonstrated growth of a pathogen nonsusceptible to penicillin, which represented 0.4% (95% CI: 0.2%–0.8%) of the children in whom a blood culture was obtained and 0.2% (95% CI: 0.1%–0.3%) of all children hospitalized with CAP. (Table 4)
TABLE 4.
Overall Yield of Blood Cultures
| N | % of Cohort (N = 7509) | % of All Blood Cultures Performed (n = 2568) | |
|---|---|---|---|
| Cohort (hospitalized with CAP) | 7509 | — | — |
| Blood culture performed | 2568 | 34.20 | — |
| Pathogen | 65 | 0.87 | 2.53 |
| Penicillin-nonsusceptible organisma | 11 | 0.15 | 0.43 |
—, not applicable.
Percent susceptible and nonsusceptible reflect the percentages among those tested (4 patients had missing susceptibility data).
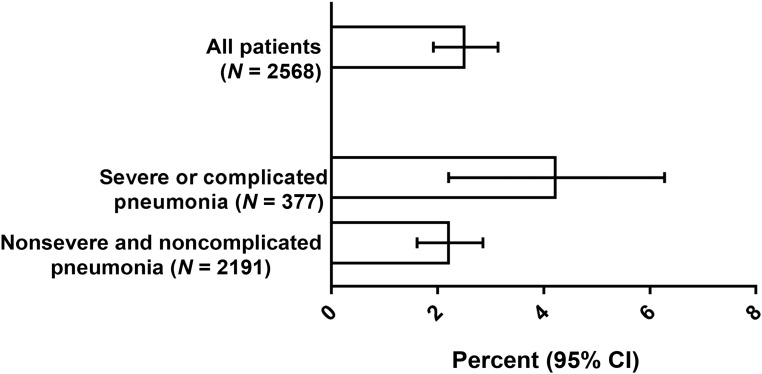
Compared with the overall cohort, the rate of bacteremia with a pathogen was not different among the subset of children treated with an antibiotic on the first or second hospital day (2.6%, 95% CI: 2.0%–3.2%). The prevalence of bacteremia among patients with severe or complicated pneumonia on presentation was 4.2% (95% CI: 2.6%–6.8%) and was 2.2% (95% CI: 1.6%–2.9%) among children presenting without severe and complicated pneumonia (Fig 1).
FIGURE 1.
Percentage of patients with bacteremia because of a pathogen, overall and stratified by the presence or absence of severe or complicated pneumonia.
Discussion
In this large, multicenter study, bacteremia was rarely identified in children hospitalized with CAP. Among the few detected pathogens, S pneumoniae was the most common, and most pathogens were susceptible to penicillin. These findings suggest that the routine performance of blood cultures has limited value in the majority of children hospitalized with CAP because the results would rarely require changes from guideline-recommended, empirical antibiotic therapy.
The rationale for obtaining blood cultures in children hospitalized with moderate to severe CAP relates to the ability to target therapy when a causative agent is identified. Although cited as a strong recommendation in the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America guideline, the evidence supporting the recommendation to obtain a blood culture in children hospitalized with CAP was graded as low quality.1 The low detection of bacteremia observed in our study should be interpreted in the context of other investigations. In a multicenter study that included 1 year of data (2010) from 2 of the hospitals included in this study, blood cultures were performed in 56% of children hospitalized with CAP with a prevalence of bacteremia of 7%.9 Although this rate of bacteremia was considerably higher than what was observed in this study, the inclusion criteria differed between the 2 studies. Most notably, the other study included only radiographically confirmed cases of pneumonia. In another study of 535 hospitalized children <36 months of age with radiographic evidence of uncomplicated pneumonia, 2.2% had positive blood culture results, all of which were considered contaminants.6 In a study of children with CAP evaluated in an emergency department, one-third had blood cultures performed, and 2.1% had bacteremia.5 We observed wide variation in the rates of obtaining blood cultures across the 6 participating centers. This variation may have influenced the pathogen detections by site, with institutions more liberal in blood culture performance demonstrating a lower rate of pathogen detection. This variation emphasizes the need to standardize care among children hospitalized with CAP.
Among a population of >7500 children hospitalized with CAP, 11 children had a blood culture result that would indicate a broadening of antibiotic coverage beyond the typically recommended, narrow-spectrum agent ampicillin. By using these estimates, 667 children would need blood cultures to identify 1 child for whom a change in antibiotic coverage would be recommended. By using the more conservative estimate of children in whom a blood culture was actually obtained, the number needed to test would be 223. Although isolating a bacterial pathogen (ie, CAP caused by a penicillin-susceptible S pneumoniae) may not necessarily lead to a change in empirical antibiotic therapy, such knowledge may be informative if the response to treatment is suboptimal.
The value of blood cultures lies primarily in the detection of a pathogen for which an empirical antibiotic regimen would not effectively cover. A blood culture with isolation of a pathogenic organism susceptible to the empirical therapy may also provide useful information to avoid broadening therapy in patients who are not improving or worsening on initial therapy. The challenge is that when blood cultures are drawn early in the course of evaluation and treatment, the severity of CAP may not be fully apparent, which highlights the challenge of identifying which children would potentially benefit from a blood culture. This marginal benefit of blood culture for targeting therapy must be weighed against the additional consequences of obtaining a blood culture, including the identification of nonpathogenic contaminants. Similar to those of other studies, the contamination rate was low across the 6 study sites.19 In our cohort, for every 5 children that had bacteremia because of a pathogen, 2 had a contaminated blood culture. Additionally, nearly 3 times as many children had a contaminated blood culture as did children with bacteremia because of a pathogen nonsusceptible to penicillin. Contaminated cultures are not without consequence; studies in adults have shown that contaminants contribute to longer hospital stays, increased exposure to unnecessary broad-spectrum antibiotics, and increased cost.2,20,21
Among the few detected pathogens, S pneumoniae was the most common, accounting for 78% of the pathogens identified. More than 90% of pneumococcal isolates were susceptible to narrow-spectrum penicillins, reinforcing the recommendation for ampicillin as a first-line agent for empirical treatment of children hospitalized with CAP. Furthermore, in the case of S pneumoniae, it should also be noted that the recommended antibiotic treatment course for nonsevere CAP is not different on the basis of the presence or absence of bacteremia, further limiting the value of a blood culture in this population.
Our study is subject to several limitations. All subjects were cared for at tertiary-care children’s hospitals that participated in our research network, and our findings may not be generalizable to other institutions. However, given the multiple participating institutions, case mix, and higher overall severity of disease in patients cared for at children’s hospitals, we would not expect the yield of blood cultures to be different in other settings. We relied on the use of administrative data augmented by microbiologic data from the PHIS+. Although we used previously validated strategies to identify children hospitalized with CAP, the lack of additional clinical information limits our ability to assess detailed clinical characteristics of patients included in our study or to differentiate bacterial from viral pneumonia.15 We are unable to ascertain antibiotic exposure before blood culture collection, which can reduce the rate of pathogen detection. Additionally, we are unable to account for other testing, including viral studies, which may have been performed in some children included in this study. Furthermore, the classification of severe or complicated pneumonia was made in retrospect and may not have been known at the time of blood culture collection.
The exclusion of children with underlying chronic conditions and those who transferred to the study institutions may limit the generalizability of our findings; the low rate of bacteremia observed in this study may not be extrapolated directly to all children hospitalized with CAP. It is important to note that nearly one-half of all cases considered were excluded on the basis of these criteria. The exclusion of children with underlying comorbidities likely results in a study cohort that more closely resembles populations served by nonchildren’s hospital facilities. Underestimation of the true rate of bacteremia may also have occurred because of the fact that many children did not have blood cultures obtained, and we cannot account for potential use of antibiotics before blood culture collection that may have precluded bacterial detections. Also, susceptibility information was missing from 4 isolates, which could impact our point estimates for the rate of nonsusceptible strains. It should also be noted that our findings of the low yield of blood cultures might not apply to children admitted to an intensive care setting or children presenting with complicated pneumonia, such as empyema, in whom the rate of bacteremia is nearly twofold higher. Lastly, if clinicians become more selective with performance of blood culture by targeting only children with more severe illness, then future studies may find a higher prevalence of bacteremia.1,22
Conclusions
The rate of bacteremia in children without comorbidities hospitalized with CAP in our study was low. When bacteremia occurred, >80% of isolates were susceptible to ampicillin, the recommended first-line treatment of children with CAP. The yield of blood cultures is low among children without comorbidities hospitalized with uncomplicated pneumonia in a non-ICU setting. The routine performance of blood cultures in these children may not be indicated. Researchers in future studies should seek to identify the clinical characteristics of children in whom obtaining blood cultures would lead to changes in clinical management, especially when identifying those patients at risk for CAP caused by organisms not susceptible to guideline-recommended, narrow-spectrum antibiotics.
Acknowledgments
Pediatric Research in Inpatient Settings Network collaborators include the following:
David Bertoch, MHA (Children’s Hospital Association, Lenexa, KS); Sean A. Frederick, MD (Newborn Medicine Program, University of Pittsburgh Medical Center, Pittsburgh, PA); Ram K. Gouripeddi, MS, MBBS (School of Medicine, University of Utah, Salt Lake City, UT); Phil Jaggard (Children’s Hospital Association, Lenexa, KS); Ron Keren, MD, MPH (Division of General Pediatrics, Center for Pediatric Clinical Effectiveness, Children’s Hospital of Philadelphia, Philadelphia, PA); Christopher P. Landrigan, MD, MPH (Division of General Pediatrics, Boston Children’s Hospital, Boston, MA); Sarah McBride (Division of General Pediatrics, Boston Children’s Hospital, Boston, MA); Stephane Meystre, MD, PhD, MS (Biomedical Informatics, School of Medicine, University of Utah, Salt Lake City, UT); Jebi Miller, PMP, MCPM (Children’s Hospital Association, Lenexa, KS); Shawn J. Rangel, MD, MSCE (Department of Surgery, Boston Children’s Hospital, Boston, MA); Jeffrey M. Simmons, MD, MSc (Division of Hospital Medicine, Cincinnati Children’s Hospital, Cincinnati, OH); Rajendu Srivastava, MD, MPH (Division of Inpatient Medicine, University of Utah Health Care, Salt Lake City, UT); Bryan L. Stone, MD, MS (Division of Inpatient Medicine, University of Utah Health Care, Salt Lake City, UT); Lauren Tanzer, MS, PMP (Division of General Pediatrics, Center for Pediatric Clinical Effectiveness, Children’s Hospital of Philadelphia, Philadelphia, PA); and Joel S. Tieder, MD, MPH (Division of Hospital Medicine, Seattle Children’s Hospital, Seattle, WA).
Glossary
- CAP
community-acquired pneumonia
- CI
confidence interval
- PHIS+
Pediatric Health Information System Plus
Footnotes
Dr Neuman conceptualized and designed the study, assisted with interpretation of the data, and drafted and revised the manuscript; Dr Hall conceptualized and designed the study, oversaw and conducted analyses, and reviewed and revised the manuscript; Drs Lipsett, Hersh, Williams, Gerber, Brogan, Blaschke, Grijalva, Parikh, Ambroggio, and Shah conceptualized and designed the study, assisted with interpretation of the data, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Center for Advancing Translational Sciences, the National Institute of Allergy and Infectious Diseases, or the Agency for Healthcare Research and Quality.
FINANCIAL DISCLOSURE: Other than the conflict of interest declared by Dr Blaschke, the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Williams received funding from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (K23AI104779), and Drs Grijalva and Shah received funding from the Agency for Healthcare Research and Quality (R03HS022342 and R01HS019862, respectively). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Blaschke collaborates with BioFire Diagnostics LLC on federally funded studies, has received research funding from BioFire Diagnostics for investigator-initiated research, has intellectual property licensed to BioFire Diagnostics, receives royalties through the University of Utah, and has acted as an advisor to BioFire Diagnostics and BioFire Defense LLC regarding risk assessment for US Food and Drug Administration–cleared products; and the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bradley JS, Byington CL, Shah SS, et al. ; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America . The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. 2011;53(7):e25–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy M, Bates DW, Wright SB, Ruiz R, Wolfe RE, Shapiro NI. Do emergency department blood cultures change practice in patients with pneumonia? Ann Emerg Med. 2005;46(5):393–400 [DOI] [PubMed] [Google Scholar]
- 3.Campbell SG, Marrie TJ, Anstey R, Dickinson G, Ackroyd-Stolarz S. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest. 2003;123(4):1142–1150 [DOI] [PubMed] [Google Scholar]
- 4.Corbo J, Friedman B, Bijur P, Gallagher EJ. Limited usefulness of initial blood cultures in community acquired pneumonia. Emerg Med J. 2004;21(4):446–448 [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SS, Dugan MH, Bell LM, et al. . Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SS, Alpern ER, Zwerling L, McGowan KL, Bell LM. Risk of bacteremia in young children with pneumonia treated as outpatients. Arch Pediatr Adolesc Med. 2003;157(4):389–392 [DOI] [PubMed] [Google Scholar]
- 9.Myers AL, Hall M, Williams DJ, et al. . Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iroh Tam P-Y, Bernstein E, Ma X, Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: a systematic review and meta-analysis. Hosp Pediatr. 2015;5(6):324–336 [DOI] [PubMed] [Google Scholar]
- 11.Ross RK, Hersh AL, Kronman MP, et al. . Impact of Infectious Diseases Society of America/Pediatric Infectious Diseases Society guidelines on treatment of community-acquired pneumonia in hospitalized children. Clin Infect Dis. 2014;58(6):834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnacho-Montero J, García-Cabrera E, Diaz-Martín A, et al. . Determinants of outcome in patients with bacteraemic pneumococcal pneumonia: importance of early adequate treatment. Scand J Infect Dis. 2010;42(3):185–192 [DOI] [PubMed] [Google Scholar]
- 13.Waterer GW, Kessler LA, Wunderink RG. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest. 2006;130(1):11–15 [DOI] [PubMed] [Google Scholar]
- 14.Gouripeddi R, Warner PB, Mo P, et al. . Federating clinical data from six pediatric hospitals: process and initial results for microbiology from the PHIS+ consortium. AMIA Annu Symp Proc. 2012;2012:281–290 [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DJ, Shah SS, Myers A, et al. . Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah SS, Srivastava R, Wu S, et al. ; Pediatric Research in Inpatient Settings Network . Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. [DOI] [PubMed] [Google Scholar]
- 17.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e99 [DOI] [PubMed] [Google Scholar]
- 18.Neuman MI, Hall M, Gay JC, et al. . Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92–96 [DOI] [PubMed] [Google Scholar]
- 20.Richter SS, Beekmann SE, Croco JL, et al. . Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory-based algorithm. J Clin Microbiol. 2002;40(7):2437–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265(3):365–369 [PubMed] [Google Scholar]
- 22.Parikh K, Hall M, Blaschke AJ, et al. . Aggregate and hospital-level impact of national guidelines on diagnostic resource utilization for children with pneumonia at children’s hospitals. J Hosp Med. 2016;11(5):317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]