In this 10-year prospective study, we capture the epidemiology and risk factors of AOM and the otitis-prone condition in the PCV era.
Abstract
OBJECTIVES:
To study the epidemiology of acute otitis media (AOM), especially the otitis-prone condition, during the pneumococcal conjugate vaccines 7 and 13 era.
METHODS:
Six hundred and fifteen children were prospectively managed from 6 to 36 months of life during a 10-year time frame (June 2006–June 2016). All clinical diagnoses of AOM were confirmed by tympanocentesis and bacterial culture of middle ear fluid.
RESULTS:
By 1 year of age, 23% of the children experienced ≥1 episode of AOM; by 3 years of age, 60% had ≥1 episodes of AOM, and 24% had ≥3 episodes. The peak incidence occurred at 6 to 12 months of life. Multivariable analysis of demographic and environmental data revealed a significantly increased risk of AOM associated with male sex, non-Hispanic white race, family history of recurrent AOM, day care attendance, and early occurrence of AOM. Risk factors for stringently defined (tympanocentesis-confirmed) otitis proneness, in which children suffered at least 3 episodes of AOM in a 6-month period or at least 4 within a year, were male sex, day care attendance, and family history of AOM, whereas breastfeeding in the first 6 months of life was protective. Stringently defined otitis prone children were also likely to experience their first AOM episode at a younger age. The proportion of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis causing AOM had dynamic changes during the past decade.
CONCLUSIONS:
We conclude that the epidemiology but not the risk factors for AOM have undergone substantial changes since the introduction of pneumococcal conjugate vaccines.
What’s Known on This Subject:
Studies of epidemiology of acute otitis media (AOM) that relied on clinical diagnosis and occurred during pneumococcal conjugate vaccination are few, and none used the American Academy of Pediatrics’ current, strict definition of AOM, or included microbiologic confirmation by tympanocentesis.
What This Study Adds:
During the pneumococcal conjugate vaccine era, AOM incidence and frequency of the otitis-prone condition have decreased. Otopathogen predominance has undergone dynamic changes. Day care attendance and family history of AOM remain the major risk factors for AOM and for becoming otitis prone.
In the 1980s, a prospective cohort study was conducted in Boston, Massachusetts, on the epidemiology of acute otitis media (AOM) in 698 children who were observed for at least the first 3 years of life.1 In that time frame, the presence of fluid behind the tympanic membrane (TM) was considered evidence of AOM, amoxicillin was the dominant antibiotic prescribed and few alternative antibiotics were available. Reexamination of children after AOM was routinely performed, and persistence of fluid behind the TM was considered evidence of antibiotic treatment failure and additional antibiotics were prescribed.2 No vaccine to prevent AOM caused by any of the predominant bacterial otopathogens was available. In the 3 decades since publication of that seminal work, major changes in the definition of AOM, demographics, modifying risk factors for AOM infection, antibiotic treatments, follow-up routines, and vaccinations have occurred. According to the American Academy for Pediatrics, over 5 000 000 AOM cases occur annually in US children, resulting in >10 000 000 annual antibiotic prescriptions and ∼30 000 000 annual visits for medical care.3–6 It is the most common condition treated with antibiotics, and increasing incidence of antibiotic resistance among the organisms responsible for AOM is a cause for concern.
Our group commenced a prospective, longitudinal study of a cohort of children in their first 3 years of life in Rochester, New York, to reassess the epidemiology, etiology, and immunobiology of AOM during the pneumococcal conjugate vaccine (PCV) era.7–16 Central to our study design has been the microbiologic confirmation of nearly every clinical diagnosis of AOM by culture of middle ear fluid (MEF) collected by tympanocentesis (a technique rarely used worldwide).6,17
Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are the 3 main otopathogens causing AOM,6,17 but the implementation of national childhood immunization programs against S pneumoniae has led to changes in the proportion of infections caused by these organisms.10,14–16
Here, we describe the epidemiology of AOM on the basis of our study in the most recent 10 years (2006–2016). Key differences that have occurred since the 1980s study include a much stricter definition of AOM and of antibiotic treatment failure, emergence of antibiotic resistance among all 3 of the dominant bacterial otopathogens, and the consequence of PCV introduction. The influence of PCVs on incidence, demographics of those experiencing AOM infection, risk factors for AOM, and etiology were determined in our large prospectively managed cohort. Similarly, characterization of factors contributing to otitis proneness, in which children suffer from recurrent otitis media with ≥3 episodes within 6 months or ≥4 in a year was accomplished.
Methods
Patient Population
Children included in this study were in their first 3 years of life, recruited as part of a prospective cohort as previously described.13 Healthy term infants were recruited at 6 months of age and managed with collection of nasopharyngeal and blood samples at regular intervals from 6 to 36 months (6-, 9-, 12-, 15-, 18-, 24-, and 36-months visits) and at every AOM episode. The study was approved by the University of Rochester and the Rochester General Hospital Research Subjects Review Boards.
Definitions
(A) AOM: The diagnosis of AOM was made by 2 validated otoscopists. AOM was diagnosed with acute onset of possible otalgia and had TMs that were: (a) mild, moderate, or severe bulging, (b) completely opacified or a cloudy or purulent effusion was observed, and (c) TM mobility was reduced and/or absent, consistent with the American Academy of Pediatrics 2013 guidelines.5 (B) Stringently defined otitis prone (sOP): A child was considered sOP if he or she experienced at least 3 AOM episodes within 6 months or at least 4 AOM episodes in a year. (C) Nonotitis prone (NOP): A child who had no AOM episodes or low frequency of AOM episodes. (D) Antibiotic treatment failure: If a child returned for medical care with symptoms and signs of AOM within 2 weeks of receiving antibiotic therapy for a previous AOM episode, that episode was considered AOM treatment-failure and a second tympanocentesis was performed.
Treatment: After diagnosis of AOM, each child received a high-dose of amoxicillin and/or clavulanate for 5 days regardless of the child’s age.18 On the basis of tympanocentesis culture results that identify the frequency of amoxicillin-resistant otopathogens in our study population (50% of H influenzae and 100% of M catarrhalis are β-lactamase producing), the patients are predominantly treated with amoxicillin-clavulanate. A shorter course of 5-day treatment is based on our own research18 and a Cochrane Database of Systematic Reviews protocol showing a marginal benefit of 10-day over 5-day antibiotic treatment.19 Children allergic to amoxicillin or who previously could not tolerate amoxicillin and/or clavulanate received cefdinir (∼15% of cases).
Risk factors for AOM: A questionnaire was administered to parents detailing sex, race and ethnicity, day care attendance, and whether the child was exposed to secondhand smoke at home (parental smoking).
Family history of recurrent AOM: A sibling and parental history of recurrent AOM was considered to have >3 episodes of AOM or ear tube insertion surgery for persistent or recurrent MEF in a sibling or either parent, occurring before the child was enrolled in study.
Breastfeeding: We defined the duration of breastfeeding by asking parents how often the child was being breastfed at 6 months of age by providing parents with the following percentages: 0%, 50%, or 100% of the time.
Atopy: A child with atopy presented with allergic hypersensitivity reactions to one or more of the following: eczema, allergic rhinitis, or allergic asthma.
Vaccine status: All the study children received 4 doses of either 7-valent-PCV (enrolled before April 2010) or 13-valent-PCV (enrolled after April 2010) at 2, 4, and 6 months of age with a booster at 15 months, along with the regular pediatric immunization schedule of vaccines.
Sample Collection and Microbiology
Details of sample collection, processing, and analysis have been described previously.13,20,21 Tympanocentesis was performed and 50 to 250 µL of MEF were obtained. Bacteria were isolated from MEF according to standard laboratory culture procedures. Antibiotic-susceptibility testing was performed as previously described.14,16
Data Analysis
Odds ratios for risk factors were calculated by using multiple logistic regressions with AOM or otitis proneness as an outcome. Starting models contained all variables with 2-way interactions, and a stepwise model selection was performed in both forward and backward directions by using the Bayesian information criterion in base R version-3.1.1. For otopathogens distribution in the MEF during AOM in both sOP and NOP populations, a difference in proportions test (χ2 or Fisher’s exact test) was used. A P value of <.05 was considered significant.
Results
Demographics and Incidence of AOM
Data were analyzed for 615 children who were managed for at least 2 years after enrollment at 6 months of age. Table 1 shows the demographic characteristics of these children. The cumulative experience of AOM to age 1, 2, and 3 to 4 years for 615 children is shown in Table 2. The mean number of AOM episodes decreased from 0.38 per child during year 1 to 0.15 per child in year 3. The incidence of AOM in each of the 3 years is shown in Table 3. By age 1, 23.0% of children had ≥1 episode of AOM and 3.6% had ≥3 episodes. The peak incidence of AOM occurred ∼12 months of age. Figure 1 shows age of first episode of AOM for children managed from birth to age 3 years. In our population, 26 children had AOM treatment failure (∼5%) in which the child was diagnosed again with AOM within 15 days of first antibiotic treatment.
TABLE 1.
Demographic Characteristics of 615 Children From Rochester, New York, Who Were Observed for AOM
| Characteristics | No. | % |
|---|---|---|
| Sex | ||
| Boy | 324 | 52.7 |
| Girl | 288 | 46.8 |
| Unknown/not reported | 3 | 0.5 |
| Siblings | ||
| Yes | 372 | 60.5 |
| No | 239 | 38.9 |
| Unknown/not reported | 4 | 0.7 |
| Family history of ear infection | ||
| Yes | 256 | 41.6 |
| No | 347 | 56.4 |
| Unknown/not reported | 12 | 2.0 |
| Exposure to smoke | ||
| Yes | 80 | 13.0 |
| No | 532 | 86.5 |
| Unknown/not reported | 3 | 0.5 |
| Breastfed at 6 mo of age (%) | ||
| 0 | 162 | 26.3 |
| 50 | 74 | 12.0 |
| 100 | 132 | 21.5 |
| Unknown/not reported | 247 | 40.2 |
| Atopy | ||
| Yes | 158 | 25.7 |
| No | 423 | 68.8 |
| Unknown/not reported | 34 | 5.5 |
| Race | ||
| Non-Hispanic white | 424 | 68.9 |
| African American | 74 | 12.0 |
| Hispanic | 22 | 3.6 |
| Asian American | 8 | 1.3 |
| Multiracial | 57 | 9.3 |
| Other | 15 | 2.4 |
| Unknown/not reported | 15 | 2.4 |
| Day care at 1 y of age | ||
| Yes | 131 | 21.3 |
| No | 308 | 50.1 |
| Unknown/not reported | 176 | 28.6 |
TABLE 2.
Subjects Experiencing Cumulative Incidence of AOM in 615 Children in Rochester, New York
| % of Children Experiencing Cumulative # of Episodes | |||
|---|---|---|---|
| Age (y) | ≥1 | ≥3 | ≥5 |
| ≤1 | 23.0 | 3.6 | 0.3 |
| ≤2 | 41.6 | 13.2 | 3.8 |
| ≤4 | 59.9 | 23.6 | 7.8 |
TABLE 3.
AOM in Children in Rochester, New York, Managed Until Age of 3 y
| Year of Life | Total Subjects | Mean (Range) # of Episodes per Subject | Percentage of Subjects With Each No. Episodes | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | >4 | |||
| 1 | 615 | 0.38 (0–5) | 76.9 | 13.2 | 6.1 | 2.3 | 1.0 | 0.3 |
| 2 | 523 | 0.48 (0–6) | 72.8 | 13.9 | 8.0 | 3.4 | 1.5 | 0.2 |
| 3–4 | 399 | 0.15 (0–3) | 87.7 | 9.8 | 2.0 | 0.5 | 0.0 | 0.0 |
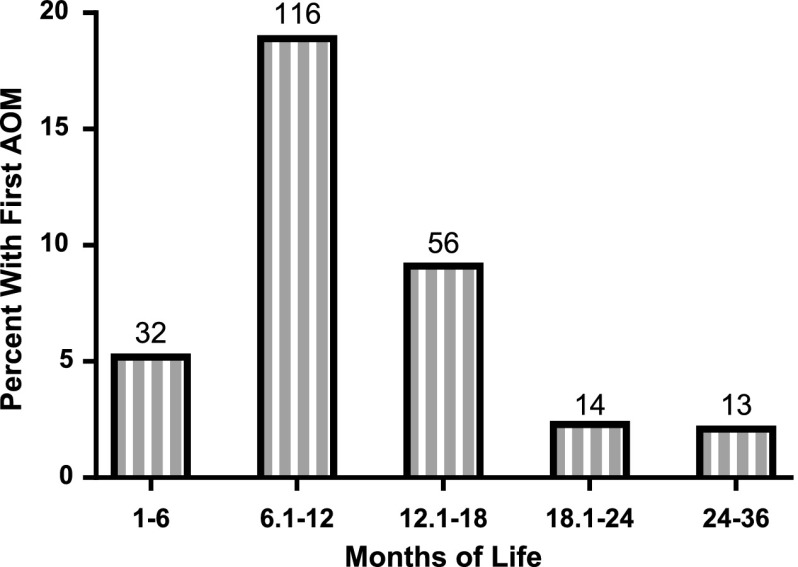
FIGURE 1.
Age at first episode of AOM in 615 children.
Variables Associated With Increased Risk of AOM
Univariate analyses of factors are shown in Table 4, which revealed a number of variables significantly associated with increased risk for experiencing AOM in the first, second, or third year of life. For ≥1 episode of AOM in the first year of life, variables included male sex, family history of ear infection, non-Hispanic white race, and attending day care. A negative association with exposure to smoke was attributable to interactions with race. In the second and third year of the children’s life, only day care attendance and family history of AOM, respectively, were significantly associated with risk of having AOM.
TABLE 4.
Univariate Analysis of Factors Potentially Associated With Risk for AOM in Children in Rochester, New York
| Factor | No. (%) With AOM | ||
|---|---|---|---|
| <1 y | 1–2 y | 2–4 y | |
| Sex | |||
| Boy | 89 (27.8)a | 83 (29.9) | 31 (14.5) |
| Girl | 49 (17.4) | 57 (23.5) | 18 (9.8) |
| P | <.01 | .116 | .204 |
| Siblings | |||
| Yes | 85 (23.1) | 80 (25.7) | 29 (12.1) |
| No | 53 (22.7) | 60 (28.7) | 20 (12.6) |
| P | 1 | .515 | 1 |
| Family history of ear infection | |||
| Yes | 73 (28.9) | 69 (29.5) | 32 (16.5) |
| No | 62 (18.2) | 68 (24.1) | 15 (7.6) |
| P | .003 | .238 | .01 |
| Exposure to smoke | |||
| Yes | 5 (6.4) | 15 (27.3) | 3 (9.1) |
| No | 133 (25.4) | 125 (26.9) | 46 (12.6) |
| P | <.001 | 1 | .756 |
| Percentage breastfed at 6 mo (%) | |||
| 0 | 72 (24.1) | 76 (29.2) | 25 (13.4) |
| 50 | 15 (17.2) | 13 (16.5) | 3 (4.6) |
| 100 | 35 (23.0) | 33 (23.9) | 17 (14.8) |
| P | .404 | .065 | .109 |
| Risk of having AOM ever with breastfeeding significant (P = .024) | |||
| Atopy | |||
| Yes | 38 (24.2) | 42 (29.6) | 15 (12.9) |
| No | 90 (21.7) | 86 (24.6) | 30 (11.5) |
| P | .604 | .31 | .822 |
| Risk of having AOM ever with atopy is significant (P = .011) | |||
| Race | |||
| Non-Hispanic white | 113 (27) | 113 (29.0) | 40 (12.5) |
| African American | 7 (9.5) | 8 (18.6) | 5 (23.8) |
| Hispanic | 1 (4.5) | 2 (13.3) | 1 (20.0) |
| Asian American | 0 (0.0) | 4 (50.0) | 0 (0.0) |
| Multiracial | 11 (19.6) | 7 (17.1) | 1 (3.7) |
| Other | 2 (15.4) | 5 (38.5) | 2 (20) |
| P | <.01 | .121 | .311 |
| Day care at 12 mo | |||
| Center | 44 (54.3) | 81 (43.2) | 13 (18.8) |
| Home | 15 (30.0) | 50 (22.0) | 11 (12.2) |
| None | 58 (18.8) | 302 (23.5) | 71 (9.9) |
| P | <.0001 | <.001 | .131 |
Not all children had every visit, so percentages will vary slightly from total demographic.
Although atopy and breastfeeding were not significantly associated with AOM risk within a particular age group, atopy significantly increased the risk of experiencing at least 1 AOM episode by 3 years of age (P = .011) and breastfeeding significantly decreased this risk (P = .024) (Table 4).
Because many of these variables were closely correlated, we used a logistic regression to explore the contribution of each variable to the risk of AOM. Multivariable analysis (Table 5) showed day care attendance, non-Hispanic white race, and atopy as associated with risk of having AOM; in addition, children who had siblings in addition to a family history of AOM had an increased risk of experiencing AOM. The decreased risk associated with exposure to smoke was eliminated from the multivariable model because of interactions with race.
TABLE 5.
Multivariable Analysis of Factors Associated With AOM
| Variable | Regression Coefficient | Odds Ratio | 95% CI | P |
|---|---|---|---|---|
| Constant | −1.6604 | — | — | — |
| Day care | 1.0214 | 2.78 | 2.19–3.52 | <.0001 |
| Race non-Hispanic white | 1.0598 | 2.89 | 1.97–4.23 | .005 |
| Atopy | 0.8148 | 2.26 | 1.78–2.87 | <.001 |
| Siblings | −0.3697 | 0.69 | 0.51–0.93 | .217 |
| Family history of AOM | −0.3541 | 0.70 | 0.50–0.99 | .304 |
| Siblings and family history of AOM | 1.3135 | 3.72 | 2.39–5.80 | .003 |
—, reference.
Age at First Diagnosis of AOM
To determine the association between the risk for later AOM and age at time of first diagnosis of AOM, we included 231 children who experienced their first episode of AOM before 3 years of age. Table 6 shows the percentages of children experiencing additional AOM episodes after first diagnosis at different age intervals. Children experiencing a first AOM episode before 12 months of age had a ∼70% chance of experiencing at least one more AOM episode. Logistic regression indicated that the age at which a child first experienced AOM was significantly associated with additional AOM episodes (P < .0001), with younger first AOM episode ages predisposing children more strongly toward future AOM episodes.
TABLE 6.
Association of Age at First Episode of AOM and Number of Additional Episodes of AOM Up to 2 y of Observation After First Episode
| Age at First Episode of AOM (mo) | Total No. of Children Having AOM (N = 231) | % Experiencing Additional Episodes of AOM | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥4 | ||
| 0–6 | 32 | 25.0 | 18.8 | 21.9 | 3.1 | 31.3 |
| 6–12 | 116 | 33.6 | 18.1 | 22.4 | 10.3 | 15.5 |
| 12–18 | 56 | 48.2 | 26.8 | 12.5 | 7.1 | 5.4 |
| 18–24 | 14 | 92.9 | 0.0 | 7.1 | 0.0 | 0.0 |
| 24–36 | 13 | 76.9 | 23.1 | 0.0 | 0.0 | 0.0 |
Variables Associated With Increased Risk of Recurrent Otitis Media
Univariate analysis identified male sex, family history of AOM, and day care attendance as significantly associated with the sOP condition (Table 7). Because many of these variables were correlated, multivariable logistic regression was performed, identifying male sex, family history of AOM, and day care attendance to be risk factors for sOP, whereas breastfeeding was protective (Table 8).
TABLE 7.
Univariate Analysis of Factors Associated With Risk of Being sOP
| Factor | sOP Children | Total Children | P |
|---|---|---|---|
| Sex | |||
| Boy | 50 | 324 | <.001 |
| Girl | 18 | 288 | — |
| Siblings | |||
| Yes | 43 | 372 | .772 |
| No | 25 | 239 | — |
| Family history of ear infection | |||
| Yes | 38 | 256 | .018 |
| No | 29 | 347 | — |
| Exposure to smoke | |||
| Yes | 4 | 80 | .094 |
| No | 64 | 532 | — |
| Breastfed at 6 mo (%) | |||
| 0 | 40 | 300 | .063 |
| 50 | 6 | 88 | — |
| 100 | 11 | 152 | — |
| Atopy | |||
| Yes | 23 | 158 | .089 |
| No | 39 | 423 | — |
| Race | |||
| Non-Hispanic white | 56 | 424 | .094 |
| African American | 4 | 74 | — |
| Hispanic | 1 | 22 | — |
| Asian | 0 | 8 | — |
| Multiracial | 3 | 57 | — |
| Other | 3 | 15 | — |
| Day care at 12 mo | |||
| Center | 29 | 81 | <.0001 |
| Home | 4 | 50 | — |
| None | 25 | 308 | — |
—, not statistically significant.
TABLE 8.
Multivariable Analysis of Factors Associated With sOP Condition
| Variable | Regression Coefficient | Odds Ratio | 95% CI | P |
|---|---|---|---|---|
| Constant | −3.15 | — | — | — |
| Male sex | 0.96 | 2.60 | 1.83–3.69 | .006 |
| Breastfed | −0.01 | 0.99 | 0.98–0.99 | .016 |
| Family history of AOM | 0.80 | 2.23 | 1.62–3.09 | .013 |
| Day care attendance | 1.17 | 3.21 | 2.34–4.40 | .0002 |
—, reference.
Otopathogens in sOP and NOP Children
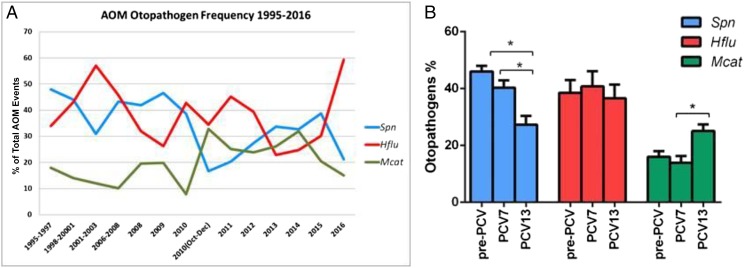
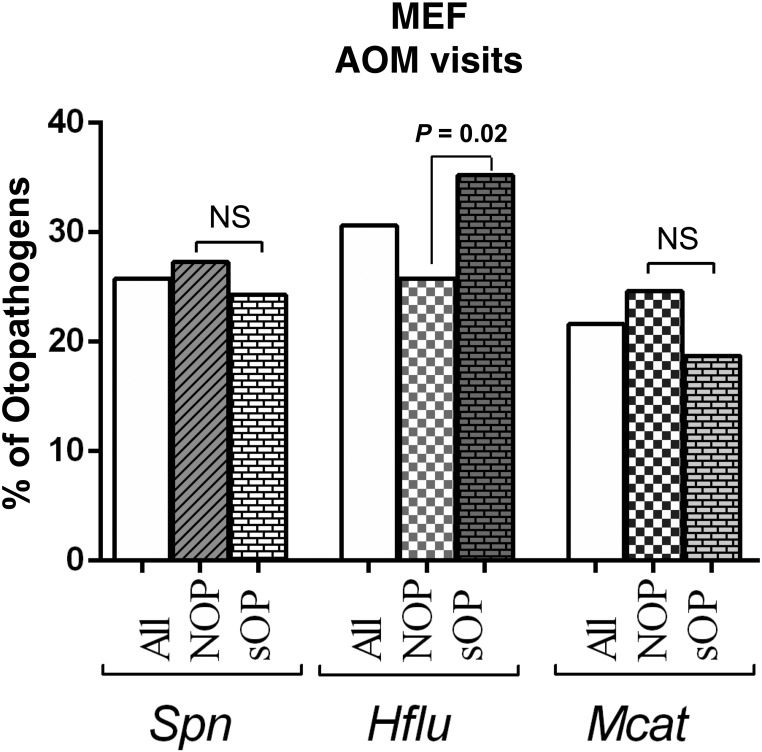
The proportion of MEF isolates yielding S pneumoniae, H influenzae and M catarrhalis have changed over time since the introduction of PCVs. Deployment of different PCV formulas has been followed by changes in the relative prevalence of S pneumoniae, H influenzae, and M catarrhalis (Fig 2A). In particular, dividing the study period into 3 eras (pre-PCV: 1995–2001, PCV7: 2001–2010, PCV13: 2010–2016) shows a significant decrease in S pneumoniae prevalence and a simultaneous increase in M catarrhalis prevalence as causative pathogens of AOM, particularly since the introduction of PCV13 (Fig 2B). H influenzae has remained a predominant otopathogen, and our latest data for 2016 reveals a surge in H influenzae prevalence (Fig 2A). From 2006 to 2016, MEF samples tested culture positive for no otopathogen in 27.6% ± 3.6% of cases. During the time frame of 2006–2016, similar otopathogen proportions in sOP and NOP children were observed except for H influenzae, which was significantly more prevalent in sOP (35.2%) than NOP (25.8%, P = .02) (Fig 3). Multiple otopathogens were only rarely isolated from the MEF in both sOP and NOP. The proportion of MEF S pneumoniae isolates that were intermediately susceptible to penicillin was 24%, whereas those resistant to penicillin was 16%; total penicillin nonsusceptible 40%. The proportion of β-lactamase producing H influenzae isolated from MEF (rendering the organisms resistant to amoxicillin) was 45%, and the proportion of β-lactamase producing M catarrhalis was 100%.
FIGURE 2.
(A) The frequency of otopathogens isolated from MEF during AOM from 1995 to 2016. (B) The changes in otopathogen prevalence in different vaccine eras (* P < .05). Spn, S pneumonia; Hflu, H influenzae; and Mcat, M catarrhalis.
FIGURE 3.
Otopathogens isolated from MEF during AOM visits in NOP (n = 256) and otitis-prone (n = 267) children. Spn, S pneumonia; Hflu, H influenzae; and Mcat, M catarrhalis. NS, not significant.
Discussion
AOM is a complex disease with many different factors contributing to its epidemiology. By applying strict inclusion and diagnostic criteria, confirming every AOM by tympanocentesis and microbiological culture over a 10-year period, we performed an unprecedented study to investigate the risk factors associated with AOM infections. We sought to update the seminal work of Teele et al1 published in 1989 after completion of their prospective cohort study in Boston, Massachusetts, regarding epidemiology of otitis media during the first years of life in children.
Incidence
In our study, by 1 year of age, 23% of children experienced ≥1 episode of AOM and 3.6% have had ≥3 episodes of AOM. By 3 years of age ∼60% of children have had ≥1 episode of AOM and ∼24% have had ≥3 episodes of AOM, with ∼11% meeting the sOP definition. In the 1989 report from the Boston pediatric cohort, by 3 years of age >80% of children had experienced ≥1 AOM and >40% had ≥3 episodes; a significant decrease of AOM cases appears to have occurred by comparing the results from the current situation. The most prominent likely explanation is the change in definition of AOM and confirmation of all diagnoses in our cohort in Rochester, New York, by tympanocentesis. However, we note that Chonmaitree et al22 in Galveston, Texas, applied a similar diagnostic criteria as we did and found that 46% of children had ≥1 episode of AOM by 1 year of age; a higher incidence than the 23% incidence we observed, probably due to populations demographics differences in our children compared with the Galveston, Texas, cohort. The observed rate of culture negative MEF results in which no otopathogens was detected during AOM in our population was ∼27% from 2003 to 2016 (PCV era). Researchers conducting studies during the pre-PCV era (1980–2000) have reported highly variable culture negative rates (7%,23 22%,24 24%25, and 42%26) in MEF samples of AOM. Our observations fall within this range, but we find no evidence of a trend toward increasing culture negative rates in MEF since the introduction of PCVs. By using a polymerase chain detection in a subset of culture negative samples we detected the presence of S pneumoniae, H influenza, or M catarrhalis in 17% of culture negative samples to the total detection of otopathogens in >88% of MEF samples during AOM.27 An overall decline in the incidence of otitis media (including AOM, otitis media with effusion, and chronic suppurative otitis media) over the past decade has been reported in a recent review.28 However, the studies included in that review include many studies that may have significant methodological flaws.29 The stringent AOM diagnostic criteria employed in our study eliminates mis- or overdiagnosis of AOM, which has been noted as a significant challenge.30,31 The mean number of episodes in our cohort was only 0.4 with a range of 0 to 5 episodes compared with 1.2 with a range of 0 to 6 episodes by Teele et al,1 a threefold reduction. The peak incidence of AOM occurred during 6 to 12 months of age in our study is consistent with Teele et al1,32,33 and various previous prospective studies.22,28,34
Risk Factors for AOM
Several meta-analyses have been published regarding the risk factors of AOM including chronic and/or recurrent otitis media.35–37 Many variables were examined in our study, including sex, race, siblings, family history of ear infection, smoke exposure, breastfeeding, atopy, and day care attendance to identify those that might be predictive for risk of AOM and recurrent AOM.
Sex
We found male sex to be a significant risk factor for developing AOM in the first year of life but not in the second or third year of life. Multivariable analysis did not identify male sex as a risk factor for AOM when the cumulative risk was estimated over the first 3 years of life. Male sex was a significant risk factor of being sOP both in univariate and multivariable analyses consistent with Teele et al1,33 and most epidemiologic studies.38–40 Boys have frequently been observed to show heightened susceptibility to infectious diseases; this has been attributed to interactions between sex hormones and T helper 1 and 2 cytokine balances.41
Family History
The strongest risk factor of AOM and sOP condition was a family history of AOM, which was consistent with the findings of Teele et al.1 Evaluation of the genetic contribution to the development of otitis media remains challenging. Researchers have evaluated the potential association between single-nucleotide polymorphisms of selected genes in determining susceptibility to acute or recurrent AOM.42–47 Esposito et al42 showed an association between variants in genes’ encoding factors of innate or adaptive immunity and recurrent AOM. Recently, genome-wide association studies of AOM were performed and identified the 6q25.3 locus associated with AOM,46 and chromosome 17q12 and 10q22.3 regions associated with recurrent AOM.47
Race
Our multivariable analysis identified a significant increase in AOM risk associated with non-Hispanic white race. Teele et al1 included only non-Hispanic white subjects in their study, so no comparison is possible. African American and Asian American infants are less likely to be diagnosed with AOM probably because of care seeking behavior; race is confounded by multiple social factors.48 In our data set, 19.2% African American and Hispanic children attended day care compared with 32.2% of white children (P = .0825).
Day Care
Day care attendance was the strongest single predictor of both AOM and becoming sOP in our study cohort, probably due to increased environmental exposure of children to otopathogen nasal colonization and upper respiratory viral infections that facilitate AOM pathogenesis. Teele et al1 did not include day care as a risk factor, but other researchers have found day care as a major risk factor for AOM.40,44,49–52
Smoking
Previous studies have revealed either high or no risk of AOM with passive smoke exposure.35,52–55 Teele et al1 did not find an association of smoking with AOM by using a multivariable analysis, but a univariate analysis showed parent smoking as a risk factor for AOM in the first year of life. Surprisingly, our univariate analysis found a significant decrease in AOM incidence among children <12 months old who were exposed to secondhand smoke, but this association was not significant after multivariable calculations. In our study, ∼13% of children were exposed to parental smoking overall. However, 28% of African American and Hispanic children and only 9% of non-Hispanic white children were exposed to household smoke (P < .0001). Because non-Hispanic white race is a significant risk factor of AOM in our study, the identification of secondhand smoke exposure as protective against AOM was found to be attributable to the significantly reduced incidence of smoking in the populations at greatest risk for AOM.
Atopy
We found atopy to be a significant risk factor for AOM but no association with sOP condition. Although Teele et al1 did not explore atopy as an AOM risk factor, researchers for other studies have revealed an association of atopy and recurrent AOM and chronic otitis media with effusion.37,56 Lack et al57 showed that obstruction of Eustachian tube can occur because of allergies.
Breastfeeding
Children who were breastfed 50% of the time up to 6 months of age were significantly less likely to experience at least 1 AOM episode, and breastfeeding was associated with an overall decreased risk of AOM. Teele et al1 also found that breastfed infants had lower risk of AOM as well as recurrent AOM, which is consistent with many other studies.35,40,44,58,59 Sabirov et al60 have shown the likely mechanism by which breastfeeding decreases risk of AOM; breastfeeding stimulates the immune response of infants, measured as higher concentrations of antibodies against otopathogens.
Age at First AOM Episode and sOP Condition
We found that younger age for a first AOM episode was a major predictor of the likelihood to become otitis prone. Teele et al1 made a similar observation as did Macintyre et al.34,61
Otopathogens in sOP and NOP Children
Before PCVs, various studies reported S pneumoniae and H influenzae as the main otopathogens of otitis media, including recurrent AOM.26,62–67 We have been prospectively obtaining MEF by tympanocentesis since 1995 and have observed many dynamic changes in the proportions of S pneumoniae, H influenza, and M catarrhalis isolates. PCVs introduction has consistently been associated with a decline of AOM infections caused by S pneumoniae followed by an increase as strains expressing nonvaccine capsular types have emerged.14–16 We also have consistently observed an increase in antibiotic resistant strains for S pneumoniae, H influenza, and M catarrhalis, more so in children frequently treated with antibiotics because they are otitis prone.
Our study has strengths and weaknesses that were inherent to our study design. The strengths included a prospective, longitudinal study design along with a large sample size; strict definitions of AOM, otitis prone, and antibiotic treatment failure; consistency of diagnosis criteria applied by only 2 highly qualified, validated otoscopists; and consistency in antibiotic treatment regimen. Another strength that is also a weakness relates to the fact that all the children had tympanocentesis to confirm the diagnosis and determine the etiology of AOM. We have previously shown that tympanocentesis is a therapeutic procedure.30,68 Not only does the procedure reduce pain as soon as the pressure of the middle ear infection is relieved but also it reduces the incidence of subsequent AOM and the need to insert tympanostomy tubes.30 Our results are likely to be generalizable to other primary care pediatric practices serving a diverse population typically seen in a suburban practice setting. Child populations that greatly differ from the demographic description of our study cohort may not have similar results.
Conclusions
The epidemiology of AOM has undergone shifts over the past 30 years, especially since the introduction of PCVs. Because the risk factors for AOM we identify in this study are predominantly similar to those described by Teele et al,1 we conclude that much of the shift in otopathogen prevalence can be attributed to the influence of the vaccine and changes in diagnostic criteria for AOM versus otitis media with effusion. In comparison with 30 years ago, (1) the number of AOM episodes and the number of otitis-prone children have decreased, and major contributors to this decrease are the introduction of PCV formulations and more stringent diagnostic criteria; (2) AOM early in life remains a predictor of otitis-prone condition; (3) day care attendance and family history of AOM are predominant risk factors in both AOM and becoming otitis prone; and (4) the otopathogens mix has undergone multiple dynamic changes that likely will continue in the years ahead.
Acknowledgments
We thank Dr Janet Casey, the nurses and staff of Legacy Pediatrics, the parents who consented, and the children who participated in this long and challenging study. We thank all of the technicians at Dr Michael Pichichero’s laboratory who performed bacterial identifications on children samples.
Glossary
- AOM
acute otitis media
- MEF
middle ear fluid
- NOP
nonotitis prone
- PCV
pneumococcal conjugate vaccine
- sOP
stringently defined otitis prone
- TM
tympanic membrane
Footnotes
Dr Kaur supervised data collection, conducted the data collection and analysis, and drafted the initial manuscript; Dr Morris conducted the initial analyses and critically reviewed the manuscript; Dr Pichichero conceptualized and designed the study and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institutes of Health (NIH), grant NIDCD-R0108671. Dr Pichichero is also supported by Sanofi Pasteur and Pfizer. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2017-1966.
References
- 1.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94 [DOI] [PubMed] [Google Scholar]
- 2.Marcy M, Takata G, Chan LS, et al. . Management of acute otitis media. Evid Rep Technol Assess (Summ). 2000;(15):1–4 [PMC free article] [PubMed] [Google Scholar]
- 3.Pichichero M. Widening differences in acute otitis media study populations. Clin Infect Dis. 2009;49(11):1648–1649 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S, Shapiro NL, Bhattacharyya N. Incremental health care utilization and costs for acute otitis media in children. Laryngoscope. 2014;124(1):301–305 [DOI] [PubMed] [Google Scholar]
- 5.Siddiq S, Grainger J. The diagnosis and management of acute otitis media: American Academy of Pediatrics Guidelines 2013. Arch Dis Child Educ Pract Ed. 2015;100(4):193–197 [DOI] [PubMed] [Google Scholar]
- 6.Lieberthal AS, Carroll AE, Chonmaitree T, et al. . The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e964 [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Casey JR, Newman E, Pichichero ME. Otitis-prone children have immunologic deficiencies in naturally acquired nasopharyngeal mucosal antibody response after Streptococcus pneumoniae colonization. Pediatr Infect Dis J. 2016;35(1):54–60 [DOI] [PubMed] [Google Scholar]
- 8.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis. 2012;205(8):1225–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204(4):645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichichero ME, Casey JR, Hoberman A, Schwartz R. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003-2006. Clin Pediatr (Phila). 2008;47(9):901–906 [DOI] [PubMed] [Google Scholar]
- 11.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29(5):1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J. 2011;30(8):645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedel V, Zilora S, Bogaard D, Casey JR, Pichichero ME. Five-year prospective study of paediatric acute otitis media in Rochester, NY: modelling analysis of the risk of pneumococcal colonization in the nasopharynx and infection. Epidemiol Infect. 2014;142(10):2186–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32(8):805–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur R, Casey JR, Pichichero ME. Emerging Streptococcus pneumoniae strains colonizing the nasopharynx in children after 13-valent Pneumococcal conjugate vaccination in comparison to the 7-valent era, 2006-2015. Pediatr Infect Dis J. 2016;35(8):901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451–1465 [DOI] [PubMed] [Google Scholar]
- 18.Pichichero ME, Marsocci SM, Murphy ML, Hoeger W, Francis AB, Green JL. A prospective observational study of 5-, 7-, and 10-day antibiotic treatment for acute otitis media. Otolaryngol Head Neck Surg. 2001;124(4):381–387 [DOI] [PubMed] [Google Scholar]
- 19.Kozyrskyj A, Klassen TP, Moffatt M, Harvey K. Short-course antibiotics for acute otitis media. Cochrane Database Syst Rev. 2010;(9):CD001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur R, Casey JR, Pichichero ME. Relationship with original pathogen in recurrence of acute otitis media after completion of amoxicillin/clavulanate: bacterial relapse or new pathogen. Pediatr Infect Dis J. 2013;32(11):1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur R, Kim T, Casey JR, Pichichero ME. Antibody in middle ear fluid of children originates predominantly from sera and nasopharyngeal secretions. Clin Vaccine Immunol. 2012;19(10):1593–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chonmaitree T, Trujillo R, Jennings K, et al. . Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137(4):e20153555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liston TE, Foshee WS, McCleskey FK. The bacteriology of recurrent otitis media and the effect of sulfisoxazole chemoprophylaxis. Pediatr Infect Dis. 1984;3(1):20–24 [DOI] [PubMed] [Google Scholar]
- 24.Leibovitz E, Dagan R, Laver JH, et al. . Interleukin 8 in middle ear fluid during acute otitis media: correlation with aetiology and bacterial eradication. Arch Dis Child. 2000;82(2):165–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chonmaitree T, Owen MJ, Patel JA, Hedgpeth D, Horlick D, Howie VM. Effect of viral respiratory tract infection on outcome of acute otitis media. J Pediatr. 1992;120(6):856–862 [DOI] [PubMed] [Google Scholar]
- 26.Pelton SI, Teele DW, Shurin PA, Klein JO. Disparate cultures of middle ear fluids. Results from children with bilateral otitis media. Am J Dis Child. 1980;134(10):951–953 [DOI] [PubMed] [Google Scholar]
- 27.Kaur R, Adlowitz DG, Casey JR, Zeng M, Pichichero ME. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr Infect Dis J. 2010;29(8):741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schilder AG, Chonmaitree T, Cripps AW, et al. . Otitis media. Nat Rev Dis Primers. 2016;2:16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichichero ME, Casey JR. Comparison of study designs for acute otitis media trials. Int J Pediatr Otorhinolaryngol. 2008;72(6):737–750 [DOI] [PubMed] [Google Scholar]
- 30.Pichichero ME, Casey JR, Almudevar A. Reducing the frequency of acute otitis media by individualized care. Pediatr Infect Dis J. 2013;32(5):473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichichero ME. Otitis media. Pediatr Clin North Am. 2013;60(2):391–407 [DOI] [PubMed] [Google Scholar]
- 32.Teele DW, Klein JO, Rosner B, et al. . Middle ear disease and the practice of pediatrics. Burden during the first five years of life. JAMA. 1983;249(8):1026–1029 [PubMed] [Google Scholar]
- 33.Teele DW, Klein JO, Rosner BA. Epidemiology of otitis media in children. Ann Otol Rhinol Laryngol Suppl. 1980;89(3 pt 2):5–6 [DOI] [PubMed] [Google Scholar]
- 34.Revai K, Dobbs LA, Nair S, Patel JA, Grady JJ, Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119(6). Available at: www.pediatrics.org/cgi/content/full/119/6/e1408 [DOI] [PubMed] [Google Scholar]
- 35.Uhari M, Mäntysaari K, Niemelä M. A meta-analytic review of the risk factors for acute otitis media. Clin Infect Dis. 1996;22(6):1079–1083 [DOI] [PubMed] [Google Scholar]
- 36.Bardach A, Ciapponi A, Garcia-Marti S, et al. . Epidemiology of acute otitis media in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2011;75(9):1062–1070 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Xu M, Zhang J, Zeng L, Wang Y, Zheng QY. Risk factors for chronic and recurrent otitis media-a meta-analysis. PLoS One. 2014;9(1):e86397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharyya N, Shapiro NL, Vakharia KT. Influence of race and ethnicity on access to care among children with frequent ear infections. Otolaryngol Head Neck Surg. 2010;143(5):691–696 [DOI] [PubMed] [Google Scholar]
- 39.Vakharia KT, Shapiro NL, Bhattacharyya N. Demographic disparities among children with frequent ear infections in the United States. Laryngoscope. 2010;120(8):1667–1670 [DOI] [PubMed] [Google Scholar]
- 40.Hatakka K, Piirainen L, Pohjavuori S, Poussa T, Savilahti E, Korpela R. Factors associated with acute respiratory illness in day care children. Scand J Infect Dis. 2010;42(9):704–711 [DOI] [PubMed] [Google Scholar]
- 41.Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014;209(suppl 3):S120–S126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito S, Marchisio P, Orenti A, et al. . Genetic polymorphisms of functional candidate genes and recurrent acute otitis media with or without tympanic membrane perforation. Medicine (Baltimore). 2015;94(42):e1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ilia S, Goulielmos GN, Samonis G, Galanakis E. Polymorphisms in IL-6, IL-10, TNF-α, IFN-γ and TGF-β1 genes and susceptibility to acute otitis media in early infancy. Pediatr Infect Dis J. 2014;33(5):518–521 [DOI] [PubMed] [Google Scholar]
- 44.Hoffman HJ, Daly KA, Bainbridge KE, et al. . Panel 1: epidemiology, natural history, and risk factors. Otolaryngol Head Neck Surg. 2013;148(suppl 4):E1–E25 [DOI] [PubMed] [Google Scholar]
- 45.Rye MS, Warrington NM, Scaman ES, et al. . Genome-wide association study to identify the genetic determinants of otitis media susceptibility in childhood. PLoS One. 2012;7(10):e48215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Ingen G, Li J, Goedegebure A, et al. . Genome-wide association study for acute otitis media in children identifies FNDC1 as disease contributing gene. Nat Commun. 2016;7:12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casselbrant ML, Mandel EM, Jung J, et al. . Otitis media: a genome-wide linkage scan with evidence of susceptibility loci within the 17q12 and 10q22.3 regions. BMC Med Genet. 2009;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vernacchio L, Lesko SM, Vezina RM, et al. . Racial/ethnic disparities in the diagnosis of otitis media in infancy. Int J Pediatr Otorhinolaryngol. 2004;68(6):795–804 [DOI] [PubMed] [Google Scholar]
- 49.McCormick DP, Jennings K, Ede LC, Alvarez-Fernandez P, Patel J, Chonmaitree T. Use of symptoms and risk factors to predict acute otitis media in infants. Int J Pediatr Otorhinolaryngol. 2016;81:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daly KA, Hoffman HJ, Kvaerner KJ, et al. . Epidemiology, natural history, and risk factors: panel report from the Ninth International Research Conference on Otitis Media. Int J Pediatr Otorhinolaryngol. 2010;74(3):231–240 [DOI] [PubMed] [Google Scholar]
- 51.Hatakka K, Piirainen L, Pohjavuori S, Poussa T, Savilahti E, Korpela R. Allergy in day care children: prevalence and environmental risk factors. Acta Paediatr. 2009;98(5):817–822 [DOI] [PubMed] [Google Scholar]
- 52.Martines F, Bentivegna D, Maira E, Sciacca V, Martines E. Risk factors for otitis media with effusion: case-control study in Sicilian schoolchildren. Int J Pediatr Otorhinolaryngol. 2011;75(6):754–759 [DOI] [PubMed] [Google Scholar]
- 53.Håberg SE, Bentdal YE, London SJ, Kvaerner KJ, Nystad W, Nafstad P. Prenatal and postnatal parental smoking and acute otitis media in early childhood. Acta Paediatr. 2010;99(1):99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacoby PA, Coates HL, Arumugaswamy A, et al. . The effect of passive smoking on the risk of otitis media in Aboriginal and non-Aboriginal children in the Kalgoorlie-Boulder region of Western Australia. Med J Aust. 2008;188(10):599–603 [DOI] [PubMed] [Google Scholar]
- 55.Hammarén-Malmi S, Saxen H, Tarkkanen J, Mattila PS. Passive smoking after tympanostomy and risk of recurrent acute otitis media. Int J Pediatr Otorhinolaryngol. 2007;71(8):1305–1310 [DOI] [PubMed] [Google Scholar]
- 56.Juntti H, Tikkanen S, Kokkonen J, Alho OP, Niinimäki A. Cow’s milk allergy is associated with recurrent otitis media during childhood. Acta Otolaryngol. 1999;119(8):867–873 [DOI] [PubMed] [Google Scholar]
- 57.Lack G, Caulfield H, Penagos M. The link between otitis media with effusion and allergy: a potential role for intranasal corticosteroids. Pediatr Allergy Immunol. 2011;22(3):258–266 [DOI] [PubMed] [Google Scholar]
- 58.Vogazianos E, Vogazianos P, Fiala J, Janecek D, Slapák I. The effect of breastfeeding and its duration on acute otitis media in children in Brno, Czech Republic. Cent Eur J Public Health. 2007;15(4):143–146 [DOI] [PubMed] [Google Scholar]
- 59.Lodge CJ, Bowatte G, Matheson MC, Dharmage SC. The role of breastfeeding in childhood otitis media. Curr Allergy Asthma Rep. 2016;16(9):68. [DOI] [PubMed] [Google Scholar]
- 60.Sabirov A, Casey JR, Murphy TF, Pichichero ME. Breast-feeding is associated with a reduced frequency of acute otitis media and high serum antibody levels against NTHi and outer membrane protein vaccine antigen candidate P6. Pediatr Res. 2009;66(5):565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macintyre EA, Karr CJ, Koehoorn M, et al. . Otitis media incidence and risk factors in a population-based birth cohort. Paediatr Child Health. 2010;15(7):437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr. 2001;160(7):407–413 [DOI] [PubMed] [Google Scholar]
- 63.Weiner R, Collison PJ. Middle ear pathogens in otitis-prone children. S D J Med. 2003;56(3):103–107 [PubMed] [Google Scholar]
- 64.Bernstein JM, Faden HF, Dryja DM, Wactawski-Wende J. Micro-ecology of the nasopharyngeal bacterial flora in otitis-prone and non-otitis-prone children. Acta Otolaryngol. 1993;113(1):88–92 [DOI] [PubMed] [Google Scholar]
- 65.Giebink GS, Canafax DM. Antimicrobial treatment of otitis media. Semin Respir Infect. 1991;6(2):85–93 [PubMed] [Google Scholar]
- 66.Karma PH, Pukander JS, Sipilä MM, Vesikari TH, Grönroos PW. Middle ear fluid bacteriology of acute otitis media in neonates and very young infants. Int J Pediatr Otorhinolaryngol. 1987;14(2–3):141–150 [DOI] [PubMed] [Google Scholar]
- 67.Ruuskanen O, Arola M, Putto-Laurila A, et al. . Acute otitis media and respiratory virus infections. Pediatr Infect Dis J. 1989;8(2):94–99 [PubMed] [Google Scholar]
- 68.Casey JR, Pichichero ME. Payment analysis of two diagnosis and management approaches of acute otitis media. Clin Pediatr (Phila). 2014;53(9):865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]