Abstract
The search for efficient radioprotective agents to protect from radiation-induced toxicity, due to planned or accidental radiation exposure, is still ongoing worldwide. Despite decades of research and development of widely different biochemical classes of natural and derivative compounds, a safe and effective radioprotector is largely unmet. In this comprehensive review, we evaluated the evidence for the radioprotective performance of classical thiols, vitamins, minerals, dietary antioxidants, phytochemicals, botanical and bacterial preparations, DNA-binding agents, cytokines, and chelators including adaptogens. Where radioprotection was demonstrated, the compounds have shown moderate dose modifying factors ranging from 1.1 to 2.7. To date, only few compounds found way to clinic with limited margin of dose prescription due to side effects. Most of these compounds (amifostine, filgratism, pegfilgrastim, sargramostim, palifermin, recombinant salmonella flagellin, Prussian blue, potassium iodide) act primarily via scavenging of free radicals, modulation of oxidative stress, signal transduction, cell proliferation or enhance radionuclide elimination. However, the gain in radioprotection remains hampered with low margin of tolerance. Future development of more effective radioprotectors requires an appropriate nontoxic compound, a model system and biomarkers of radiation exposure. These are important to test the effectiveness of radioprotection on physiological tissues during radiotherapy and field application in cases of nuclear eventualities.
Keywords: Radioprotector, Free radicals, Antioxidants, Oxidative stress, Radioprotection, Ionizing radiation, Radiotoxicity
Background
The quest for agents that can protect organisms from radiation-induced damage began shortly aftershock of the nuclear detonation in Hiroshima and Nagasaki (Japan, 1945) during the Second World War. This was followed by several major radiological accidents, such as Three-Miles island (USA, 1979), Chernobyl (Russia, 1986), Fukushima (Japan, 2011) in addition to many isolated incidents (Singh et al. 2015; Gupta et al. 2013; Coeytaux et al. 2015). Furthermore, radiation preparedness plan anticipates disposing of low toxicity radioprotectors as countermeasures following threats of nuclear detonation, radiological terrorisms, and accidental radiation contamination. Radioprotectors may also be useful for staff working at different facilities of radiation sources, radiographers, patients undergoing lengthy radiological procedures, naval cadets in nuclear submarine, armed forces, flight pilots and astronauts (Stone et al. 2004). In addition, increasing number of cancer patients undergoing radiotherapy can benefit from such applications, to protect normal tissues from radiation-induced side effects, which otherwise may compromise the quality of life of cancer survivors (Hall and Giaccia 2006; Nair et al. 2001; Zelefsky et al. 2002; Kry et al. 2005).
There are two distinct mechanisms by which ionizing radiation damages DNA and causes cell death. One involves ionization of atoms in the DNA (direct effect) while the other involves attacks by free radicals produced by the radiolysis of surrounding water molecules (indirect effect). The total biological effects result in damaging cellular and tissue structures that lead to dysfunction of organs. In case of planned or inadvertent, whole or large body radiation exposure, the physiologic failures of organ systems are known as acute radiation syndromes (hematopoietic, gastrointestinal, neuro-vascular), which may generally develop after doses of as low as 1–2 Gy. The lethal dose that will result in the death of 50% of exposed individuals (LD50) is 3.5–4 Gy. With optimal supportive care and bone marrow transplantation, this dose can be increased by two-fold. Interestingly, saving only 10% of bone marrow during whole body exposure may shun the effects of LD50 dose (Kumar et al. 2012). Thus, the protection of hematopoietic system is important to rescue individuals where radioprotectors with ability to protect hematopoietic system can come into play as major radiation countermeasures. The desired properties of a radioprotector include: low toxicity in the therapeutic concentrations, ability to reduce damage to numerous organs with high-dose reduction factor, economical, abundant, and orally administered.
Radioprotective agents are broadly classified into three groups: radioprotectors, radiation mitigators and therapeutic agents. Historically, radioprotectors are referred to the agents that protect organisms from cellular and molecular damage during irradiation, predominantly by enhancing antioxidant defense mechanism through scavenging of free radicals (Singh and Hauer-Jensen 2016). Mitigators are agents administered after radiation exposure but before the appearance of symptoms and generally protect organism by enhancing DNA repair, cellular signaling and modulating thiols redox system of cells. Radiation therapeutic agents are administered after appearance of symptoms to regenerate tissues by stimulating division of functional undamaged cells (Citrin et al. 2010). Alternatively, radioprotective agents are also categorized as radioprotectors, adaptogens and absorbents (Nair et al. 2001). Radioprotectors are composed of antioxidant and sulphahydryl compounds. Adaptogens are compounds that enhance radioresistance by acting as stimulator of defense system via boosting antioxidants and repair. Lastly, absorbents are compounds that likely to perform action as chelating agents to protect individual from ingested radionuclides.
Appraisal of various classes of radioprotective agents
Although radioprotective agents can be grouped into radioprotectors, radiation mitigators and therapeutic agents or alternatively into radioprotectors, adaptogens and absorbents, there is no unified system that can unambiguously classify various compounds. There is no doubt that the number of agents studied for their radioprotective potential is overwhelmingly large. Here we sought to render a comprehensive, yet brief, current account of these diverse compounds. We evaluated the evidence for their radioprotective efficacy and the future developments in this field. Therefore, in this review we have grouped known radioprotective agents according to their biochemical classes along with the potential clinical applications as illustrated in the schematic presentation (Fig. 1). To assess the radioprotective ability, the efficacy of different radioprotectors is expressed in terms of dose modifying factor (DMF). The DMF or reduction factor is defined as the ratio of radiation dosage producing similar effects in the presence or absence of the compound. Since human experiment is not feasible, investigations were carried out using in vitro cell cultures and in vivo animal models. The 30 days survival of irradiated mice is considered the gold standard endpoint to test the efficiency of a radioprotector. The various biochemical classes of radioprotectors and derivatives were discussed below and agents with quantified level of radioprotection (DMF or % survival) are listed in Table 1 along with the mechanisms of action (Table 1).
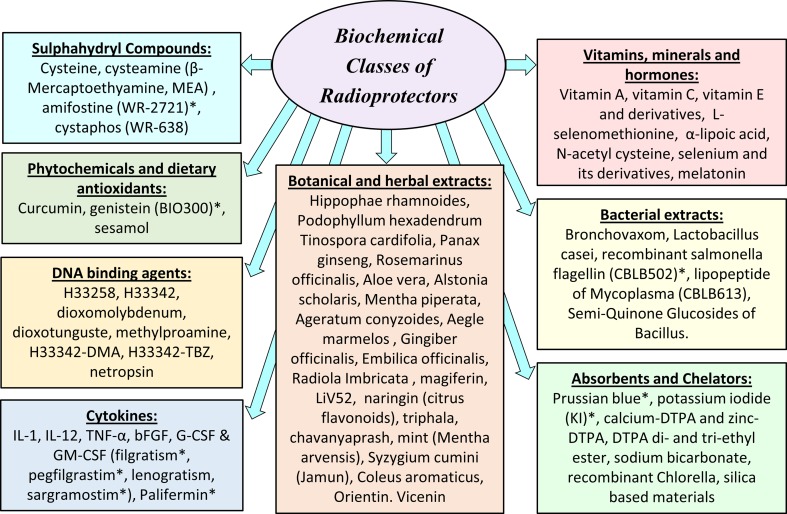
Fig. 1.
Schematic representation of various classes of radioprotectors citing most studied compounds. Asterisks indicate agents approved or under considerations for clinical use as follows: Amifostine (Ethyol) is specifically approved by FDA as a radioprotector that prevents cumulative normal tissues toxicity associated with cancer treatments, and offers significant reduction of radiation-induced xerostomia in head and neck radiotherapy patients. Genistein (BIO300) has currently an investigational new drug (IND) status as radioprotector of normal tissues to prevent acute radiation syndromes. While FDA has approved Neupogen (filgratism), Neulasta (pegfilgrastim) and Leukine (sargramostim) to ameliorate neutropenia induced by cancer treatment, these compounds are currently under investigation as radiation countermeasure agents. Palifermin (Kepivance) is an FDA-approved recombinant derivative of human keratinocyte growth factor (KGF) that is used to treat oral mucositis in patient undergoing hematopoietic stem cell transplantation. Recombinant salmonella flagellin CBLB502 (Entilimod) is FDA approved as off-label drug that can be used during nuclear or radiological accidents to protect against acute radiation syndromes. Prussian blue (Radiogardase) and potassium iodide (KI) are FDA-approved decorporating agents to increase the rate of elimination of radionuclides in internal contamination
Table 1.
List of main radioprotective compounds studied with their dose modifying factor (DMF) or the percentage (%) of survival after 30 days following its application along with the probable mechanism of action and chemical structure where available
| Group and name of compounds | DMF or % survival | Mechanism of action | Chemical structure (pubchem) | References |
|---|---|---|---|---|
| Sulfhydryl compounds | ||||
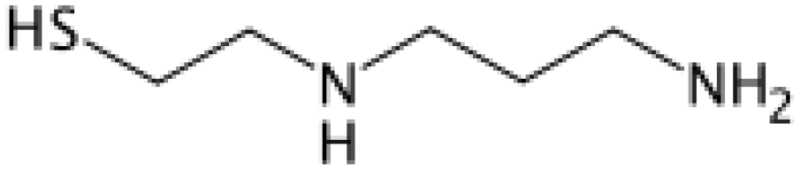
| Amifostine (WR-2721) | 2.7 | Free radical scavenging, repair |
|
Hall and Giaccia (2006) |
| Cystaphos (WR-638) | 2.1 | Free radical scavenging, repair |
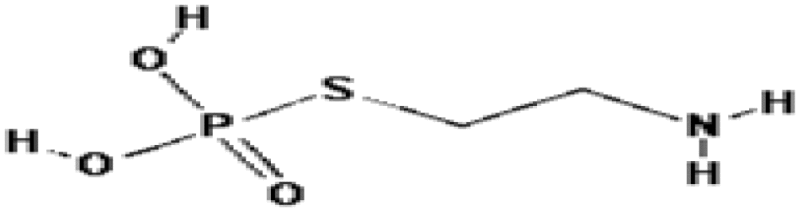
|
Hall and Giaccia (2006) |
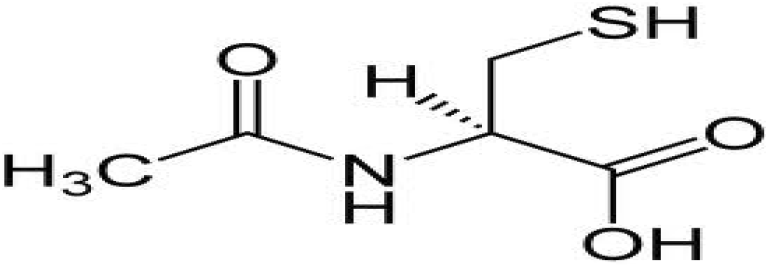
| N-Acetylcysteine | 1.1 | Free radical scavenging |
|
Landauer et al. (1988) |
| Vitamins and hormones | ||||
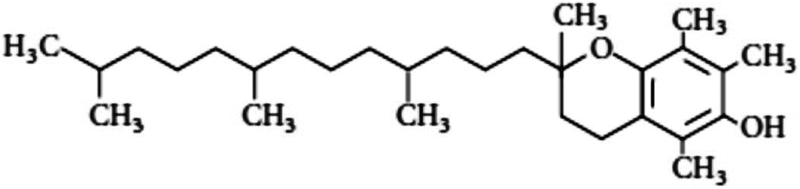
| Vitamin E (tocopherol) | 1.1 | Antioxidant, free radical scavenging |
|
Srinivasan et al. (1997) |
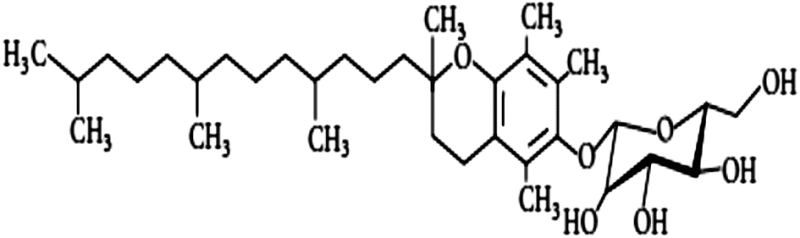
| Tocopherol monoglucoside | 1.23 | Antioxidant, free radical scavenging |
|
Satyamitra et al. (2003) |
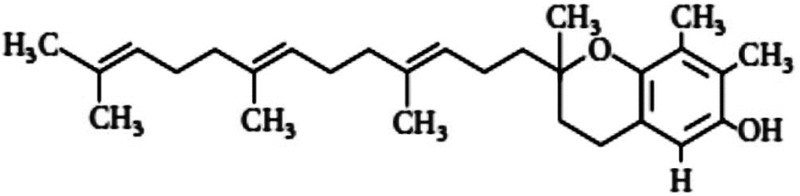
| Gamma-tocotrienol (GT3) | 1.29 | Antioxidant, free radical scavenging, stimulation of G-CSF |
|
Ghosh et al. (2009) |
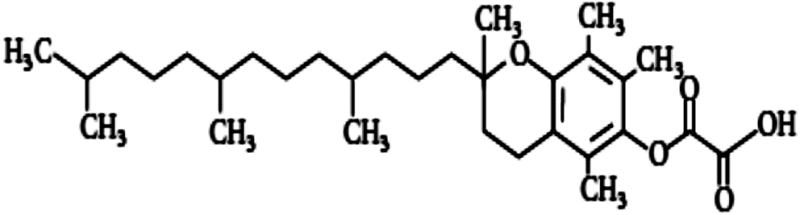
| Tocopherol succinate | 1.28 | Free radical scavenging, modulation of antioxidant enzymes |
|
Landauer et al. (1988) |
| Vitamin A | 1.11 | Free radical scavenging |
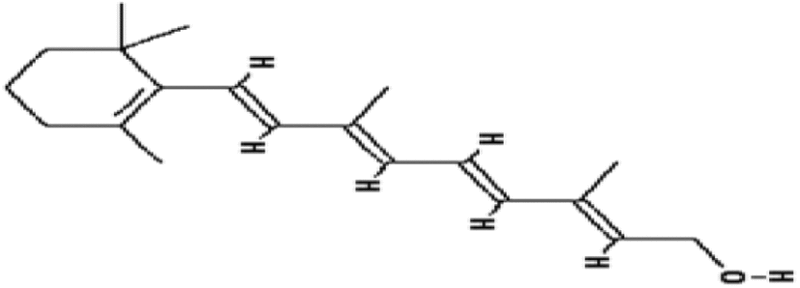
|
Seifter et al. (1984) |
| Vitamin C | No protection | Free radical scavenging |
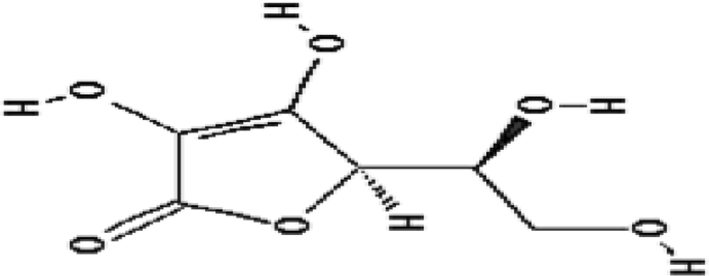
|
Harapanhalli et al. (1996) |
| Lipoic acid | 1.26 | Free radical scavenging |
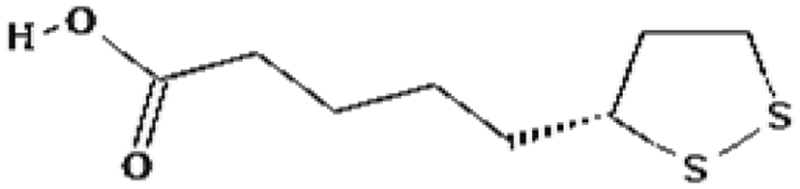
|
Ramakrishnan et al. (1992) |
| l-Selenomethionine, vitamin C, vitamin E succinate, α-lipoic acid and N-acetylcysteine (mixed) | 1.6 | Free radical scavenging |
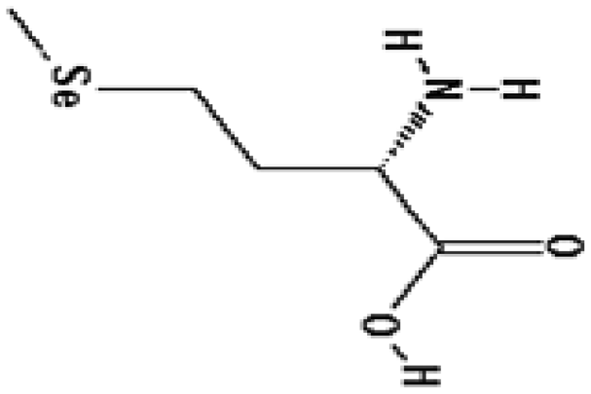
|
Wambi et al. (2008) |
| Melatonin | 86 | Free radical scavenging |
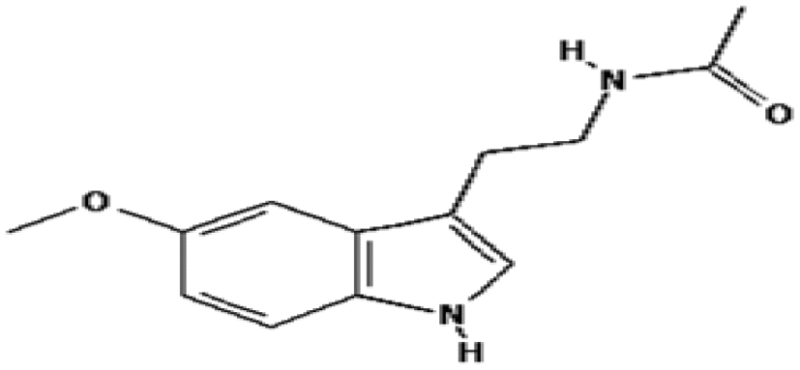
|
Vijayalaxmi et al. (1999b) |
| Phytochemicals and dietary antioxidants | ||||
| Orientin and vicenin | 1.3–1.37 | Free radical scavenging |
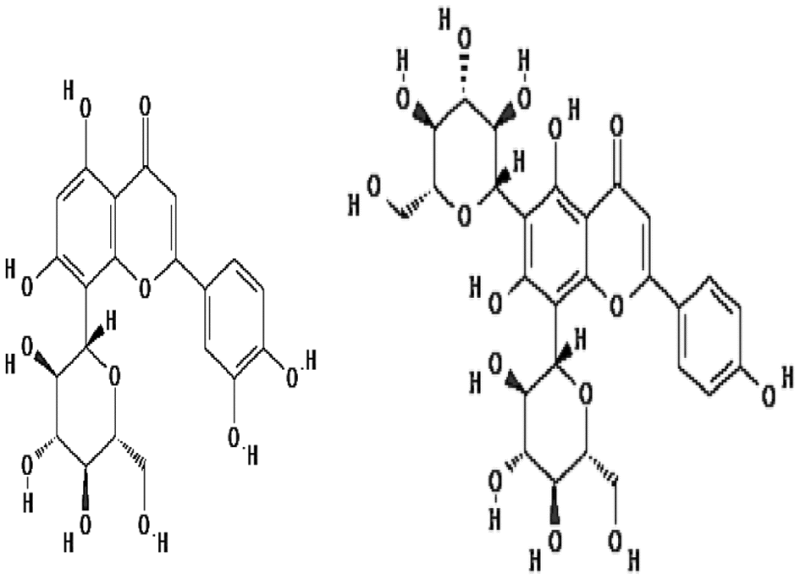
|
Uma Devi et al. (1999) |
| Genistein | 1.16 | Free radical scavenging |
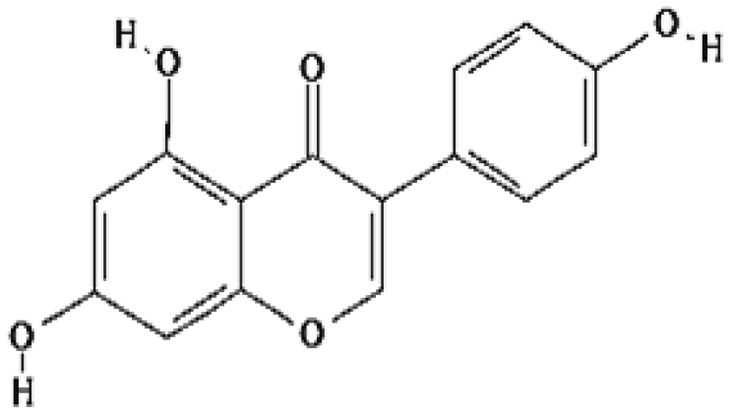
|
Landauer et al. (2003) |
| Sesamol | 100 | Free radical scavenging |
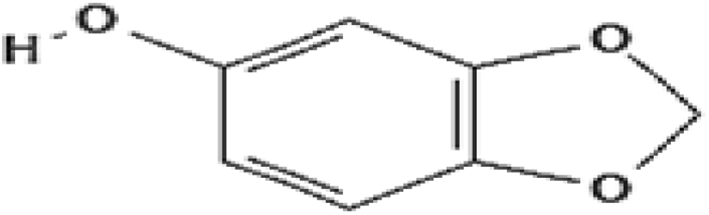
|
Khan et al. (2015) |
| Botanical and herbal extracts | ||||
| Podophyllum hexadendrum | 1.62 | Free radical scavenging | N/A | Lata et al. (2009), Gupta et al. (2007) |
| Ageratum conyzoides | 1.3 | Free radical scavenging | N/A | Jagetia et al. (2003b) |
| Aegle marmelos | 1.2 | Inhibition of lipid peroxidation | N/A | Jagetia et al. (2004b) |
| Zingiber officinale | 1.15 | Free radical scavenging | N/A | Jagetia et al. (2003a, 2004a) |
| Hippophae rhamnoides | 80 | Free radical scavenging | N/A | Prakash et al. (2005) |
| Radiola Imbricata | 90 | Free radical scavenging | N/A | Goel et al. (2006) |
| Bacterial extracts | ||||
| CBLB502 (recombinant salmonella flagellin) | 1.6 | Binding to toll-like receptor 5 and activation of nuclear factor-kB | N/A | Burdelya et al. (2008) |
| CBLB613 (lipopeptide from Mycoplasma arginini) | 1.25 | Induction of interleukins and chemokines | N/A | Singh et al. (2012) |
| Cytokines | ||||
| IL-1 | 88–100 1.05–1.12 |
Regulates the proliferation of hematopoietic cells | N/A | Neta et al. (1986), Dorie et al. (1989) |
N/A not available
Sulphahydryl compounds
The marathon search for agents that can protect humans from radiation was initiated by U.S. Army due to vivid memory of victims of Hiroshima and Nagasaki nuclear detonation. Cysteine, a thiol compound, was the first agent to confer radiation protection of mice from whole body exposure in 1949 (Patt et al. 1949). However, cysteine and its derivatives N-acetylcysteine (DMF = 1.1) had shown toxicity (nausea and vomiting) that limited its usefulness (Landauer et al. 1988). To reduce toxicity, a series of chemical compounds were synthesized and tested in Walter Reed Institute of Research (USA). The discovery of cysteamine (β-mercaptoethyamine, MEA) further accelerated the research and an analog compound named amifostine (WR-2721) was synthesized with a DMF of 2.7 (Hall and Giaccia 2006). Marketed under the trade name of Ethyol, it is the only cytoprotective agent specifically approved by the FDA as a radioprotector. Amifostine selectively protects normal tissues in multiple organs against the toxic effects of radiation and various cytotoxic drugs in cancer patients with advanced stages (Brizel et al. 2000). The compound offers significant reduction of radiation-induced xerostomia and is currently being used during radiotherapy of head and neck cancers. A related phosphocysteamine compound (WR-638, cystaphos, DMF = 2.1) was carried by Russians army for infield radioprotection. Amisfostine is an injectable non-reactive phosphorothioate prodrug that does not readily permeate cells because of phosphorothioic acid group. It is believed that dephosphorylation by alkaline phosphatase enhances the uptake of amifostine and its conversion to the active metabolite WR-1065. The free radical scavenging (hydrogen donation), DNA protection and enhanced repair properties of thiols are believed to be the mechanisms of protection. However, similar to other thiols drugs, amifostine also exhibits toxicity and causes nausea, vomiting and hypotension (Kouvaris et al. 2007). This limits the use of amifostine in fields for mass radiation casualties.
The significant toxicity and short half-life (>90% clearance in 6 min) of sulphahydryl compounds fueled the search for non-thiols-based compounds (van der Vijgh and Korst 1996). Several chemicals such as alcohols, glycerol, and glycol were also tested but failed again due to toxicity. Different antioxidant mimetic agents, nitroxide, superoxide dismutase (SOD) were also studied and boosted the exploration of natural nontoxic compounds for their radioprotective properties (Weiss and Landauer 2003).
Vitamins, minerals and hormones
Varieties of vitamins and minerals are present in the nature. Most of them are natural antioxidants owing to their abilities to neutralize free radicals, and act as cofactors in different metabolic processes. The radioprotective properties of most studied vitamins, hormones, and minerals are described here.
Vitamin E
The study on vitamin E (tocopherol, C29H50O2) and its isoforms started in early 1980, when it had been shown that either injected (100 IU/kg) or fed as diet enhances the survival of CD2F1 mice exposed to lethal dose of radiation (Srinivasan et al. 1997). A DMF of 1.06–1.1 was obtained when vitamin E was injected 1 h before irradiation. In addition, vitamin E has been shown to reduce oral mucositis in cancer patients during radiotherapy (Srinivasan and Weiss 1992). Furthermore, tocopherol monoglucosides (a vitamin E derivative) protected plasmid from radiation-induced DNA strand breaks (Rajagopalan et al. 2002). It also provided relative radiation protection of animals from lethal dose when injected intraperitoneally (600 mg/kg), with a DMF of 1.09 (Satyamitra et al. 2003). However, low DMF and requirement of high concentration limit the application of these molecules in clinics.
A series of studies on gamma-tocotrienol (GT3), a member of vitamin E family and inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA), suggested a protective effect in mice, with a DMF of 1.29, at 100 and 200 mg/kg body weight when injected 24 h before irradiation (Ghosh et al. 2009). GT3 significantly increased granulocyte colony-stimulating factor (G-CSF), interleukin-6 (IL-6) and reduced radiation-induced cytopenia (Kulkarni et al. 2010, 2012). Tocopherol succinate (TS) inhibited apoptosis in various cells and had protected mice from lethal radiation dose when it was administered (400 mg/kg) 24 h before irradiation with a DMF of 1.28 (Singh et al. 2009). The mechanism of protection is believed to be due to the stimulation of G-CSF, a glycoprotein that stimulates bone marrow to produce granulocytes and stem cells (Singh et al. 2013). Recently, TS has been suggested to mobilize progenitor cells (Singh et al. 2014). Although TS and tocotrienols had higher DMF compared to classical tocopherol (alpha), recent studies suggest that injection of high dose (75 mg/kg) of these drugs causes adverse effects in non- human primates and could increase mortality with no significant protection compared to irradiated control (Singh et al. 2016). These results suggest that vitamin E and its derivatives at high concentrations might be toxic to humans.
Ascorbic acid (Vitamin C)
Many studies have been carried out to investigate the radioprotective effect of vitamin C (C6H8O6). Although a role as antioxidant and radical scavenger is established, conflicting results envelop its effect in improving the overall survival after lethal radiation dose. Ascorbic acid was shown to reduce the formation of micronuclei and dicentrics in mice (Sarma and Kesavan 1993), and protect radionuclides-mediated DNA damage in mice sperm with a DMF of 2.2 (Narra et al. 1993). Monoglucosides’ derivatives of ascorbic acid have been shown to scavenge different free radicals and protect plasmid from radiation-induced DNA strand breaks (Mathew et al. 2007). Although supplemental vitamin C improved the survival of mice’ nucleated bone marrow cells following whole body sublethal irradiation (3.5 Gy, DMF = 1.7), it had no significant effect after lethal irradiation (9 Gy) on 30-day survival outcome (Harapanhalli et al. 1996). Nonetheless, pre-treatment of ascorbic acid (150 mg/kg/day) orally for 3 days before a lethal radiation dose (14 Gy) followed by bone marrow transplantation had reduced gastrointestinal syndrome and resulted in an enhancement of mice survival (Yamamoto et al. 2010). Post-treatment with ascorbic acid at high dose (3 g/kg) immediately after exposure (7–8 Gy) resulted in a recovery of hematopoietic system, reduced apoptosis in bone marrow cells and effectively improved survival of mice (Sato et al. 2015). It appears that ascorbic acid at high dosage could provide protection to mice after whole body irradiation. However, a high dose of 3 g/kg for 60 kg humans will be impracticable. Therefore, it has been concluded that the requirement of high pharmacologic effective doses limits the application of this molecules in clinics (Du et al. 2015).
The reason for failure of such classic antioxidants and vitamins as radioprotectors is due to their poor efficacy in whole body irradiation. Low reduction factors (DMF 1.11–1.26) were also observed for vitamin A and lipoic acid (Seifter et al. 1984; Harapanhalli et al. 1996; Ramakrishnan et al. 1992). However, a mixture of vitamins (l-selenomethionine, vitamin C, vitamin E succinate, α-lipoic acid and N-acetyl cysteine) provided improved protection with a DMF of 1.6 (Wambi et al. 2008). Clearly, vitamins and minerals act as cofactors and regulate many physiological systems in addition to their anti-radical activities. These essential nutrients are required in small amounts by living organisms for normal function. Thus, they may harm rather than help at high concentration needed a priory for radiation protection purposes.
Melatonin
The pineal hormone melatonin (C13H16N2O2) had attracted much attention when it was shown to reduce radiation-induced genetic damage and protect mice from a lethal radiation dose (8.15 Gy) in a series of work by Vijyalaxmi et al. (1999a, b). The increase in survival was dose dependent and improved from 45% without treatment to 60% (DMF = 1.33) and 85% (DMF = 1.78) for mice pretreated with 125 and 250 mg/kg melatonin, respectively. Furthermore, clinical reports indicated that melatonin administration results in a favorable ratio of radiotherapy efficacy versus toxicity during the treatment of human cancers (Vijayalaxmi et al. 2004). The mechanism of radiation protection by melatonin has been attributed to scavenging of varieties of oxygen-based radicals and DNA protective properties (Reiter et al. 2010). It has also been reported that melatonin possesses the unique property of selectively sensitizing tumor (Um et al. 2011) while protecting normal cells (Mishra et al. 2011). Further, it was suggested that melatonin could protect antioxidative enzymes including catalase (CAT), glutathione peroxidase (GPx), and SOD from depletion following UV radiation (Fischer et al. 2013).
Melatonin is highly tolerated over a wide range of doses and recent studies suggest that up to 8000 mg/kg of body weight is not toxic in mice (Ali et al. 2012). Assessing the efficacy and safety of melatonin as adjuvant therapy in concurrent chemo-radiotherapy for solid tumors, a meta-analysis of 8 randomized controlled trials concluded that 20 mg orally, once a day, led to substantial improvements in tumor remission, 1-year survival, and alleviation of therapy-related side effects (Wang et al. 2012). Although melatonin has shown some characteristics of a promising candidate radioprotector, its implication in regulating physiological functions is still inadequately understood. In addition, its short active half-life (20–50 min) and the requirement of rather high doses for radiation protection are still challenging for developing melatonin as radiation countermeasures.
Selenium
Selenium (Se) is an important chemical element that exists as cofactor for many enzymes such as GPx, thioredoxin reductase and ribonucleotide reductase in different prokaryotes and eukaryotes. Early report using selenium and its derivatives had described radioprotective effect in mice (Weiss et al. 1992). Intraperitoneal injection either before (≤1 h) or shortly after (15 min) 9 Gy radiation has enhanced the 30-day survival. Selenomethionine (SLM) has lower lethal and behavioral toxicity (locomotor activity depression) compared to sodium selenite (Weiss et al. 1992). A derivative of selenium, 3, 3, di-selenopropionic acid had also protected mice by reducing DNA damage and apoptosis (Kunwar et al. 2010). Furthermore, dietary selenium (100 µg) had mitigated the radiation-induced nephropathy in mice (Sieber et al. 2011). The combination of selenium with vitamin E has also been reported to protect gastrointestinal system from ionizing radiation (Mutlu-Turkoglu et al. 2000). A recent review of clinical trials has concluded that selenium supplementation (200–500 µg/day) improves the general conditions and the quality of life of patients and reduces the side effects without reducing the effectiveness of radiotherapy (Puspitasari et al. 2014). The authors, however; warned that high-dose and long-term supplementation may be unsafe due to selenium toxicity and that more evidence-based information and research are needed to ensure its therapeutic benefits. In addition, in a more recent randomized phase II clinical trial on 18 radiotherapy patients, selenomethionine did not lower the incidence of severe mucositis or improve quality of life or survival outcomes (Mix et al. 2015).
Phytochemicals and dietary antioxidants
Many dietary compounds from a wide range of foods and beverages have been screened for their potential modulatory effects in various human ailments on the belief that they do not only provide us with nutrients, but also enhance our capacity to fight against oxidative stress and diseases. Different natural compounds possess hepatoprotective, cardioprotective, neuroprotective, and anti-tumorigenic properties (Omar 2010; Alqasoumi 2012; Shareef et al. 2016) and these are desired traits for effective medicinal agents. The radioprotective properties of the most common natural dietary compounds are discussed here.
Curcumin
The phenolic compound curcumin (C21H20O6) present in spices (curcuma longa) was shown to protect radiation-induced genotoxicity (micronuclei and dicentrics) in cultured rat lymphocytes and hepatocytes and decreased lipid peroxidation, particularly at the highest dose (10 µg/ml) used (Srinivasan et al. 2006, 2007). An in vivo study, on the lens of rats fed with 100 mg/kg curcumin for 28 days, had reported significant decrease (from 100 to 40%) in radiation-induced (15 Gy) cataract (Ozgen et al. 2012). In addition, pre-treatment with curcumin (25–200 mg/kg) led to a dose-dependent increase in wound healing following 6 Gy whole body irradiation of mice (Jagetia and Rajanikant 2004).
In contrast to these radioprotective effects in normal cells and tissues, curcumin was reported to radiosensitize variety of tumor cells in vitro, especially by blocking NF-kB pathways (Chendil et al. 2004; Qiao et al. 2012), modulating p53, thioredoxin reductase-1, enhancing reactive oxygen species (ROS) and MAP kinase pathways (Javvadi et al. 2008, 2010; Veeraraghavan et al. 2010). The apparent differential effect of curcumin between normal (radioprotective) and tumor (radiosensitizer) cells is difficult to interpret in radiobiological terms. Curcumin is traditionally known for its anti-inflammatory effects. It has been shown to be a potent immunomodulatory agent that can regulate the activation of T cells, B cells, macrophages, neutrophils, natural killer cells, dendritic cells and can enhance antibody responses (Jagetia and Aggarwal 2007). It remains to be demonstrated whether this dissimilarity in effects pertains to a potential metamorphic ability of curcumin to modulate the immune system in such a way that it distinguishes between good (normal) and bad (cancer) cells or simply by harnessing the differences in cellular homeostasis? Thus, the studies on the radioprotective effects of curcumin are inconclusive. In addition, its poor aqueous solubility, hydrolytic degradation, poor bioavailability and metabolism may introduce technical bias in various studies (Chidambaram and Krishnasamy 2014). Although these limitations preclude the use of curcumin as radioprotector or radiosensitizer in clinic, its immunomodulatory roles merit in depth exploration for a well-defined use in medicine (Srivastava et al. 2011).
Genistein
Genistein (C15H10O5) is a natural isoflavone from soybeans that has drawn wide attention for its bioactivity and potential role in modulating radiation damage (Song et al. 2015). According to in vivo studies, genistein was reported to have a protective effect on radiation-induced intestinal damage in tumor-bearing mice and to also delay tumor growth (Son et al. 2013). A study by Calveley and colleagues on rat lung tissue had shown that although genistein treatment decreased the levels of many inflammatory cytokines and protected from DNA damage, it provided partial protection against pneumonitis and fibrosis (Calveley et al. 2010). Subcutaneous pre-administration of genistein (25–400 mg/kg), 24 h before irradiation (9.5 Gy) enhanced the 30-day survival of mice (DMF = 1.16), whereas survival was not different from controls when it was administered one hour before irradiation (Landauer et al. 2003). In addition, enhanced hematopoietic cell recovery and 30-day survival of mice (97% compared to 31%) were described following subcutaneous administration of genistein (200 mg/kg) 24 h prior to lethal radiation (8.75 Gy) dose (Davis et al. 2007).
As for in vitro studies, it has been shown that low concentration of genistein (1.5 µM) could protect L-02 cells from radiation-induced injury via inhibition of apoptosis, DNA damage and chromosome aberration; meanwhile, high concentration (20 µM) demonstrated radiosensitizing effect via opposite increase in apoptosis, chromosome aberration and impaired DNA repair along with up-regulation and down-regulation of certain genes (Song et al. 2015). Genistein at 10 µM had also radiosensitized breast cancer cell strains via G2/M cell cycle arrest, increased DNA damage and apoptosis (Liu et al. 2013). In addition, genistein had potentiated radiation-induced cell killing in vitro and in vivo orthotopic model of PC-3 prostate carcinoma (Hillman et al. 2004). Again, these apparent differential effects of genistein on normal and tumor cells are difficult to interpret. Currently genistein has an investigational new drug (IND) status as radioprotector of normal tissues for acute radiation syndrome under name BIO300 (Singh et al. 2016). However, its ability to inhibit many enzymes (tyrosine kinase) and modulate different signal transduction pathways infers a complex role in biological systems and call for further investigation to establish genistein as a radioprotective agent (Grabowski et al. 2015; Qian et al. 2015).
Sesamol
Sesamol (C7H6O3) is a natural compound found in sesame (sesamum indicum) oil. Although sesame seeds are popular nutritional food, it is a relatively new antioxidant molecule having potential radioprotective effect with strong anti-radical properties (Mishra 2012; Kumar et al. 2015). Sesamol exhibited more free radicals scavenging capacity compared to melatonin and demonstrated greater radioprotective efficacy in plasmid and calf thymus DNA (DMF = 10 at 100 µM), human lymphocytes, and V79 cell survival (DMF = 2) in vitro (Prasad et al. 2005; Mishra et al. 2011). Sesamol has been shown to protect C57BL/6 male mice (100 mg/kg) in vivo following 7.5 Gy radiation dose by protecting hematopoietic and gastrointestinal systems (Khan et al. 2015). Radioprotection was also described in the jejunum of Swiss albino mice irradiated with 15 Gy, where it showed increased crypt cells, maintained villus height, and prevented mucosal erosion (Parihar et al. 2006). In addition, sesamol was also reported to reduce radiation-induced cytogenetic damage in bone marrow cells of mice (Kumar et al. 2015). These studies are encouraging and put forth to consider sesamol as a potent candidate radioprotector.
Botanical and herbal extracts
Worldwide, different botanical, herbal, and Ayurvedic preparations have been traditionally used to treat different ailments including stress-induced disorders. Therefore, it was assumed that these preparations might be effective against radiation-induced mortality (Jagetia 2007). Many botanical and herbal extracts have been studied to determine their capability to demonstrate radioprotective effect. The crude extracts of different parts of plants such as root, leaf, bark, seeds, flower were examined. The radioprotective properties were revealed for some botanical or herbal plants, such as Tinospora cardifolia, Panax ginseng, Rosemarinus officinalis, Aloe vera, Embilica officinalis, Alstonia scholaris and Mentha piperata. Extracts of these plants have showed significant protection against radiation-induced syndromes (hematopoietic, gastrointestinal), as well as cellular and molecular damages in mice (Goyal and Gehlot 2009; Jindal et al. 2010; Samarth et al. 2004).
The high-altitude plant Hippophae rhamnoides (seabuckthorn) was studied in different model systems for potential radioprotective properties. It was shown to protect hematopoietic and gastrointestinal systems when administered 30 min before irradiation (Goel et al. 2002; Bala et al. 2015). A 30 mg/kg of this botanical extracts (RH-3) rendered a more than 80% survival after whole body lethal irradiation (10 Gy) in mice (Prakash et al. 2005). The hippophae extracts had also protected mitochondrial and genomic DNA from radiation damage (Shukla et al. 2006). In contrast, it showed enhanced apoptosis in thymocytes (Goel et al. 2004). Another high-altitude plant that has been studied is Podophyllum hexadendrum. The crude extracts are rich in podophylotoxins, such as 4-O-α-(d)-6-acetylglucopyranoside and podophyllotoxin-4-O-β-(d)-6-acetylglucopyranosid (Puri et al. 2006). A single dose (10–15 mg/kg body weight) of a semi-purified extract (REC-2001) conferred greater than 90% of survival when administered before whole body irradiation (10 Gy) with a DMF of 1.6 (Lata et al. 2009). Conversely, high dose (115 mg/kg body weight) was toxic and killed 100% of the animals. At pharmacological doses, the extract protected different organs such as liver, testis, gastrointestinal, brain and reduced genotoxicity in peripheral blood lymphocytes (Dutta and Gupta 2014; Dutta et al. 2015). The extract had also protected HePG2 cells from radiation-induced cell death (Gupta et al. 2003). Another extract of P. hexadendrum (REC-2006) had protected 90% of the mice from lethal whole body irradiation (10 Gy) at significantly lower dose of 6–8 mg/kg (Gupta et al. 2007). The names of these extracts were based on the years they were isolated and the active constituents provided protection even after years of extraction from the plants. The mechanism of radioprotection was attributed to scavenging of free radicals and immunomodulation (Rajesh et al. 2007).
The radioprotective properties of many traditional medicinal plants were widely studied by various groups of investigators (Table 1). The overall outcome indicates that dietary supplements of Ageratum conyzoides (DMF = 1.3), Aegle marmelos (DMF = 1.2), Zingiber (Ginger) officinalis (DMF = 1.15), Radiola Imbricata (90% survival), magiferin (Mangifera indica), LiV52, naringin (citrus flavonoids), triphala, chavanyaprash, mint (Mentha arvensis), Syzygium cumini (Jamun), Coleus aromaticus can confer protection to mice from radiation-induced cell death (Jagetia et al. 2003a, 2004b; Goel et al. 2006; Jagetia 2007). Active flavonoids Orientin and Vicenin from basil plants (Ocimum sanctum) have also demonstrated potential radioprotective properties (Uma Devi et al. 1999). The results also showed that most of these herbal extracts possess strong antioxidants and immunomodulatory properties.
Plant products have great potentials as pharmacological agents. However; these herbal preparations are mixtures of compounds. The scientific communities believe that it is very difficult in such situation to predict the effects of a single constituent alone (Ramana et al. 2014). In addition, the limited knowledge regarding dosing of different herbals and botanicals preparations during treatment of various pathological conditions restricts their universal applications and carries the risk of inefficiency or toxicity due to miss-dosing. Also, the relative availability of specific constituents in plants at specific geographical areas further complicates their study as radioprotective drugs.
Bacterial extracts
It has been observed that many bacterial species may survive at extreme conditions of heat and/or radiation (thermophiles and radiophiles). These species must be adapted to these environmental conditions by changing their physiological or genetic constitution (Satoh et al. 2016). It is assumed that understanding the mechanism of radioresistance and mimicking the condition may provide protection to humans against ionizing radiation. A few bacterial species are radioresistant (such as Deinococcus radiodurans, radiophilus, and grandis), and evidence suggests that extracts of those bacteria could confer radioprotective properties in different experimental models (Fedorocko and Mackova 1996; Daly et al. 2010).
Various other bacterial extracts were also tested for radioprotective potential. Intraperitoneal administration of 250 and 500 µg endotoxin-free extract of bronchovaxom (bacteria responsible for respiratory infection, available as drug) at 24 h before whole body irradiation (9.5 Gy) protected mice from death with DMFs of 1.12 and 1.25; respectively (Fedorocko et al. 1992). A single subcutaneous injection of extract prepared from heat killed Lactobacillus casei protected mice from a lethal radiation dose of 8.5 Gy (Tsuneoka et al. 1994). The effect was attributed to enhanced protection of hematopoietic tissues due to activation of macrophage stimulating factor. Also, a single injection of recombinant polypeptide derived from salmonella flagellin (CBLB502) 30 min before lethal total body irradiation had protected mice from hematopoietic and gastrointestinal syndromes and brought about two-fold (DMF = 1.6) improvement in mice survival and mortality in rhesus macaques (Burdelya et al. 2008). CBLB502 protected animals by binding to toll-like receptor 5 and activation of nuclear factor-kB, which presents a landmark work that changed the way of earlier thinking considering only radical scavengers as good radioprotectors. CBLB502 (marketed as Entilimod) is FDA approved as off-label drug that can be used during nuclear or radiological incidents for acute radiation syndrome (Singh et al. 2015). In addition, CBLB613, a toll-like receptor 2/6 (TLR 2/6) agonist and natural lipopeptide obtained from Mycoplasma arginini, had also shown significant radioprotective capacity in CD2F1 mice against hematopoietic syndrome (DMF = 1.25), and stimulated induction of various interleukins and chemokines (Singh et al. 2012). Intraperitoneal administration of aqueous solution of semi-quinone glucosides (SQGD) obtained from bacillus species INM-1 showed antioxidant and radioprotective properties in vitro and enhanced the expression of immuno-stimulatory cytokines such as IL-12p40, IL-12p35, IL-23p19, and Rel A genes in human peripheral blood mononuclear cells (Kumar et al. 2011). Alteration of host immunomodulatory activity seems to be the primary mode of action of many bacterial products. However, immune responses in humans are very complex in nature and the mechanisms of different bacterial products as drug are not well understood.
Cytokines
Cytokines, which include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factors, are proteins of small molecular weight that regulate the interaction among lymphoid, inflammatory and hematopoietic cells (Schaue et al. 2012). Interleukins were the first to demonstrate radioprotective effect. Intraperitoneal injection of 2000 units of synthetic interlukin-1 (IL-1) showed to protect 88 and 100% of C57BL/6 mice following LD100/17 and LD50/30 radiation doses, respectively (Neta et al. 1986). Other study, however, showed rather modest DMFs (1.05–1.12) for 100 ng/mouse (3 µg/kg) in C57BL/Ka and C3H/Km mice (Dorie et al. 1989). In addition, the administration of antibodies against interleukins and tumor necrosis factor (TNFα) reduced survival of CD2F1 mice following whole body irradiation due to myeloid suppression in exposed animals (Neta et al. 1991). Furthermore, many cytokines, such as IL-3, IL-6, IL-12, IL-11, G-CSF, granulocytes-macrophages colony-stimulating factors (GM-CSF), erythropoietin, and thrombopoietin that regulate the proliferation of hematopoietic cells at various stages, were clinically tested for their ability to restore bone marrow after chemotherapy or radiotherapy of cancer patients. However, only IL-1, IL-12, TNF-α, basic fibroblast growth factor (bFGF), and G-CSF had protected animals when given prior to irradiation [(Singh and Yadav 2005) and references therein]. Although cytokines can potentially be used as candidate radioprotectors due to decreased toxicity, few cytokines such as TNF-α sensitize cells and, therefore, their mechanism and role in radiation-induced cell death need to be explored in more detail before being used as radioprotectors. Furthermore, the natural compound curcumin downregulates the expression of pro-inflammatory cytokines such as TNF, IL-1, IL-2, IL-6, IL-8 and IL-12, mostly through an activation of Nf-kB transcription factor (Jagetia 2007).
Interleukins such as G-CSF and GM-CSF, and their pharmaceutical analogs, filgratism, lenogratism sargramostim, regulate maturation and differentiation of progenitor cells in the bone marrow to granulocytes, macrophages and T cells. Interleukins G-CSF and GM-CSF are also shown to ameliorate cancer therapy-induced neutropenia for which FDA has approved Neupogen (filgratism), Neulasta (pegfilgrastim) and Leukine (sargramostim) and currently under investigation as radiation countermeasure agents (Singh et al. 2015). In this context, palifermin (Kepivance), a recombinant derivative of human keratinocyte growth factor (KGF), is also an FDA-approved drug (Fig. 1) that is used to treat oral mucositis in patient undergoing hematopoietic stem cell transplantation (Vadhan-Raj et al. 2013). In contrast to amifostine, the mechanism of action of palifermin is related to epithelial cell proliferation leading to significant reduction in oral mucositis in head and neck cancer patients. Further studies are required to explore its usefulness as radiation countermeasure agent after radiological events (Johnke et al. 2014).
Thus, cytokines are diversified in their nature and functions. The intricate actions of interleukins and their targets during irradiation of animals are poorly understood in the scenarios of whole body exposure. Since these signaling molecules are produced during different physiological processes and each interleukin is involved in more than one pathway, it will be difficult to demonstrate the mechanism of action responsible for the desired radioprotective effect. On the other side, it is a fact that these interleukins promote also tumor progression and are responsible for the poor prognosis in many cases of cancers (Rastogi et al. 2015).
DNA-binding agents
DNA-binding or damaging agents are broadly anticancer drugs. In general, there are three modes of binding of small-molecule with double-stranded DNA: intercalation, groove binding and covalent binding. The minor groove of DNA is of particular interest due to sequence-specific interactions with a large number of small molecules. These minor groove ligands bind typically to AT-rich sequences and either induce permanent DNA damage or cause only reversible inhibition of DNA-dependent functions (Baraldi et al. 2004). Although most of these small molecules have cytotoxic activities, a few modulates cellular pathways that promote cell survival and exhibit radioprotective properties (Gurova 2009). The mechanism of radioprotection seems to be hydrogen or electron transfer from the ligands to the DNA radical at binding sites leading to DNA structural stabilization.
The Hoechst minor groove ligands H33258 (C25H24N6O·3HCl), H33342 (C27H28N6O·3HCl) and amino derivatives methylproamine had protected V79 cells from radiation-induced DNA damage and death with a DMF of 2.1 (Martin et al. 2004). Also, dioxomolybdenum and dioxotungusten hydroxamato had protected DNA from radiation-induced strand breaks (Paul et al. 2014). Netropsin (C18H26N10O3·2HCl), which is another minor groove binding ligand, had radioprotective properties through DNA structural stability mechanism (Mishra et al. 2009). In contrast with H33258, the netropsin protection was at specific DNA site. H33342 derivatives such as tris (TBZ), and dimethoxty (DMA) benzimidazole were also studied for their radioprotective efficacy in vivo. DMA showed an effective radioprotection in mice at single nontoxic oral dose with a DMF of 1.28 (Nimesh et al. 2015). DMA-treated mice showed delayed radiation sickness, such as weight loss, irritability and lethargy.
DNA-binding drugs raise, however, a serious concern due to potential mutagenic effects. Many classes of DNA minor groove binding ligands induce aneuploidy, polyploidy, chromosome decondensation at heterochromatic regions rich in AT content, fragile sites, which may have a propensity to develop particular cancers (Turner and Denny 1996). Nonetheless, pentamidine (a minor groove binder used in the treatment of AIDS-related pneumocystis pneumonia) did not show so far mutagenic effects in nuclear DNA. Thus, minor groove binding does not necessarily lead to mutagenesis and further studies are required to ascertain their role as safe radioprotectors.
Absorbents and chelators
In general, absorbent is defined as a substance endowed with the property of attaching other substances to its surface without covalent bonding, while chelator is a chemical compound that bonds with a metal ion to form a chelate. Yet, the exact distinction between the two terms is not unanimous and often used interchangeably. In radiation protection, absorbents do often include chelating and are the compounds that can sequester radionuclides after internal contamination and nullify toxic effects to protect biological systems (Nair et al. 2001). During a nuclear explosion, distinctive radioactive materials may be produced and released to the atmosphere where they could be inhaled either as gases, ingested as particulates by mouth, or through skin contamination. Hence, suitable decorporating agents are required to remove internal contamination of different radionuclides (Rump et al. 2016). Radionuclides have unstable nucleus and in the process of stabilization, they emit gamma radiation, alpha or beta particles which are ionizing radiations causing a high risk of stochastic and sometimes deterministic health effects. Radioactive nuclides, such as strontium 90, cesium 137, plutonium 239, cobalt 60, radium 226, polonium 210, iridium 192, iodine 131 and americium 241, might be also a component of potential radioactive dirty bombs (Rump et al. 2016).
Radioprotective agents can be used as decorporating agents to remove radionuclides from the whole body. Fast and effective removal of radionuclides from biological system are the properties required from these agents. Prussian blue (FDA approved decorporating agents) has been used as radioprotector to eliminate cesium and thallium ingested radionuclides (Bhardwaj et al. 2006). Prussian blue, a non-absorbable resin dye, acts as laxative agent that promotes the elimination of radionuclides from the digestive system. The absorption of certain divalent radionuclides, such as strontium, may be inhibited using calcium chloride in large quantity. Iodine radionuclides accumulate in the thyroid gland and potassium iodide is used to eliminate the incorporated isotopes. Chelating agents, such as calcium-DTPA and zinc-DTPA, may be administered to chelate a number of radioactive materials and promote their elimination via urinary system. Changes in the alkalinity by sodium bicarbonate can be used to eliminate uranium poisoning (Dominguez-Gadea and Cerezo 2011).
A variety of synthetic and natural products have been studied for decorporation of radionuclides from the body. Orally administered dietary recombinant Chlorella algae were shown to inhibit the absorption of strontium (90Sr) into the blood and enhance its elimination from mice body through adsorption in intestine (Ogawa et al. 2016). The transdermal delivery of synthetic DTPA di- and tri-ethyl ester has been reported to enhance decorporation in a dose-dependent manner (Zhang et al. 2013). Different silica-based materials have also been tested to capture various radionuclides of Plutonium, Americium, Uranium, and Thorium (Yantasee et al. 2010). The mechanisms of radioprotection in case of radionuclide’s toxicity might be chelating, inhibiting uptake by changing chemical state, diluting, and flooding of active compounds (Dominguez-Gadea and Cerezo 2011). However, most of these absorbents are not well tolerated due to their side effects. Research is still in progress for more suitable agents with less toxicity to various organs.
Conclusions
An overwhelming number of natural agents and derivatives were studied for their radioprotective potentials. These compounds belong to a broad range of unrelated chemical groups. Although the mechanisms of radioprotection differ widely among groups, most compounds act primarily via scavenging of radiation-induced free radicals or by quenching of secondary biomolecular reactive species. Where radioprotection was demonstrated, the compounds showed modest dose reduction factor (DMF) ranging from 1.1 to 2.7. However, the resulting number of useful radioprotectors that can be used in human remains surprisingly limited. To date, not a single drug is ready to be used with acceptable degree of confidence except amifostine. Several drugs obtained an Investigational New Drug (IND) status; however, their use in clinical setting is still to be confirmed. Hence, the ideal radioprotector remedy has not been achieved yet despite the extensive research for last five decades.
Future development of ideal radioprotectors requires taking into consideration the following points. The interaction of radiation with biological systems involves physico-chemical and molecular processes and, hence, these are complex in nature. A radioprotector comes into play as an intermediate neutralizing the produced free radicals, thus preventing their consequences on the biological system. Whether the intent of their use is for initial radiation protection, mitigation or therapeutic, they are often required to be present in effective concentrations during or shortly after the radiation exposure. The development of suitable radiation countermeasures that could possibly be used as prophylactic and/or therapeutic agents is in nascent stage. The quest is still ongoing to discover less toxic compounds that could be used under minimal medical supervision during radiotherapy and field applications.
Varieties of molecules have shown their radioprotective properties and potential (Fig. 1; Table 1), but their detailed molecular, physiological and pharmacological mechanisms need to be fully studied and understood before being applied. Attempts to repurpose suitable nontoxic drug for the management of radiation victims are also an attractive approach and that can save time, money, and risk associated with new drug development. It is evident that natural compounds are currently the most attractive source due to their potential lower toxicity than synthetic compounds. The major concerns of high dosage and limited bioavailability and efficacy can be improved by designing more efficient derivatives of selected potential compounds.
One of the important challenges of radioprotector development is the model system where these promising molecules could be tested. Therefore, the identification of biomarkers that can be used to test putative agents is also needed. Although the ability to reduce radiation-induced xerostomia was tested, many more test systems are needed for different category of radioprotective agents. The risk of radiation-induced secondary malignancy and cardiovascular diseases due to the use of radiotherapy for cancer patients is also legitimate targets for radiation protection. The risk of cardiovascular diseases during breast cancer therapy is of growing concern and has attracted more attention recently. Therefore, suitable agents with the ability to protect cardiovascular system will be an achievement of great importance.
In summary, the future development of ideal radioprotector requires an appropriate nontoxic drug, a model system and biomarkers of radiation exposure to test and verify the effects on physiological systems. Multifaceted mechanistic understanding is essential in this development. Such approach is essential to ensure the efficacy and safety of customized agents for use during radiotherapy and field application in cases of nuclear eventualities.
Acknowledgements
We would like to extend sincere gratitude and appreciation to the Dr. B. Moftah, N. Al-Harbi, and S. Bin Judia for their supports, and Farah Yamak for helping in editing the manuscript.
Abbreviations
- bFGF
Beta fibroblast growth factors
- CD2F1
Cluster of differentiations 2F1
- DMA
(5-2-[2′-(3, 4-Dimethoxyphenyl)-5′-benzimidazolyl]
- DMF
Dose modifying factor
- DTPA
Diethylenetriaminepentaacetic acid
- FDA
Food and Drug Administration
- G-CSF
Granulocyte colony-stimulating factor
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- GPx
Glutathione peroxidase
- GT3
Gamma tocotrienol
- Gy
Gray (dose of ionizing radiation)
- HMG-CoA
3-Hydroxy-3-methyl-glutaryl-coenzyme A
- IL
Interleukin
- MAP kinases
Mitogen activated protein kinases
- MEA
β-Mercaptoethyamine
- NF-kB
Nuclear factor kappa beta
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- SQGD
Semiquinone glucoside
- TBZ
2-(4-Thiazolyl) benzimidazole
- TS
Tocopherol succinate
- LD50
Lethal dose for 50% killing
- TLR 2/6
Toll-like receptor 2/6
- TNF-α
Tumor necrosis factor-α
Author contributions
KM collected information and drafted the manuscript and GA conceived the review and critically revised and edited the content.
Compliance with ethical standards
Funding
This work was partially supported the National Science, Technology and Innovation Plan (NSTIP, KACST) Grants 12-MED2945-20 and 15-MED4114-20 (KFSHRC, RAC 2130-025 and 2170 005).
Conflict of interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Availability of data and material
Data sharing is not applicable to this review manuscript as no datasets were generated or analyzed during the current study.
Contributor Information
Krishnanand Mishra, Email: kmishra9@kfshrc.edu.sa.
Ghazi Alsbeih, Phone: +966-11-4427891, Email: galsbeih@kfshrc.edu.sa.
References
- Ali R, Ali R, Jaimini A, Nishad DK, Mittal G, Chaurasia OP, Kumar R, Bhatnagar A, Singh SB. Acute and sub acute toxicity and efficacy studies of Hippophae rhamnoides based herbal antioxidant supplement. Indian J Pharmacol. 2012;44(4):504–508. doi: 10.4103/0253-7613.99329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqasoumi SI. ‘Okra’ Hibiscus esculentus L.: a study of its hepatoprotective activity. Saudi Pharm J. 2012;20(2):135–141. doi: 10.1016/j.jsps.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala M, Gupta M, Saini M, Abdin MZ, Prasad J. Sea buckthorn leaf extract protects jejunum and bone marrow of (60)cobalt-gamma-irradiated mice by regulating apoptosis and tissue regeneration. Evid Based Complement Altern Med. 2015;2015:765705. doi: 10.1155/2015/765705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi PG, Bovero A, Fruttarolo F, Preti D, Tabrizi MA, Pavani MG, Romagnoli R. DNA minor groove binders as potential antitumor and antimicrobial agents. Med Res Rev. 2004;24(4):475–528. doi: 10.1002/med.20000. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Bhatnagar A, Pathak DP, Singh AK. Dynamic, equilibrium and human studies of adsorption of 201Tl by Prussian blue. Health Phys. 2006;90(3):250–257. doi: 10.1097/01.HP.0000180771.66884.d3. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W, Sauer R. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, Feinstein E, Gudkov AV. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320(5873):226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, Hill RP. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173(5):602–611. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23(8):1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- Chidambaram M, Krishnasamy K. Drug-drug/drug-excipient compatibility studies on curcumin using non-thermal methods. Adv Pharm Bull. 2014;4(3):309–312. doi: 10.5681/apb.2014.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010;15(4):360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coeytaux K, Bey E, Christensen D, Glassman ES, Murdock B, Doucet C. Reported radiation overexposure accidents worldwide, 1980-2013: a systematic review. PLoS One. 2015;10(3):e0118709. doi: 10.1371/journal.pone.0118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MJ, Gaidamakova EK, Matrosova VY, Kiang JG, Fukumoto R, Lee DY, Wehr NB, Viteri GA, Berlett BS, Levine RL. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS One. 2010;5(9):e12570. doi: 10.1371/journal.pone.0012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Clarke TK, Mog SR, Landauer MR. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int J Radiat Biol. 2007;83(3):141–151. doi: 10.1080/09553000601132642. [DOI] [PubMed] [Google Scholar]
- Dominguez-Gadea L, Cerezo L. Decontamination of radioisotopes. Rep Pract Oncol Radiother. 2011;16(4):147–152. doi: 10.1016/j.rpor.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorie MJ, Allison AC, Zaghloul MS, Kallman RF. Interleukin 1 protects against the lethal effects of irradiation of mice but has no effect on tumors in the same animals. Proc Soc Exp Biol Med. 1989;191(1):23–29. doi: 10.3181/00379727-191-42884. [DOI] [PubMed] [Google Scholar]
- Du J, Cieslak JA, 3rd, Welsh JL, Sibenaller ZA, Allen BG, Wagner BA, Kalen AL, Doskey CM, Strother RK, Button AM, Mott SL, Smith B, Tsai S, Mezhir J, Goswami PC, Spitz DR, Buettner GR, Cullen JJ. Pharmacological ascorbate radiosensitizes pancreatic cancer. Can Res. 2015;75(16):3314–3326. doi: 10.1158/0008-5472.CAN-14-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Gupta ML. Alleviation of radiation-induced genomic damage in human peripheral blood lymphocytes by active principles of Podophyllum hexandrum: an in vitro study using chromosomal and CBMN assay. Mutagenesis. 2014;29(2):139–147. doi: 10.1093/mutage/get071. [DOI] [PubMed] [Google Scholar]
- Dutta A, Gupta ML, Kalita B. The combination of the active principles of Podophyllum hexandrum supports early recovery of the gastrointestinal system via activation of Nrf2-HO-1 signaling and the hematopoietic system, leading to effective whole-body survival in lethally irradiated mice. Free Radic Res. 2015;49(3):317–330. doi: 10.3109/10715762.2015.1004328. [DOI] [PubMed] [Google Scholar]
- Fedorocko P, Mackova O. Radioprotective effects of combination broncho-vaxom, a macrophage activator, and indomethacin, an inhibitor of prostaglandin production: relationship to myelopoiesis. Eur J Haematol. 1996;56(1–2):54–61. doi: 10.1111/j.1600-0609.1996.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Fedorocko P, Brezani P, Mackova NO. Radioprotection of mice by the bacterial extract broncho-vaxom: haemopoietic stem cells and survival enhancement. Int J Radiat Biol. 1992;61(4):511–518. doi: 10.1080/09553009214551271. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54(3):303–312. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, Hauer-Jensen M, Kumar KS. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85(7):598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- Goel HC, Prasad J, Singh S, Sagar RK, Kumar IP, Sinha AK. Radioprotection by a herbal preparation of Hippophae rhamnoides, RH-3, against whole body lethal irradiation in mice. Phytomedicine. 2002;9(1):15–25. doi: 10.1078/0944-7113-00077. [DOI] [PubMed] [Google Scholar]
- Goel HC, Indraghanti P, Samanta N, Ranaz SV. Induction of apoptosis in thymocytes by Hippophae rhamnoides: implications in radioprotection. J Environ Pathol Toxicol Oncol. 2004;23(2):123–137. doi: 10.1615/JEnvPathToxOncol.v23.i2.50. [DOI] [PubMed] [Google Scholar]
- Goel HC, Bala M, Prasad J, Singh S, Agrawala PK, Swahney RC. Radioprotection by Rhodiola imbricata in mice against whole-body lethal irradiation. J Med Food. 2006;9(2):154–160. doi: 10.1089/jmf.2006.9.154. [DOI] [PubMed] [Google Scholar]
- Goyal PK, Gehlot P. Radioprotective effects of Aloe vera leaf extract on Swiss albino mice against whole-body gamma irradiation. J Environ Pathol Toxicol Oncol. 2009;28(1):53–61. doi: 10.1615/JEnvironPatholToxicolOncol.v28.i1.60. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Banecki B, Kadzinski L, Jakobkiewicz-Banecka J, Kazmierkiewicz R, Gabig-Ciminska M, Wegrzyn G, Wegrzyn A, Banecka-Majkutewicz Z. Genistein inhibits activities of methylenetetrahydrofolate reductase and lactate dehydrogenase, enzymes which use NADH as a substrate. Biochem Biophys Res Commun. 2015;465(3):363–367. doi: 10.1016/j.bbrc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Gupta D, Arora R, Garg AP, Goel HC. Radiation protection of HepG2 cells by Podophyllum hexandrum Royale. Mol Cell Biochem. 2003;250(1–2):27–40. doi: 10.1023/A:1024925612233. [DOI] [PubMed] [Google Scholar]
- Gupta ML, Tyagi S, Flora SJ, Agrawala PK, Choudhary P, Puri SC, Sharma A, Devi M, Haksar A, Qazi GN, Tripathi RP. Protective efficacy of semi purified fraction of high altitude Podophyllum hexandrum rhizomes in lethally irradiated Swiss albino mice. Cell Mol Biol (Noisy-le-grand) 2007;53(5):29–41. [PubMed] [Google Scholar]
- Gupta ML, Srivastava NN, Dutta S, Shukla SK, Dutta A, Verma S, Devi M. Blood biomarkers in metal scrap workers accidentally exposed to ionizing radiation: a case study. Hum Exp Toxicol. 2013;32(12):1311–1322. doi: 10.1177/0960327113482477. [DOI] [PubMed] [Google Scholar]
- Gurova K. New hopes from old drugs: revisiting DNA-binding small molecules as anticancer agents. Future Oncol. 2009;5(10):1685–1704. doi: 10.2217/fon.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 6. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- Harapanhalli RS, Yaghmai V, Giuliani D, Howell RW, Rao DV. Antioxidant effects of vitamin C in mice following X-irradiation. Res Commun Mol Pathol Pharmacol. 1996;94(3):271–287. [PubMed] [Google Scholar]
- Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M, Joiner MC, Marples B, Forman JD, Sarkar FH. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3(10):1271–1279. [PubMed] [Google Scholar]
- Jagetia GC. Radioprotective Potential of plants and herbs against the effects of ionizing radiation. J Clin Biochem Nutr. 2007;40(2):74–81. doi: 10.3164/jcbn.40.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27(1):19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Rajanikant GK. Effect of curcumin on radiation-impaired healing of excisional wounds in mice. J Wound Care. 2004;13(3):107–109. doi: 10.12968/jowc.2004.13.3.26589. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Baliga MS, Venkatesh P, Ulloor JN. Influence of ginger rhizome (Zingiber officinale Rosc) on survival, glutathione and lipid peroxidation in mice after whole-body exposure to gamma radiation. Radiat Res. 2003;160(5):584–592. doi: 10.1667/RR3057. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Shirwaikar A, Rao SK, Bhilegaonkar PM. Evaluation of the radioprotective effect of Ageratum conyzoides Linn. extract in mice exposed to different doses of gamma radiation. J Pharm Pharmacol. 2003;55(8):1151–1158. doi: 10.1211/0022357021576. [DOI] [PubMed] [Google Scholar]
- Jagetia G, Baliga M, Venkatesh P. Ginger (Zingiber officinale Rosc.), a dietary supplement, protects mice against radiation-induced lethality: mechanism of action. Cancer Biother Radiopharm. 2004;19(4):422–435. doi: 10.1089/cbr.2004.19.422. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Venkatesh P, Baliga MS. Evaluation of the radioprotective effect of bael leaf (Aegle marmelos) extract in mice. Int J Radiat Biol. 2004;80(4):281–290. doi: 10.1080/09553000410001679776. [DOI] [PubMed] [Google Scholar]
- Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73(5):1491–1501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javvadi P, Hertan L, Kosoff R, Datta T, Kolev J, Mick R, Tuttle SW, Koumenis C. Thioredoxin reductase-1 mediates curcumin-induced radiosensitization of squamous carcinoma cells. Can Res. 2010;70(5):1941–1950. doi: 10.1158/0008-5472.CAN-09-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal A, Agrawal A, Goyal PK. Influence of Rosemarinus officinalis extract on radiation-induced intestinal injury in mice. J Environ Pathol Toxicol Oncol. 2010;29(3):169–179. doi: 10.1615/JEnvironPatholToxicolOncol.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- Johnke RM, Sattler JA, Allison RR. Radioprotective agents for radiation therapy: future trends. Future Oncol. 2014;10(15):2345–2357. doi: 10.2217/fon.14.175. [DOI] [PubMed] [Google Scholar]
- Khan S, Kumar A, Adhikari JS, Rizvi MA, Chaudhury NK. Protective effect of sesamol against (6)(0)Co gamma-ray-induced hematopoietic and gastrointestinal injury in C57BL/6 male mice. Free Radic Res. 2015;49(11):1344–1361. doi: 10.3109/10715762.2015.1071485. [DOI] [PubMed] [Google Scholar]
- Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12(6):738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, Rosen II. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62(4):1195–1203. doi: 10.1016/j.ijrobp.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, Gambles K, Toles R, Kao TC, Hauer-Jensen M, Kumar KS. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res. 2010;173(6):738–747. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Cary LH, Gambles K, Hauer-Jensen M, Kumar KS, Ghosh SP. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int Immunopharmacol. 2012;14(4):495–503. doi: 10.1016/j.intimp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Kumar R, Bansal DD, Patel DD, Mishra S, Karamalakova Y, Zheleva A, Gadjeva V, Sharma RK. Antioxidative and radioprotective activities of semiquinone glucoside derivative (SQGD) isolated from Bacillus sp. INM-1. Mol Cell Biochem. 2011;349(1–2):57–67. doi: 10.1007/s11010-010-0660-x. [DOI] [PubMed] [Google Scholar]
- Kumar KS, Kiang JG, Whitnall MH, Hauer-jensen M. Perspectives in radiological and nuclear countermeasures. In: Mickelson AB, editor. Medical consequences of radiological and nuclear weapons. Washington DC: U.S. Government Printing Office; 2012. pp. 239–266. [Google Scholar]
- Kumar A, Selvan TG, Tripathi AM, Choudhary S, Khan S, Adhikari JS, Chaudhury NK. Sesamol attenuates genotoxicity in bone marrow cells of whole-body gamma-irradiated mice. Mutagenesis. 2015;30(5):651–661. doi: 10.1093/mutage/gev026. [DOI] [PubMed] [Google Scholar]
- Kunwar A, Bansal P, Kumar SJ, Bag PP, Paul P, Reddy ND, Kumbhare LB, Jain VK, Chaubey RC, Unnikrishnan MK, Priyadarsini KI. In vivo radioprotection studies of 3,3′-diselenodipropionic acid, a selenocystine derivative. Free Radic Biol Med. 2010;48(3):399–410. doi: 10.1016/j.freeradbiomed.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Landauer MR, Davis HD, Dominitz JA, Weiss JF. Comparative behavioral toxicity of four sulfhydryl radioprotective compounds in mice: WR-2721, cysteamine, diethyldithiocarbamate, and N-acetylcysteine. Pharmacol Ther. 1988;39(1–3):97–100. doi: 10.1016/0163-7258(88)90046-0. [DOI] [PubMed] [Google Scholar]
- Landauer MR, Srinivasan V, Seed TM. Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol. 2003;23(6):379–385. doi: 10.1002/jat.904. [DOI] [PubMed] [Google Scholar]
- Lata M, Prasad J, Singh S, Kumar R, Singh L, Chaudhary P, Arora R, Chawla R, Tyagi S, Soni NL, Sagar RK, Devi M, Sharma RK, Puri SC, Tripathi RP. Whole body protection against lethal ionizing radiation in mice by REC-2001: a semi-purified fraction of Podophyllum hexandrum. Phytomedicine. 2009;16(1):47–55. doi: 10.1016/j.phymed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun C, Jin X, Li P, Ye F, Zhao T, Gong L, Li Q. Genistein enhances the radiosensitivity of breast cancer cells via G(2)/M cell cycle arrest and apoptosis. Molecules. 2013;18(11):13200–13217. doi: 10.3390/molecules181113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RF, Broadhurst S, Reum ME, Squire CJ, Clark GR, Lobachevsky PN, White JM, Clark C, Sy D, Spotheim-Maurizot M, Kelly DP. In vitro studies with methylproamine: a potent new radioprotector. Can Res. 2004;64(3):1067–1070. doi: 10.1158/0008-5472.CAN-03-2423. [DOI] [PubMed] [Google Scholar]
- Mathew D, Nair CK, Jacob JA, Biswas N, Mukherjee T, Kapoor S, Kagiya TV. Ascorbic acid monoglucoside as antioxidant and radioprotector. J Radiat Res. 2007;48(5):369–376. doi: 10.1269/jrr.07007. [DOI] [PubMed] [Google Scholar]
- Mishra K, Bhardwaj R, Chaudhury NK. Netropsin, a minor groove binding ligand: a potential radioprotective agent. Radiat Res. 2009;172(6):698–705. doi: 10.1667/RR1815.1. [DOI] [PubMed] [Google Scholar]
- Mishra K, Srivastava PS, Chaudhury NK. Sesamol as a potential radioprotective agent: in vitro studies. Radiat Res. 2011;176(5):613–623. doi: 10.1667/RR2661.1. [DOI] [PubMed] [Google Scholar]
- Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chem. 2012;130(4):1036–1043. doi: 10.1016/j.foodchem.2011.07.127. [DOI] [Google Scholar]
- Mix M, Singh AK, Tills M, Dibaj S, Groman A, Jaggernauth W, Rustum Y, Jameson MB. Randomized phase II trial of selenomethionine as a modulator of efficacy and toxicity of chemoradiation in squamous cell carcinoma of the head and neck. World J Clin Oncol. 2015;6(5):166–173. doi: 10.5306/wjco.v6.i5.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu-Turkoglu U, Erbil Y, Oztezcan S, Olgac V, Toker G, Uysal M. The effect of selenium and/or vitamin E treatments on radiation-induced intestinal injury in rats. Life Sci. 2000;66(20):1905–1913. doi: 10.1016/S0024-3205(00)00516-6. [DOI] [PubMed] [Google Scholar]
- Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42(1):21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- Narra VR, Howell RW, Sastry KS, Rao DV. Vitamin C as a radioprotector against iodine-131 in vivo. J Nucl Med. 1993;34(4):637–640. [PubMed] [Google Scholar]
- Neta R, Douches S, Oppenheim JJ. Interleukin 1 is a radioprotector. J Immunol. 1986;136(7):2483–2485. [PubMed] [Google Scholar]
- Neta R, Oppenheim JJ, Schreiber RD, Chizzonite R, Ledney GD, MacVittie TJ. Role of cytokines (interleukin 1, tumor necrosis factor, and transforming growth factor beta) in natural and lipopolysaccharide-enhanced radioresistance. J Exp Med. 1991;173(5):1177–1182. doi: 10.1084/jem.173.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimesh H, Tiwari V, Yang C, Gundala SR, Chuttani K, Hazari PP, Mishra AK, Sharma A, Lal J, Katyal A, Aneja R, Tandon V. Preclinical evaluation of DMA, a bisbenzimidazole, as radioprotector: toxicity, pharmacokinetics, and biodistribution studies in Balb/c mice. Mol Pharmacol. 2015;88(4):768–778. doi: 10.1124/mol.115.098376. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Fukuda T, Han J, Kitamura Y, Shiba K, Odani A. Evaluation of Chlorella as a decorporation agent to enhance the elimination of radioactive strontium from body. PLoS One. 2016;11(2):e0148080. doi: 10.1371/journal.pone.0148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar SH. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi Pharm J. 2010;18(3):111–121. doi: 10.1016/j.jsps.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgen SC, Dokmeci D, Akpolat M, Karadag CH, Gunduz O, Erbas H, Benian O, Uzal C, Turan FN. The protective effect of curcumin on ionizing radiation-induced cataractogenesis in rats. Balkan Med J. 2012;29(4):358–363. doi: 10.5152/balkanmedj.2012.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Prabhakar KR, Veerapur VP, Kumar MS, Reddy YR, Joshi R, Unnikrishnan MK, Rao CM. Effect of sesamol on radiation-induced cytotoxicity in Swiss albino mice. Mutat Res. 2006;611(1–2):9–16. doi: 10.1016/j.mrgentox.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Patt HM, Tyree EB, Straube RL, Smith DE. Cysteine protection against X irradiation. Science. 1949;110(2852):213–214. doi: 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- Paul SS, Selim M, Saha A, Mukherjea KK. Synthesis and structural characterization of dioxomolybdenum and dioxotungsten hydroxamato complexes and their function in the protection of radiation induced DNA damage. Dalton Trans. 2014;43(7):2835–2848. doi: 10.1039/C3DT52434E. [DOI] [PubMed] [Google Scholar]
- Prakash H, Bala M, Ali A, Goel HC. Modification of gamma radiation induced response of peritoneal macrophages and splenocytes by Hippophae rhamnoides (RH-3) in mice. J Pharm Pharmacol. 2005;57(8):1065–1072. doi: 10.1211/0022357056668. [DOI] [PubMed] [Google Scholar]
- Prasad NR, Menon VP, Vasudev V, Pugalendi KV. Radioprotective effect of sesamol on gamma-radiation induced DNA damage, lipid peroxidation and antioxidants levels in cultured human lymphocytes. Toxicology. 2005;209(3):225–235. doi: 10.1016/j.tox.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Puri SC, Handa G, Bhat BA, Dhar KL, Spiteller M, Qazi GN. Characterization of two epimers, 4alpha and 4beta, of a novel podophyllotoxin-4-O-(d)-6-acetylglucopyranoside from Podophyllum hexandrum by LC-ESI-MS-MS. J Chromatogr Sci. 2006;44(5):239–243. doi: 10.1093/chromsci/44.5.239. [DOI] [PubMed] [Google Scholar]
- Puspitasari IM, Abdulah R, Yamazaki C, Kameo S, Nakano T, Koyama H. Updates on clinical studies of selenium supplementation in radiotherapy. Radiat Oncol. 2014;9:125. doi: 10.1186/1748-717X-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Cao L, Guan T, Chen L, Xin H, Li Y, Zheng R, Yu D. Protection by genistein on cortical neurons against oxidative stress injury via inhibition of NF-kappaB, JNK and ERK signaling pathway. Pharm Biol. 2015;53(8):1124–1132. doi: 10.3109/13880209.2014.962057. [DOI] [PubMed] [Google Scholar]
- Qiao Q, Jiang Y, Li G. Curcumin improves the antitumor effect of X-ray irradiation by blocking the NF-kappaB pathway: an in vitro study of lymphoma. Anticancer Drugs. 2012;23(6):597–605. doi: 10.1097/CAD.0b013e3283503fbc. [DOI] [PubMed] [Google Scholar]
- Rajagopalan R, Wani K, Huilgol NG, Kagiya TV, Nair CK. Inhibition of gamma-radiation induced DNA damage in plasmid pBR322 by TMG, a water-soluble derivative of vitamin E. J Radiat Res. 2002;43(2):153–159. doi: 10.1269/jrr.43.153. [DOI] [PubMed] [Google Scholar]
- Rajesh A, Sagar R, Singh S, Kumar R, Sharma AK, Prasad J, Singh S, Gupta M, Sharma RK, Puri SC, Krishna B, Siddiqui MS, Lahiri SS, Tripathi RP, Qazi GN. Cytoprotective effect of Podophyllum hexandrum against gamma radiation is mediated via hemopoietic system stimulation and up-regulation of heme-oxygenase-1 and the prosurvival multidomain protein Bcl-2. Integr Cancer Ther. 2007;6(1):54–65. doi: 10.1177/1534735406298303. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan N, Wolfe WW, Catravas GN. Radioprotection of hematopoietic tissues in mice by lipoic acid. Radiat Res. 1992;130(3):360–365. doi: 10.2307/3578382. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Singhal SS, Reddy AB. Therapeutic potential of natural pharmacological agents in the treatment of human diseases. Biomed Res Int. 2014;2014:573452. doi: 10.1155/2014/573452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Pandey MM, Rawat AK. Traditional herbs: a remedy for cardiovascular disorders. Phytomedicine. 2015 doi: 10.1016/j.phymed.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Manchester LC, Tan DX. Neurotoxins: free radical mechanisms and melatonin protection. Curr Neuropharmacol. 2010;8(3):194–210. doi: 10.2174/157015910792246236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump A, Stricklin D, Lamkowski A, Eder S, Abend M, Port M. The impact of time on decorporation efficacy after a “dirty bomb” attack studied by simulation. Drug Res. 2016 doi: 10.1055/s-0042-112809. [DOI] [PubMed] [Google Scholar]
- Samarth RM, Goyal PK, Kumar A. Protection of swiss albino mice against whole-body gamma irradiation by Mentha piperita (Linn.) Phytother Res. 2004;18(7):546–550. doi: 10.1002/ptr.1483. [DOI] [PubMed] [Google Scholar]
- Sarma L, Kesavan PC. Protective effects of vitamins C and E against gamma-ray-induced chromosomal damage in mouse. Int J Radiat Biol. 1993;63(6):759–764. doi: 10.1080/09553009314552161. [DOI] [PubMed] [Google Scholar]
- Sato T, Kinoshita M, Yamamoto T, Ito M, Nishida T, Takeuchi M, Saitoh D, Seki S, Mukai Y. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS One. 2015;10(2):e0117020. doi: 10.1371/journal.pone.0117020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Onodera T, Omoso K, Takeda-Yano K, Katayama T, Oono Y, Narumi I. Draft genome sequence of the radioresistant bacterium Deinococcus grandis, isolated from freshwater fish in Japan. Genome Announc. 2016 doi: 10.1128/genomeA.01631-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamitra M, Uma Devi P, Murase H, Kagiya VT. In vivo postirradiation protection by a vitamin E analog, alpha-TMG. Radiat Res. 2003;160(6):655–661. doi: 10.1667/RR3077. [DOI] [PubMed] [Google Scholar]
- Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178(6):505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifter E, Rettura G, Padawer J, Stratford F, Weinzweig J, Demetriou AA, Levenson SM. Morbidity and mortality reduction by supplemental vitamin A or beta-carotene in CBA mice given total-body gamma-radiation. J Natl Cancer Inst. 1984;73(5):1167–1177. [PubMed] [Google Scholar]
- Shareef M, Ashraf MA, Sarfraz M. Natural cures for breast cancer treatment. Saudi Pharm J. 2016;24(3):233–240. doi: 10.1016/j.jsps.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SK, Chaudhary P, Kumar IP, Samanta N, Afrin F, Gupta ML, Sharma UK, Sinha AK, Sharma YK, Sharma RK. Protection from radiation-induced mitochondrial and genomic DNA damage by an extract of Hippophae rhamnoides. Environ Mol Mutagen. 2006;47(9):647–656. doi: 10.1002/em.20251. [DOI] [PubMed] [Google Scholar]
- Sieber F, Muir SA, Cohen EP, Fish BL, Mader M, Schock AM, Althouse BJ, Moulder JE. Dietary selenium for the mitigation of radiation injury: effects of selenium dose escalation and timing of supplementation. Radiat Res. 2011;176(3):366–374. doi: 10.1667/RR2456.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Hauer-Jensen M. gamma-Tocotrienol as a promising countermeasure for acute radiation syndrome: current status. Int J Mol Sci. 2016 doi: 10.3390/ijms17050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Yadav VS. Role of cytokines and growth factors in radioprotection. Exp Mol Pathol. 2005;78(2):156–169. doi: 10.1016/j.yexmp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Singh VK, Brown DS, Kao TC. Tocopherol succinate: a promising radiation countermeasure. Int Immunopharmacol. 2009;9(12):1423–1430. doi: 10.1016/j.intimp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Singh VK, Ducey EJ, Fatanmi OO, Singh PK, Brown DS, Purmal A, Shakhova VV, Gudkov AV, Feinstein E, Shakhov A. CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini, as a novel radiation countermeasure. Radiat Res. 2012;177(5):628–642. doi: 10.1667/RR2657.1. [DOI] [PubMed] [Google Scholar]
- Singh VK, Beattie LA, Seed TM. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 2013;54(6):973–988. doi: 10.1093/jrr/rrt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Romaine PL, Newman VL, Seed TM. Tocols induce G-CSF and mobilise progenitors that mitigate radiation injury. Radiat Prot Dosim. 2014;162(1–2):83–87. doi: 10.1093/rpd/ncu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Romaine PL, Seed TM. Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the strategic national stockpile. Health Phys. 2015;108(6):607–630. doi: 10.1097/HP.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Kulkarni S, Fatanmi OO, Wise SY, Newman VL, Romaine PL, Hendrickson H, Gulani J, Ghosh SP, Kumar KS, Hauer-Jensen M. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat Res. 2016;185(3):285–298. doi: 10.1667/RR14127.1. [DOI] [PubMed] [Google Scholar]
- Son TG, Gong EJ, Bae MJ, Kim SD, Heo K, Moon C, Yang K, Kim JS. Protective effect of genistein on radiation-induced intestinal injury in tumor bearing mice. BMC Complement Altern Med. 2013;13:103. doi: 10.1186/1472-6882-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Ma L, Cong F, Shen X, Jing P, Ying X, Zhou H, Jiang J, Fu Y, Yan H. Radioprotective effects of genistein on HL-7702 cells via the inhibition of apoptosis and DNA damage. Cancer Lett. 2015;366(1):100–111. doi: 10.1016/j.canlet.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23(4):841–845. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Weiss JF, Kumar S. Radioprotection by misoprostol (PGE1 methyl analog) in combination with vitamin E, selenomethionine and WR-3689794. Adv Exp Med Biol. 1997;400B:791–797. [PubMed] [Google Scholar]
- Srinivasan M, Rajendra Prasad N, Menon VP. Protective effect of curcumin on gamma-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat Res. 2006;611(1–2):96–103. doi: 10.1016/j.mrgentox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Modulatory effects of curcumin on gamma-radiation-induced cellular damage in primary culture of isolated rat hepatocytes. Environ Toxicol Pharmacol. 2007;24(2):98–105. doi: 10.1016/j.etap.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol. 2011;11(3):331–341. doi: 10.1016/j.intimp.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, Macvittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins ME, Schiestl RH, Seed TM, Tomaszewski JE, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162(6):711–728. doi: 10.1667/RR3276. [DOI] [PubMed] [Google Scholar]
- Tsuneoka K, Ishihara H, Dimchev AB, Nomoto K, Yokokura T, Shikita M. Timing in administration of a heat-killed Lactobacillus casei preparation for radioprotection in mice. J Radiat Res. 1994;35(3):147–156. doi: 10.1269/jrr.35.147. [DOI] [PubMed] [Google Scholar]
- Turner PR, Denny WA. The mutagenic properties of DNA minor-groove binding ligands. Mutat Res. 1996;355(1–2):141–169. doi: 10.1016/0027-5107(96)00027-9. [DOI] [PubMed] [Google Scholar]
- Um HJ, Park JW, Kwon TK. Melatonin sensitizes Caki renal cancer cells to kahweol-induced apoptosis through CHOP-mediated up-regulation of PUMA. J Pineal Res. 2011;50(4):359–366. doi: 10.1111/j.1600-079X.2010.00851.x. [DOI] [PubMed] [Google Scholar]
- Uma Devi P, Ganasoundari A, Rao BS, Srinivasan KK. In vivo radioprotection by ocimum flavonoids: survival of mice. Radiat Res. 1999;151(1):74–78. doi: 10.2307/3579750. [DOI] [PubMed] [Google Scholar]
- Vadhan-Raj S, Goldberg JD, Perales MA, Berger DP, van den Brink MR. Clinical applications of palifermin: amelioration of oral mucositis and other potential indications. J Cell Mol Med. 2013;17(11):1371–1384. doi: 10.1111/jcmm.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vijgh WJ, Korst AE. Amifostine (Ethyol): pharmacokinetic and pharmacodynamic effects in vivo. Eur J Cancer. 1996;32A(Suppl 4):S26–S30. doi: 10.1016/S0959-8049(96)00332-2. [DOI] [PubMed] [Google Scholar]
- Veeraraghavan J, Natarajan M, Herman TS, Aravindan N. Curcumin-altered p53-response genes regulate radiosensitivity in p53-mutant Ewing’s sarcoma cells. Anticancer Res. 2010;30(10):4007–4015. [PubMed] [Google Scholar]
- Vijayalaxmi, Meltz ML, Reiter RJ, Herman TS. Melatonin and protection from genetic damage in blood and bone marrow: whole-body irradiation studies in mice. J Pineal Res. 1999;27(4):221–225. doi: 10.1111/j.1600-079X.1999.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Meltz ML, Reiter RJ, Herman TS, Kumar KS. Melatonin and protection from whole-body irradiation: survival studies in mice. Mutat Res. 1999;425(1):21–27. doi: 10.1016/S0027-5107(98)00246-2. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Reiter RJ, Tan DX, Herman TS, Thomas CR., Jr Melatonin as a radioprotective agent: a review. Int J Radiat Oncol Biol Phys. 2004;59(3):639–653. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko YH, Sayers CM, Baran M, Ware JH, Kennedy AR. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat Res. 2008;169(4):384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Jin BZ, Ai F, Duan CH, Lu YZ, Dong TF, Fu QL. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol. 2012;69(5):1213–1220. doi: 10.1007/s00280-012-1828-8. [DOI] [PubMed] [Google Scholar]
- Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189(1–2):1–20. doi: 10.1016/S0300-483X(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Weiss JF, Srinivasan V, Kumar KS, Landauer MR. Radioprotection by metals: selenium. Adv Space Res. 1992;12(2–3):223–231. doi: 10.1016/0273-1177(92)90112-B. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kinoshita M, Shinomiya N, Hiroi S, Sugasawa H, Matsushita Y, Majima T, Saitoh D, Seki S. Pretreatment with ascorbic acid prevents lethal gastrointestinal syndrome in mice receiving a massive amount of radiation. J Radiat Res. 2010;51(2):145–156. doi: 10.1269/jrr.09078. [DOI] [PubMed] [Google Scholar]
- Yantasee W, Sangvanich T, Creim JA, Pattamakomsan K, Wiacek RJ, Fryxell GE, Addleman RS, Timchalk C. Functional sorbents for selective capture of plutonium, americium, uranium, and thorium in blood. Health Phys. 2010;99(3):413–419. doi: 10.1097/HP.0b013e3181ce5f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, Amols H, Venkatraman ES, Leibel SA. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53(5):1111–1116. doi: 10.1016/S0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sadgrove MP, Mumper RJ, Jay M. Radionuclide decorporation: matching the biokinetics of actinides by transdermal delivery of pro-chelators. AAPS J. 2013;15(4):1180–1188. doi: 10.1208/s12248-013-9527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]