Abstract
Introduction
Patient eczema severity time (PEST) is a new atopic dermatitis (AD) scoring system based on patients’ own perception of their disease. Conventional scales such as SCORing of atopic dermatitis (SCORAD) reflect the clinician’s observations during the clinic visit. Instead, the PEST score captures eczema severity, relapse and recovery as experienced by the patient or caregiver on a daily basis, promoting patient engagement, compliance with treatment and improved outcomes. This study aims to determine the correlation between carer-assessed PEST and clinician-assessed SCORAD in paediatric AD patients after 12 weeks of treatment using a ceramide-dominant therapeutic moisturizer.
Methods
Prospective, open-label, observational, multi-centre study in which children with AD aged 6 months to 6 years were treated with a ceramide dominant therapeutic moisturizer twice daily for 12 weeks; 58 children with mild-to-moderate AD were included. Correlation between the 7-day averaged PEST and SCORAD scores for assessment of AD severity was measured within a general linear model. PEST and SCORAD were compared in week 4 and week 12.
Results
At week 12, a moderate correlation was found between the SCORAD and PEST scores (r = 0.51). The mean change in SCORAD and PEST scores from baseline to week 12 was −11.46 [95% confidence interval (CI) −14.99 to −7.92, p < 0.0001] and −1.33 (95% CI −0.71 to −0.10, p < 0.0001) respectively. PEST demonstrated greater responsiveness to change (33.3% of scale) compared to SCORAD (13.8% of scale).
Conclusion
The PEST score correlates well with the SCORAD score and may have improved sensitivity when detecting changes in the severity of AD. The ceramide-dominant therapeutic moisturizer used was safe and effective in the management of AD in young children.
Funding
Hyphens Pharma Pte Ltd.
Trial Registration
clinicaltrials.gov identifier, NCT02073591.
Keywords: Atopic dermatitis, Ceramide, Children, SCORAD, PEST
Introduction
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disorder with onset commonly occurring in infancy and childhood [1–3]. AD-prone patients may develop allergic rhinitis, food allergy and asthma [1, 4–6]. AD arises as a result of a complex interaction of environmental and genetic factors. Breakdown of the skin barrier is common to all forms of AD. At the mild end of the severity spectrum, this predominantly arises as a result of outside-inside interactions, while at the severe end of the spectrum, it is predominantly inside-outside with immune dysregulation playing a major role [1, 7]. Meanwhile, there is increasing evidence supporting a barrier-initiated pathogenesis of AD [7–10].
The severity of AD can be assessed using various scoring systems such as the scoring of atopic dermatitis index (SCORAD), eczema area and severity index (EASI) and patient-oriented eczema measure (POEM), each of which is sensitive and widely accepted [11–14]. SCORAD was developed by the European Task Force on Atopic Dermatitis (ETFAD) [15] to assess the extent and severity of AD. Considered an objective measure, SCORAD’s strengths include its validity, responsiveness and inter-observer reliability. Its limitations were previously reported [13, 16, 17].
Patient eczema severity time (PEST) is a new, simple daily measure, which supports the patients’ own perception and experience of the severity of their disease [18]. Patients or carers rate the severity of AD on a daily basis, thus reflecting disease fluctuations over a period of time instead of at discrete time intervals. Such insights may promote effective management of AD.
This study sought to investigate the correlation between SCORAD and PEST scores over 12 weeks in children between 6 months and 6 years of age.
Methods
Study Design
This was a prospective, open-label, single-arm, observational and multicentre study.
Study Population
Patients were recruited from two centres in Singapore. Male and female children between 6 months to 6 years of age, with evidence of mild to moderate AD, as determined by dermatitis with baseline PEST scores of 3–4, and grading of 3–11 as per the Nottingham Eczema Severity Scale (NESS), were eligible. Other inclusion criteria were diagnosis of current flare (increased dryness, itching, redness, swelling and general irritability) at the baseline visit according to the investigator’s judgement and dermatologist-naïve for treatment of AD. Informed consent was obtained from the parent or guardian (and assent as well, if applicable).
Patients with clinical signs of skin infection, history of severe episodes of AD, known reaction or allergy to the test drug, excipients, steroids, history of cutaneous or systemic diseases or medication that might interfere in the study results were excluded from study.
Procedure
Participants received treatment with a ceramide-dominant therapeutic moisturizer (Ceradan® Cream; Hyphens Pharma Pte Ltd, Singapore) for topical application at least twice daily for 12 weeks. Use of permitted topical steroid(s) as rescue treatment was allowed according to standard local practice and according to investigator discretion. A non-soap based wash once or twice daily was also allowed during the study period. Patients were evaluated for severity of AD at baseline, week 4 (visit 2) and week 12 (visit 3) using SCORAD. PEST was recorded daily using a patient diary containing pictorials ranging from ‘not at all unhappy’ to ‘extremely unhappy’ and scoring 1–5 (Fig. 1).
Fig. 1.
PEST scoring tool. PEST patient eczema severity time
Outcome Measures
The primary outcome was the correlation between the PEST and SCORAD scores after 12 weeks of treatment. Secondary outcomes included changes in SCORAD and PEST scores during follow-up, progress of AD over time evaluated by changes in SCORAD and PEST scores, and counts of relapse or flare of AD. Using SCORAD as a basis, the severity grading was defined as mild (<20), moderate (20–40) and severe (≥40). Relapse was defined as exacerbation of AD to the level where a topical corticosteroid or alternative therapy was required.
The study also evaluated subject satisfaction. At the end of the study (week 12), patients were asked whether they would like to continue with the emollient regimen. Options to choose were (1) definitely, (2) most likely, (3) maybe and (4) no.
Adverse Events
All treatment-emergent adverse events (TEAEs) were recorded at each study visit. Number, dose and duration of rescue medications used for adverse events were also documented.
Sample Size
Sample size was determined by considering an anticipated correlation of 0.4 between the mean PEST and SCORAD scores providing 80% power at a 5% significance level, applying Fisher’s z test for Pearson correlation. Thus, 46 evaluable patients were required.
Statistical Analyses
Pearson’s correlation (r) between PEST and SCORAD scores at week 12 was estimated using a general linear model (GLM) of standardized scores. Values of |r| ≥ 0.5 signified moderate correlation and |r| ≥ 0.7 signified high correlation. PEST scores were averaged over the 7-day period corresponding to the 1-week recall of the SCORAD, e.g. the 4th or 12th week. For continuous variables, descriptive statistics were presented as counts, mean, median, standard deviation, minimum and maximum. Categorical variables were presented as counts and percentages.
For patients who were withdrawn early from the study, analyses conservatively applied the last observation carried forward principle. The primary analysis was by modified intention to treat (mITT), although efficacy was also explored as patients completing treatment per protocol and excluding patients with major protocol deviations. Safety was assessed for all patients who applied the study medication at least once. All statistical analyses were performed with SAS version 9.2 or higher.
This study was approved by the institutional review board (IRB) of KK Women’s and Children’s Hospital and National Skin Centre Singapore and conducted according to Good Clinical Practice guidelines and applicable local regulatory requirements. All procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1964, as revised in 2013. Written informed consent was obtained from all patients or their caregivers.
Results
Subjects
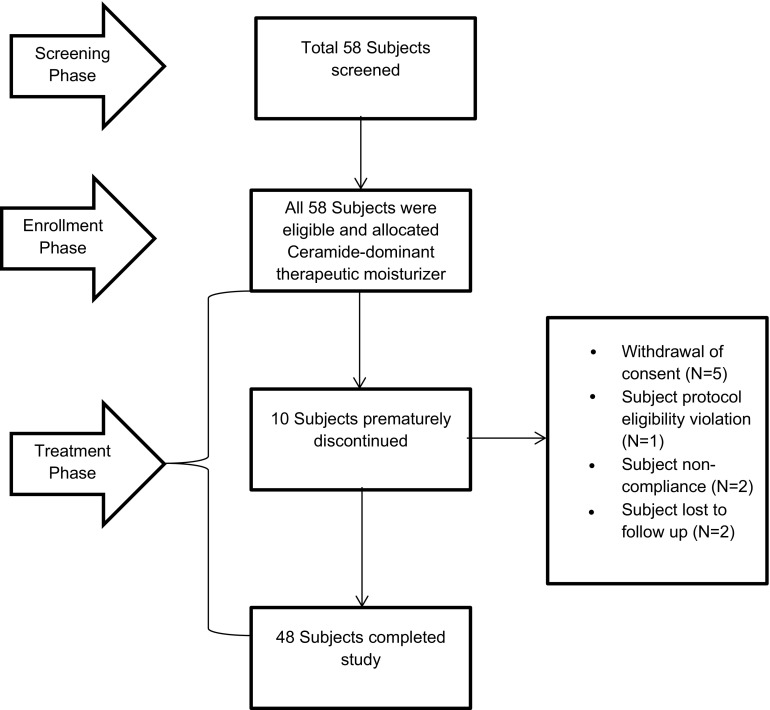
A total of 58 subjects were screened between March 2014 and August 2014. All subjects received a ceramide-dominant therapeutic moisturizer at least twice daily for 12 weeks. Ten subjects were withdrawn prematurely for a range of reasons, although all contributed to the safety analysis (Fig. 2). The modified intent-to-treat population consisted of 55 (94.8%) patients: 3 patients had five major protocol deviations of the following nature: three counts of eligibility criteria violation, one incomplete patient diary and one missing diary. The most common minor deviation was follow-up attendance outside of the acceptable window period: 13 patients for visit 2 and 11 patients for visit 3. The per-protocol population included 46 (79.3%) patients.
Fig. 2.
Flow diagram of subject disposition
Subjects’ age ranged from 0.5 to 6.4 years; 48.3% were male and 51.7% were female. The majority of subjects were Chinese (79.3%). At baseline, the mean SCORAD score was 24.4 (8.5 SD) and mean PEST score was 3.11 (0.52 SD) for the mITT population with the last observation carried forward (LOCF) (Table 1).
Table 1.
Demographic and disease characteristics at baseline
| Variable | N = 58 |
|---|---|
| Mean age in years (range in years) | 2.61 (0.51–6.44) |
| Number of subjects | % | |
|---|---|---|
| Gender | ||
| Male | 28 | 48.28 |
| Female | 30 | 51.72 |
| Race | ||
| Chinese | 46 | 79.31 |
| Malay | 6 | 10.34 |
| Indian | 3 | 5.17 |
| Others | 3 | 5.17 |
| Height (cm): mean (SD) | 89.82 (17.06) | |
| Weight (kg): mean (SD) | 13.01 (4.60) | |
| SCORAD score for mITT population with LOCF (N = 55) at baseline mean (SD) | 24.42 (8.46) | |
| PEST score for mITT population with LOCF (N = 55) at baseline mean (SD) | 3.11 (0.52) | |
cm centimetres, kg kilograms, SD standard deviation, mITT modified intention to treat
Correlation Between PEST and SCORAD Scores
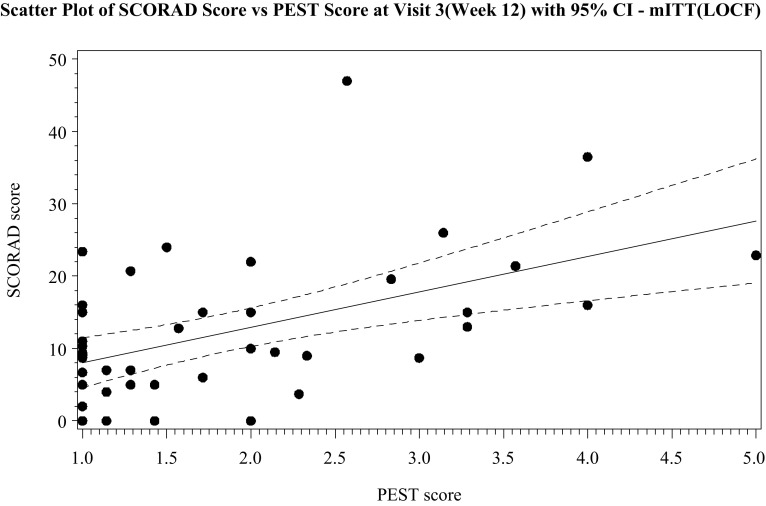
The primary finding was a moderate correlation between the SCORAD and PEST score (r = 0.51) at week 12 (Fig. 3). Correlation was similar at week 4 (r = 0.52), but at baseline, the correlation was weak (r = 0.22) (Table 2). Analysis of correlation stratified by level of ceramide-dominant therapeutic moisturizer usage at week 12 showed moderate correlation (r = 0.48) in the lowest range of therapeutic moisturizer used (20–399.99 g). The use of more therapeutic moisturizer showed higher correlation, i.e. 400–799.9 g (r = 0.50) and 800–1200 g (r = 0.71).
Fig. 3.
Scatter plot of the SCORAD score vs. PEST score at visit 3 (week 12) with 95% CI. PEST patient eczema severity time, SCORAD scoring of atopic dermatitis, mITT modified intention-to-treat, LOCF last observation carried forward, CI confidence interval
Table 2.
Correlation between the PEST and SCORAD scores at baseline, week 4 and week 12 (mITT population with LOCF imputation)
| (N = 55) n |
Correlation coefficient | |
|---|---|---|
| Visit 1 (baseline) | 54 | 0.22 |
| Visit 2 (week 4) | 53 | 0.52 |
| Visit 3 (week 12) | 43 | 0.51 |
Correlation coefficient calculated from the generalized linear model
PEST patient eczema severity time, SCORAD scoring of atopic dermatitis, mITT modified intention to treat, LOCF last observation carried forward, N number of subjects in the mITT population, n number of subjects in the specified category
Efficacy by Change in SCORAD and PEST Scores
SCORAD decreased from the baseline during the study period. Mean change from baseline was −8.63 (95% CI −11.68 to −5.57) and −11.46 (95% CI −14.99 to −7.92) at visit 2 and visit 3 respectively; both changes were statistically significant (p < 0.0001). Mean PEST decreased from baseline by −0.93 (95% CI −1.18 to −0.67) at visit 2 (average score of 2.2) and −1.33 (95% CI −1.62 to −1.04) at visit 3 (average score of 2.0); both changes were statistically significant (p < 0.0001).
The mean change in SCORAD, with a range of 0–83, was 13.8% of scale (95% CI 9.5–18.1%); the mean change in PEST (with a range of 1–5) was 33.3% of scale (95% CI 26.0–40.5%).
Changes in PEST Score Over Time
All subjects started with scores of 3 or 4 (quite and very unhappy); improvement in the severity of AD was observed over time (Fig. 4). After 12 weeks of treatment, of 48 patients in the quite unhappy category at baseline, 39 (81.3%) showed improvement in AD severity, 3 (6.25%) observed no change in AD severity and 4 (8.33%) showed worsening in AD severity; 2 (4.17%) patients’ records were not available. Of the seven patients who were very unhappy at baseline, after 12 weeks of treatment, all of the patients showed improvement in AD severity: four (57.1%) patients were found in the not at all unhappy group, one (14.3%) patient was in the a little unhappy and two (28.6%) patients were in the quite unhappy category; none of the subjects were reported as very unhappy or extremely unhappy.
Fig. 4.
Progress of the condition using an average PEST score per week over 12 weeks. PEST patient eczema severity time
Changes in the SCORAD Score over Time
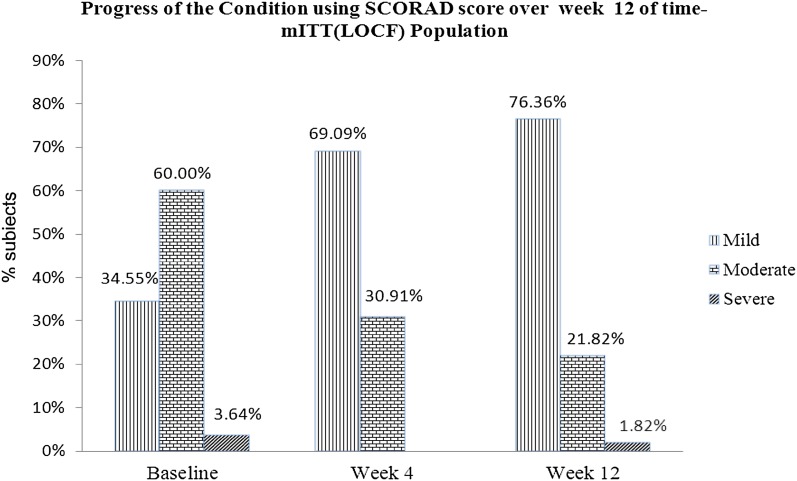
Subjects using the ceramide-dominant therapeutic moisturizer showed general improvement in AD (Fig. 5). None of the patients’ severity had worsened from the mild to severe category after 12 weeks of treatment. Thirty-three subjects with a moderate AD category either improved to the mild category (26 subjects) or remained in the moderate category (6 subjects), while one patient showed increased severity from moderate to severe AD after 12 weeks of treatment. Two subjects with the severe category at baseline showed improvement in AD severity; one subject was found to be in the mild AD category, and another was found to be in the moderate AD category.
Fig. 5.
Progression of the condition of AD using the SCORAD score over week 12 of the time-mITT (LOCF) population. SCORAD scoring of atopic dermatitis, mITT modified intention-to-treat, LOCF last observation carried forward
Relapse of AD
Use of the ceramide-dominant therapeutic moisturizer prevented relapse of AD in 25 (45.5%) patients.
Subject Satisfaction
More than half of the patients, 32 (58.2%), indicated that they would like to continue the use of the ceramide-dominant therapeutic moisturizer regimen, 15 (27.3%) patients responded that they may continue with the ceramide-dominant therapeutic moisturizer and only 2 (3.6%) patients answered that they would not use this regimen (Table 3).
Table 3.
Subject satisfaction with the ceramide-dominant therapeutic moisturizer at week 12
| Subject satisfaction for ceramide-dominant therapeutic moisturizer | (N = 55) n (%) |
|---|---|
| Definitely + most likely | 32 (58.18%) |
| Maybe | 15 (27.27%) |
| No | 2 (3.64%) |
| Not available | 6 (10.91%) |
N number of subjects in the mITT population, n number of subjects at the specified category
Safety
During the study, 26 (44.8%) patients reported at least one adverse event (AE). Overall, 40 AEs were reported (Table 4); none were reported as severe. The causality of the AEs was categorized as ‘certain/definite, probable, possible, unlikely and not related’ to the use of the ceramide-dominant therapeutic moisturizer. Two (3.45%) of the patients had ‘probable’ AEs, none of the patients had experienced ‘possible’ AEs and two (3.45%) of the patients had ‘unlikely’ AEs, while the remaining 23 (39.66%) patients experienced ‘not related’ AEs. The most common AEs reported are respiratory disorders and skin disorders. No death was reported in this study. One patient was discontinued from treatment because of AE/SAE. Only one SAE, a viral infection, occurred in one (1.72%) patient, and this was evaluated to be not related to the use of the ceramide-dominant therapeutic moisturizer.
Table 4.
Overall summary of adverse events
| Category | (N = 58) n (%) |
|---|---|
| Total number of adverse events (AE) | 40 |
| Total number of subjects with at least one AE | 26 (44.83%) |
| Total number of serious adverse events (SAE) | 1 |
| Total number of subjects with at least one SAE | 1 (1.72%) |
| Death | 0 (0.00%) |
| Subjects discontinued because of AE/SAE | 1 (1.72%) |
| Mild | 24 (41.38%) |
| Moderate | 3 (5.17%) |
| Severe | 0 (0.00%) |
None of the patients experienced AE that was ‘certainly/definitely’ related to the ceramide-dominant therapeutic moisturizer
N number of subjects in the safety population, n number of subjects at a specified category
Discussion
Charman and colleagues revealed that use of valid and clinically meaningful patient outcome measures is gaining importance as more stress is being placed on the results of patient/carer measures in clinical trials [19]. Clinical assessments of disease may be inadequate to express disease impact, and subjective measures may help to provide a more complete and appropriate assessment of the effect of treatment on patients’ lives [12, 20]. Mason and colleagues reported a new patient-centric scoring system, PEST for assessment of atopic dermatitis [18]. PEST is a simple system, which assesses a patient’s (or carer’s) global feeling about the severity of disease instead of clinical opinion, while its ease of use as a daily record allows important changes over time to be captured. The pictorial design helps the parents to assess the PEST score in patients who are too young to vocalize their own experience.
PEST was used concurrently with POEM in an educational programme aimed at increasing emollient use and reducing AD symptoms in 136 children aged 3 months to 6 years with mild to moderate atopic eczema [18]. The POEM scale uses a questionnaire of seven questions, each with a 5-point scale (0–4) to assess symptom frequency and the pattern of remission and relapse. Topical emollient application reduced AD severity: on average, the POEM score reduced by 5.38 (95% CI 4.36–6.41, p = 0.001), a 47% reduction from baseline, while the PEST score reduced by 0.61 (95% CI 0.47–0.75, p = 0.001), a 48% reduction from baseline. POEM and PEST scores were strongly correlated with Pearson correlation coefficients. At week 12, the correlation coefficient was 0.71 (p < 0.01). While POEM assesses and aggregates the frequency of signs and symptoms experienced in the past week, PEST captures changes over time of the summative experience of the patient. Thus, a major advantage of the PEST score is that it takes only a couple of seconds for the patient/carer to give a score.
In the present study, we evaluated the use of SCORAD and PEST scores in the management of AD in children aged 6 months to 6 years who used a ceramide-dominant therapeutic moisturizer. SCORAD and PEST scores were moderately correlated at week 4 (r = 0.52) and at week 12 (r = 0.51), providing some cross validation, although the two measures may be capturing different aspects of the disease. SCORAD provides detailed clinical assessments at a point in time; PEST describes and summates the time-varying patient experience of AD. Interestingly, the weak correlation at baseline could be due to a disconnect between an objective scoring (SCORAD) and patient score when there is acute flare. This phenomenon could be unique in Singapore where patients are generally demanding, resulting in them giving higher PEST scores than the corresponding SCORAD score.
Also, the proportionate scale changes suggest the discrimination provided by daily assessment may make PEST a more sensitive measure with greater proportionate use of the scale range.
In contrast, SCORAD is not used as a daily measure, but is used to characterise the area and intensity of AD with minor weight given to the patient experience. Individual patients may only use a small part of its range of scores, e.g. mild disease. Thus, the sensitivity of SCORAD is constrained by the frequency of use and area of involvement. However, in contrast to PEST, SCORAD remains useful to compare severity between patients and individual patient changes during a clinical trial.
PEST, as a patient-reported outcome measure (PROM), may have particular utility as a sensitive measure for use in the clinic or clinical trials because of its range and time characteristics. The score range expands to fit an individual patient: score 1 is the best the AD is for that individual patient; score 5 is the worst it is for that individual patient. In the time domain, PEST has the ability to capture the relapsing-remitting nature of AD as experienced by the patient (or their carer) on a daily basis and thus is important in the long-term management.
The study was designed to recruit patients with current flares; thus, topical steroid(s) were used to rapidly control the inflammation. The European Guidelines for treatment of atopic dermatitis clearly state that emollient use alone on inflamed skin is poorly tolerated and recommend treating the acute flare first [21]. Therefore, one possible limitation is that the effect of the steroid on PEST in the early days cannot be ruled out. Hence, the degree of severity improvement in the early days must be interpreted carefully.
Conclusions
The PEST score correlates moderately with the SCORAD score and has improved sensitivity when detecting changes in the severity of AD in an individual patient in a clinical trial or in the clinic. Measuring the PEST score daily may provide insights for better patient prognosis and concordance. The ceramide-dominant therapeutic moisturizer used within the study was safe and effective in the management of AD in young children.
Acknowledgements
The study design, data collection, analysis, interpretation of results, medical writing, study-approved ceramide-dominant therapeutic moisturizer, topical steroids, non-soap wash used by the subjects and article processing charges were funded by Hyphens Pharma Pte Ltd. ClinActis Pte Ltd. provided assistance in medical writing. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published. The authors would like to thank the hospital staff who managed the clinical trial and patients enrolled in the trial.
Disclosures
Mark Jean-Ann Koh, Yoke Chin Giam, Hui Min Liew, Alice Yee-Wah Foong, Jin Ho Chong, Sharon Mun Yee Wong, Mark Boon Yang Tang, Madeline Sheun Ling Ho, Lucinda Siyun Tan, James M. Mason and Michael J. Cork have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the institutional review board (IRB) of KK Women’s and Children’s Hospital and National Skin Centre Singapore and conducted according to Good Clinical Practice guidelines and applicable local regulatory requirements. All procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1964, as revised in 2013. Written informed consent was obtained from all patients or their caregivers.
Data Availability
The data supporting the conclusion are included within the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
The original version of this article was revised: The given name and family name of the co-author Dr. Jin Ho Chong were incorrectly published in the original publication. The correct given name and family name should read as ‘Jin Ho’ and ‘Chong’, respectively.
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/9998F0604CCE08B7.
An erratum to this article is available at https://doi.org/10.1007/s13555-017-0190-5.
Change history
7/4/2017
An erratum to this article has been published.
References
- 1.Watson W, Kapur S. Atopic dermatitis. Allergy asthma. Clin Immunol. 2011;7(Suppl 1):S4. doi: 10.1186/1710-1492-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:354250. doi: 10.1155/2014/354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz RA. Pediatric atopic dermatitis. http://emedicine.medscape.com/article/911574-overview. Assessed on 28 Apr 2015.
- 4.De Benedetto A, Agnihothri R, McGirt LY, et al. Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol. 2009;129:14–30. doi: 10.1038/jid.2008.259. [DOI] [PubMed] [Google Scholar]
- 5.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 6.Zheng T, Yu J, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cork MJ, Robinson DA, Vasilopoulos Y, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:282–295. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O’Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 10.Danby SG, Cork MJ. The skin barrier in atopic dermatitis. In: Irvine A, Hoeger P, Yan A, editors. Textbook of pediatric dermatology. 3. Oxford: Blackwell Publishing Ltd; 2011. [Google Scholar]
- 11.Rullo V, Segato A, Kirsh A, et al. Severity scoring of atopic dermatitis: a comparison of two scoring systems. Allergol Immunopathol. 2008;36:205–211. doi: 10.1016/S0301-0546(08)72551-5. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt J, Langan S, Williams HC, et al. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol. 2007;120:1389–1398. doi: 10.1016/j.jaci.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Wolkerstorfer A, De Waard van der Spek FB, Glazenburg EJ, et al. Scoring the severity of atopic dermatitis: three item severity score as a rough system for daily practice and as a pre-screening tool for studies. Acta Derm Venereol. 1999;79:356–359. doi: 10.1080/000155599750010256. [DOI] [PubMed] [Google Scholar]
- 14.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513–1519. doi: 10.1001/archderm.140.12.1513. [DOI] [PubMed] [Google Scholar]
- 15.European Task Force on Atopic Dermatitis Severity scoring of atopic dermatitis: the SCORAD index (consensus report of the European Task Force on Atopic Dermatitis) Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 16.Oranje AP, Glazenburg EJ, Wolkerstorfer A. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157:645–648. doi: 10.1111/j.1365-2133.2007.08112.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt J, Spuls PI, Thomas KS, et al. The harmonising outcome measures for eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800–807. doi: 10.1016/j.jaci.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Mason JM, Carr J, Buckley C, et al. Improved emollient use reduces atopic eczema symptoms and is cost neutral in infants: before-and-after evaluation of a multifaceted educational support programme. BMC Dermatol. 2013;13:7. doi: 10.1186/1471-5945-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charman C, Chambers C, Williams H. Measuring atopic dermatitis severity in randomized controlled clinical trials: what exactly are we measuring? J Invest Dermatol. 2003;120:932–941. doi: 10.1046/j.1523-1747.2003.12251.x. [DOI] [PubMed] [Google Scholar]
- 20.Murray CS, Rees JL. Are subjective accounts of itch to be relied on? The lack of relation between visual analogue itch scores and actigraphic measures of scratch. Acta Derm Venereol. 2011;91:18–23. doi: 10.2340/00015555-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26(8):1045–1060. doi: 10.1111/j.1468-3083.2012.04635.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusion are included within the manuscript.