Abstract
Introduction
Intense nocturnal pruritus as well as the complex pathophysiology of atopic dermatitis (AD) can severely affect sleep and become a major factor in negatively impacting quality of life in adults. However, much of the literature on sleep disturbance in AD patients is on the pediatric population, and it is not well studied in adults. Furthermore, limited studies are available to guide effective management of sleep disturbance in AD in general. We review the literature to present the studies that have investigated the relationship between AD and its effect on sleep in adults and provide an approach for clinicians caring for this population.
Methods
A systematic literature search was conducted through the PubMed and EMBASE databases using the search terms “atopic dermatitis” OR “eczema” AND “sleep.” The articles generated by the search and their references were reviewed.
Results
A high prevalence of sleep disturbance is experienced by adults with AD. The likelihood of sleep disturbance is much higher in patients with AD compared to those without AD. Sleep disturbance appears to worsen with AD severity. Pruritus and scratching appear to be large contributors to sleep disturbance in adult patients with AD.
Conclusion
It is important that clinicians evaluate the severity of AD and ask general questions about itching, sleep, impact on daily activities, and persistence of disease during each patient visit and follow-up with the complaint of sleep disturbance. Management of sleep disturbance in AD should focus on adequate disease control of AD as well as possible medical interventions to help improve sleep. The pathophysiology of sleep disturbance in AD is extremely complex, and further research is needed to better understand the interplay of the immune system, circadian rhythm, and environmental factors implicated in both AD and sleep.
Keywords: Adults, Atopic, Dermatitis, Disturbance, Eczema, Sleep
Introduction
Atopic dermatitis (AD), commonly known as eczema, is a chronic relapsing inflammatory skin disease characterized by pruritus, erythema, and lichenified lesions of the skin. The pathogenesis of AD is complex and involves genetic predisposition, a hyperactive immune system, and environmental factors [1]. While AD most frequently affects children, it is a disease that can persist into adulthood or have adult onset. The prevalence in US adults is estimated to be 7.2% [2, 3].
Pruritus is an early characteristic symptom of AD, so much so that AD is known as “the itch that rashes” [4]. AD is commonly associated with intense nocturnal pruritus, which can affect sleep and quality of life (QoL) [5, 6]. Sleep insufficiency can lead to excessive daytime sleepiness, mood disturbance, and impaired cognition [7], which can then have an impact on work or school productivity, accidents, and adverse health outcomes including metabolic, endocrine, and immune dysregulation such as type 2 diabetes, hypertension, and infection [8–12].
Both qualitative and quantitative analyses of sleep disturbance in patients with AD are valuable for the evaluation of disease activity and response to therapy. Much of the current literature focuses on sleep disturbance in pediatric patients with AD using qualitative assessments based on subjective surveys [13]. In general, discrepancies between subjective and objective measurements of sleep have been previously reported [14, 15]. For example, adults with nocturnal asthma reported reduced overall sleep quality compared to control subjects but not increases in frequency of awakenings (subjective) even though polysomnographic measurement revealed differences in sleep latency, efficiency, and awakenings (objective) [16]. The topic of sleep disturbance in adult AD patients is not well studied. We reviewed the literature for studies that have investigated the relationship between AD and its effect on sleep in adults.
Methods
A systematic literature search was conducted through the PubMed and EMBASE databases using the search terms “atopic dermatitis” OR “eczema” AND “sleep.” The search terms were used to find peer-reviewed journal articles published between 1980 and 20 December 2016. The literature search was limited to human adult (≥18 years of age) studies written in English. Each article resulting from the literature search was screened primarily by three authors for content by reading the abstract. Original investigations focusing on sleep disturbance in adults with AD were chosen for further review. The references of these articles were searched for other relevant articles and reviewed. Review articles, case series/reports, and commentaries were excluded. Articles were organized under five sections: “I. Methods Used to Assess Sleep Disturbance”, “II. Association of AD with Sleep Disturbance”, “III. Specific Alterations of Sleep Identified in AD”, “IV. Association of AD with Impaired Functioning and Quality of Life”, and “V. Treatment Strategies for Sleep Disturbance in Adults with AD”. Some articles fell under multiple categories. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
The PubMed and EMBASE database search yielded a total of 250 articles. Of these, 39 articles were deemed relevant and were original investigations on sleep disturbance in adults with AD that focused on at least one of five parameters outlined above. The summary of the results is shown in Table 1.
Table 1.
Summary of results of original investigations on sleep disturbance in adults with atopic dermatitis (Sections I–IV)
| References | I. Methods Used to Assess Sleep Disturbance | II. Association of AD with Sleep Disturbance | III. Specific Alterations of Sleep Identified in AD | IV. Association of AD with Impaired Functioning and Quality of Life |
|---|---|---|---|---|
| Aoki et al. [31] | PSG | Scratching mainly occurs in N1 and N2 stages, the act of scratching during sleep brought the patients to a more superficial stage of sleep | ||
| Beikert et al. [25] | Subjective surveys, PSQI, DLQI | Significant correlation between PSQI and DLQI | ||
| Bringhurst et al. [30] | Actigraphy | The median number of movements during sleep is twice as high in AD vs. controls | ||
| Bender et al. [27] | PSQI, actigraphy, PSG | No significant differences in sleep latency in AD vs. controls using PSQI; AD patients slept less, awoke more often, and spent more time awake during waking episodes vs. controls using actigraphy | ||
| Bender et al. [26] | PSQI, actigraphy, PSG | Correlation between AD and sleep disturbance | Scratching mainly occurs in N1 and N2 stages, scratching correlates with lower sleep efficiency, no statistically significant difference in sleep efficiency | More daytime dysfunction in AD patients with sleep disturbance vs. control subjects |
| Ebata et al. [35] | Video monitoring | Longer durations of scratching and being awake after a scratching bout, patients usually turned over or woke up after a scratching bout | ||
| Ebata et al. [32] | PSG, video monitoring | Longer durations of scratching and being awake after a scratching bout in AD vs. controls | ||
| Misery et al. [22] | Subjective surveys | No significant correlation between SCORAD and daytime sleepiness | ||
| Noro et al. [34] | A novel wristwatch sound detector, overnight video observation | Higher ratio of scratching duration to total sleeping time in AD vs. controls, the mean scratching duration is more than 10 times higher in AD, no significant difference in the mean sleeping time between AD and controls | ||
| Jernelov et al. [21] | Subjective surveys | Longer sleep latencies | ||
| Kong et al. [24] | Subjective surveys, PSQI, DLQI | Correlation between only the pruritus score of SCORAD and PSQI | Significant correlation between PSQI and DLQI | |
| Sanchez-Perez et al. [20] | Subjective surveys | Difficulty falling asleep and waking up from sleep | ||
| Sandoval et al. [29] | PSQI, actigraphy | Correlation between AD and sleep disturbance | ||
| Simpson et al. [18] | POEM, SCORAD, 5D Pruritus Scale | 68.2% of patients reported itch delayed falling asleep and occasionally or frequently woke them up at night (5D Pruritus Scale); 36.1% reported sleep was disturbed every night (POEM). Sleep was disrupted an average of 4.4 nights over the previous week (POEM); the mean score on the self-reported sleep loss VAS was 4.8 (SCORAD) | ||
| Silverberg et al. [2] | Subjective surveys, DLQI | More likely to suffer from insomnia, found association between AD and sleep disturbance using DLQI | AD patients with sleep disturbance had higher numbers of missed workdays, days in bed and doctor visits than those with either AD or sleep disturbances alone | |
| Terreehorst et al. [23] | Subjective surveys | Severity of sleepiness has significant negative effect on QoL | ||
| Yano et al. [19] | Subjective surveys, PSQI, DLQI | Correlation between SCORAD and PSQI | Subjective sleep quality, latency, habitual sleep efficiency, sleep disturbance, and daytime dysfunction correlate with lower DLQI scores; duration of sleep and sleep medication use do not correlate with DLQI | |
| Yu et al. [17] | Subjective surveys, DLQI | Shorter sleep time, trouble falling asleep, nighttime awakenings, early morning awakenings, feeling unrested, feeling overly sleepy, feeling as if they did not get enough sleep, association between AD and sleep disturbance using DLQI | Higher occurrence of leg jerks and leg cramps, no significant association between AD and snoring or cessation of breathing | AD patients who reported being tired were more likely to have difficulty with IADLs |
AD atopic dermatitis, DLQI dermatology life quality index, IADLs instrumental activities of daily living, POEM patient-oriented eczema measure, PSG polysomnography, PSQI Pittsburg Sleep Quality Assessment, QoL quality of life, SCORAD SCORing atopic dermatitis, VAS visual analog scale
I. Methods Used to Assess Sleep Disturbance
Questionnaires
A number of studies in the literature assessed for sleep disturbance using qualitative assessments based on subjective surveys [2, 17–23]. However, a more in-depth assessment of sleep was reported in other studies using a specific sleep questionnaire using the Pittsburg Sleep Quality Assessment (PSQI) [19, 24–27]. This is a validated, self-reporting questionnaire to measure the quality and patterns of sleep in adults [28]. It is composed of seven components that evaluate the quality of sleep in adults: (1) subjective sleep quality, (2) sleep latency, (3) sleep duration, (4) habitual sleep efficiency, (5) sleep disturbance, (6) use of sleeping medication, and (7) daytime dysfunction (Table 2). Ranging from 0 to 21 points, a higher PSQI score is indicative of more disturbed sleep. A limitation of PSQI is the reliance on self-reported data, which may be affected by recall bias.
Table 2.
The seven components of the Pittsburg Sleep Quality Assessment (PSQI)
| Pittsburg sleep quality assessment (PSQI) |
|---|
| 1. Subjective sleep quality |
| 2. Sleep latency |
| 3. Sleep duration |
| 4. Habitual sleep efficiency |
| 5. Sleep disturbance |
| 6. Use of sleeping medication |
| 7. Daytime dysfunction |
Actigraphy
Actigraphy is a small, wrist-worn device that estimates sleep-wake patterns (sleep latency, efficiency, and periods of awakening) by using activity-based monitoring. It has been validated to assess sleep parameters and highly correlates with polysomnography [24]. Actigraphy may be the preferred method of assessing sleep in AD because it reflects the home environment, is easy to use, is cost-effective, and assesses the most common parameters of sleep affected in patients with AD (e.g., sleep disruption secondary to nocturnal awakenings and scratching) [29]. In this review, we identified a small number of studies using actigraphy [26, 27, 29, 30].
Polysomnography
The components of polysomnography (PSG) include the electroencephalogram (EEG), electrooculogram (EOG), and electromyogram (EMG), which together make up the gold standard of sleep examinations. From this literature search, only a small number of studies used PSG to assess sleep in adults with AD [26, 27, 31, 32]. Utilizing PSG can be difficult because of the inconvenience of having to stay overnight at a sleep center and the discomfort of having multiple leads and equipment attached, especially for patients with AD who may experience more skin irritation.
II. Association of AD with Sleep Disturbance
Estimated Prevalence of Sleep Disturbance in AD
Current published data based on surveys of AD patients suggest that 33–87.1% of adults with AD suffer from sleep disturbance based on surveys of AD patients [17, 18, 20].
Risk of Sleep Disturbance in AD
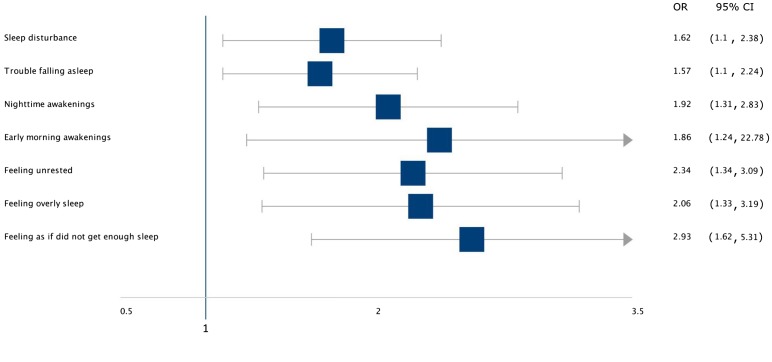
A survey-based study by Yu et al. [17] demonstrated that adult patients with AD had a higher likelihood of sleep disturbance compared to those without AD, including specific measures such as shorter sleep time, trouble falling asleep, nighttime awakenings, and early morning awakenings (Fig. 1). Adult AD patients also have higher odds of feeling unrested, overly sleepy, and as if they did not get enough sleep. This study did not report any significant association between AD and clinical diagnosis of a sleep disorder. Another survey-based study by Silverberg et al. [2] found that adult AD patients are more likely to suffer from insomnia (OR: 2.36; P < 0.0001) compared to adults without AD. A number of studies using general QoL surveys [e.g., the Dermatology Quality Life Index (DLQI)] also found an association between AD and sleep disturbance [2, 17, 19, 24, 25].
Fig. 1.
Risk of sleep disturbance in atopic dermatitis according to Yu et al. [17]
Correlation Between Sleep Disturbance and AD Severity
Studies that investigated the relationship between AD severity and degree of sleep disturbance showed mixed results. Yano et al. [19] found significant correlation between SCORing Atopic Dermatitis (SCORAD) and PSQI (P < 0.0003), while Kong et al. [24] found only the pruritus score of SCORAD to correlate with PSQI (P < 0.001). However, Misery et al. [22] found no significant correlation with between SCORAD and daytime sleepiness (P < 0.15).
Sanchez-Perez et al. [20] reported difficulty falling asleep in up to 76.2% of adult AD patients with mild disease, 89% with moderate disease, and 100% with severe disease, based on the modified Eczema Area and Severity Index (mEASI). Furthermore, 54.8% of patients with mild disease, 76.2% with moderate disease, and 92.6% with severe disease reported waking up from sleep because of pruritus.
Studies measuring sleep using actigraphy showed a positive correlation between AD disease severity and degree of sleep disturbance [26, 29].
III. Specific Alterations of Sleep Identified in AD
Sleep Latency, Sleep Efficiency, and Normal Sleep Stages
The term “sleep latency” refers to the time between going to bed and sleep onset. The term “sleep efficiency” refers to the proportion of time in bed that is spent sleeping. After entering sleep, there is a cyclic alteration every 90 min between two states: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep [33]. The first third of the night has a higher proportion of NREM and is essential for restorative sleep. NREM has stages N1, N2, and N3. The arousal threshold is lowest in N1 and highest in N3. The arousal threshold in N2 is similar to the REM state. There is a higher proportion of REM sleep during the last third of the night, which is associated with dreaming and minimal muscle tone activity.
Sleep Latency in AD Patients
Jernelov et al. [21] reported that the adult AD group had longer sleep latencies based on self-reported surveys (P < 0.05). On the other hand, Bender et al. [27] demonstrated that there were no significant differences in sleep latency (P = 0.10) in adult AD patients using PSQI.
Scratching During Sleep in AD Patients
Studies using multiple methods for assessing sleep show that scratching mainly occurs in earlier stages of sleep, specifically the N1 and N2 stages [26, 31]. Bender et al. [26] used PSG to demonstrate that scratching correlates with lower sleep efficiency (P = 0.01). Noro et al. [34], using a novel wristwatch sound detector, showed that the ratio of scratching duration to total sleeping time is significantly higher in AD patients than in control subjects (7.51% vs. 0.40%; P < 0.05) and the mean scratching duration is more than ten times higher in AD patients (P < 0.05). Ebata et al. [32, 35] used video monitoring and observed longer durations of scratching and being awake after a scratching bout rather than increased frequency of scratching during sleep in AD patients. Furthermore, using PSG, Aoki et al. [31] demonstrated that the act of scratching during sleep brought the patients to a more superficial stage of sleep. Similarly, Ebata et al. [35] found that patients usually turned over or woke up after a scratching bout. Bringhurst et al. [30] found that the median number of movements during sleep is twice as high in AD patients vs. control subjects (P < 0.001) using actigraphy.
Sleeping Time in AD Patients
A number of studies have shown conflicting results on sleep time in AD. Bender et al. [27], using actigraphy, demonstrated that AD patients slept less (402 vs. 441 min; P < 0.05), awoke more often (13.9 episodes vs. 7.2 episodes; P < 0.03), and spent more time awake during waking episodes (4 vs. 2 min; P = 0.02) compared to control subjects. However, the authors did not find significant differences in sleep duration using PSQI (P = 0.10). In addition, Bender et al. [26] demonstrated that differences in sleep efficiency were not statistically significant (P = 0.09). Similarly, another study by Noro et al. [34] using overnight video observation observed no significant difference in the mean sleeping time between AD patients and controls.
Sleep Quality in AD Patients
Bender et al. [27] demonstrated that the mean PSQI score of adult AD patients was significantly higher than controls (P < 0.05) and reported lower sleep quality and more awakenings. In contrast, based on survey data, Jernelov et al. [21] reported that sleep quality between the AD group and control group was similar.
Other Findings
A study by Yu et al. [17] using self-reported surveys found that higher occurrence of leg jerks (OR: 2.49; P = 0.004) and leg cramps (OR: 3.96; P = 0.001) during sleep were reported by AD patients vs. control subjects. There was no significant association between AD and snoring or cessation of breathing (OR: 1.64; P = 0.18).
IV. Association of AD with Impaired Functioning and Quality of Life
Impact of Sleep Disturbance on Functioning
Using the PSQI, Bender et al. [26] showed that there was more daytime dysfunction in AD patients with sleep disturbance vs. control subjects (P < 0.05). According to survey data by Silverberg et al. [2], AD patients with sleep disturbance had higher numbers of missed workdays, days in bed, and doctor visits than those with either eczema or sleep disturbances alone (P < 0.0001 for all). Furthermore, Yu et al. [17] demonstrated that AD patients who reported being tired were more likely to have difficulty with instrumental activities of daily living (IADLs), including concentrating (OR: 1.61; P = 0.01), remembering (OR: 1.85; P = 0.004), eating (OR: 2.90; P = 0.001), performing hobbies (OR: 2.36; P = 0.001), doing finances (OR: 2.56; P = 0.001), and driving and/or navigating public transportation (OR: 1.99; P = 0.004).
Impact of Sleep Disturbance on QoL
Kong et al. [24] and Beikert et al. [25] found a significant correlation between PSQI and DLQI in AD patients (P = 0.04, P = 0.00, respectively). Yano et al. [19] found that subjective sleep quality (P < 0.001), latency (P < 0.001), habitual sleep efficiency (P < 0.05), sleep disturbance (P < 0.01), and daytime dysfunction (P < 0.001) are correlated with lower DLQI scores. Duration of sleep and sleep medication use did not correlate with DLQI. Furthermore, survey data by Terreehorst et al. [23] demonstrated that the severity of sleepiness has a significant negative effect on QoL, including mental health (P < 0.01) and social functioning (P < 0.01).
V. Treatment Strategies for Sleep Disturbance in Adults with AD
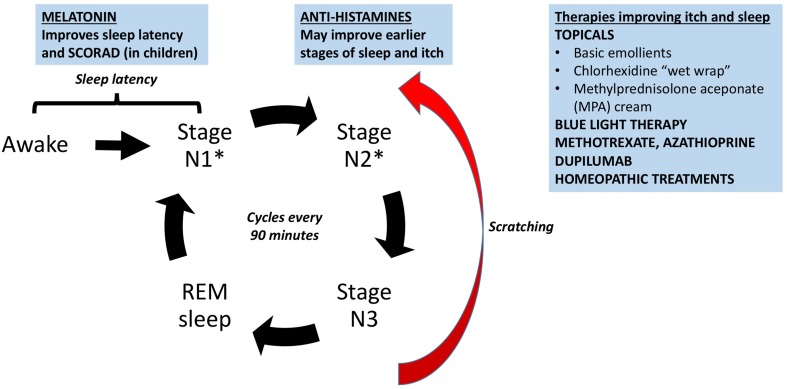
In general, most studies examining treatment of AD do not specifically address the “sleep” component; thus, the data are very limited. Our findings on the treatment of sleep from the literature are summarized in Fig. 2.
Fig. 2.
Summary of the treatment of sleep in atopic dermatitis (AD) patients from the literature. Asterisk affected by AD. SCORAD SCORing Atopic Dermatitis
Topical Therapy
Weber et al. [36] demonstrated that emollients are important in the daily skin care of adult patients with AD and can significantly improve itch (P < 0.001). Abeck et al. [37] demonstrated that basic emollients in combination with chlorhexidine-soaked dressing “wet wrap” improved itch and sleep loss in AD patients (adults and children).
Another study by Peserico et al. [38] demonstrated that the use of methylprednisolone aceponate (MPA) cream combined with an emollient as maintenance therapy is an effective regimen with reduction in risk of relapse (HR: 3.5; P < 0.0001), stable maintenance of low intensity of itching (P < 0.0001), and relatively stable maintenance of sleep quality (significance of data was not shown) compared to the emollient alone in moderate-to-severe AD adult and adolescent patients.
Light Therapy
Becker et al. [39] demonstrated that blue light total body irradiation of severe, chronic AD significantly improved pruritus and sleep disturbance (P < 0.003 for both).
Antihistamines
Antihistamines are widely used as a therapeutic adjunct in patients with AD to treat pruritus [40]. First-generation antihistamines are often used for sleep problems in AD patients, most likely because of their sedating effects [41]. However, the evidence to support their use in AD patients is relatively weak since no large, randomized, placebo-controlled clinical trials with definitive conclusions have been performed [42]. For adults, O’Donoghue et al. [43] reported that sedating antihistamines (e.g., hydroxyzine, diphenhydramine) are often beneficial in patients who suffer from exacerbations of pruritus at night. Furthermore, Endo et al. [44] demonstrated that azelastine hydrochloride, a nonsedating antihistamine, can alleviate nocturnal scratching and sleep disturbance in the early period of sleep of patients, especially in patients with a serum immunoglobulin (Ig) E ≥1000 IU/ml. However, ‘sleep time’ or ‘pre-sleep’ time did not differ significantly. Doxepin is a tricyclic antidepressant with high histamine H1 receptor antagonist activity and sedative effects. A small study in adults with AD found that tricyclic antidepressants decrease sleep latency and wakefulness after sleep onset, but did not help with nocturnal pruritus [45].
Systemic Immunomodulatory Therapy
Weatherhead et al. [46] showed that methotrexate is an effective, well-tolerated treatment for moderate-to-severe AD and can result in significant improvements in pruritus and sleep loss (P < 0.05 for both). Similarly, Berth-Jones et al. [47] demonstrated that azathioprine can significantly improve pruritus (P < 0.003) and sleep disturbance (P < 0.01).
Dupilumab (DUPIXENT®) was approved by the US Food and Drug Administration (FDA) for treatment of moderate-to-severe atopic dermatitis on 28 March 2017 [48]. During clinical trials, significant improvement in sleep were reported with dupilumab in 1–2 weeks relative to placebo (P < 0.05) and were maintained over the treatment duration (except for the 100-mg q4w dose) based on sleep items from the SCORAD and Patient-Oriented Eczema Measure (POEM) [18]. For pruritus, significant improvements relative to placebo (P < 0.05) were reported on the pruritus Numeric Rating Scale (NRS) at all doses as early as week 1 and remained significant throughout the study.
Supplements
Though no data are available for adults, Chang et al. [49] recently evaluated the effectiveness of melatonin supplementation in children with AD and demonstrated that it is a safe and effective way to improve sleep-onset latency (sleep-onset latency shortened by 21.4 min after melatonin treatment compared with after placebo; P = 0.02) and disease severity by SCORAD (SCORAD decreased by 9.1 compared with after placebo; P < 0.001).
Mehrbani et al. [50] demonstrated that the use of whey with the aqueous extract of field dodder (Cuscuta campestris Yunck) seeds in severe and refractory AD patients can significantly improve pruritus (P < 0.001) and sleep disturbance (P < 0.05). A study by Itamura et al. [51] found that individualized homeopathic treatment provokes good response in AD patients, including improvement in pruritus and sleep (significance of data was not shown).
Other
Barbeau et al. [52] reported that 18 out of 41 patients with severe AD take medication for sleep. However, Yu et al. [17] did not find statistically significant odds of increased sleeping pill (unspecified type) use in AD patients vs. the control group (OR: 1.40; P = 0.18). Sanchez-Perez et al. [20] found no specific pattern of sleeping pill use (unspecified type) based on AD severity. A double-blind, placebo-controlled, crossover study in adults with AD showed that benzodiazepines can increase total sleep time but can disrupt the sleep architecture and did not reduce total nocturnal scratching [53].
Discussion
A comprehensive review of the literature showed that there is a high prevalence (33–87.1%) of sleep disturbance in adults with AD [17, 18, 20]. The likelihood of sleep disturbance is much higher in patients with AD compared to healthy controls [2, 17]. Sleep disturbance appears to worsen with AD severity [19, 20, 24, 26, 29]. Although various studies showed mixed results, overall, adults with AD experience lower sleep quality as evidenced by difficulty falling asleep, greater frequency and duration of waking episodes, and shorter sleep duration. Such disturbances can consequently lead to daytime sleepiness, fatigue, and dysfunction. Adult patients with AD and associated sleep disturbance can experience difficulties with IADLs and may have more missed workdays, days in bed, and doctor visits [2, 17, 26, 27], which may contribute to decreased QoL. These findings illustrate the importance of addressing sleep disturbances in patients with AD as part of routine disease management.
The current literature review found pruritus and scratching to be a large contributor to sleep disturbance in adult patients with AD. Pruritus appears to be associated with lower quality of sleep [24] with more frequent and longer duration of awakenings. Scratching also brings one to a more superficial stage of sleep. Scratching mainly occurs in N1 and N2 stages of NREM sleep [26, 31], which have the lowest arousal threshold but are essential for “restorative” sleep. Pruritus in AD is associated with a negative impact on QoL as measured by validated instruments such as the DLQI and EuroQoL-5 dimensions visual analog scale (EQ-5d VAS) [23]. A better understanding of the mechanism of pruritus and therapies may improve QoL in adult patients with AD and sleep disturbance.
Small numbers of studies suggest that sleep disturbance is associated with increased type 2 T helper (Th2) cell pathway activation in AD patients, which can worsen skin disease, leading to a self-perpetuating cycle of itch-scratch-sleep loss. However, current evidence does not clearly describe the extremely complex relationship between sleep disturbance and cytokines in AD. Many cytokines have been implicated in the pathogenesis of AD, and a small number of studies show that these cytokines may overlap or interact with those that regulate or influence sleep. Bender et al. [26] found that the morning-evening change of interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-10 produced by peripheral mononuclear cells and stimulated by anti-CD3 correlates with sleep efficiency as measured by actigraphy in adults with AD. This suggests that higher levels of inflammatory cytokines are associated with poor sleep efficiency, a finding that is also seen in other diseases such as rheumatoid arthritis and inflammatory bowel disease [54, 55]. Other studies have found that the ratio of interferon (IFN)-γ to IL-4 is lower in children with AD experiencing poor sleep efficiency and that morning blood plasma levels of IL-31 correlated with sleep disturbance, specifically in N1 sleep [56, 57].
Interestingly, inflammation and sleep are linked by central and peripheral circadian mechanisms [58], which not only regulate melatonin and sleep-wake cycles, but also affect inflammatory cells, cytokines [59, 60], and intrinsic skin properties such as pH, water loss, and permeability [61–63]. In AD, cyclical changes in melatonin and cortisol release may promote inflammation and exacerbate itch [41]. Nighttime changes in skin physiology and inflammatory cytokines can, in turn, exacerbate atopic inflammation, pruritus, and sleep disturbance [58, 61, 64].
Several environmental factors have also been associated with sleep disturbance in AD. For example, serum IgE antibodies specific for dust mite allergens, Derf and Derp, were significantly correlated with reduced sleep efficiency, longer sleep onset latency, increased sleep fragmentation, and decreased non-REM sleep in children with eczema [65]. High concentrations of dust mite allergen are found on beds and may be a source of exposure during sleep. In children with AD from the German LISAplus cohort, exposure to mold or visible dampness was also associated with increased risk of sleep problems, including difficulty falling asleep, difficulty staying asleep, and decreased overall sleep time [66]. Currently, there is a lack of studies examining the impact of environmental factors on sleep in adults with AD. Future research in this area may identify modifiable aspects of the living environment that can improve sleep and QoL in adults.
Despite the obvious importance of evaluating sleep, there is currently no reliable tool designed specifically for AD patients to assess sleep in the clinical setting. It is important to note that sleep disturbance is a parameter that is included in the calculation of total points on the SCORAD; this can give rise to bias in favor of reported correlations between both sleep disturbance and AD activity in the clinical and research settings. PSG and actigraphy are not practical for utilization in a dermatology practice. Widely used, validated dermatology QoL surveys such as DLQI [67] identify sleep disturbance only as a secondary measure and do not focus on assessing the degree of sleep disturbance or offer much insight into treatment options. Furthermore, available patient QoL measurement scales are considered less practical and are not recommended for routine clinical practice [68]. However, administering a brief sleep questionnaire (e.g., PSQI) prior to the first visit in all new patients and then on subsequent follow-up visits may be worthwhile to follow improvement with management of AD [69]. At a minimum, clinicians should evaluate the patients’ severity of AD and ask general questions about itch, sleep, impact on daily activities, and persistence of disease. If a patient complains of sleep disturbance, clinicians can take a basic sleep history focusing on the five major domains of sleep: sleep onset, latency, quality, efficiency, snoring, or difficulty breathing. A useful mnemonic used as a screening tool in children is BEARS (B = bedtime problems, E = excessive daytime sleepiness, A = awakenings during the night, R = regularity and duration of sleep, S = snoring or difficulty breathing), which can be useful to quickly assess different domains of sleep [70]. Furthermore, it may also help rule in or out other possible causes that may be contributing to sleep disturbance, such as an unhealthy sleep hygiene or an underlying sleep disorder, and make appropriate recommendations if necessary. A referral to a specialist for sleep evaluation can be considered when a patient regularly has trouble with sleep disturbance despite addressing one or more of these factors.
Optimal management of sleep health in AD requires a multidisciplinary approach. One aspect of improving sleep involves targeting pruritus, which can drive sleep disturbance in AD. Generally, the least invasive treatments should be tried first. Patient education on breaking the itch-scratch cycle has been used to address sleep disruption [71]. Topical emollients can significantly improve itch and consequently sleep quality [35]. Addition of chlorhexidine-soaked dressing “wet wrap” may also be helpful [37]. Similarly, topical corticosteroids with or without use of emollients and systemic agents for AD such as methotrexate [46] and azathioprine [47] are also shown to be beneficial for improving itch and sleep in AD patients. Dupilumab is a new biologic agent that also has been shown to significantly improve pruritus and sleep during clinical trials [18]. Antihistamines used to treat pruritus have sedative effects and can therefore also improve sleep [40, 42–44, 50]. On the other hand, the use of benzodiazepines is not well studied and may not improve sleep in adult AD patients [51]. Other interventions focus directly on sleep disturbance itself. For example, psychological therapy aimed at improving sleep hygiene, promoting relaxation, and establishing behavior plans for addressing sleep problems may reduce loss of sleep in chronic itch [72, 73]. In addition to behavioral interventions, melatonin has been shown to be a safe and effective way to improve sleep-onset latency in pediatric patients with AD [49].
Conclusions
A review of the current literature shows that there is a high prevalence of sleep disturbance experienced by adults with AD, which can have a significant impact on QoL. It is important that clinicians evaluate the severity of AD and ask general questions about itch, sleep, impact on daily activities, and persistence of disease during each patient visit and follow-up with complains of sleep disturbance. Management of sleep disturbance in AD should focus on adequate disease control of AD as well as possible medical interventions to help improve sleep. The pathophysiology of sleep disturbance in AD is extremely complex, and further research is needed to better understand the interplay of the immune system, circadian rhythm, and environmental factors implicated in both AD and sleep.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Wilson Liao, MD, is supported in part by grants from the National Institutes of Health (R01AR065174, U01AI119125). Tina Bhutani, MD, conducts research for Abbvie, Janssen, Mela, and Merck. Caleb Jeon, Di Yan, Mio Nakamura, Sahil Sekhon, and Timothy Berger have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/BDD8F06053CF2490.
References
- 1.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg JI, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Investig Dermatol. 2015;135(1):56–66. doi: 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 3.Margolis JS, et al. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150(6):593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeo SP. Atopic dermatitis: the itch that rashes. Pediatr Nurs. 1995;21(2):157–163. [PubMed] [Google Scholar]
- 5.Yosipovitch G, Papoiu AD. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep. 2008;8(4):306–311. doi: 10.1007/s11882-008-0049-z. [DOI] [PubMed] [Google Scholar]
- 6.Hong J, et al. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30(2):71–86. doi: 10.1016/j.sder.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinges DF, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 8.Daley M, et al. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427–438. doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 10.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann NY Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tochikubo O, et al. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27(6):1318–1324. doi: 10.1161/01.HYP.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 12.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 13.Camfferman D, et al. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14(6):359–369. doi: 10.1016/j.smrv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Corkum P, et al. Actigraphy and parental ratings of sleep in children with attention-deficit/hyperactivity disorder (ADHD) Sleep. 2001;24(3):303–312. doi: 10.1093/sleep/24.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick MF, et al. Morbidity in nocturnal asthma: sleep quality and daytime cognitive performance. Thorax. 1991;46(8):569–573. doi: 10.1136/thx.46.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu SH, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27(2):50–58. doi: 10.1097/DER.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 18.Simpson EL, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498. doi: 10.1016/j.jaad.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Yano C, et al. Impact of disease severity on sleep quality in Japanese patients with atopic dermatitis. J Dermatol Sci. 2013;72(2):195–197. doi: 10.1016/j.jdermsci.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Perez J, et al. Impact of atopic dermatitis on health-related quality of life in Spanish children and adults: the PSEDA study. Actas Dermosifiliogr. 2013;104(1):44–52. doi: 10.1016/j.ad.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Jernelov S, et al. Effects of examination stress on psychological responses, sleep and allergic symptoms in atopic and non-atopic students. Int J Behav Med. 2009;16(4):305–310. doi: 10.1007/s12529-008-9020-6. [DOI] [PubMed] [Google Scholar]
- 22.Misery L, et al. Atopic dermatitis: impact on the quality of life of patients and their partners. Dermatology. 2007;215(2):123–129. doi: 10.1159/000104263. [DOI] [PubMed] [Google Scholar]
- 23.Terreehorst I, et al. The unfavorable effects of concomitant asthma and sleeplessness due to the atopic eczema/dermatitis syndrome (AEDS) on quality of life in subjects allergic to house-dust mites. Allergy. 2002;57(10):919–925. doi: 10.1034/j.1398-9995.2002.23708.x. [DOI] [PubMed] [Google Scholar]
- 24.Kong TS, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28(3):321–326. doi: 10.5021/ad.2016.28.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beikert FC, et al. Willingness to pay and quality of life in patients with atopic dermatitis. Arch Dermatol Res. 2014;306(3):279–286. doi: 10.1007/s00403-013-1402-1. [DOI] [PubMed] [Google Scholar]
- 26.Bender BG, et al. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol. 2008;58(3):415–420. doi: 10.1016/j.jaad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Bender BG, Leung SB, Leung DY. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. J Allergy Clin Immunol. 2003;111(3):598–602. doi: 10.1067/mai.2003.174. [DOI] [PubMed] [Google Scholar]
- 28.Cole JC, et al. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 29.Sandoval LF, et al. Measure of atopic dermatitis disease severity using actigraphy. J Cutan Med Surg. 2014;18(1):49–55. doi: 10.2310/7750.2013.13093. [DOI] [PubMed] [Google Scholar]
- 30.Bringhurst C, et al. Measurement of itch using actigraphy in pediatric and adult populations. J Am Acad Dermatol. 2004;51(6):893–898. doi: 10.1016/j.jaad.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 31.Aoki T, et al. Nocturnal scratching and its relationship to the disturbed sleep of itchy subjects. Clin Exp Dermatol. 1991;16(4):268–272. doi: 10.1111/j.1365-2230.1991.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 32.Ebata T, et al. The characteristics of nocturnal scratching in adults with atopic dermatitis. Br J Dermatol. 1999;141(1):82–86. doi: 10.1046/j.1365-2133.1999.02924.x. [DOI] [PubMed] [Google Scholar]
- 33.Hirshkowitz M. Normal human sleep: an overview. Med Clin North Am. 2004;88(3):551–565. doi: 10.1016/j.mcna.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Noro Y, et al. Novel acoustic evaluation system for scratching behavior in itching dermatitis: rapid and accurate analysis for nocturnal scratching of atopic dermatitis patients. J Dermatol. 2014;41(3):233–238. doi: 10.1111/1346-8138.12405. [DOI] [PubMed] [Google Scholar]
- 35.Ebata T, Aizawa H, Kamide R. An infrared video camera system to observe nocturnal scratching in atopic dermatitis patients. J Dermatol. 1996;23(3):153–155. doi: 10.1111/j.1346-8138.1996.tb03990.x. [DOI] [PubMed] [Google Scholar]
- 36.Weber TM, et al. Steroid-free emollient formulations reduce symptoms of eczema and improve quality of life. J Drugs Dermatol. 2014;13(5):589–595. [PubMed] [Google Scholar]
- 37.Abeck D, et al. Treatment of acute exacerbated atopic eczema with emollient-antiseptic preparations using the “wet wrap” (“wet pajama”) technique. Hautarzt. 1999;50(6):418–421. doi: 10.1007/s001050050934. [DOI] [PubMed] [Google Scholar]
- 38.Peserico A, et al. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol. 2008;158(4):801–807. doi: 10.1111/j.1365-2133.2008.08436.x. [DOI] [PubMed] [Google Scholar]
- 39.Becker D, et al. Clinical efficacy of blue light full body irradiation as treatment option for severe atopic dermatitis. PLoS One. 2011;6(6):e20566. doi: 10.1371/journal.pone.0020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuovo J, Ellsworth AJ, Larson EB. Treatment of atopic dermatitis with antihistamines: lessons from a single-patient, randomized clinical trial. J Am Board Fam Pract. 1992;5(2):137–141. [PubMed] [Google Scholar]
- 41.Fishbein AB, et al. Nocturnal eczema: review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol. 2015;136(5):1170–1177. doi: 10.1016/j.jaci.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135(12):1522–1525. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- 43.O’Donoghue M, Tharp MD. Antihistamines and their role as antipruritics. Dermatol Ther. 2005;18(4):333–340. doi: 10.1111/j.1529-8019.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 44.Endo K, et al. Objective scratch monitor evaluation of the effect of an antihistamine on nocturnal scratching in atopic dermatitis. J Dermatol Sci. 1999;22(1):54–61. doi: 10.1016/S0923-1811(99)00048-1. [DOI] [PubMed] [Google Scholar]
- 45.Savin JA, et al. Further studies of scratching during sleep. Br J Dermatol. 1975;93(3):297–302. doi: 10.1111/j.1365-2133.1975.tb06495.x. [DOI] [PubMed] [Google Scholar]
- 46.Weatherhead SC, et al. An open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol. 2007;156(2):346–351. doi: 10.1111/j.1365-2133.2006.07686.x. [DOI] [PubMed] [Google Scholar]
- 47.Berth-Jones J, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002;147(2):324–330. doi: 10.1046/j.1365-2133.2002.04989.x. [DOI] [PubMed] [Google Scholar]
- 48.Administration, U.S.F.a.D. FDA approves new eczema drug Dupixent. FDA News Release March 28, 2017 April 04, 2017]; Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm549078.htm.
- 49.Chang YS, et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: a randomized clinical trial. JAMA Pediatr. 2016;170(1):35–42. doi: 10.1001/jamapediatrics.2015.3092. [DOI] [PubMed] [Google Scholar]
- 50.Mehrbani M, et al. The efficacy of whey associated with dodder seed extract on moderate-to-severe atopic dermatitis in adults: a randomized, double-blind, placebo-controlled clinical trial. J Ethnopharmacol. 2015;172:325–332. doi: 10.1016/j.jep.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Itamura R. Effect of homeopathic treatment of 60 Japanese patients with chronic skin disease. Complement Ther Med. 2007;15(2):115–120. doi: 10.1016/j.ctim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45(1):31–36. doi: 10.1111/j.1365-4632.2004.02345.x. [DOI] [PubMed] [Google Scholar]
- 53.Ebata T, et al. Effects of nitrazepam on nocturnal scratching in adults with atopic dermatitis: a double-blind placebo-controlled crossover study. Br J Dermatol. 1998;138(4):631–634. doi: 10.1046/j.1365-2133.1998.02174.x. [DOI] [PubMed] [Google Scholar]
- 54.Ali T, et al. Sleep, immunity and inflammation in gastrointestinal disorders. World J Gastroenterol. 2013;19(48):9231–9239. doi: 10.3748/wjg.v19.i48.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raap T, et al. Neurotransmitter modulation of interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol. 2000;27(11):2558–2565. [PubMed] [Google Scholar]
- 56.Hon KL, et al. Pathophysiology of nocturnal scratching in childhood atopic dermatitis: the role of brain-derived neurotrophic factor and substance P. Br J Dermatol. 2007;157(5):922–925. doi: 10.1111/j.1365-2133.2007.08149.x. [DOI] [PubMed] [Google Scholar]
- 57.Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann NY Acad Sci. 2012;1261:88–96. doi: 10.1111/j.1749-6632.2012.06634.x. [DOI] [PubMed] [Google Scholar]
- 58.Gupta MA, Gupta AK. Sleep-wake disorders and dermatology. Clin Dermatol. 2013;31(1):118–126. doi: 10.1016/j.clindermatol.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Geiger SS, Fagundes CT, Siegel RM. Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology. 2015;146(3):349–358. doi: 10.1111/imm.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Born J, et al. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158(9):4454–4464. [PubMed] [Google Scholar]
- 61.Yosipovitch G, et al. Circadian and ultradian (12 h) variations of skin blood flow and barrier function in non-irritated and irritated skin-effect of topical corticosteroids. J Investig Dermatol. 2004;122(3):824–829. doi: 10.1111/j.0022-202X.2004.22313.x. [DOI] [PubMed] [Google Scholar]
- 62.Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol. 2007;87(4):295–298. doi: 10.2340/00015555-0280. [DOI] [PubMed] [Google Scholar]
- 63.Geyfman M, Andersen B. How the skin can tell time. J Investig Dermatol. 2009;129(5):1063–1066. doi: 10.1038/jid.2008.384. [DOI] [PubMed] [Google Scholar]
- 64.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Sidenius KE, et al. House dust mites and their allergens at selected locations in the homes of house dust mite-allergic patients. Clin Exp Allergy. 2002;32(9):1299–1304. doi: 10.1046/j.1365-2222.2002.01472.x. [DOI] [PubMed] [Google Scholar]
- 66.Tiesler CM, et al. Exposure to visible mould or dampness at home and sleep problems in children: results from the LISAplus study. Environ Res. 2015;137:357–363. doi: 10.1016/j.envres.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 67.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 68.Eichenfield LF, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bloom HG, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57(5):761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6(1):63–69. doi: 10.1016/j.sleep.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 71.Spielman SC, et al. A review of multidisciplinary interventions in atopic dermatitis. J Clin Med. 2015;4(5):1156–1170. doi: 10.3390/jcm4051156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lavery MJ, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17(3):425. doi: 10.3390/ijms17030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bae BG, et al. Progressive muscle relaxation therapy for atopic dermatitis: objective assessment of efficacy. Acta Derm Venereol. 2012;92(1):57–61. doi: 10.2340/00015555-1189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.