Abstract
Abstract
Fixed-dose combination topical therapy with corticosteroid and vitamin D analog provides effective treatment and possible long-term management of psoriasis. The anti-inflammatory and immunomodulatory effects of corticosteroids and vitamin D analogs in treating psoriasis are well investigated; their complementary effects lead to the disruption of the inflammatory feedback loop underlying psoriasis pathogenesis. Recent preclinical data showed that combination therapy is more effective than monotherapies of the active ingredients in preventing activation of resting pro-inflammatory cells, inducing immunomodulation, reducing inflammatory responses by regulating T cell production, and normalizing keratinocytes. The increased understanding of the mechanism of action of fixed-dose combination therapy from preclinical studies is supported by several clinical studies. As the efficacy of topical therapy is correlated with the skin penetration of the active ingredients, new drug delivery systems have been developed. The fixed-dose combination Cal/BD aerosol foam creates a modified supersaturated formulation when applied to the skin, which is maintained for at least 26 h in the laboratory setting. Clinical studies have demonstrated superior efficacy of fixed-dose combination calcipotriol (Cal) 50 µg/g and betamethasone dipropionate (BD) 0.5 mg/g aerosol foam compared with monotherapies of the active ingredients. Furthermore, Cal/BD aerosol foam has shown significantly improved efficacy compared with more traditional formulations, such as Cal/BD ointment and gel, in other studies. Calcipotriol also mitigates risks associated with betamethasone dipropionate and vice versa, resulting in the favorable safety profile observed with fixed-dose combination treatment. Recent data also suggest that fixed-dose combination treatment could provide long-term management of psoriasis, although further clinical investigations are needed. Overall, these data support the value of fixed-dose combination therapy of corticosteroid and vitamin D analog and highlight the added potential of innovative drug delivery for the treatment of psoriasis.
Funding
LEO Pharma.
Keywords: Anti-inflammatory, Betamethasone dipropionate, Calcipotriol, Fixed-dose combination, Plaque psoriasis
Introduction
Plaque psoriasis is a chronic, inflammatory, immune-mediated skin disorder characterized by recurrent itchy, scaly, erythematous plaques [1]. The burden of psoriasis on a patient’s quality of life (QoL) may be considerable; this burden is not only physical (e.g., painful and debilitating effects of plaques), but also psychologic, with effects including depression, alexithymia, coping with feelings of stigmatization, and suicide ideation [2].
Topical treatments containing corticosteroids and vitamin D analogs, used separately, together, or in a fixed combination, are essential and well-established first-line treatments for patients with mild-to-moderate psoriasis [3, 4]. Although patients with moderate-to-severe psoriasis are commonly treated with phototherapy and systemic therapies, including biologic agents [5, 6], the combination of these therapies with topical treatments can help to individually optimize disease control and long-term management [7].
The increased understanding of psoriasis pathogenesis has provided further insights into therapeutic targets, and new treatments for psoriasis are becoming available [5, 6, 8]. That being said, critical issues related to topical therapies are still unresolved, including the poor treatment adherence due to perceived lack of efficacy and the cumbersome nature of available treatments [9, 10]. Although there are no new compounds available, considerable research has been undertaken for topical treatments to: (1) improve efficacy by enhancing the drug delivery and bioavailability of active ingredients, without compromising safety [11, 12], (2) enhance the convenience of application, and (3) increase the variety of available formulations [11]. New topical formulations with greater efficacy and more convenient application could lead to better adherence and provide subsequent long-term maintenance of a disease-free state.
This review will examine the anti-inflammatory and immunomodulatory mechanisms of action of fixed-dose combination corticosteroid and vitamin D analog versus monotherapies of the active ingredients, the drug delivery challenges, and the clinical relevance of these data. The article presented here is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
The Current Understanding of Psoriasis Pathogenesis
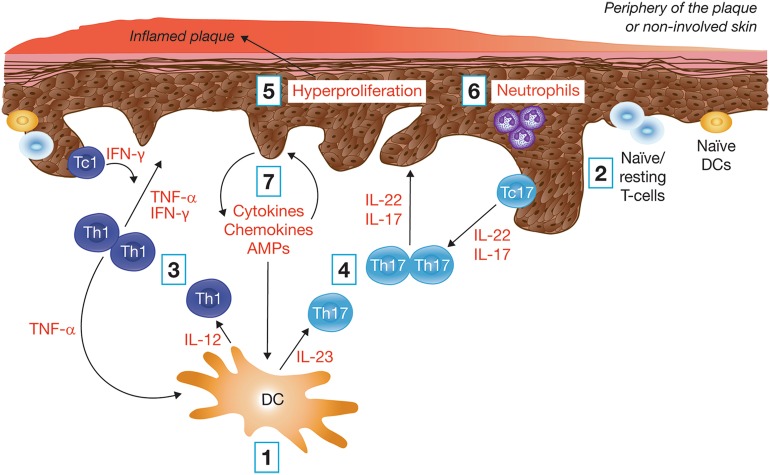
In individuals with a genetic predisposition, environmental factors, such as physical and psychologic stress, may trigger the initiation of psoriasis, beginning with the activation of dendritic cells. The key steps in psoriasis pathogenesis are summarized in Fig. 1, with further descriptions below [1, 13].
Dendritic cell cytokine release The skin inflammatory response is elicited by activated inflammatory dendritic cells, which secrete pro-inflammatory cytokines, such as interleukin (IL)-23, IL-12, and tumor necrosis factor (TNF)-α [1, 13, 14].
Activation of the adaptive immune response Cytokines produced by dendritic cells also promote the differentiation and activation of naïve, or resting, T-cells to effector, helper (Th1/17), and cytotoxic (Tc1/17) T-cells. Evidence also suggests that the altered balance between helper T-cells (Th1 and Th2) contributes to psoriasis pathogenesis by further promoting autoimmune reactions [1, 15–18].
Type 1 T-cell cytokine release The activated Th1 and Tc1 cells within the skin release TNF-α and interferon (IFN)-γ, which further support the activation and maturation of dendritic cells and also promote keratinocyte activation [1, 13, 15, 16, 19].
Type 17 T-cell cytokine release Similarly, activated Th17 and Tc17 cells within the skin release IL-17 and IL-22, which promote keratinocyte activation [13, 15, 16, 19, 20].
Keratinocyte activation The activation of keratinocytes promotes their hyperproliferation and atypical differentiation, causing psoriatic plaque formation on the skin [16, 21–23].
Activation of the innate immune response Innate immune cells, such as neutrophils, may respond quickly to IL-17 [21].
Keratinocyte pro-inflammatory mediator release This in turn leads to the production of additional inflammatory mediators, including IL-6, IL-8, IL-17C, IL-20, TNF-α, IFN-γ, and antimicrobial peptides (AMPs), which recruit and activate cells of the innate and adaptive immune system [16, 21, 24–26]. Interactions between the keratinocytes and extracellular matrix (ECM) also promote tissue reorganization and the deposition of the ECM [1, 13, 17, 26, 27].
Fig. 1.
Key components in the pathogenesis of psoriasis. AMPs antimicrobial peptides, DC dendritic cell, IL interleukin, IFN interferon, Tc cytotoxic T-cell, Th T-helper cell, TNF tumor necrosis factor
A pro-inflammatory feedback loop is then established among keratinocytes, immune cells, and components of the extracellular matrix (e.g., collagen), leading to sustained, active skin inflammation and subsequent reorganization of the ECM [1, 15, 16].
Corticosteroid and Vitamin D Analog Combination Therapy Results in Increased Effectiveness Versus Monotherapy in the Treatment of Psoriasis
Corticosteroids and vitamin D analogs complement one another in treating the key pathogenic factors of plaque psoriasis [16, 28–30]. Corticosteroids have been used for more than 50 years for the treatment of psoriasis [31, 32], and their immunosuppressive effects are critical for inhibiting the pro-inflammatory environment and T-cell activation [15]. Vitamin D analogs exert normalizing effects on the hyperproliferation and abnormal differentiation of keratinocytes and also have immunomodulatory effects [15, 28].
The treatment goal is to clear the psoriatic plaques by inhibiting the underlying inflammation and normalizing skin homeostasis, keratinocyte proliferation, and differentiation and to provide immunomodulation. Combination therapy, unlike monotherapies, has been shown to address all these treatment goals as supported by preclinical data and is further discussed below.
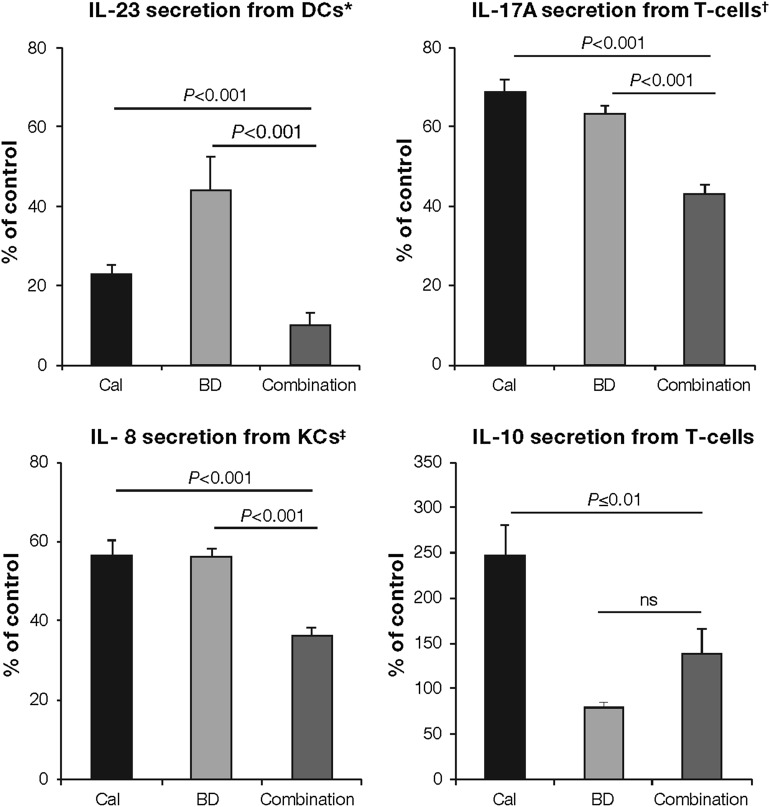
Dendritic cell cytokine release Corticosteroids and vitamin D analogs both inhibit the release of cytokines, such as IL-23, which are known to stimulate the innate and adaptive immune systems. Furthermore, studies with combination treatment in in vitro cultured dendritic cells have shown additive effects, leading to greater inhibition compared with monotherapies of the active ingredients (Fig. 2, adapted from Lovato et al. [27]).
Th1-cell cytokine release Combination treatment inhibits TNF-α secretion from cytotoxic Th1-cells, preventing further activation and maturation of dendritic cells and activation of keratinocytes (Fig. 3, adapted from Bailey et al. [33]) [17, 27].
Th17-cell cytokine release Data have shown that pro-inflammatory cytokines (e.g., IL-17A) were inhibited significantly more with combination treatment than monotherapies of the active ingredients in cultured and activated cytotoxic and helper T-cells (Figs. 2, 3) [17, 27]. Reduction of these pro-inflammatory cytokines can inhibit keratinocyte hyperproliferation and abnormal differentiation.
Th2-cell cytokine release Although corticosteroids, such as betamethasone dipropionate, suppress IL-10 secretion, vitamin D analogs, such as calcipotriol, induce Th2-cell production (Fig. 2) [27].
Keratinocyte pro-inflammatory mediator release Keratinocytes in psoriatic plaques release inflammatory mediators, such as IL-6, IL-8, IL-17C, IL-20, IFN-γ, and AMPs, which leads to the initiation of the pro-inflammatory feedback loop. Combination of corticosteroids and vitamin D analogs inhibits all of the aforementioned pro-inflammatory cytokines more than monotherapies of the active ingredients (Fig. 2) [27].
Fig. 2.
Combination treatment in vitro is significantly more effective than monotherapies of the active ingredients in inhibiting cytokine released from key cells involved in psoriasis pathogenesis [27]. Pro-inflammatory (IL-23, IL-17A, and IL-8) and immunomodulatory (IL-10) cytokine levels released by dendritic cells, T-cells, and keratinocytes are expressed as percentage of vehicle-treated control (100%). Treatment was applied before DC activation, after (IL-17A) and before (IL-10) Th-cell (CD4+) differentiation, and on stimulated keratinocytes. DC cultures were differentiated from CD14+ cells. CD4+ cells were differentiated into Th1 and Th17 cells and were processed for ribonucleic acid extraction and quantitative real-time polymerase chain reaction. KC cells were obtained from primary human epidermal KCs. *Combination treatment also led to TNF-α inhibition (both P < 0.001). †Similar results were found for the inhibition of IL-22, IL-8, and TNF-α (all P < 0.001). ‡Similar results were observed for IL-6, IL-17C, and IL-20 (all P < 0.001). BD betamethasone dipropionate, Cal calcipotriol, DC dendritic cells, IL interleukin, KCs keratinocytes, ns not significant
Adapted from Lovato et al. [27]
Fig. 3.
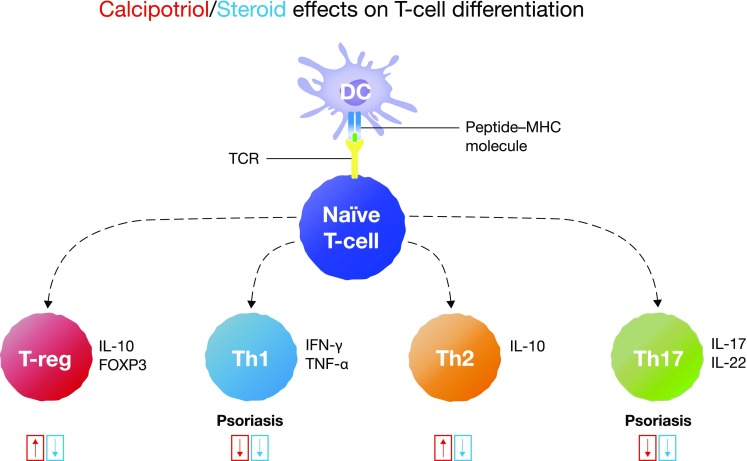
Summary of the effects of corticosteroids and vitamin D analogs on T-cell subsets involved in psoriasis pathogenesis [33]. Th17-cells are more involved in psoriasis pathogenesis than Th1 cells. The upward and downward arrows indicate induction and downregulation of T-cells, respectively, by calcipotriol and steroid. DC dendritic cells, FOXP3 Forkhead box P3, IFN interferon, IL interleukin, MHC major histocompatibility complex, TCR T-cell receptor, Th T-helper cell, T-reg regulatory T-cell
Adapted from Bailey et al. [33]
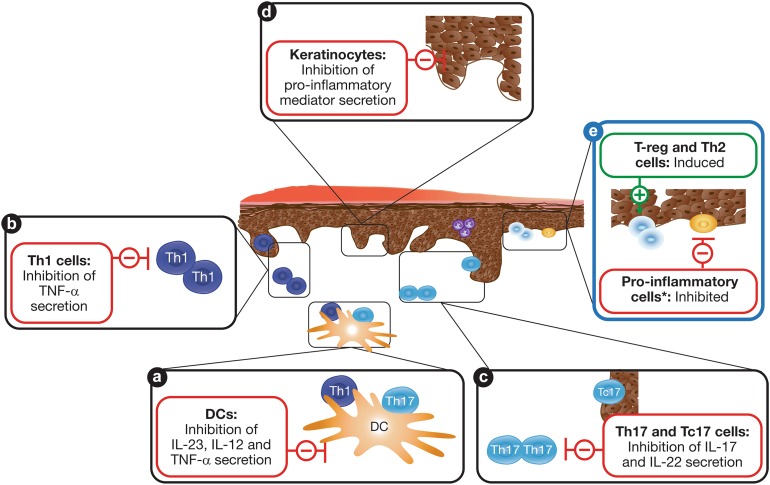
In summary, corticosteroid and vitamin D analog combination therapy inhibits the effects of Th1 and Th17 cytokines in an additive way (Fig. 3). Corticosteroids can also suppress immunomodulatory Th2-cells and IL-10 secretion when used alone [17, 18, 27]; additionally, altering the Th1/Th2 balance has been suggested to further promote autoimmune reactions [17, 18]. The vitamin D analog calcipotriol complements the effects of corticosteroids by inducing Th2- and T-reg cell production (Fig. 3) [17, 27]. Equilibrating the Th1 and Th2 ratio could therefore provide immunomodulation and may prevent rebound [18], while T-reg cells are central to actively attenuating inflammatory responses via inhibition of effector T-cells (Fig. 3) [1]. The effect of combination therapy on cellular targets in the pathophysiology of psoriasis is summarized in Fig. 4a–d.
Fig. 4.
Summary of the complementary and additive actions of corticosteroid and vitamin D analog combination treatment on cellular targets in the pathophysiology of psoriasis. a–d Therapeutic targets for inhibiting the pro-inflammatory environment. e Possible key targets for long-term maintenance therapy. Asterisk: Resting and naïve dendritic cells and T-cells; plus and minus signs: indicate induction and inhibition, respectively. DC dendritic cell, IL interleukin, Tc cytotoxic T-cell, Th T-helper cell, TNF tumor necrosis factor, T-reg regulatory T-cell
Corticosteroid and Vitamin D Analog Combination Topical Treatment May Be Able to Provide Long-Term Management of Psoriasis
Upon clearance of psoriatic plaques and normalization of skin homeostasis, the therapeutic objective shifts to the maintenance of a relapse-free state. Psoriatic inflammation tends to recur in the previously affected skin locations, which may be caused by the expression and reactivation of inflammatory cytokines present in the apparently normalized, plaque-free skin after treatment [34, 35]. New data indicate that combination treatment is able to induce T-reg cells as well as counteract the activation and differentiation of cytotoxic T-cells more effectively than corticosteroids alone [27]. Further clinical studies are required to explore the possibility of combination treatment for the long-term management of psoriasis (see Fig. 4e).
Summary: Combination Therapy has Complementary Effects on the Underlying Pathophysiology of Psoriasis, Resulting in Increased Therapeutic Response.
As well as their normalizing effect on keratinocytes, vitamin D analogs, such as calcipotriol, exert immunomodulatory effects on Th1, Th2, Th17, and T-reg cells.
Corticosteroids, such as betamethasone dipropionate, combined with vitamin D analogs, additively inhibit Th1 and Th17 pro-inflammatory effects.
Calcipotriol induces an immunomodulatory Th2/T-reg cellular response, whereas corticosteroids suppress this effect, and combination treatment yields mild induction.
The preclinical results support the superior antipsoriatic effect of corticosteroid and vitamin D analog combination treatment compared with monotherapies.
Corticosteroid and Vitamin D Analog Combination Therapy Attenuates Side Effects Associated with their Individual Monotherapies
Corticosteroid and vitamin D analog monotherapy is associated with increased risks of skin atrophy and perilesional skin irritation, respectively [4, 16, 36]. As the frequency of application of corticosteroids and vitamin D analogs used in combination therapy is lower than in monotherapy [4], the risk of adverse events associated with monotherapy is reduced [16, 37, 38].
Long-term continuous use of topical corticosteroid treatment alone can lead to skin atrophy. As a result, the thickness of the skin is reduced and transepidermal water loss increases, causing loss of skin barrier function [37, 39]. Recent studies in cultured skin cells have demonstrated that the addition of calcipotriol reduces the early signs of betamethasone and clobetasol-induced skin atrophy by modulating key ECM components [39]. The effects of corticosteroid, vitamin D analog, and combination treatment during skin atrophy are summarized in Table 1.
Table 1.
| Mechanism | Effect of corticosteroids | Effect of vitamin D analogs | Overall clinical effect of combination treatment |
|---|---|---|---|
| Lipid synthesis | ⇩ | ⇧ | Prevents skin barrier and water loss impairment caused by corticosteroids |
| AMPs, e.g., LL-37 | ⇩ | ⇧ | |
| KC proliferation* | ⇩ | = | Attenuates epidermal thinning by corticosteroid-induced reduction of epidermal cells |
|
Change in tissue modeling and structure: –Hyaluronic acid –Matrix metalloproteinases |
⇩ | ⇧ | Limits epidermal thinning from corticosteroid-induced loss of cellular volume |
| Collagen synthesis and turnover | ⇩ | ⇧ | Reduces dermal thinning caused by corticosteroid induced decrease in matrix network |
| Glycosamine synthesis | ⇩ | ⇧ | Increases water-binding capacity of the skin, decreasing corticosteroid-induced dermal thinning |
| Elastic fiber synthesis | ⇩ | ⇧ | Attenuates reduced skin flexibility/elasticity observed in topical steroidal monotherapy |
Downward arrow indicates downregulation; upward arrow indicates upregulation; equal sign indicates no effect; AMPs antimicrobial peptides; KC keratinocytes
* KC proliferation is psoriasis activity-dependent. The data presented here are based on non-inflamed skin
Calcipotriol as monotherapy (50 μg/g twice daily doses) may cause perilesional skin irritation [16, 36]; however, the addition of a corticosteroid has shown a beneficial effect: a 52-week study demonstrated that daily treatment with Cal/BD ointment (Cal 50 µg/g and BD 0.5 mg/g) significantly reduced the overall number of adverse events—particularly burning, itching, or erythema of the skin—compared with vitamin D analog monotherapy (Cal ointment, 50 μg/g) [40].
Authors’ clinical opinion The improved safety profile of fixed-dose topical combination treatment, together with the once-daily treatment use, offers improved convenience and better acceptance compared with monotherapies [42, 43]. These factors are essential in the therapeutic armamentarium for psoriasis and are likely to facilitate adherence to treatment, which may subsequently lead to faster and greater improvements in patient QoL.
Summary: Combination Treatment Minimizes Skin Atrophy and Decreases Other Monotherapy-Related Risks.
Long-term continuous use of corticosteroids leads to skin atrophy. Vitamin D analogs can reduce corticosteroid-induced skin atrophy by modulating key ECM components.
Combination treatment reduces the frequency of application of corticosteroid and vitamin D analogs used to treat psoriasis compared with monotherapy. This leads to a significant reduction of adverse events associated with monotherapies of both active ingredients.
Challenges of Drug Delivery in Topical Formulations
Poor penetration of active ingredients into the skin can result in low, or lack of, clinical efficacy; therefore, considerable research has been undertaken to improve drug delivery in topical formulations. The ability for an active ingredient to penetrate the skin depends primarily on (1) the condition of the skin barrier, (2) the physicochemical properties of the combined drug and vehicle, and (3) the concentration of active ingredients dissolved into the vehicle.
Condition of the skin barrier Skin permeability depends on hydration levels on the outer surface [44, 45]. Occlusion of the skin by plastic wraps, impermeable dressings, or vehicles containing fats or polymer oils—such as oils, gels, and ointments—are well-known methods to increase skin hydration levels and thus influence the permeability of active ingredients of topical treatments [44].
Physicochemical properties of combined drug and vehicle Physicochemical properties, such as the shape, lipophilicity, viscosity, occlusive properties, charge, and size, of both active ingredients, as well as excipients, influence the partitioning of the active ingredients between the vehicle and skin and their subsequent ability to penetrate the skin [11, 45].
Concentration of active ingredients dissolved into the vehicle The rate of skin penetration is proportional to the concentration of active ingredient dissolved in the vehicle because of the resulting increase in thermodynamic activity [12, 46, 47]. This is a rate-limiting step for most topical treatments, whose active ingredients have limited solubilities in their vehicles. One potential method to enhance the rate of skin penetration is to increase the concentration of active ingredients dissolved in the applied product beyond the normal solubility limit—i.e., to create a supersaturated solution. A recent study demonstrated that a supersaturated environment was created and maintained with Cal/BD when applied as an aerosol foam; this state was created after rapid evaporation of the propellants during application [11]. A supersaturated solution is only clinically relevant if it is stable, as crystallization of ingredients will decrease penetrative properties of the treatment [46]. Results from the same study showed that the supersaturated solution of Cal/BD aerosol foam was maintained in the laboratory setting, post-application, for clinically relevant time periods (at least 26 h in the laboratory setting) [11], which may explain the observed increase in bioavailability of Cal/BD aerosol foam versus Cal/BD ointment [11].
Summary: Challenges in Drug Delivery.
Enhancing the penetration of active ingredients into the skin is one of the main challenges in topical drug delivery.
The success of drug delivery depends on skin hydration levels, physicochemical properties of the combined drug and vehicle, and the concentration of active ingredients dissolved into the vehicle.
A modified, stable supersaturated solution—such as the one observed with Cal/BD aerosol foam—may contribute to the superior efficacy observed when compared with more traditional vehicles, e.g., ointment and gel.
Clinical Benefits of Corticosteroid and Vitamin D Analog Fixed-Dose Combination Treatment
Corticosteroids and vitamin D analogs are directed at different targets in the pathogenesis of psoriasis. Their complementary and additive effects observed in preclinical data have been translated into effective fixed-dose combination therapies. The corticosteroid component largely allows for fast and efficacious anti-inflammatory effects, whereas the vitamin D analog ensures durability of the treatment and possible maintenance of a relapse-free state. The current clinical evidence for corticosteroid and vitamin D analog combination treatment, and what this means for the treating physician, are discussed below.
Corticosteroid and Vitamin D Analog Fixed-Dose Combination Treatment is More Efficacious than Monotherapies in Treating the Underlying Psoriasis
The superior efficacy of corticosteroid and vitamin D analog combination treatment compared with monotherapy is supported by randomized, double-blind controlled clinical studies [48, 49]. A three-arm, multicenter study demonstrated that fixed-dose combination Cal/BD aerosol foam was significantly more efficacious in improving the mean modified Psoriasis Area and Severity Index score (mPASI; excluding the head, which was not treated) than the individual active ingredients after 4 weeks (P < 0.001 versus both Cal foam and BD foam) [48]. These data were supported in a plaque test study where the total clinical score, which measures the changes in erythema, scaling, and plaque thickness, was significantly improved with Cal/BD foam compared with BD foam after 4 weeks of treatment (P = 0.005) [49].
As well as providing efficient anti-inflammatory effects, the use of vitamin D analogs may also lead to long-term management of psoriasis by inducing immunomodulatory responses. Combination therapy with calcipotriol and betamethasone butyrate propionate ointment was associated with a decrease in T-cell production of the pro-inflammatory cytokines IL-17 and IFN-γ, which correlated with a significant improvement in mean PASI score in patients with moderate-to-severe psoriasis [50].
Authors’ clinical opinion Targeting specific molecules involved in the pathophysiology of psoriasis, as observed in fixed-dose combination treatment, results in an early and rapid response. This helps build patient confidence in the treatment and positively impacts QoL and adherence to therapy. These aspects could, in turn, help in developing a mutually trusting and successful patient-dermatologist partnership. Additionally, patients can personally adapt fixed-dose combination treatment during long-term management. For example, in cases of flares, patients can increase the frequency of application and reduce it accordingly once the flare has been controlled.
An Innovative Drug Delivery Formulation Results in Improved Efficacy
A number of studies have demonstrated the superior efficacy of Cal/BD aerosol foam formulation compared with traditional formulations, such as ointments, gels, and lotions (Table 2) [49, 51–53]. For example, in two 4-week Phase II studies, Cal/BD aerosol foam demonstrated significant improvements in mPASI (P = 0.005) [51] and plaque symptom severity (P = 0.038) [49] compared with Cal/BD ointment. In a Phase III study designed to compare Cal/BD aerosol foam and Cal/BD gel based on the recommended US/European treatment periods (4 weeks for Cal/BD aerosol foam, and 8 for Cal/BD gel), significantly more patients treated with aerosol foam than gel had clear/almost clear skin at weeks 4 and 8, respectively (P < 0.001; defined as ≥2 grade improvement according to the Physician’s Global Assessment of disease severity) [53]. Results from health-related QoL questionnaires, such as Dermatology Life-Quality Index (DLQI), were also significantly improved with Cal/BD aerosol foam than with Cal/BD gel [52]. Notably, significantly more patients treated with Cal/BD aerosol foam reported that psoriasis no longer impacted their daily lives (i.e., achieved DLQI scores of 0/1) at week 4 compared with patients treated with Cal/BD gel (45.7% vs. 32.4%; P = 0.013) [52]. One aspect of psoriasis that has a strong negative impact on patient QoL is itch [54]. Improvements in DLQI scores after 4 weeks of Cal/BD aerosol foam treatment were significantly correlated with improvements in itch VAS scores [55]. Cal/BD aerosol foam also provided fast and significantly greater itch relief than placebo (P = 0.013 at day 3) [56]. Furthermore, the superior efficacy of Cal/BD aerosol foam is associated with a similar safety profile, as demonstrated in a pooled safety analysis of three clinical studies comparing fixed combination Cal/BD aerosol foam with BD foam, Cal foam, Cal/BD ointment, and vehicles (foam and ointment) [57].
Table 2.
Summary of studies comparing Cal/BD aerosol foam with Cal/BD gel or ointment
| References | Study identifier | Design | Duration (weeks) | N | Comparator(s) | Outcomes |
|---|---|---|---|---|---|---|
| Queille-Roussel [49] | NCT01347255 | Phase IIa, exploratory, single-center, intra-individual comparison | 4 | 24 | Cal/BD foam vs. Cal/BD ointment vs. BD foam vs. foam vehicle (all n = 24) | TCS decrease: −6.00 vs. −5.25 (Cal/BD ointment; P = 0.038), vs. −4.96 (BD foam; P = 0.005) |
| Koo et al. [51] | NCT01536886 | Phase II, randomized, multicenter | 4 | 376 | Cal/BD foam (n = 141) vs. Cal/BD ointment (n = 135) vs. foam (n = 49) and ointment (n = 51) vehicle | Treatment success rates: 54.6% vs. 43.0% (Cal/BD ointment; P = 0.025); mPASI mean difference: −0.6 vs. Cal/BD ointment (P = 0.005) |
| Paul et al. [52] | NCT02132936 | Phase III, randomized, parallel-group (PSO-ABLE) | 12 | 463 | Cal/BD foam (n = 185) vs. Cal/BD gel (n = 188) vs. foam (n = 47) and gel (n = 43) vehicle | Treatment success rates: 38% vs. 22% (Cal/BD gel; P < 0.001); mPASI mean difference: −0.6 vs. Cal/BD gel (P = 0.028) |
| Paul et al. [53] | NCT02132936 | Phase III, randomized, parallel-group (PSO-ABLE secondary, HRQoL analysis) | 12 | 463 | Cal/BD foam (n = 185) vs. Cal/BD gel (n = 188) | DLQI scores of 0/1: 61% vs. 44% (Cal/BD gel; P = 0.003); EQ-5D utility index: 0.09 vs. 0.03 (Cal/BD gel; P < 0.001) |
All studies were investigator-blinded
BD bethamethasone dipropionate 0.5 mg/g, Cal calcipotriol 50 μg/g, DLQI Dermatology Life Quality Index, EQ-5D EuroQoL-5D-5L-PSO, HRQoL health-related quality of life, mPASI modified Psoriasis Area and Severity Index (excluding the head, which was not measured), TCS total clinical score (sum of erythema, scaling and plaque thickness)
Authors’ clinical opinion Cal/BD aerosol foam treatment can significantly reduce the burden of the disease, as demonstrated by the superior efficacy and ability to provide significant and measurable improvement in QoL compared with conventional Cal/BD formulations, such as gel and ointment. The rapid itch relief associated with Cal/BD aerosol foam is crucial for treatment success as patients perceive itch to be one of the most bothersome symptoms of psoriasis. Due to its improved efficacy and positive impact on QoL, with no additional concerns about tolerability, Cal/BD aerosol foam is not just an improvement on previous formulations, but could also be considered a new stand-alone treatment.
Conclusions
Fixed-dose combination of corticosteroids and vitamin D analog has demonstrated superior efficacy over monotherapies with topical steroids in both preclinical and clinical studies, as well as in daily practice. Updated knowledge on the mechanism of action of the active ingredients demonstrates how combination treatment successfully and significantly inhibits active inflammation and supports the maintenance of a relapse-free state. The rationale for fixed-dose combination treatment is further supported by the fact that adverse events associated with corticosteroid and vitamin D analog monotherapy, such as skin atrophy and perilesional skin irritation, respectively, can be effectively minimized by combining the two active ingredients. Improved delivery of the active ingredients via innovative formulations, such as an aerosol foam, has shown improved clinical response and QoL, while providing patients with more therapeutic options suited to their lifestyle. Additional research is still required to understand how long-term maintenance of a disease-free state can be achieved in the clinical setting. For this reason, a randomized clinical trial with Cal/BD aerosol foam has recently been initiated to examine the long-term management of plaque psoriasis (PSO-LONG; NCT02899962).
Acknowledgements
This study was sponsored by LEO Pharma. Article-processing charges were funded by LEO Pharma. Medical writing support was provided by Mai Kurihara, PhD, from Mudskipper Business Limited, funded by LEO Pharma. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work and the interpretation of the data from the reviewed publications, and have given final approval to the version to be published.
Disclosures
Siegfried Segaert has been a speaker/consultant for Abbott, Amgen, Biogen, Boehringer-Ingelheim, Celgene, Galderma, Janssen, LEO Pharma, Lilly, Merck Serono, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Sun Pharma, and UCB. Neil H. Shear has received honoraria related to consultations or services provided by LEO Pharma and support from the Canadian Dermatology Foundation (of which he is President) and LEO Pharma. Andrea Chiricozzi has been a scientific consultant for LEO Pharma, Novartis, Biogen, Eli-Lilly, and AbbVie. Diamant Thaçi has been an advisor, received speaker's honoraria and grant support, and participated in clinical trials for AbbVie, Almiral, Amgen, Biogen-Idec, Boehringer-Ingelheim, Celgene, Dignitiy, Dr. Reddy, Elli-Lilly, Forward-Pharma, GlaxoSmithKline, LEO Pharma, Janssen-Cilag, Maruho, MSD, Mundipharma, Novartis, Pfizer, Regeneron, Roche, Sanofi, Sandoz, and Xenoport. Jose-Manuel Carrascosa has been an advisor, received speaker’s honoraria and grant support, and participated in clinical trials for AbbVie, Almirall, Biogen, Celgene, Eli-Lilly, Janssen, LEO Pharma, Novartis, and Pfizer. Helen Young has received grant support and acted as scientific consultant for AbbVie, Amgen, Jansen, LEO Pharma, Lilly, MEDA, Novartis, Stiefel, and UCB Pharma. Vincent Descamps is a scientific consultant for Elli-Lilly and has been advisor/received speaker’s honoraria/participated in clinical trials for AbbVie, Celgene, Janssen, Novartis, and Pfizer.
Compliance with Ethics Guidelines
The article presented here is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/A9F8F0605484360A.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Hrehorow E, Salomon J, Matusiak L, Reich A, Szepietowski JC. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67–72. doi: 10.2340/00015555-1193. [DOI] [PubMed] [Google Scholar]
- 3.Laws PM, Young HS. Topical treatment of psoriasis. Expert Opin Pharmacother. 2010;11:1999–2009. doi: 10.1517/14656566.2010.492778. [DOI] [PubMed] [Google Scholar]
- 4.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Higgins E, Markham T. Current treatment options in the management of psoriasis. Prescriber. 2010;21:31–44. doi: 10.1002/psb.640. [DOI] [Google Scholar]
- 6.Salgo R, Thaci D. Treatment of moderate-to-severe plaque psoriasis. G Ital Dermatol Venereol. 2009;144:701–711. [PubMed] [Google Scholar]
- 7.Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416–430. doi: 10.1016/j.jaad.2002.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Gordon KM. Update on new and emerging therapies in the management of psoriasis. Semin Cutan Med Surg. 2015;34:S34–S36. doi: 10.12788/j.sder.2015.0136. [DOI] [PubMed] [Google Scholar]
- 9.Feldman SR, Horn EJ, Balkrishnan R, Basra MK, Finlay AY, McCoy D, et al. Psoriasis: improving adherence to topical therapy. J Am Acad Dermatol. 2008;59:1009–1016. doi: 10.1016/j.jaad.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Thorneloe RJ, Bundy C, Griffiths CE, Ashcroft DM, Cordingley L. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol. 2013;168:20–31. doi: 10.1111/bjd.12039. [DOI] [PubMed] [Google Scholar]
- 11.Lind M, Nielsen KT, Schefe LH, Norremark K, Eriksson AH, Norsgaard H, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther (Heidelb). 2016;6:413–425. doi: 10.1007/s13555-016-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surber C, Smith EW. The mystical effects of dermatological vehicles. Dermatology. 2005;210:157–168. doi: 10.1159/000082572. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33:13–23. doi: 10.1016/j.det.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb A. Immune modulation with combined vitamin D analogs and corticosteriods in psoriasis. Psoriasis Forum. 2015;21:35–41. [Google Scholar]
- 16.Segaert S, Røpke M. The biological rationale for use of vitamin D analogs in combination with corticosteroids for the topical treatment of plaque psoriasis. J Drugs Dermatol. 2013;12:e129–e137. [PubMed] [Google Scholar]
- 17.Fujiyama T, Ito T, Umayahara T, Ikeya S, Tatsuno K, Funakoshi A, et al. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion. J Allergy Clin Immunol. 2016;138:517–528. doi: 10.1016/j.jaci.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Jadali Z, Eslami MB. T cell immune responses in psoriasis. Iran J Allergy Asthma Immunol. 2014;13:220–230. [PubMed] [Google Scholar]
- 19.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015;24:529–535. doi: 10.1111/exd.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- 23.Chiricozzi A, Nograles KE, Johnson-Huang LM, Fuentes-Duculan J, Cardinale I, Bonifacio KM, et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One. 2014;9:e90284. doi: 10.1371/journal.pone.0090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9:302–309. doi: 10.1038/cmi.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen C, Funding AT, Otkjaer K, Kragballe K, Jensen UB, Madsen M, et al. Protein expression of TNF-alpha in psoriatic skin is regulated at a posttranscriptional level by MAPK-activated protein kinase 2. J Immunol. 2006;176:1431–1438. doi: 10.4049/jimmunol.176.3.1431. [DOI] [PubMed] [Google Scholar]
- 26.Howie SE, Aldridge RD, McVittie E, Forsey RJ, Sands C, Hunter JA. Epidermal keratinocyte production of interferon-gamma immunoreactive protein and mRNA is an early event in allergic contact dermatitis. J Invest Dermatol. 1996;106:1218–1223. doi: 10.1111/1523-1747.ep12348507. [DOI] [PubMed] [Google Scholar]
- 27.Lovato P, Norsgaard H, Tokura Y, Ropke MA. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell-Th17 cell axis in psoriasis. J Dermatol Sci. 2016;81:153–164. doi: 10.1016/j.jdermsci.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Bikle DD. 1,25(OH)2D3-regulated human keratinocyte proliferation and differentiation: basic studies and their clinical application. J Nutr. 1995;125:1709S–1714S. doi: 10.1093/jn/125.suppl_6.1709S. [DOI] [PubMed] [Google Scholar]
- 29.Lange K, Kleuser B, Gysler A, Bader M, Maia C, Scheidereit C, et al. Cutaneous inflammation and proliferation in vitro: differential effects and mode of action of topical glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2000;13:93–103. doi: 10.1159/000029913. [DOI] [PubMed] [Google Scholar]
- 30.Norris DA. Mechanisms of action of topical therapies and the rationale for combination therapy. J Am Acad Dermatol. 2005;53:S17–S25. doi: 10.1016/j.jaad.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(Suppl 3):47–51. doi: 10.1111/j.1468-3083.2012.04523.x. [DOI] [PubMed] [Google Scholar]
- 32.Uva L, Miguel D, Pinheiro C, Antunes J, Cruz D, Ferreira J, et al. Mechanisms of action of topical corticosteroids in psoriasis. Int J Endocrinol. 2012;2012:561018. doi: 10.1155/2012/561018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark RA. Gone but not forgotten: lesional memory in psoriatic skin. J Invest Dermatol. 2011;131:283–285. doi: 10.1038/jid.2010.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol. 2011;131:391–400. doi: 10.1038/jid.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott LJ, Dunn CJ, Goa KL. Calcipotriol ointment. A review of its use in the management of psoriasis. Am J Clin Dermatol. 2001;2:95–120. doi: 10.2165/00128071-200102020-00008. [DOI] [PubMed] [Google Scholar]
- 37.Schoepe S, Schäcke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006;15:406–420. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 38.Augustin M, Mrowietz U, Bonnekoh B, Rosenbach T, Thaci D, Reusch M, et al. Topical long-term therapy of psoriasis with vitamin D(3) analogues, corticosteroids and their two compound formulations: position paper on evidence and use in daily practice. J Dtsch Dermatol Ges. 2014;12:667–682. doi: 10.1111/ddg.12396. [DOI] [PubMed] [Google Scholar]
- 39.Norsgaard H, Kurdykowski S, Descargues P, Gonzalez T, Marstrand T, Dunstl G, et al. Calcipotriol counteracts betamethasone-induced decrease in extracellular matrix components related to skin atrophy. Arch Dermatol Res. 2014;306:719–729. doi: 10.1007/s00403-014-1485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kragballe K, Austad J, Barnes L, Bibby A, de la Brassinne M, Cambazard F, et al. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet®/Daivobet®/Taclonex®) in the treatment of psoriasis vulgaris. Br J Dermatol. 2006;154:1155–1160. doi: 10.1111/j.1365-2133.2006.07236.x. [DOI] [PubMed] [Google Scholar]
- 41.Jensen JD, Fujita M, Dellavalle RP. Validation of psoriasis clinical severity and outcome measures: searching for a gold standard. Arch Dermatol. 2011;147:95–98. doi: 10.1001/archdermatol.2010.242. [DOI] [PubMed] [Google Scholar]
- 42.Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607–613. doi: 10.1016/j.jaad.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Lambert J, Hol CW, Vink J. Real-life effectiveness of once-daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: final analysis of the 52-week PRO-long study. J Eur Acad Dermatol Venereol. 2015;29:2349–2355. doi: 10.1111/jdv.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjorklund S, Engblom J, Thuresson K, Sparr E. A water gradient can be used to regulate drug transport across skin. J Control Release. 2010;143:191–200. doi: 10.1016/j.jconrel.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer H, Redelmeier TE. Skin barrier: principles of percutaneous absorption. New York: Karger; 1996. [Google Scholar]
- 46.Hadgraft J, Lane ME. Drug crystallization—implications for topical and transdermal delivery. Expert Opin Drug Deliv. 2016;13:817–30. doi: 10.1517/17425247.2016.1140146. [DOI] [PubMed] [Google Scholar]
- 47.Moser K, Kriwet K, Froehlich C, Kalia YN, Guy RH. Supersaturation: enhancement of skin penetration and permeation of a lipophilic drug. Pharm Res. 2001;18:1006–1011. doi: 10.1023/A:1010948630296. [DOI] [PubMed] [Google Scholar]
- 48.Lebwohl M, Tyring S, Bukhalo M, Alonso-Llamazares J, Olesen M, Lowson D, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase II study. J Clin Aesthet Dermatol. 2016;9:34–41. [PMC free article] [PubMed] [Google Scholar]
- 49.Queille-Roussel C, Olesen M, Villumsen J, Lacour JP. Efficacy of an innovative aerosol foam formulation of fixed combination calcipotriol plus betamethasone dipropionate in patients with psoriasis vulgaris. Clin Drug Investig. 2015;35:239–245. doi: 10.1007/s40261-015-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hino R, Kabashima R, Kawakami C, Sugita K, Nakamura M, Tokura Y. Circulating Th17 cell fluctuation in psoriatic patients treated with topical calcipotriol and betamethasone butyrate propionate. J Eur Acad Dermatol Venereol. 2011;25:242–244. doi: 10.1111/j.1468-3083.2010.03714.x. [DOI] [PubMed] [Google Scholar]
- 51.Koo J, Tyring S, Werschler WP, Bruce S, Olesen M, Villumsen J, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris—a randomized phase II study. J Dermatol Treat. 2016;27:120–127. doi: 10.3109/09546634.2015.1083935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul C, Stein Gold L, Cambazard F, Kalb R, Lowson D, Moller AH et al. More rapid improvement in quality of life with fixed combination calcipotriene plus betamethasone dipropionate aerosol foam versus topical suspension (PSO-ABLE study in patients with psoriasis vulgaris). J Am Acad Dermatol. 2016;74:AB260 (abst P3712).
- 53.Paul C, Stein Gold L, Cambazard F, Kalb RE, Lowson D, Bang B, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy versus gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31:119–126. doi: 10.1111/jdv.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Global report on PSORIASIS. 2016. http://apps.who.int/iris/bitstream/10665/204417/1/9789241565189_eng.pdf. Accessed 11 Apr 2017.
- 55.Leonardi C, Bagel J, Yamauchi P, Pariser D, Xu Z, Moeller A, et al. The aerosol foam formulation of the fixed combination calcipotriene plus betamethasone dipropionate improves the health-related quality of life in patients with psoriasis vulgaris: results from the randomized PSO-FAST study. J Drugs Dermatol. 2016;15:981–987. [PubMed] [Google Scholar]
- 56.Leonardi C, Bagel J, Yamauchi P, Pariser D, Xu Z, Olesen M, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris—a randomized phase III study (PSO-FAST) J Drugs Dermatol. 2015;14:1468–1477. [PubMed] [Google Scholar]
- 57.Menter A, Stein GL, Koo J, Villumsen J, Rosen M, Lebwohl M. Fixed combination calcipotriene plus betamethasone dipropionate aerosol foam is well tolerated in patients with psoriasis vulgaris (pooled data from three randomized controlled studies) Skinmed. 2017;15:119–24. [PubMed] [Google Scholar]