Abstract
The exopolysaccharide succinoglycan is produced mainly by a large number of soil microbes of Agrobacterium, Rhizobium or Pseudomonas genera etc. Structural properties of succinoglycan are unique in terms of its thermal stability and superior viscosifying property. Unlike the other highly commercialized bacterial exopolysaccharides like dextran or xanthan, mass scale application of succinoglycan has not been that much broadly explored yet. Bacterial succinoglycan is found suitable as a viscosifying and emulsifying agent in food industry, in gravel packing or fluid-loss control agent etc. In this present review, the key aspects of succinoglycan study, in particular, developments in structural characterizations, exo/exs operon system involved in biosynthesis pathway, commercial applications in food and other industries and patenting trends have been discussed.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0655-3) contains supplementary material, which is available to authorized users.
Keywords: Biosynthesis, Industrial applications, Patents, Succinoglycan, Structural properties
Introduction
Succinoglycan, an acidic, water soluble heteropolysaccharide is first purified by Tokuya Harada in 1965 [1]. The name of this exopolysaccharide (EPS) has been initially coined as succinoglucan because succinic acid is a present as a main moiety in the biopolymer and succinic acid is primarily composed of several glucose residues. Later, the name is changed to succinoglycan as one galactose residue per seven glucose residues are also present in the structure along with some non-carbohydrate substituents, namely pyruvate, succinate and acetate [2, 3]. So far yield of bacterial succinoglycan up to 13.7 g/l [4] has only been reported from soil inhabiting bacteria like Agrobacterium tumefaciens, Agrobacterium radiobacter, Agrobacterium rhizogenes [5], Rhizobium radiobacter [6], Sinorhizobium meliloti [7], Ensifer meliloti [8] and Pseudomonas sp. [9]. Various uses of bacterial succinoglycan include brine based treatment fluids, laundry detergent compositions, cosmetic additives etc. based on its properties as a thickener, texture enhancer, emulsifier, plasticizer, stabilizer or flocculating agent [6, 10–12]. Recently, succinoglycan has also been used as stabilizing agent in metal nanoparticle green synthesis, where the size of silver nanoparticle remains uniformly below 10 nm. These metal nanoparticles are a potent alternative of antimicrobials too [7, 13, 14].
Structural Properties of Succinoglycan
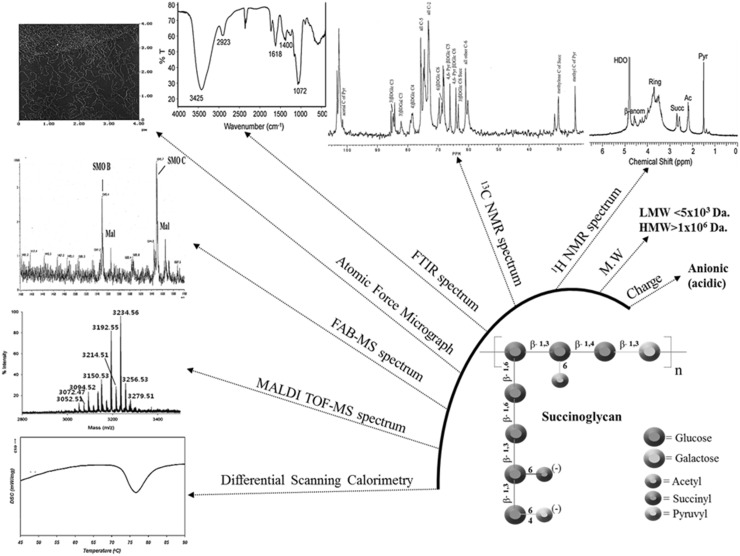
The first work on structural revelation of succinoglycan has been reported by Masaki et al. [15]. Molecular weight of succinoglycan using gel filtration chromatography is reported in both low and high molecular weight forms. Low molecular weight succinoglycan is <5 × 103 Da in size, where as high molecular weight succinoglycan is >1 × 106 Da of size. Molecular weight of the native form is 4.2 × 106 Da but after a single heating cycle, molecular weight of the disordered EPS is reduced to 3.0 × 106 Da [16]. Atomic force microscopy of aqueous succinoglycan has been imaged either as an individual single chain or as an association of at least two individual chains. Increasing the ionic strength of solution, increase in intrinsic chain rigidity is observed which is probably due to formation of a single helical structure [17]. Thermal characteristics by Differential Scanning Calorimetry (DSC) supports the view that succinoglycan is a double helical structure at 25 °C which melts into a single strand above 65 °C and thus the structural arrangement is a semi-flexible one. Molecular weight of succinoglycan at 25 °C is double that of the value at 75 °C. Thermogram (TGA) of succinoglycan obtained under nitrogen atmosphere and heating rate 10 °C/min and it shows approximately 48% mass stability at 600 °C. 1–2% of succinoglycan acts like non-Newtonian shear-thinning fluid under 25–55 °C temperature range. Increasing solution concentrations, viscosity and pseudoplasticity proportionally increases; while temperature increase is inversely proportional to viscosity and pseudoplasticity. The coil overlap parameter for succinoglycan is consistent as a rod-like polymer [18]. But with the changes in electrolyte, pH and ionic strength, conformational changes also take place [19].
FT-IR spectrum of succinoglycan shows characteristic peaks of polysaccharides through O–H stretching frequency of sugar poly-hydroxyl groups, C–H and asymmetric C–O–C stretching of sugar backbone, symmetric stretching of carboxyl groups due to the bending of symmetric CH3 groups of acetyl and pyruvylresidues. The chemical shifts in 1H NMR spectrum has represented methyl protons of 1-carboxyethylidene (pyruvate) and acetyl groups, methylene protons of succinyl group; protons of sugar backbone. 1H NMR spectroscopic analysis also indicates that high molecular weight fractions of fully acylated succinoglycan contain about each one succinyl, acetyl and pyruvate alteration per oligosaccharide sub-unit [20]. Simultaneously, 13C NMR spectrum shows chemical shifts at 25.7 ppm assigning the CH3 of pyruvate, 102.2 ppm as acetyl carbon and two CH2 signals of succinate at 32.4 and 31.2 ppm [21].
FAB-MS spectrum of succinoglycan has revealed the presence of additional salt ions too. In Fig. 1, ions have been detected at m/z 1565 and m/z 1665 from an additional octasaccharide in each oligosaccharide, which is probably due to the presence of malate instead of succinate [22]. MALDI TOF-MS studies of low molecular weight succinoglycan monomers, dimers and trimers clearly indicate galactosyl linkages are more labile than glucosyl linkages. Additionally, loss of more acetyl groups than succinyl/pyruvyl ester linkages induces more instability to acetyl linkages than pyruvyl/succinyl linkages [7].
Fig. 1.
General characteristic features of bacterial succinoglycan. The figure is showing the linear repeating unit of succinoglycan octasaccharide composed of glucose and galactose; charge; molecular weight; 1H NMR and FT-IR spectrum [19]; 13C NMR spectrum [4]; AFM micrograph [16]; FAB-MS spectrum [22]; MALDI TOF-MS [7] and DSC [17]
Succinoglycan Biosynthesis Pathway and Its Role in Symbiosis
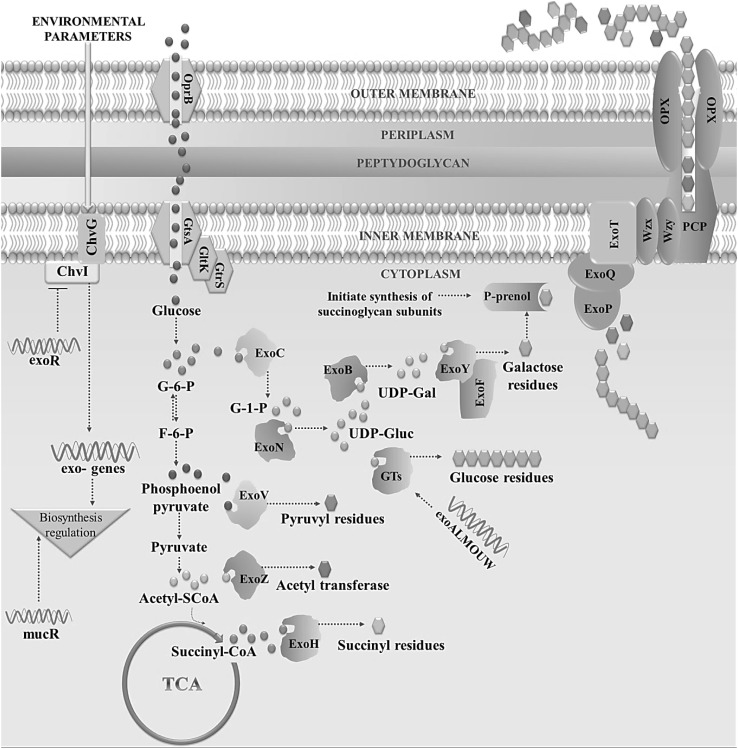
The pSymBmega plasmid and 28 exo/exs genes shows a large exo/exs cluster in most of the succinoglycan biosynthesis related operons. The exo genes of linear chromosome encode enzymes responsible for sugar synthesis, repeating unit assembly, modification, polymerization and export of EPS; whereas in the circular chromosome exoD, exoR, exoI, and chvG (exoS) genes are EPS biosynthesis regulators. Three genes (exoC, exoB and exoN) are essential for sugar precursor synthesis, whereas exoY and exoF are for galactose addition to lipid carrier. exoN, a UDP-glucose pyrophosphorylase phosphorylate the tyrosine residue resulting into initial establishment of symbiosis [23]. Glycosyltransferases (GTs) encoded by exoALMOUW gene adds glucose residues and is also responsible for glycosidic bond formation. exoPQT encoded proteins catalyze polymerization of repeating units and EPS secretion; whereas ExoP autophosphorylating protein tyrosine kinase forms dimers of octasaccharide units. High and low molecular weight succinoglycan production is respectively controlled by ExoQ and ExoT gene products. Different other modifications of growing EPS are done by exoZ, exoH and exoV proteins addition to the sugar chain. Among these, ExoH provides succinyl, ExoV pyruvyl groups and exoZ encodes acetyl transferase enzyme. About 25 two-component regulatory systems control this pathway with a key controlling acid-activated ChvG/ChvI system. ChvG produces a sensor kinase which monitors environmental parameters, finally activating response regulator. As a response regulator, ChvI controls succinoglycan biosynthesis by influencing overall exo expression and establishment of symbiosis. In some studies, it is reported that the mucoidy nature of rhizobacteria are due to succinoglycan type galactoglucan and the EPS synthesis is sensitive to concentration of Mg2+, K+ ion or deoxycholate. Magnesium homeostasis related mhrA are tentatively behind this action [8]. Apart from this, exoR produces a negative biosynthesis regulator too, genetically attached with ChvG/ChvI system. Both mucR and ros genes also play a key role in positive control of succinoglycan biosynthesis [24].
Succinoglycan along with galactoglucan produced by different types of rhizospheric bacteria, play a crucial role in establishment of host plant specific symbiosis through nodule invasion. Rhizobial EPS attribute different roles in symbiosis such as inducing bacterial adhesion and invasion to host roots inside infection threads and protection against plant defence mechanisms [24]. One most important feature in symbiosis is succinylation of succinoglycan to initiate invasion. A recent study has showed that exoH mutants of S. meliloti becomes unable to succinylate succinoglycan which finally failed to form infection threads, even though they make large quantities of succinoglycan [25–27]. Mechanism of biosynthesis pathway of succinoglycan is described and represented schematically in Fig. 2 [24, 28, 29].
Fig. 2.
Biosynthesis pathway of succinoglycan. Schematic diagram of succinoglycan biosynthesis pathway in soil inhabiting bacteria demonstrating the genes involved in production of succinoglycan [24, 28, 29]
Different Applications of Bacterial Succinoglycan
Viscosifying Activity
High viscosity in aqueous solutions is one of the major characteristic features of succinoglycan [6]. It shows an unusually high viscosity due to the presence of about 10% succinic acid [1]. The unique gelation property of EPS leads soil particle aggregation and thus soil stability and improved water retention [10]. On respective heating and cooling, succinoglycan behaves like polyelectrolyte chain having salt concentration influenced viscosity.
Emulsification Property
Like xanthan, succinoglycan is also used for stabilizing hydrocarbon oil-in-water emulsions at neutral pH. Presence of even small concentration of this EPS can produce large increases in creaming. Increasing the polymer concentration, improved creaming stability is observed due to the greatly increased viscosity in the continuous phase [11]. Properties of adsorbed protein layers influence food emulsions stability greatly. Creaming and flocculation of oil-in-water emulsions are also affected by the nature and strength of biopolymer–biopolymer, biopolymer–surfactant interactions in aqueous phase and interface. Addition of small quantities of Ca2+ ions in systems containing protein-coated droplets exhibits improved stability [12]. Thus bacterial succinoglycan can be used as a potent EPS in commercial food emulsification.
Pseudo-Plasticizing Activity
Aqueous succinoglycan solution is reversibly pseudoplastic in nature under continuous heating and cooling [9]. It has been observed that removal of succinyl groups improved the pseudoplasticity of the solution; whereas removal of acetyl groups leads to a decrease in the pseudoplastic nature [30]. Shiseido Company Limited has been frequently used bacterial succinoglycan as a pseudo-plasticizer (Suppl. Table 1).
Cross-Linking Property
Aqueous succinoglycan composition is also used as gel-forming polymer and it is capable of cross-linking with water-soluble polyvalent metal cations. It is also capable of cross-linking with Al3+ ion. Pfizer International Corporation is the path leading company which is using bacterial succinoglycan as an industrial cross-linker (Suppl. Table 1). In addition, iron (II) chelation property is reported in case of LMW succinoglycan which is important for nodulation [31].
Brine Solution Stabilizing Agent
Succinoglycan remains surprisingly stable in calcium bromide brine solutions above 2 M concentration. Halliburton Energy Services, International Corporations have commercialized this formula successfully (Suppl. Table 1). Unfortunately the transition temperature is below 60 °C and therefore the temperature factor is a major hindrance behind showing mass scale industrial interest on it.
Fluid-Loss Controlling Agent
Bacterial succinoglycan is a unique amalgamation of different enviable properties of fluid-loss control; like easy mixing, purity, shear-thinning rheological property, temperature-insensitive viscosity below transition temperature, variable melting point etc. As this EPS forms a viscous fluid, it completely relies on viscosity for fluid loss decrease. Also, it does not form any hard-to-remove lump in fluid thus no considerable formation damage occurred (Suppl. Table 1). Employing any slow acting internal breaker can reduce the minimal damage formed from incomplete back-production of viscous, fluid-loss control pills. Succinoglycan-based pill with HCl internal breaker ensures sustained control and delayed break of fluid-loss system (Suppl. Table 1). However, optimum formulation of succinoglycan and adding an acid breaker are needed for wide use of succinoglycan as a commercial fluid-loss controller.
Gravel Packing
Succinoglycan causes least formation spoil and has exclusive rheological properties of high shear-thinning behaviour along with temperature-induced viscosity break back. Thus it mayalso be used without addition of any breakers. Shell Oil Company is main user of this formulation (Suppl. Table 1).
Cosmetic Additive
Some regularly formulated succinoglycan composition in cosmetic preparations are water-in-oil milky lotion, ultraviolet ray protecting whitening essence, sun screen cream, water-in-oil foundation, sun screen milky lotion etc. Its oil-in-water type emulsification, thickening and plasticizing activity has made it a popular choice as cosmetic additive (Suppl. Table 1).
Commercial Availability of Bacterial Succinoglycan
Succinoglycan (INCI name) or 73667-50-2 is commercially available under the trade name Rheozan® SH and the company Solvay Novecare is the only producer and supplier of bacterial succinoglycan throughout the world. The company has reported succinoglycan production by fermentation of Agrobacterium tumeficiens and purifying it by precipitating with isopropanol, followed by drying and milling.
Patents Related to Bacterial Succinoglycan
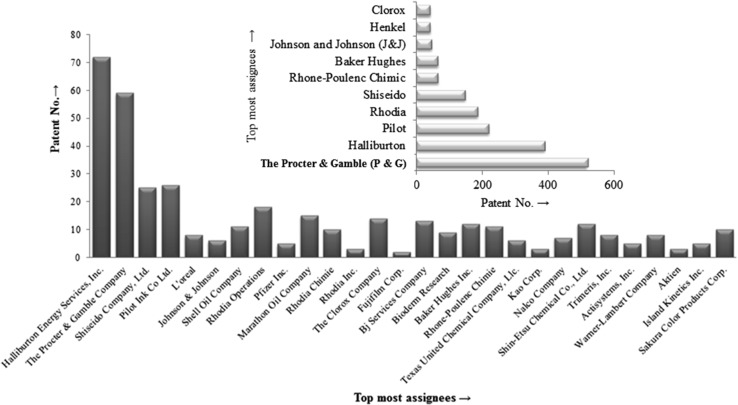
There are number of patents on succinoglycan are documented in Suppl. Table 1. The first patent on succinoglycan has come on 1986 which was almost 20 years after the first published research on succinoglycan. Other publications related to succinoglycan and its trends have been summarised manually and also using the software Relecura in Suppl. Fig. 1. This is probably due to the fact that initial 20 years passed on exploring production, optimization of its production and structural characterization of it including some of the other basic researches. From 1990 onwards, the number of patents increases with a peak of maximum patenting in 2013 as shown in Suppl. Fig. 2. The main hindrances behind exponential growth on succinoglycan patenting may be probably attributed to the fact that only soil inhabiting rhizobacteria are commercially exploited for the production. Also more commercialization of this EPS is needed as we can see only a couple of companies are dominating succinoglycan research, mainly, Halliburton Energy Services. Inc. and The Procter and Gamble Company (Fig. 3). Thus involvement of new companies may broaden the dimension of succinoglycan research (http://patft.uspto.gov/netacgi/nphParser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearchbool.html&r=0&f=S&l=50&TERM1=succinoglycan&FIELD1=&co1=AND&TERM2=&FIELD2=&d=PTXT. Accessed on 11/01/2017 and http://worldwide.espacenet.com/searchResults?ST=singleline&locale=en_EP&submitted=true&DB=&query=succinoglycan. Accessed on 11/04/2017).
Fig. 3.
Comparative patent studies of different assignees of succinoglycan from bacterial sources using Relecura patent search tool (in blue), Espacenet patent search (in red) and US Patent full-text database Boolean search (in red) (color figure online)
Conclusion
Bacterial succinoglycan is a uniquely important rhizobacterial exopolysaccharide with varied interesting industry friendly physico-chemical properties which should be explored more in terms of production and commercialization. In the last 50 years of succinoglycan research staring from 1965, researchers have extensively studied its nodule invasion property and role in symbiosis. Commercialization of succinoglycan for better nodule establishment in leguminous plants may open a new era in sustainable agriculture. Among the other notable points, succinoglycan can be used as a nano-biomaterial and may be explored in different biomedical applications; including drug delivery, biomedical imaging, nano-biosensor etc. A number of studies on biomedical applications of dextran-nanoparticle conjugate indicated a paradigm shift in bacterial exopolysaccharide based nanobiotechnology. These conjugates are widely used in organ specific drug delivery, biosensor, drug carrier and encapsulation, haemoglobin-conjugate as blood substitute etc. [32]. Recently, modifications of succinoglycan using alginate beads with functionalized polydiacetylene vesicles are developed to assess barium (II) as a tangible fluorogenic sensor system [33]. As bacterial exopolysaccharides are unique group of biopolymer which is both biodegradable and non-toxic, thus more research on application of succinoglycan as nanobiomaterial may open a new era in biomedical field. Till date, due to the non-toxic and good viscosifying nature, succinoglycan is only used in food or cosmetic industry as commercial emulsifying agent. Production and commercialization of raw EPS is an intensive process; thus easy downstream processing technique is needed for fast and better marketization. Last 50 years of bacterial succinoglycan research has been dynamic and promising. Succinoglycan, an anionic, highly water soluble bacterial EPS with prominent cross-linking capacity thus needs much attention from researchers in this field for further exploitation of the potential aspects which are beginning to emerge.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to UGC-Centre of Advanced Study, Department of Botany, The University of Burdwan for pursuing research activities and Relecura patent search tool for patented documents analysis. Aparna Banerjee is also thankful for the financial assistance of SRF (State Funded) [Fc (Sc.)/RS/SF/BOT./2014-15/103 (3)].
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0655-3) contains supplementary material, which is available to authorized users.
References
- 1.Harada T. Succinoglucan 10C3: a new acidic polysaccharide of Alcaligenes faecalis var. myxogenes. Arch Biochem Biophys. 1965;112:65–69. doi: 10.1016/0003-9861(65)90010-X. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Kwon S, Kwon C, Jung S. Low-energy collision-activated dissociation electrospray ionization tandem mass spectrometric analysis of Sinorhizobial succinoglycan monomers. Carbohydr Res. 2009;344:1127–1129. doi: 10.1016/j.carres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Simsek S, Mert B, Campanella OH, Reuhs B. Chemical and rheological properties of bacterial succinoglycan with distinct structural characteristics. Carbohydr Polym. 2009;76:320–324. doi: 10.1016/j.carbpol.2008.10.033. [DOI] [Google Scholar]
- 4.Stredansky M, Conti E, Bertocchi C, Matulova M, Zanetti F. Succinoglycan production by Agrobacterium tumefaciens. J Ferment Bioeng. 1998;85:398–403. doi: 10.1016/S0922-338X(98)80083-4. [DOI] [Google Scholar]
- 5.Hisamatsu M, Abe JI, Amemura A, Harada T. Structural elucidation on succinoglycan and related polysaccharides from Agrobacterium and Rhizobium by fragmentation with two special β-d-glycanases and methylation analysis. Agric Biol Chem. 1980;44:1049–1055. [Google Scholar]
- 6.Andhare P, Delattre D, Pierre G, Michaud P, Pathak H. Characterization and rheological behaviour analysis of the succinoglycan produced by Rhizobium radiobacter strain CAS from curd sample. Food Hydrocoll. 2017;64:1–8. doi: 10.1016/j.foodhyd.2016.10.008. [DOI] [Google Scholar]
- 7.Kwon C, Lee S, Jung S. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometric behavior of succinoglycan monomers, dimers, and trimers isolated from Sinorhizobium meliloti 1021. Carbohydr Res. 2011;346:2308–2314. doi: 10.1016/j.carres.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins JP, Oresnik IJ. Characterization of a gene encoding a membrane protein that affects exopolysaccharide production and intracellular Mg2+ concentrations in Ensifer meliloti. FEMS Microbiol Lett. 2017;364:fnx061. doi: 10.1093/femsle/fnx061. [DOI] [PubMed] [Google Scholar]
- 9.Gravanis G, Milas M, Rinaudo M, Clarke-Sturman AJ. Conformational transition and polyelectrolyte behaviour of a succinoglycan polysaccharide. Int J Biol Macromol. 1990;12:195–200. doi: 10.1016/0141-8130(90)90032-6. [DOI] [PubMed] [Google Scholar]
- 10.Morris VJ, Brownsey GJ, Gunning AP, Harris JE. Gelation of the extracellular polysaccharide produced by Agrobacterium rhizogenes. Carbohydr Polym. 1990;13:221–225. doi: 10.1016/0144-8617(90)90085-7. [DOI] [Google Scholar]
- 11.Cao Y, Dickinson E, Wedlock DJ. Creaming and flocculation in emulsions containing polysaccharide. Food Hydrocoll. 1990;4:185–195. doi: 10.1016/S0268-005X(09)80151-3. [DOI] [Google Scholar]
- 12.Dickinson E. Emulsion stability. Food hydrocolloids. New York: Springer; 1994. pp. 387–398. [Google Scholar]
- 13.Sathiyanarayanan G, Dineshkumar K, Yang YH. Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Crit Rev Microbiol. 2017;43:1–22. doi: 10.3109/1040841X.2015.1136261. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee A, Halder U, Bandopadhyay R. Preparations and applications of polysaccharide based green synthesized metal nanoparticles: a state-of-the-art. J Clust Sci. 2017;28:1–11. doi: 10.1007/s10876-017-1219-8. [DOI] [Google Scholar]
- 15.Misaki A, Saito H, Ito T, Harada T. Structure of succinoglucan and exocellular acidic polysaccharide of Alcaligenes faecalis var myxogenes. Biochemistry. 1969;8:4645–4650. doi: 10.1021/bi00839a062. [DOI] [PubMed] [Google Scholar]
- 16.Freitas F, Alves VD, Reis MA. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–398. doi: 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Balnois E, Stoll S, Wilkinson KJ, Buffle J, Rinaudo M, Milas M. Conformations of succinoglycan as observed by atomic force microscopy. Macromolecules. 2000;33:7440–7447. doi: 10.1021/ma0002951. [DOI] [Google Scholar]
- 18.Bakhtiyari M, Moosavi-Nasab M, Askari H. Optimization of succisnoglycan hydrocolloid production by Agrobacterium radiobacter grown in sugar beet molasses and investigation of its physicochemical characteristics. Food Hydrocoll. 2015;45:18–29. doi: 10.1016/j.foodhyd.2014.11.002. [DOI] [Google Scholar]
- 19.Hill RJ. On the electrophoretic mobility of succinoglycan modelled as a spherical polyelectrolyte: from Hermans–Fujita theory to charge regulation in multi-component electrolytes. J Colloid Interface Sci. 2016;482:131–134. doi: 10.1016/j.jcis.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Kang S, Lee S, Kyung S, Jung S. Catalytic methanolysis induced by succinoglycan, a Rhizobial exopolysaccharide. Bull Korean Chem Soc. 2006;27:921. doi: 10.5012/bkcs.2006.27.6.921. [DOI] [Google Scholar]
- 21.Matulova M, Toffanin R, Navarini L, Gilli R, Paoletti S, Cesaro A. NMR analysis of succinoglycans from different microbial sources: partial assignment of their 1H and 13C NMR spectra and location of the succinate and the acetate groups. Carbohydr Res. 1994;265:167–179. doi: 10.1016/0008-6215(94)00227-4. [DOI] [PubMed] [Google Scholar]
- 22.Simsek S, Wood K, Reuhs BL. Structural analysis of succinoglycan oligosaccharides from Sinorhizobium meliloti strains with different host compatibility phenotypes. J Bacteriol. 2013;195:2032–2038. doi: 10.1128/JB.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medeot DB, Rivero MR, Cendoya E, Contreras-Moreira B, Rossi FA, Fischer SE, Becker A, Jofré E. Sinorhizobium meliloti low molecular mass phosphotyrosine phosphatase SMc02309 modifies activity of the UDP-glucose pyrophosphorylaseExoN involved in succinoglycan biosynthesis. Microbiology. 2016;162:552–563. doi: 10.1099/mic.0.000239. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Li A, Ma F, Yang J, Xie Y. Genetic control and regulatory mechanisms of succinoglycan and curdlan biosynthesis in genus Agrobacterium. Appl Microbiol Biotechnol. 2016;100:6183–6192. doi: 10.1007/s00253-016-7650-1. [DOI] [PubMed] [Google Scholar]
- 25.Mendis HC, Madzima TF, Queiroux C, Jones KM. Function of succinoglycan polysaccharide in Sinorhizobium meliloti host plant invasion depends on succinylation, not molecular weight. mBio. 2016;7:00606–00616. doi: 10.1128/mBio.00606-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobialsymbionts invade plants: the Sinorhizobium–Medicago model. Nat Rev Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skorupska A, Janczarek M, Marczak M, Mazur A, Król J. Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb Cell Fact. 2006;5:7. doi: 10.1186/1475-2859-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janczarek M, Rachwał K, Kopcińska J. Genetic characterization of the Pss region and the role of PssS in exopolysaccharide production and symbiosis of Rhizobium leguminosarum bv. trifolii with clover. Plant Soil. 2015;396:257–275. doi: 10.1007/s11104-015-2567-5. [DOI] [Google Scholar]
- 29.Becker A. Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front Microbiol. 2015;6:687. doi: 10.3389/fmicb.2015.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridout MJ, Brownsey GJ, York GM, Walker GC, Morris VJ. Effect of o-acyl substituents on the functional behaviour of Rhizobium meliloti succinoglycan. Int J Biol Macromol. 1997;20:1–7. doi: 10.1016/S0141-8130(96)01140-3. [DOI] [PubMed] [Google Scholar]
- 31.Cho E, Choi JM, Kim H, Tahir MN, Choi Y, Jung S. Ferrous iron chelating property of low-molecular weight succinoglycans isolated from Sinorhizobium meliloti. Biometals. 2013;26:321–328. doi: 10.1007/s10534-013-9615-5. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee A, Bandopadhyay R. Use of dextran nanoparticle: a paradigm shift in bacterial exopolysaccharide based biomedical applications. Int J Biol Macromol. 2016;87:295–301. doi: 10.1016/j.ijbiomac.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 33.Yun D, Cho E, Dindulkar SD, Jung S. Succinoglycan octasaccharide conjugated polydiacetylene-doped alginate beads for barium (II) detection. Macromol Mater Eng. 2016;301:805–811. doi: 10.1002/mame.201600060. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.