Abstract
Ergosterol, an important component of the fungal cell membrane, is not only essential for fungal growth and development but also very important for adaptation to stress in fungi. Ergosterol is also a direct precursor for steroid drugs. The biosynthesis of ergosterol can be divided into three modules: mevalonate, farnesyl pyrophosphate (farnesyl-PP) and ergosterol biosynthesis. The regulation of ergosterol content is mainly achieved by feedback regulation of ergosterol synthase activity through transcription, translation and posttranslational modification. The synthesis of HMG-CoA, catalyzed by HMGR, is a major metabolic check point in ergosterol biosynthesis. Excessive sterols can be subsequently stored in lipid droplets or secreted into the extracellular milieu by esterification or acetylation to avoid toxic effects. As sterols are insoluble, the intracellular transport of ergosterol in cells requires transporters. In recent years, great progress has been made in understanding ergosterol biosynthesis and its regulation in Saccharomyces cerevisiae. However, few reviews have focused on these studies, especially the regulation of biosynthesis and intracellular transport. Therefore, this review summarizes recent research progress on the physiological functions, biosynthesis, regulation of biosynthesis and intracellular transportation of ergosterol in S. cerevisiae.
Keywords: Ergosterol, Biosynthesis, Regulation, Transportation, Saccharomyces cerevisiae
Introduction
Sterols, macromolecule alcohol compounds that are types of steroids exist widely in nature, and play a major role in the composition of the cell membrane, which can regulate cell membrane fluidity and permeability, membrane-bound enzyme activity and membrane integrity [1]. Depending on their source, natural sterols can be divided into animal sterols, phytosterols or fungisterols. Often, animal sterols refers to cholesterol, which is an important and indispensable component of animal cells; cholesterol is not only involved in cell membrane formation, but also required for the synthesis of bile acids, vitamin D and steroid hormones, and can also be converted into bile acid or steroid hormones by metabolism [1]. Phytosterols mainly include stigmasterol, sitosterol, campesterol, oat sterols and spinach sterols. These are not only essential for plant growth and development but also important for stress adaptations. Beyond phytosterols, cholesterol has also been found to exist in plant seeds, roots, stems and leaves [2]. Fungisterol mainly refers to ergosterol, which is an important and specific component of the fungal cell membrane [3] and has been widely used as a marker to assess fungal biomass [4]. More importantly, ergosterol and some of its biosynthetic intermediates are important metabolites of great economic value. In the pharmaceutical industry, ergosterol is a precursor of vitamin D2 and steroid hormone drugs [5]. For example, cortisone and progesterone can be produced from ergosterol [6, 7]. Steroid drugs have important physiological activities and are the second most used clinical drugs after antibiotics; converted vitamin D2 can also be used as a feed additive to increase the oviposition and hatching rate of poultry [6, 7]. In recent years, new functions of ergosterol have been found. For example, 11-dehydroergosterol peroxide has significant antitumor activity and several compounds with anti-HIV activity are structural analogues of ergosterol [8, 9]. Therefore, ergosterol has broad application as an important precursor for the development of new anti-cancer and anti-HIV drugs, promoting further study of the biosynthesis, metabolism and regulation of fungal sterols. In this review, the latest advances in the ergosterol biosynthesis, metabolism, transportation and its regulation are summarized.
The Physiological Function of Ergosterol
Ergosterol is an important component of the fungal cell membrane, where it stabilizes membrane structure through binding to phospholipids and regulating membrane structure fluidity, permeability, and membrane-bound enzyme activities, as well as substance transportation [10, 11]. Ergosterol can also affect the absorption and utilization of nutrients by regulating membrane-bound ATPase activities and regulate transportation efficiency of phospholipases by affecting the mobility of the cell membrane [12]. Ergosterol can be stored in lipid droplets in the cytoplasm in the form of steryl ester, which can serve as a sterol pool to maintain the balance of intracellular sterols [13]. Moreover, ergosterol can stimulate the growth and proliferation of fungi, and is regarded as a ‘fungal hormone’ [14]. Meanwhile, ergosterol also plays an essential role in stress adaptation during fermentation. It has been found that the ability of yeast to tolerate stress is closely related to ergosterol levels. For example, the ergosterol content of yeast that are resistant to freezing and low-sugar conditions is higher than that of common yeast; and under alcohol treatment, S. cerevisiae can increase ergosterol content in the cell membrane to suppress the membrane damage and maintain normal membrane permeability [15]. Similar results were obtained in S. cerevisiae erg6 mutant, where ergosterol content in the cell membrane was decreased and the cells became more sensitive to alcohol stress [16]. Exogenous application of ergosterol during the brewing process can significantly increase the tolerance of S. cerevisiae to alcohol [17]. Another example is the expression of mushroom C-5 sterol desaturase in fisson yeast, which can enhance its tolerance to ethanol and increased temperature [18]. In yeast, when the ergosterol biosynthesis pathway is blocked by drug treatment or mutation of biosynthesis genes, its salt and drug tolerance is significantly decreased. For example, ergosterol biosynthesis defective yeast are more sensitive to lactones and oxidative stress [19, 20], while the addition of exogenous ergosterol in the medium can increase the resistance of S. cerevisiae to oxidative stress [21, 22]. Studies have also revealed that the exogenous application of ergosterol can increase the tolerance of S. cerevisiae to D-limonene [23]. Furthermore, sterol levels are also very important for the hypoxic response and temperature stress in S. cerevisiae. Hypoxic conditions result in the disruption of S. cerevisiae growth and fermentation and yeast can activate their ergosterol biosynthesis pathway to increase sterol biosynthesis and promote its growth [24]. When ergosterol biosynthesis was blocked in a range of mutants, including erg10, erg11, erg19 and erg24, S. cerevisiae was temperature-sensitive lethal [25].
Ergosterol Synthesis Pathway
Great progress in the understanding of ergosterol biosynthesis has been achieved in yeast and other fungi. This pathway is a complex process and involves the participation of many enzymes. The ergosterol biosynthesis pathway consumes a considerable amount of energy. In yeast, the biosynthesis of one molecule of ergosterol requires the consumption of at least 24 molecules of ATP and 16 molecules of NADPH. The ergosterol biosynthetic pathway in S. cerevisiae is shown in Fig. 1 and all the enzymes involved in this process are shown in Table 1.
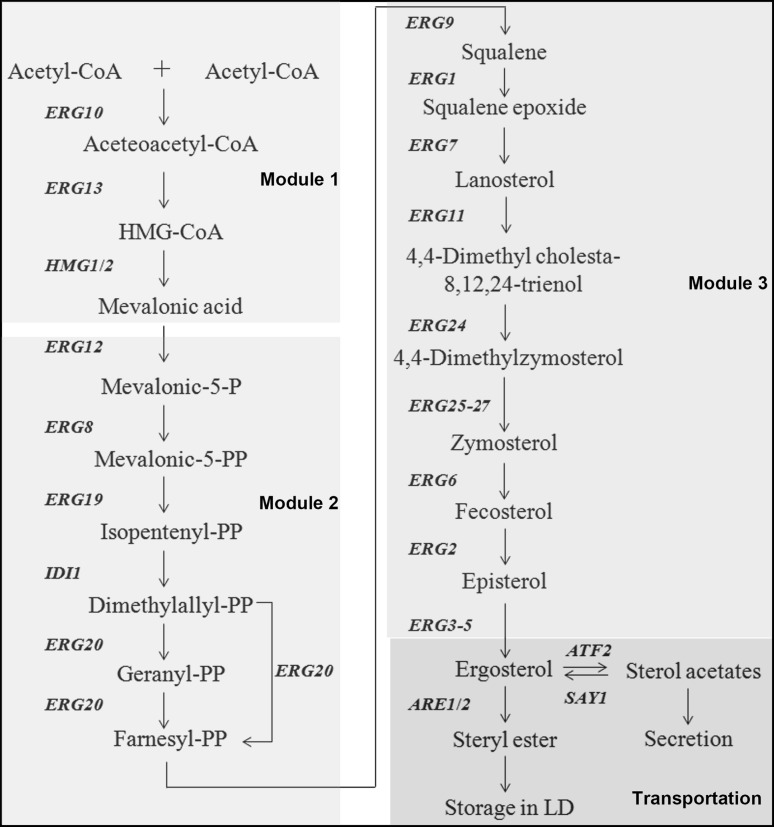
Fig. 1.
The biosynthesis pathway of ergosterol in S. cerevisiae. Synthesis intermediates, end products, and enzymes involved in ergosterol biosynthesis are indicated. Different colour indicates different modules. CoA Coenzyme A, HMG-CoA 3-hydroxy-3-methylglutaryl-CoA, P phosphate, LD lipid droplet. This figure was modified form Klug and Daum [27]
Table 1.
Genes involved in ergosterol biosynthesis in S. cerevisiae
| Gene | Gene ID | EC number | Function |
|---|---|---|---|
| Mevalonate biosynthesis | |||
| ERG10 | YPL028W | EC:2.3.1.9 | Acetoacetyl-CoA thiolase |
| ERG13 | YML126C | EC:2.3.3.10 | Hydroxymethylglutaryl-coenzyme A synthase |
| HMG1 | YML075C | EC:1.1.1.34 | Hydroxymethylglutaryl-coenzyme A reductase |
| HMG2 | YLR450W | EC:1.1.1.34 | |
| Farnesylpyrophosphate biosynthesis | |||
| ERG12 | YMR208W | EC:2.7.1.36 | Mevalonate kinase |
| ERG8 | YMR220W | EC:2.7.4.2 | Phosphomevalonate kinase |
| ERG19 | YNR043W | EC:4.1.1.33 | Diphosphomevalonate decarboxylase |
| IDI1 | YPL117C | EC:5.3.3.2 | Isopentenyl diphosphate isomerase |
| ERG20 | YJL167W | EC:2.5.1.10 | Polyprenyl synthetase |
| Ergosterol iosynthesis | |||
| ERG9 | YHR190W | EC:2.5.1.21 | Squalene synthetase |
| ERG1 | YGR175C | EC:1.14.13.132 | Squalene epoxidase |
| ERG7 | YHR072W | EC:5.4.99.7 | lanosterol cyclase/lanosterol synthase |
| ERG11 | YHR007C | EC:1.14.13.70 | Cytochrome P450 lanosterol 14a-demethylase |
| ERG24 | YNL280C | EC:1.3.1.70 | Sterol C-14 reductase |
| ERG25 | YGR060W | EC:1.14.13.72 | C-4 methyl sterol oxidase |
| ERG26 | YGL001C | EC:1.1.1.170 | Sterol C-4 decarboxylases |
| ERG27 | YLR100W | EC:1.1.1.270 | 3-Keto-steroid reductase |
| ERG6 | YML008C | EC:2.1.1.41 | C-24 sterol methyltransferase |
| ERG2 | YMR202W | EC:5.3.3.5 | C-8 sterol isomerase |
| ERG3 | YLR056W | EC:1.3.3.- | C-5 sterol desaturase |
| ERG5 | YMR015C | EC:1.14.-.- | C-22 sterol desaturase |
| ERG4 | YGL012W | EC:1.3.1.71 | C24 (28) sterol reductase |
According to the characteristics of the intermediate products, the biosynthesis pathway can be divided into three modules, which are mevalonate biosynthesis, farnesyl-PP biosynthesis and ergosterol biosynthesis. In the first module, there are three steps. Ergosterol biosynthesis starts with condensation of two acetyl-CoA molecules to produce acetoacetyl-CoA and this step is catalyzed by acetoacetyl-CoA thiolase (ERG10) and takes place in the vacuole. The condensation of a third acetyl-CoA to acetoacetyl-CoA is catalyzed by hydroxymethylglutaryl-CoA synthase (ERG13) to yield 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). HMG-CoA is further reduced to mevalonate by the HMG-CoA reductases (HMG1 and HMG2) and these two reactions take place in the mitochondria [26, 27]. The first module is conserved across all eukaryotes [28]. The second module involves the biosynthesis of farnesyl pyrophosphate from mevalonate. This process involves six reactions carried out in vacuoles and this process is catalyzed by ERG12, ERG8, ERG19, IDI and ERG20, successively [27]. ERG20 can catalyze 2 reactions shown in Fig. 1. All the functions of these enzymes are listed in Table 1. Farnesyl-PP is a very important intermediate metabolite in cells, which can be used for the synthesis of various substances in different metabolic pathways catalyzed by different enzymes. For example, farnesyl-PP is a common intermediate for the biosynthesis of sterols, benzoquinone and hemoglobin [28]. Inhibition of the biosynthesis of farnesyl-PP results in cells failing to synthesize many important metabolites, causing cell death [29]. The third module involves the steps from farnesyl-PP to ergosterol, which contains 15 steps and these reactions mainly occur in the endoplasmic reticulum (ER) [28]. The first is the conversion of farnesyl-PP to squalene, followed by the formation of lanosterol by squalene cyclization and after a series of reactions, lanosterol is transformed into ergosterol [27].
Compared with the first two modules, the third module is more complex and requires more enzymes. Based on whether the biosynthesis gene is required for yeast survival, the ergosterol biosynthesis genes are divided into essential and non-essential genes [30]. Some genes involved in the early steps of ergosterol biosynthesis, such as ERG9, ERG1, ERG7, ERG11, ERG24, ERG25, ERG26, ERG27 are essential genes, while others are regarded as non-essential genes. Of the essential genes, ERG9 encodes squalene synthase, which uses two farnesyl-PP molecules to form one molecule squalene, the first sterol-structured molecule and also the direct precursor of ergosterol biosynthesis. ERG1 and ERG7 encode squalene epoxidase and lanosterol synthase, respectively, which are two important, unique and essential enzymes in the ergosterol synthesis pathway. ERG11, also known as Cyp51, encodes a microsomal and membrane-bound protein, which functions as a lanosterol 14 alpha demethylase of the cytochrome P450 family. ERG24 encodes C-14 reductase localized on the ER and plasma membrane, which catalyzes the reduction of 4,4-dimerhylcholesta-8,14,24-trienol to 4,4-dimethylzymosterol. ERG25 (encoding C4 sterol methyl oxidase), ERG26 (encoding sterol C-4 decarboxylase), and ERG27 (encoding sterol C-3 keto reductase) are oxidoreductases that are localized to the ER, and are likely to form a demethylation complex to catalyze the final steps of zymosterol synthesis [30]. At present, mutants in these essential yeast genes have been isolated, but most of these mutants are temperature-sensitive and only grow in the medium supplied with exogenous ergosterol under aerobic conditions.
Although the non-essential genes are not essential for yeast survival, recent studies revealed that these genes, including ERG28, ERG2, ERG6 and ERG3-5, also regulate ergosterol biosynthesis and yeast growth and development. For example, mutation of ERG6, which encodes a C-24 methyltransferase that catalyzes the conversion of zymosterol to coprosterol, is not lethal, but results in a severely deficient growth and development phenotype, while overexpression of ERG6 increases ergosterol content [31]. The disruption of ERG28, which encodes a scaffold protein that mediates the formation of the ERG25/ERG26/ERG27 enzyme complex, also leads to the slow growth of yeast [32]. The last steps of ergosterol biosynthesis are catalyzed by ERG3/ERG4/ERG5. Probably because the previously biosynthesized intermediates can partially perform the functions of ergosterol, mutations of these genes do not affect yeast survival. However, they do show some interesting phenotypes. For example, erg3 mutant is not lethal, butthe strain cannot grow on media without a fermentable carbon source, while other some of erg3 mutants are sensitive to low temperature but insensitive to sterol synthesis inhibitors, suggesting that there may be another compensatory branch that can synthesize ergosterol [33]. While mutations of non-essential genes do not affect the survival of yeast, they do change the composition of the cell membrane, which also affects membrane potential, salt tolerance and drug resistance [34].
Regulation of Ergosterol Biosynthesis
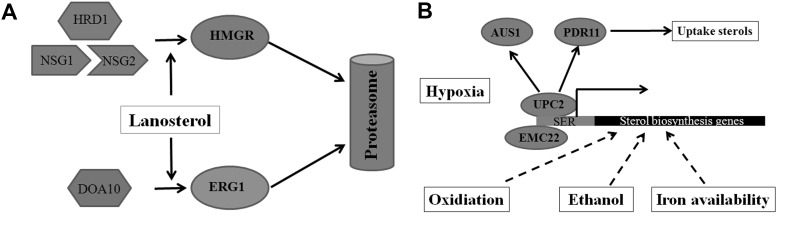
Ergosterol is an essential component of the fungal cell membrane and cellular levels of ergosterol directly affect various functions of the membrane. Ergosterol is the main sterol of fungi and it carries out a variety of important cellular functions. The implementation of each function occurs over a specific concentration range. Therefore, ergosterol content is strictly regulated so that it is maintained at an appropriate level in cells. The regulation of ergosterol content is mainly achieved by feedback regulation of ergosterol synthetase activities at the transcriptional, translational and posttranslational levels. The synthesis of HMG-CoA catalyzed by HMGR is a major metabolic check point for ergosterol biosynthesis [35]. In S. cerevisiae, excessive sterols can induce the degradation of HMG-CoA reductase (HMGR) through the proteasome degradation pathway, decreasing the synthesis of mevalonate and resulting in the down-regulation of sterol synthesis. The proteasome recognition process of HMGR is mediated by the ER-related degradation (ERAD) pathway, and the ERAD process activates HMGR degradation mainly through the recognition of specific sterols by HRD1 and the chaperone proteins NSG1 and NSG2 [36]. The activity of ubiquitin ligase DOAl0 in the ERAD process is regulated by lanosterol levels and is essential for the degradation of ERGl [37]. Thus, ERAD plays an important role in maintaining cellular sterol homeostasis. A simple diagram of ERAD regulation of sterol biosynthesis is shown in Fig. 2a.
Fig. 2.
A simple diagram of ERAD and environmental factors regulating ergosterol biosynthesis. a Excessive sterol (lanosterol and oxysterols) promotes the two ubiquitin ligases HRD1 and DOA10 to target HMGR and ERG1, respectively. The reorganization of HMGR by HRD1 requires the molecular chaperones NSG1 and NSG2. b The effects of environmental factors including oxidation, ethanol stimulation and iron availability on sterol biosynthesis. Under hypoxic conditions, transcription factors ECM22 and UPC2 bind to the sterol regulatory element (SRE), promoting expression of sterol biosynthesis genes; UPC2 can also induce AUS1 and PDR11 expression, promoting yeast to uptake sterols from the environment. Oxidation, ethanol stimulation and iron availability can also promote expression of sterol biosynthesis genes to increase ergosterol content. Dotted arrow indicates unknown mechanism
As the process of sterol biosynthesis requires oxygen, the biosynthesis of sterols is also affected by oxygen concentration. Under low oxygen conditions, cells cannot synthesize ergosterol and sterols are absorbed from the external culture environment. However, when oxygen is sufficient, cells only use ergosterol that they biosynthesize [38]. Recent studies have also found that under hypoxic conditions, the transcription factors ECM22 and UPC2 can bind to the promoter region (also known as sterol regulatory element, SRE) of the sterol biosynthesis genes to promote their expression [24]. Meanwhile, UPC2 can also induce the expression of ATP-binding transporter, AUS1 and PDR11, thereby promoting yeast to take up sterols from the environment [39]. Other environmental factors including oxidation, ethanol stimulation and iron availability also affect ergosterol biosynthesis. For example, ergosterol biosynthesis was disrupted when S. cerevisiae was grown in iron-deficient media, as squalene and lanosterol accumulated, and further studies showed that the expression levels of ERG1, ERG11, ERG3 and ERG25 were altered in iron-deficient media [40–42]. A simple diagram of environmental factors that regulate sterol biosynthesis is shown in Fig. 2b.
The various enzymes in the ergosterol biosynthesis pathway mutually cooperate to regulate ergosterol content. For example, when ERG27 is blocked, the accumulated intermediates are squalene, epoxy squalene and polyepoxyl squalene but not lanosterol, which is similar to that in erg7 mutant, indicating that there is an interesting relationship between ERG7 and ERG27 [35]. Further studies revealed that ERG27 can interact with ERG7 and promote an association between ERG7 and lipid particles to prevent ERG7 from being digested; and in lipid particles ERG7 activity is regulated by ERG27 [43]. Similarly, erg24mutant cannot grow in nutrient-rich media such as YEPD, but can grow in synthetic complete medium rich with Ca2+. However, when ERG24 and ERG4 are mutated simultaneously, the double mutant can grow neither in YEPD nor in synthetic complete medium rich with Ca2+, and similar phenomena also exist between ERG24 and three other genes ERG3, ERG5 and ERG6 [44]. Moreover, intracellular transportation of ergosterol can also regulate the expression of ergosterol synthetase (described below).
As ergosterol biosynthesis is both regulated by environmental factors and biosynthesis regulated genes, the engineering of metabolic pathways and optimization of culture conditions are the two main methods to increase ergosterol productivity [45]. For example, the overexpression of sterol biosynthesis genes (such as ERG1, ERG4, EGR9 and ERG11) or the sterol acyltransferase ARE2 (described below) can significantly increase ergosterol biosynthesis [46], and oxidative-fermentative growth combined with ethanol stimulation can also increase ergosterol productivity [45]. Thus, the regulation of ergosterol biosynthesis is a complex process regulated by multiple factors.
Intracellular Transportation of Ergosterol
There are a variety of regulatory mechanisms that regulate the homeostasis of intracellular sterols. However, under aerobic conditions, yeast cells can still synthesize excessive sterols. Yeast cells are unable to degrade sterol. Therefore, to maintain sterol homeostasis and eliminate the toxic effects of excessive sterols, sterols can be converted to steryl esters (SE) and stored in lipid droplets or used to form sterol acetates and secreted into the extracellular matrix [13]. The formation of SE is catalyzed by two enzymes, ARE1 and ARE2, which share about 50% sequence homology but have different substrate specificities. Both enzymes can use ergosterol as a substrate, but ARE1 prefers to use esterified sterol intermediates, such as lanosterol, as a substrate [46]. Normally, ARE2 is the major SE synthase, but under anaerobic conditions, its biosynthesis is blocked and esterification catalyzed by ARE1 is enhanced [47]. In an ARE1 ARE2 double mutant, the biosynthesis of SE is completely inhibited. However, the double mutant does not show any growth defects despite changes in the total sterol pattern. In the double mutant, overall sterol biosynthesis is decreased, but the level of free sterols is increased indicating that SE formation can also regulate sterol biosynthesis [45]. Indeed, further studies have shown the expression of ERG3 is down-regulated and ERG1 is destabilized, leading to a block in sterol synthesis in the double mutant [48]. It was also found that overexpression of sterol ERG4 and ARE2 can significantly increase ergosterol biosynthesis [45]. Acetylation of ergosterol is a reversible process. Acetylation is catalyzed by alcohol acetyltransferase (ATF2) and deacetylation is catalyzed by sterol deacetylase (SAY1). Both ATF2 and SAY1 are localized to the ER [49]. During the process of extracellular secretion, acetylated sterols need to be transported to the plasma membrane. The transportation process requires PRY (pathogen-related yeast) protein which can combine with acetylated sterols, making them soluble and facilitating their secretion [13].
Newly formed sterols are transported to different organelles. Usually, the transportation of newly synthesized sterols from the ER to the plasma membrane (PM) is performed by vesicle flux through the secretory pathway [50]. However, in yeast vesicle transport defective mutant SEC18, the transportation of ergosterol from ER to PM was normal, indicating that there are two sterol transportation pathways in the cell, including vesicular transport and non-vesicular transport [50], and both types of sterol transportation consume ATP [51]. As sterols are insoluble in aqueous solutions, the intracellular transport is performed by direct contact of the endomembrane system or in combination with transporters. In mammals, oxysterol-binding proteins (OSBP) can serve as transporters to make sterols transiently ‘‘soluble’ and move them to their target sites [52]. In yeast, seven proteins homologous to mammalian oxysterol-binding proteins have been identified, named OSH1–OSH7, and most of the OSH proteins are located at membrane contact sites [53]. When all the seven genes are deleted, yeast cells cannot survive and the intracellular ergosterol content is increased [54]. Recent studies have also found that OSH proteins are involved in the regulation of intracellular sphingolipid homeostasis. For example, when OSH4 was inactivated, the intracellular sphingolipid composition changed significantly, resulting in significant changes in cell membrane structure [55].
Another protein associated with sterol transportation and exogenous sterol uptake is ARV1. In mammals, ARV1 deletion results in increased steroid content in the ER and vesicular membrane [56]. An ARV1 homologue is also present in yeast and the sphingolipid composition can also be altered in arv1 mutant. Studies have also found that ARV1 can act as a protective factor against fat toxicity caused by changes in fat metabolism [57]. Studies in human cells have identified two steroid transporters, NPC and NPC1. Mutation of these transporters leads to cholesterol accumulation in lysosomes, resulting in degenerative C-type Niemann disease [58]. In yeast, the homologous proteins, NCR1 and NCR2 have also been identified. They can complement the corresponding phenotype of human cells; however, NCR1 and NCR2 knockout yeast cells do not display any growth defect or ergosterol distribution defect phenotypes [59–61].
Conclusion and Future Perspectives
Ergosterol is not only essential for the growth and reproduction of fungi, but also important for stress adaptation and can be used as a direct precursor for the production of steroidal drugs. Thus, it is important to study the ergosterol biosynthesis pathway. Studies on ergosterol biosynthesis in yeast and fungi have greatly improved our understanding of this pathway. This review has summarized current progress on the physiological functions, biosynthesis, biosynthesis regulation and intracellular transportation of ergosterol in S. cerevisiae. At present, yeast fermentation is the main method for ergosterol production. Changing metabolic flux of ergosterol by molecular biology is an effective way to enhance ergosterol yields by fungal fermentation [62]. Various strategies have been used to enhance the ergosterol content in yeast. These strategies include genetic manipulation of the ergosterol biosynthesis pathway, screening for high-ergosterol strains, or the optimization of fermentation conditions [45]. Besides ergosterol, intermediates of the ergosterol pathway, such as lanosterol and zymosterol, are also economically interesting sterol metabolites that can be used as emulsifiers for cosmetics and precursors for the production of cholesterol lowering substances [63]. Genetic modification of the ergosterol pathway can be used for the production of lanosterol and zymosterol. For example, overexpression of ERG1 and HMG1 resulted in accumulation of lanosterol and deletion of ERG2 and ERG6 resulted in the accumulation of zymosterol [64]. By studying the ergosterol biosynthesis pathway in S. cerevisiae, metabolic engineering has been used for steroids and terpenoid production. For example, overexpression of HMG1, ERG20, and UPC2 can be used to produce all terpene classes; replacement of ERG9 promoter with a repressive methionine promoter can be used to produce mono-, di- and sesquiterpenes. Site-directed mutagenesis of ergosterol biosynthesis or biosynthesis regulatory genes can also be used to produce these products [63, 64]. For example site-directed mutagenesis of UPC2 (G888A), ERG20 (K197G), and HMG2p (K6R) can be used to produce all classes of terpenes, monoterpenes, and mono-, di-, sesquiterpenes, respectively [63, 64]. Therefore, the study of ergosterol biosynthesis not only provides new ideas for enhancing ergosterol production, but can also be used for the production of other economically interesting steroids and terpenoid molecules.
Acknowledgements
This study was supported by National Natural Science Foundation of China (NSFC) (Grant Nos. 31171731 and 31460447), International S&T Cooperation Project of Jiangxi Provincial (Grant No. 20142BDH80003), General Science and Technology Project of Nanchang City (Grant No. 3000035402), “555 Talent Project” of Jiangxi Province, Science and Technology Research Project of Jiangxi Provincial Department of Education (Grant Nos. GJJ160765 and GJJ160794) and Natural Science Foundation of Jiangxi Province (20171BAB214004).
References
- 1.Wollam J, Antebi A. Sterol regulation of metabolism, homeostasis and development. Annu Rev Biochem. 2011;80:885–916. doi: 10.1146/annurev-biochem-081308-165917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarkowska D, Strnad M. Plant ecdysteroids: plant sterols with intriguing distributions, biological effects and relations to plant hormones. Planta. 2016;244:545–555. doi: 10.1007/s00425-016-2561-z. [DOI] [PubMed] [Google Scholar]
- 3.Prasad R, Shah AH, Rawal MK. Antifungals: mechanism of action and drug resistance. Adv Exp Med Biol. 2016;892:327–349. doi: 10.1007/978-3-319-25304-6_14. [DOI] [PubMed] [Google Scholar]
- 4.Beni A, Soki E, Lajtha K, Fekete I. An optimized HPLC method for soil fungal biomass determination and its application to a detritus manipulation study. J Microbiol Methods. 2014;103:124–130. doi: 10.1016/j.mimet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Matteo F, Massimo F, Massimo M, Carmela C. The red seaweed Gracilaria gracilisas a multi products source. Mar Drugs. 2013;11:3754. doi: 10.3390/md11103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang G, Cai W, Xu B. Vitamin D2, ergosterol, and vitamin B2 content in commercially dried mushrooms marketed in China and increased vitamin D2 content following UV-C irradiation. Int J Vitam Nutr Res. 2016 doi: 10.1024/0300-9831/a000294. [DOI] [PubMed] [Google Scholar]
- 7.Karpova NV, Andryushina VA, Stytsenko TS, Druzhinina AV, Feofanova TD, Kurakov AV. A search for microscopic fungi with directed hydroxylase activity for the synthesis of steroid drugs. Appl Biochem Microbiol. 2016;52:316–323. doi: 10.1134/S000368381603008X. [DOI] [PubMed] [Google Scholar]
- 8.Kobori M, Yoshida M, Ohnishi-Kameyama M, Shinmoto H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br J Pharmacol. 2007;150:209–219. doi: 10.1038/sj.bjp.0706972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitchawalit S, Kanokmedhakul K, Kanokmedhakul S, Soytong K. A new benzyl ester and ergosterol derivatives from the fungus Gymnoascus reessii. Nat Prod Res. 2014;28:1045–1051. doi: 10.1080/14786419.2014.903478. [DOI] [PubMed] [Google Scholar]
- 10.Dupont S, Lemetais G, Ferreira T, Cayot P, Gervais P, Beney L. Ergosterol biosynthesis: a fungal pathway for life on land? Evolution. 2012;66:2961–2968. doi: 10.1111/j.1558-5646.2012.01667.x. [DOI] [PubMed] [Google Scholar]
- 11.Krumpe K, Frumkin I, Herzig Y, Rimon N, Özbalci C, Brügger B, Rapaport D, Schuldiner M. Ergosterol content specifies targeting of tail-anchored proteins to mitochondrial outer membranes. Mol Biol Cell. 2012;23:3927–3935. doi: 10.1091/mbc.E11-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YQ, Gamarra S, Garciaeffron G, Park S, Perlin DS, Rao R. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog. 2010;6:e1000939. doi: 10.1371/journal.ppat.1000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhary V, Schneiter R. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proc Natl Acad Sci USA. 2012;109:16882–16887. doi: 10.1073/pnas.1209086109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik P, Chaudhry N, Kitawat SB, Kumar R, Mukherjee T. Relationship of azole resistance with the structural alteration of the target sites: novel synthetic compounds for better antifungal activities. Nat Prod J. 2014;4:131–139. [Google Scholar]
- 15.Aguilera F, Peinado RA, Millán C, Ortega JM, Mauricio JC. Relationship between ethanol tolerance, H+-ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int J Food Microbiol. 2006;110:34–42. doi: 10.1016/j.ijfoodmicro.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Iefuji H, Fujii T, Soga H, Satoh K. Cloning and characterization of a gene complementing the mutation of an ethanol-sensitive mutant of sake yeast. Biosci Biotechnol Biochem. 2000;64:229–236. doi: 10.1271/bbb.64.229. [DOI] [PubMed] [Google Scholar]
- 17.Henderson CM, Block DE. Examining the role of membrane lipid composition in determining the ethanol tolerance of saccharomyces cerevisiae. Appl Environ Microbiol. 2014;80:2966. doi: 10.1128/AEM.04151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamthan A, Kamthan M, Datta A. Expression of C-5 sterol desaturase from an edible mushroom in fisson yeast enhances its ethanol and thermotolerance. PLoS ONE. 2017;12:e0173381. doi: 10.1371/journal.pone.0173381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodedová M, Sychrová H. Changes in the sterol composition of the plasma membrane affect membrane potential, salt tolerance and the activity of multidrug resistance pumps in Saccharomyces cerevisiae. PLoS ONE. 2015;10:e0139306. doi: 10.1371/journal.pone.0139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montañés FM, Pascual-Ahuir A, Proft M. Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors. Mol Microbiol. 2011;79:1008–1023. doi: 10.1111/j.1365-2958.2010.07502.x. [DOI] [PubMed] [Google Scholar]
- 21.Marisco G, Saito ST, Ganda IS, Brendel M, Pungartnik C. Low ergosterol content in yeast adh 1 mutant enhances chitin maldistribution and sensitivity to paraquat-induced oxidative stress. Yeast. 2011;28:363–373. doi: 10.1002/yea.1844. [DOI] [PubMed] [Google Scholar]
- 22.Landolfo S, Zara G, Zara S, Budroni M, Ciani M, Mannazzu I. Oleic acid and ergosterol supplementation mitigates oxidative stress in wine strains of Saccharomyces cerevisiae. Int J Food Microbiol. 2010;141:229–235. doi: 10.1016/j.ijfoodmicro.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Zhu Y, Du G, Zhou J, Chen J. Exogenous ergosterol protects Saccharomyces cerevisiae from d -limonene stress. J Appl Microbiol. 2013;114:482–491. doi: 10.1111/jam.12046. [DOI] [PubMed] [Google Scholar]
- 24.Davies BS, Rine J. A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics. 2006;174:191–201. doi: 10.1534/genetics.106.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishimoto T, Yamamoto T, Tanaka K. Defects in structural integrity of ergosterol and the cdc50p-drs2p putative phospholipid translocase cause accumulation of endocytic membranes, onto which actin patches are assembled in yeast. Mol Biol Cell. 2005;16:5592–5609. doi: 10.1091/mbc.E05-05-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505:131–143. doi: 10.1016/j.abb.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klug L, Daum G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014;14:369–388. doi: 10.1111/1567-1364.12141. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa H, Sobue F, Motoyama K, et al. Identification of enzymes involved in the mevalonate pathway of Flavobacterium johnsoniae. Biochem Biophys Res Commun. 2017;487:702–708. doi: 10.1016/j.bbrc.2017.04.120. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Raboune S, Walker JM, Bradshaw HB. Distribution of endogenous farnesyl pyrophosphate and four species of lysophosphatidic acid in rodent brain. Int J Mol Sci. 2010;11:3965–3976. doi: 10.3390/ijms11103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristan K, Rizner TL. Steroid-transforming enzymes in fungi. J Steroid Biochem Mol Biol. 2012;129:79–91. doi: 10.1016/j.jsbmb.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Konecna A, Toth Hervay N, Valachovic M, Gbelska Y. ERG6 gene deletion modifies Kluyveromyces lactis susceptibility to various growth inhibitors. Yeast. 2016;33:621–632. doi: 10.1002/yea.3212. [DOI] [PubMed] [Google Scholar]
- 32.Mo C, Bard M. Erg28p is a key protein in the yeast sterol biosynthetic enzyme complex. J Lipid Res. 2005;46:1991–1998. doi: 10.1194/jlr.M500153-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Clay L, Caudron F, Denothlippuner A, Boettcher B, Frei SB, Snapp EL, Barral Y. A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. Elife. 2014;3:e01883. doi: 10.7554/eLife.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kodedova M, Sychrova H. Changes in the sterol composition of the plasma membrane affect membrane potential, salt tolerance and the activity of multidrug resistance pumps in Saccharomyces cerevisiae. PLoS ONE. 2015;10:e0139306. doi: 10.1371/journal.pone.0139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet. 2007;41:401–427. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- 36.Burg JS, Espenshade PJ. Regulation of HMG-CoA reductase in mammals and yeast. Prog Lipid Res. 2011;50:403–410. doi: 10.1016/j.plipres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foresti O, Ruggiano A, Hannibalbach HK, Ejsing CS, Carvalho P. Sterol homeostasis requires regulated degradation of squalene monooxygenase by the ubiquitin ligase Doa10/Teb4. Elife. 2012;2:1600–1613. doi: 10.7554/eLife.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhingra S, Cramer RA. Regulation of sterol biosynthesis in the human fungal pathogen Aspergillus fumigatus: opportunities for therapeutic development. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zavrel M, Hoot SJ, White TC. Comparison of sterol import under aerobic and anaerobic conditions in three fungal species, Candida albicans, Candida glabrata, and Saccharomyces cerevisiae. Eukaryot Cell. 2013;12:725–738. doi: 10.1128/EC.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shakouryelizeh M, Protchenko O, Berger A, Cox J, Gable K, Dunn TM, Prinz WA, Bard M, Philpott CC. Metabolic response to iron deficiency in Saccharomyces cerevisiae. J Biol Chem. 2010;285:14823–14833. doi: 10.1074/jbc.M109.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puig S, Askeland E, Thiele JD. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell. 2005;120:99. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 42.Craven RJ, Mallory JC, Hand RA. Regulation of iron homeostasis mediated by the heme-binding protein Dap1 (damage resistance protein 1) via the P450 protein Erg11/Cyp51. J Biol Chem. 2007;282:36543–36551. doi: 10.1074/jbc.M706770200. [DOI] [PubMed] [Google Scholar]
- 43.Layer JV, Barnes BM, Yamasaki Y, Barbuch R, Li L, Taramino S, Balliano G, Bard M. Characterization of a mutation that results in independence of oxidosqualene cyclase (Erg7) activity from the downstream 3-ketoreductase (Erg27) in the yeast ergosterol biosynthetic pathway. Biochim Biophys Acta. 2013;1831:361–369. doi: 10.1016/j.bbalip.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Luna-Tapia A, Peters BM, Eberle KE, Kerns ME, Foster TP, Marrero L, Noverr MC, Fidel PL, Jr, Palmer GE. ERG2 and ERG24 are required for normal vacuolar physiology as well as Candida albicans pathogenicity in a murine model of disseminated but not vaginal candidiasis. Eukaryot Cell. 2015;14:1006–1016. doi: 10.1128/EC.00116-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Náhlík J, Hrnčiřík P, Mareš J, Rychtera M, Kent CA. Towards the design of an optimal strategy for the production of ergosterol from Saccharomyces cerevisiae yeasts. Biotechnol Progr. 2017 doi: 10.1002/btpr.2436. [DOI] [PubMed] [Google Scholar]
- 46.Ploier B, Korber M, Schmidt C, Koch B, Leitner E, Daum G. Regulatory link between steryl ester formation and hydrolysis in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2015;1851:977–986. doi: 10.1016/j.bbalip.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Grillitsch K, Connerth M, Köfeler H, Arrey TN, Rietschel B, Wagner B, Karas M, Daum G. Lipid particles/droplets of the yeast Saccharomyces cerevisiae revisited: lipidome meets proteome. Biochim Biophys Acta. 2011;1811:1165–1176. doi: 10.1016/j.bbalip.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorger D, Athenstaedt K, Hrastnik C, Daum G. A yeast strain lacking lipid particles bears a defect in ergosterol formation. J Biol Chem. 2004;279:31190–31196. doi: 10.1074/jbc.M403251200. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari R, Köffel R, Schneiter R. An acetylation/deacetylation cycle controls the export of sterols and steroids from S. cerevisiae. EMBO J. 2007;26:5109–5119. doi: 10.1038/sj.emboj.7601924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan DP, Ohvorekilä H, Baumann NA, Beh CT, Menon AK. Sterol trafficking between the endoplasmic reticulum and plasma membrane in yeast. Biochem Soc Trans. 2006;34:356–358. doi: 10.1042/BST0340356. [DOI] [PubMed] [Google Scholar]
- 51.Jacquier N, Schneiter R. Mechanisms of sterol uptake and transport in yeast. J Steroid Biochem Mol Biol. 2012;129:70–78. doi: 10.1016/j.jsbmb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Perry RJ, Ridgway ND. Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol Biol Cell. 2006;17:2604–2616. doi: 10.1091/mbc.E06-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. osh proteins regulate phosphoinositide metabolism at er-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 54.Schulz TA, Prinz WA. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta. 2007;1771:769–780. doi: 10.1016/j.bbalip.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leblanc MA, Fairn GD, Russo SB, Czyz O, Zaremberg V, Cowart LA, Mcmaster CR. The yeast oxysterol binding protein Kes1 maintains sphingolipid levels. PLoS ONE. 2013;8:e60485. doi: 10.1371/journal.pone.0060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tong F, Billheimer J, Shechtman CF, Liu Y, Crooke R, Graham M, Cohen DE, Sturley SL, Rader DJ. Decreased expression of ARV1 results in cholesterol retention in the endoplasmic reticulum and abnormal bile acid metabolism. J Biol Chem. 2010;285:33632–33641. doi: 10.1074/jbc.M110.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruggles KV, Garbarino J, Liu Y, Moon J, Schneider K, Henneberry A, Billheimer J, Millar JS, Marchadier D, Valasek MA. A functional, genome-wide evaluation of liposensitive yeast identifies the “ARE2 required for viability” (ARV1) gene product as a major component of eukaryotic fatty acid resistance. J Biol Chem. 2014;289:4417–4431. doi: 10.1074/jbc.M113.515197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munkacsi AB, Porto AF, Sturley SL. Niemann–Pick type C disease proteins: orphan transporters or membrane rheostats? Future Lipidol. 2007;2:357–367. doi: 10.2217/17460875.2.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malathi K, Higaki K, Tinkelenberg AH, Balderes DA, Almanzar-Paramio D, Wilcox LJ, Erdeniz N, Redican F, Padamsee M, Liu Y. Mutagenesis of the putative sterol-sensing domain of yeast Niemann Pick C-related protein reveals a primordial role in subcellular sphingolipid distribution. J Cell Biol. 2004;164:547–556. doi: 10.1083/jcb.200310046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang S, Ren J, Li H, Zhang Q, Armstrong JS, Munn AL, Yang H. Ncr1p, the yeast ortholog of mammalian Niemann Pick C1 protein, is dispensable for endocytic transport. Traffic. 2004;5:1017–1030. doi: 10.1111/j.1600-0854.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 61.Berger AC, Vanderford TH, Gernert KM, Nichols JW, Faundez V, Corbett AH. Saccharomyces cerevisiae Npc2p is a functionally conserved homologue of the human Niemann-Pick disease type C 2 protein, hNPC2. Eukaryot Cell. 2005;4:1851–1862. doi: 10.1128/EC.4.11.1851-1862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Ke, Tong Mengmeng, Gao Kehui, Di Yanan, Wang Pinmei, Zhang Chunfang, Wu Xuechang, Zheng Daoqiong. Genomic reconstruction to improve bioethanol and ergosterol production of industrial yeast Saccharomyces cerevisiae. J Ind Microbiol Biot. 2015;42:207–218. doi: 10.1007/s10295-014-1556-7. [DOI] [PubMed] [Google Scholar]
- 63.Wriessnegger T, Pichler H. Yeast metabolic engineering–targeting sterol metabolism and terpenoid formation. Prog Lipid Res. 2013;52:277–293. doi: 10.1016/j.plipres.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Paramasivan K, Mutturi S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit Rev Biotechnol. 2017 doi: 10.1080/07388551.2017.1299679. [DOI] [PubMed] [Google Scholar]