Abstract
Mandibular condylar cartilage (MCC) exhibits dual roles both articular cartilage and growth center. Of many growth factors, TGF-β has been implicated in the growth of articular cartilage including MCC. Recently, Asporin, decoy to TGF-β, was discovered and it blocks TGF-β signaling. Asporin is expressed in a variety of tissues including osteoarthritic articular cartilage, though there was no report of Asporin expression in MCC. In the present study, we investigated the temporal and spatial expression of Asporin in MCC.
Gene expression profile of MCC and epiphyseal cartilage in tibia of 5 weeks old ICR mice were firstly compared with microarray analysis using the laser capture microdissected samples. Variance of gene expression was further confirmed by real-time RT-PCR and immunohistochemical staining at 1,3,10, and 20 weeks old. TGF-β and its signaling molecule, phosphorylated Smad-2/3 (p-Smad2/3), were also examined by immunohistochemical staining.
Microarray analysis revealed that Asporin was highly expressed in MCC. Real-time RT-PCR analysis confirmed that the fibrous layer of MCC exhibited stable higher Asporin expression at any time points as compared to epiphyseal cartilage. This was also observed in immunohistochemical staining. Deeper layer in MCC augmented Asporin expression with age. Whereas, TGF-β was stably highly observed in the layer. The fibrous layer of MCC exhibited weak staining of p-Smad2/3, though the proliferating layer of MCC was strongly stained as compared to epiphyseal cartilage of tibia at early time point. Consistent with the increase of Asporin expression in the deeper layer of MCC, the intensity of p-Smad-2/3 staining was decreased with age.
In conclusion, we discovered that Asporin was stably expressed at the fibrous layer of MCC, which makes it possible to manage both articular cartilage and growth center at the same time.
Abbreviations: MCC, mandibular condylar cartilage; MF, mandibular fibrous layer; MP, mandibular proliferating layer; MH, mandibular hypertrophic layer; TR, tibial reserve layer; TP, tibial proliferating layer; TH, tibial hypertrophic layer
Keywords: Mandibular condylar cartilage, Growth plate, Asporin, TGF-β, Endochondral growth
Highlights
-
•
Asporin gene and protein were highly expressed in mandibular condylar cartilage as compared to tibial epiphyseal cartilage.
-
•
Asporin in mandibular condylar cartilage was augmented with age.
-
•
TGF-β signaling is suppressed by augmented Asporin and decreased TGF-β production in mandibular condylar cartilage.
1. Introduction
During growth period, shaft bones such as limb have two cartilage tissue, articular cartilage and epiphyseal cartilage (Vasan and Lash, 1978). Both cartilage has distinct function, articular cartilage exhibits ablation resistance and epiphyseal cartilage plays a role as a growth center (Hunziker et al., 2007, Hellstadius, 1950). However, mandibular condylar cartilage (MCC) plays dual roles, both articular cartilage and growth center (Copray et al., 1988).
Cartilage formation in MCC and subsequent osteogenesis are regulated by many soluble factors such as IGF-1 (Masoud et al., 2012), TGF-β (Iwasaki et al., 1993), BMP (Chen et al., 1991), Ihh (Chung et al., 2001), and Shh (Murtaugh et al., 1999). TGF-β, one of the most abundant cytokines in the bone matrix (Bismar et al., 1999), has been proven to play a central role in bone remodeling by regulating migration, proliferation, and differentiation of osteoprogenitors (Jian et al., 2006, Tang et al., 2009, Janssens et al., 2005). In addition, TGF-β has been associated with the development and maintenance of articular cartilage (Moroco et al., 1997), and may regulate condylar cartilage proliferation, differentiation (Li et al., 1998, Hinton et al., 2015), and development (Oka et al., 2007).
Asporin, a novel member of the leucine-rich repeat family of proteins (Yamada et al., 2001), is expressed in a variety of mouse (Henry et al., 2001) and human tissues including osteoarthritic articular cartilage with higher levels (Lorenzo et al., 2001). A significant association between a polymorphism in the aspartic acid repeat of the gene encoding Asporin and osteoarthritis was reported (Kizawa et al., 2005). In this report, a direct interaction between Asporin and TGF-β, in other word, Asporin neutralizes the function of TGF-β, was also reported. Furthermore, Asporin blocks chondrogenesis and inhibits TGF-β-induced expression of chondrocyte phenotypes (Nakajima et al., 2007, Kou et al., 2010).
To date, there is no report about the expression of Asporin in MCC nor the relation to endochondral growth of mandible. In the present study, we investigated the temporal and spatial expression of Asporin in MCC to clarify the role of Asporin in the regulation of endochondral growth of MCC using mice.
2. Materials and methods
2.1. Animals
Male ICR mice (1, 3, 5, 10 and 20-week(s)-old; (n = 4): CLEA Japan, Inc. Tokyo Japan) were used for this study. All procedures were approved by Animal Research Committee of Tsurumi University (approved numbers: 24P065, 25P013, 26P074, and 27P070). All animal research is reported in accordance with ARRIVE guidelines.
2.2. Tissue preparation
Mice were sacrificed by cervical dislocation. Mandible and tibia were immediately excised, and the specimen were embedded in OCT compound (Sakura Fineteck Japan, Tokyo, Japan) and rapidly frozen in isopentane cooled by liquid nitrogen. Undecalcified nonfixed serial frozen sections (5 μm-thick) were prepared using a super-hard tungsten steel knife (Meiwa Shoji Ltd., Tokyo, Japan) in − 25 °C cryostat (Leica Microsystems, Wetzlar, Germany) (Nakamura et al., 2007). The sections were collected individually on a 1.35 μm thick polyethylene naphthalene membrane with an adhesive (SECTION-LAB Ltd., Hiroshima, Japan) (Kawamoto and Shimizu, 1986) and stuck onto slides. Then the sections were slightly fixed with nuclease-free 95% and 75% ethanol for 30–40 s. each, and 50% for 25–30 s. Next, they were stained with Cresyl Violet which provided as LCM Staining Kit (Ambion, Austin, TX) for 40 s., and they were washed through nuclease-free 50% and 75% ethanol for 25–30 s. each, and 95% and 100% for 30–40 s. each.
2.3. Laser capture microdissection
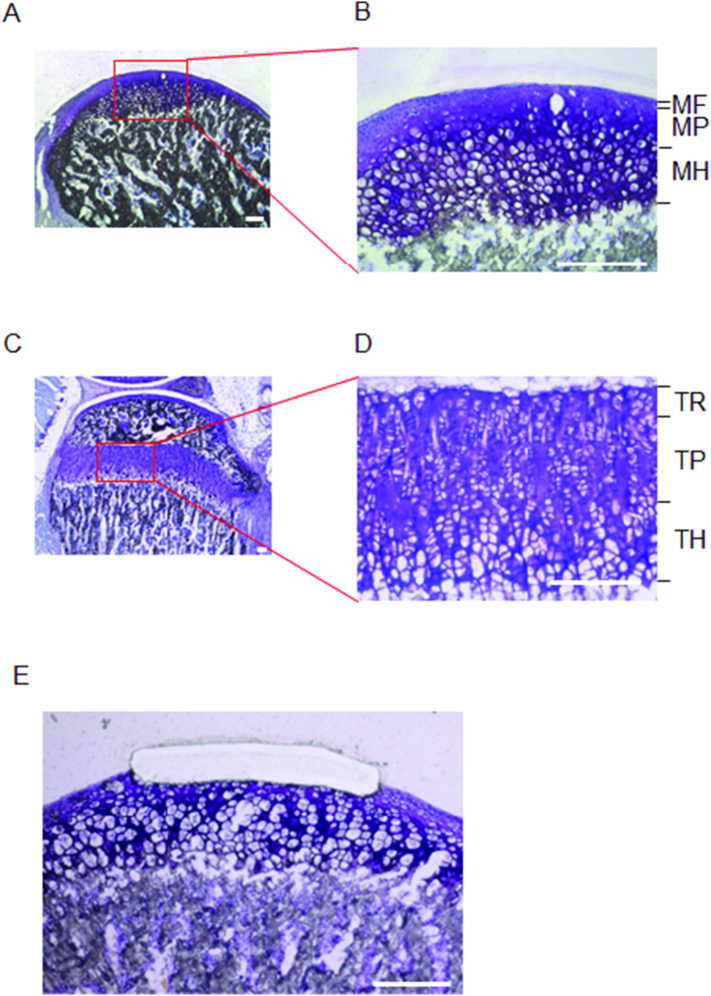
Each layers of MCC, tibial epiphyseal cartilage were microdissected and collected using laser capture microdissection (P.A.L.M. Microlaser Technologies, Bernried, Germany). Mandibular condylar cartilage and tibial growth plate were divided into three layers; mandibular fibrous layer (MF), mandibular proliferating layer (MP), mandibular hypertrophic layer (MH), tibial reserve layer (TR), tibial proliferating layer (TP), and tibial hypertrophic layer (TH), respectively (Fig. 1).
Fig. 1.
Laser capture microdissection of cartilage tissue.
Representative images of mandibular condyle of 5w ICR mouse (n = 4) in lower magnification (A) and higher magnification (B). MF: mandibular fibrous layer. MP: mandibular proliferating layer. MH: mandibular hypertrophic layer. Representative images of tibial mesial condyle of 5w ICR mouse (n = 4) in lower magnification (C) and higher magnification (D). TR: tibial reserve layer. TP: tibial proliferating layer. TH: tibial hypertrophic layer. Representative image of after microdissection at MF (E). Bar: 100 μm.
Pooled microdissected cartilage tissues (5 sections, approximately 140 μm2) were used for RNA extraction.
2.4. Extraction of total RNA
Total RNAs were extracted using RNeasy Micro Kit (QIAGEN, Hilden, Germany) with complete removal of residual genomic DNA through RNase-free DNase I (QIAGEN, Hilden, Germany) treatment according to the manufacture's protocol.
2.5. Microarray analysis
RNA samples of MF and TR from 5-weeks-old mice were collected, and were analyzed by Whole Mouse Genome Microarray 4x44K (Agilent Technologies, Santa Clara, CA). Whole gene expression levels in CF were relatively compared to that in TR, and sorted with expression ratio.
2.6. Reverse transcription and Real-time RT PCR
cDNA was synthesized using iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories, CA) and was diluted (× 2) with Tris-EDTA buffer. Real-time RT-PCR was performed with SsoFast EvaGreen Supermix (Bio-Rad Laboratories) with the following oligonucleotide primers: Asporin (accession number: NM_025711), Forward: GGGAGTGAATGACTTCTGTC, Reverse: ACTCATTCTGCCAAGAACAC; TGF-β1 (accession number: NM_011577), Forward: CTCCCGTGGCTTCTAGTGC, Reverse: GCCTTAGTTTGGACAGGATCTG; GAPDH (accession number: NM_008085), forward: ACTTTGTCAAGCTCATTTCC, reverse: GTGAGGGGAGGAGTCTCAA. Fold changes of gene of interest were calculated with ΔΔCt method using GAPDH as reference gene.
2.7. Immunohistochemistry
Briefly, undecalcified nonfixed serial frozen sections were fixed in 100% ethanol for 1 min, incubated in 3% H2O2 to quench endogenous peroxidase activity for 30 min, and then blocked with normal goat serum for 1 h at room temperature. The sections were then incubated with primary antibody diluted 1:500 with 1.5% goat serum in PBS overnight at 4 °C. The primary antibodies we used were as follows; anti Asporin antibody (Acris, San Diego, CA), anti p-Smad2/3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and anti TGF-β antibody (Cusabio, Collage Park, MD). After wash, biotinylated secondary antibody followed by avidin-horseradish peroxidase reactions (goat ABC staining system, Santa Cruz Biotechnology, CA) was performed. Color development was obtained using diaminobenzidine (DAB) for 2 min, and they were observed by a microscope (BZ9000; KEYENCE, Osaka, Japan).
2.8. Immuno-fluorescent staining
Undecalcified nonfixed serial frozen sections were fixed in 100% ethanol for 1 min and blocked with Block ACE (DS farma biomedical, Saitama, Japan) for 10 min at room temperature. The sections were then incubated with primary antibody diluted 1:500 with Antibody Diluent Solution (Invitrogen, Carlsbad, CA) for 2 h at 4 °C. The primary antibodies we used were as follows; rabbit IgG anti Asporin antibody (Acris) and mouse IgG anti TGF-β antibody (R&D SYSTEMS, Minneapolis, Minnesota). After wash, the sections were then incubated with secondary antibody diluted 1:3000 with Antibody Diluent Solution for 2 h at 4 °C. The secondary antibodies we used were as follows; Alexa 568-conjugated anti rabbit IgG antibody (Thermo Fisher Scientific, Kanagawa, Japan) for anti Asporin antibody and DyLight 488-conjugated anti mouse IgG antibody (Merck, Darmstadt, Germany) for anti TGF-β antibody.
2.9. Statistical analysis
The groups were compared using non-paired Student t-test. The multigroup of each layers and time points was compared using Tukey tests. p < 0.05 was considered statistically significant.
3. Results
3.1. Gene expression analysis in MCC
First of all, we compared gene expression between MF layer in MCC and TR in tibia at 5-weeks old. Table 1 shows the top 30 gene probes which exhibited high expression ratio in MF as compared to those of TR in tibia. Dual oxidase maturation factor 1 (NM_145395) was the highest induced gene in MF layer of MCC, followed by LIM homeobox protein 8 (NM_010713), Goosecoid (NM_010351), Semaphorin 3E (NM_011348), and Angiopoietin-like 1 (NM_028333). Of interest, osteoarthritis-related gene, Asporin, was 293-fold expression in MF layer of MCC. TGF-βs, BMPs, Ihh, and Shh exhibited almost stable gene expression within 3-fold change (data not shown).
Table 1.
Upregulated gene in MF as compared to TR by microarray.
| Gene name | Description | Accession no. | Expression levels |
Fold change | ||
|---|---|---|---|---|---|---|
| MF | TR | |||||
| 1 | Duoxa1 | Dual oxidase maturation factor 1 | NM_145395 | 21,106.9 | 4.8 | 4366.8 |
| 2 | Lhx8 | LIM homeobox protein 8 | NM_010713 | 14,481.8 | 4.6 | 3154.6 |
| 3 | Gsc | Goosecoid | NM_010351 | 13,625.4 | 5.3 | 2577.3 |
| 4 | Sema3e | Sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3E | NM_011348 | 6667.0 | 4.5 | 1466.3 |
| 5 | Angptl1 | Angiopoietin-like 1 | NM_028333 | 6751.5 | 4.8 | 1410.4 |
| 6 | Dlx2 | Distal-less homeobox 2 | NM_010054 | 5038.2 | 5.5 | 924.2 |
| 7 | Creb5 | CAMP responsive element binding protein 5 | NM_172728 | 5445.4 | 6.1 | 891.3 |
| 8 | 5830417I10 Rik | RIKEN cDNA 5830417I10 gene | AY040842 | 4044.1 | 4.8 | 845.3 |
| 9 | Duox1 | Dual oxidase 1 | XM_130483 | 10,431.4 | 18.2 | 573.4 |
| 10 | Wnt10a | Wingless related MMTV integration site 10a | NM_009518 | 2475.8 | 4.7 | 525.2 |
| 11 | Duox1 | Dual oxidase 1 | XM_130483 | 10,235.4 | 20.4 | 500.5 |
| 12 | Duox1 | Dual oxidase 1 | XM_130483 | 10,436.6 | 21.9 | 476.0 |
| 13 | Duox1 | Dual oxidase 1 | XM_130483 | 9701.3 | 20.9 | 464.0 |
| 14 | Duox1 | Dual oxidase 1 | XM_130483 | 10,264.1 | 22.8 | 450.7 |
| 15 | Duox1 | Dual oxidase 1 | XM_130483 | 10,239.3 | 22.9 | 446.4 |
| 16 | Duox1 | Dual oxidase 1 | XM_130483 | 10,185.3 | 22.8 | 446.2 |
| 17 | Kera | Keratocan | NM_008438 | 2166.2 | 4.9 | 440.1 |
| 18 | Ptges | Prostaglandin E synthase | NM_022415 | 11,840.5 | 27.5 | 430.7 |
| 19 | Duox1 | Dual oxidase 1 | XM_130483 | 10,194.0 | 25.7 | 396.2 |
| 20 | Duox1 | Dual oxidase 1 | XM_130483 | 9779.7 | 24.8 | 394.8 |
| 21 | Masp1 | Mannan-binding lectin serine peptidase 1 | AK031598 | 2172.5 | 5.5 | 393.6 |
| 22 | Casp3 | Caspase 3 | NM_009810 | 9965.0 | 26.7 | 373.0 |
| 23 | 5830417I10 Rik | RIKEN cDNA 5830417I10 gene | XM_001000961 | 1684.5 | 5.0 | 338.1 |
| 24 | Nr2f2 | Nuclear receptor subfamily 2, group F, member 2 | × 76653 | 1754.5 | 5.3 | 328.5 |
| 25 | Tnn | Tenascin N | NM_177839 | 27,913.0 | 85.9 | 325.0 |
| 26 | 9530026P05 Rik | RIKEN cDNA 9530026P05 gene | AK020576 | 1461.8 | 4.9 | 295.5 |
| 27 | Aspn | Asporin | NM_025711 | 1624.3 | 5.5 | 293.1 |
| 28 | D430019H16 Rik | RIKEN cDNA D430019H16 gene | BC058677 | 1314.3 | 4.8 | 275.9 |
| 29 | Dpt | Dermatopontin | NM_019759 | 1279.0 | 4.6 | 275.9 |
| 30 | Efhb | EF hand domain family, member B | NM_172497 | 1336.5 | 4.9 | 272.1 |
3.2. Asporin mRNA is extensively expressed in MCC as compared to tibial growth cartilage
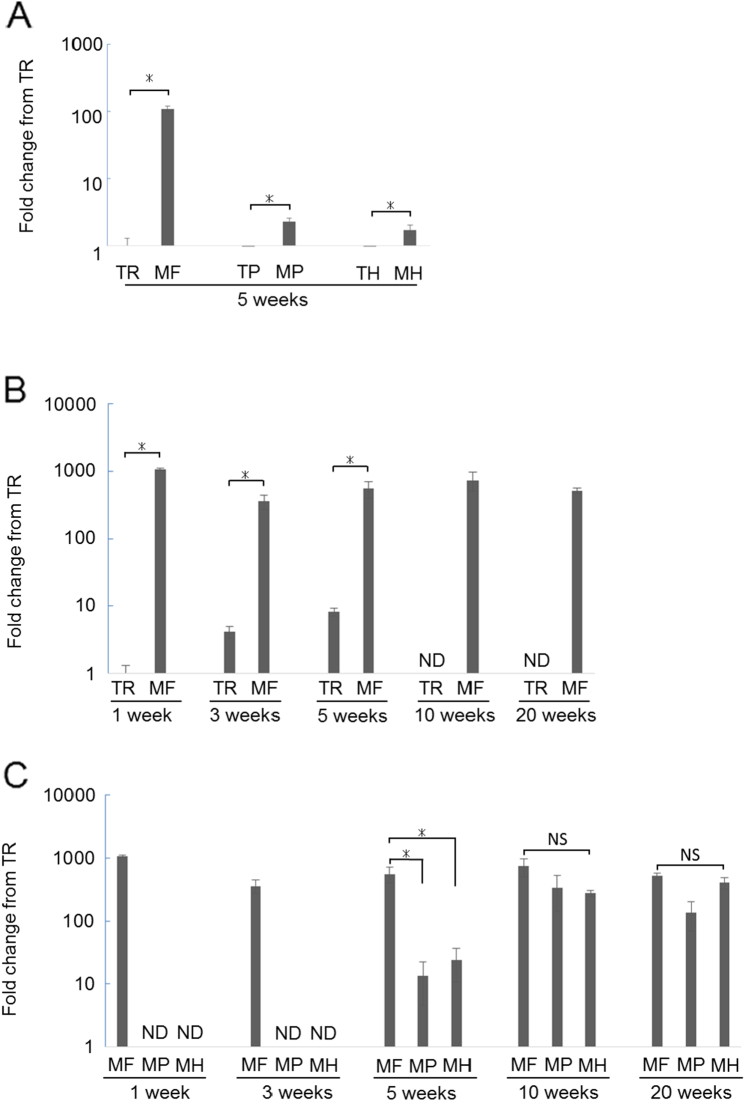
To confirm the upregulated Asporin expression in MCC obtained by microarray analysis, we performed real-time RT-PCR. Consisted with microarray analysis, surface layer of MCC (MF) exhibited robust Asporin expression as compared to the surface layer of tibia (TR) at 5 weeks (Fig. 2A). Not only surface layer of MCC, but also another layers of MCC expressed much higher Asporin gene expression as compared to tibia.
Fig. 2.
Real-time RT-PCR analysis for Asporin expression.
Asporin mRNA levels were analyzed using Real-time RT-PCR. (A) Comparison at 5 weeks-old (n = 4). (B) Temporal change at surface layer of tibia and mandibular condyle is shown (n = 4). ND: not detectable (C) Temporal and spatial change of tibia and mandibular condyle is shown (n = 4). ND: not determined NS: not significant difference between groups *: p < 0.05.
Then we examined temporal Asporin gene expression in the surface layer of MCC (Fig. 2B). MF exhibited higher Asporin gene expression as compared to tibia at all time point we examined.
To further analyse temporal and spatial Asporin gene expression in each layer of MCC, namely, mandibular fibrous layer (MF), mandibular proliferating layer (MP), and mandibular hypertrophic layer (MH), real-time RT-PCR were performed (Fig. 2C). Deeper layer of MCC, MP and MH, expressed no detectable Asporin at early stage, and then, Asporin expression was observed with age in deeper layer of MCC.
3.3. Asporin protein is extensively expressed in MCC as compared to tibial growth cartilage
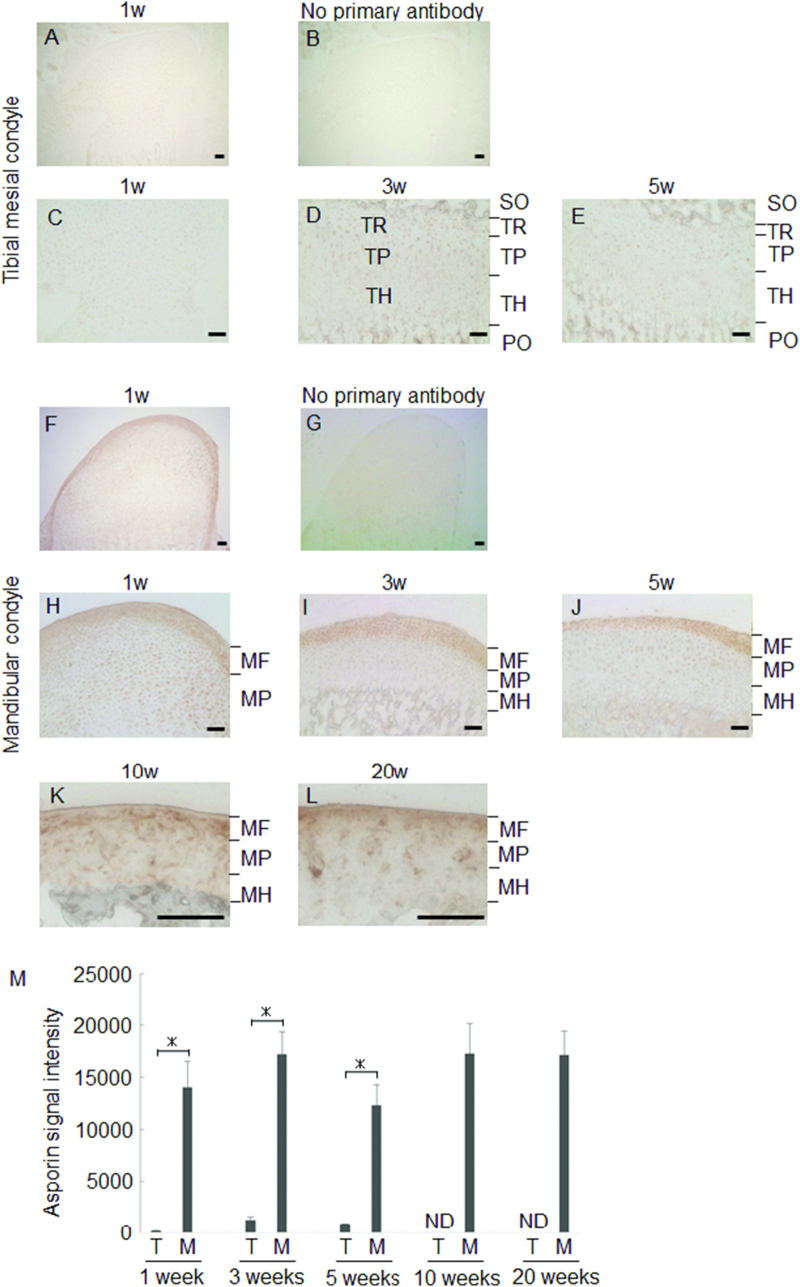
We next examined protein level expression of Asporin in MCC. Consistent with the results of real-time RT-PCR, MF exhibited intense Asporin expression as compared to tibia (Fig. 3). Augmented Asporin expression in MF was stable during experimental period (Fig. 3F–L). On the other hand, Asporin expression in the deeper layer of MCC was increased with age (Fig. 3M). Interestingly, MCC thickness was largest in 1 week old, and it gradually reduced with age.
Fig. 3.
Immunohistochemical staining for Asporin.
Representative images of temporal change of Asporin expression in tibia and MCC are shown. Tibial mesial condyle at 1 week-old in lower magnification (A) and negative control of no primary antibody (B). Tibial epiphyseal cartilage at 1, 3, 5 week(s)-old in higher magnification (C–E). Mandibular condyle at 1 week-old in lower magnification (F) and negative control of no primary antibody (G). Mandibular condylar cartilage at 1, 3, 5, 10, 20 week(s)-old in higher magnification (H–L).Signal intensity of immune-reactivity are shown (n = 3). The mean value from three images was calculated using ImageJ (M). PO: primary ossification center SO: secondary ossification center TR: Tibial reserve layer TP: tibial proliferating layer TH: tibial hypertrophic layer MF: mandibular fibrous layer MP: Mandibular proliferating layer MH: Mandibular hypertrophic layer ND: not detectable *: p < 0.05 bar: 50 μm.
As Asporin was reported to inhibit TGF-β signaling (Duval et al., 2011), these results suggest that augmented Asporin in MCC would negatively regulate TGF-β-mediated chondrogenesis.
3.4. TGF-β in MCC was relatively weak as compared to tibial growth cartilage
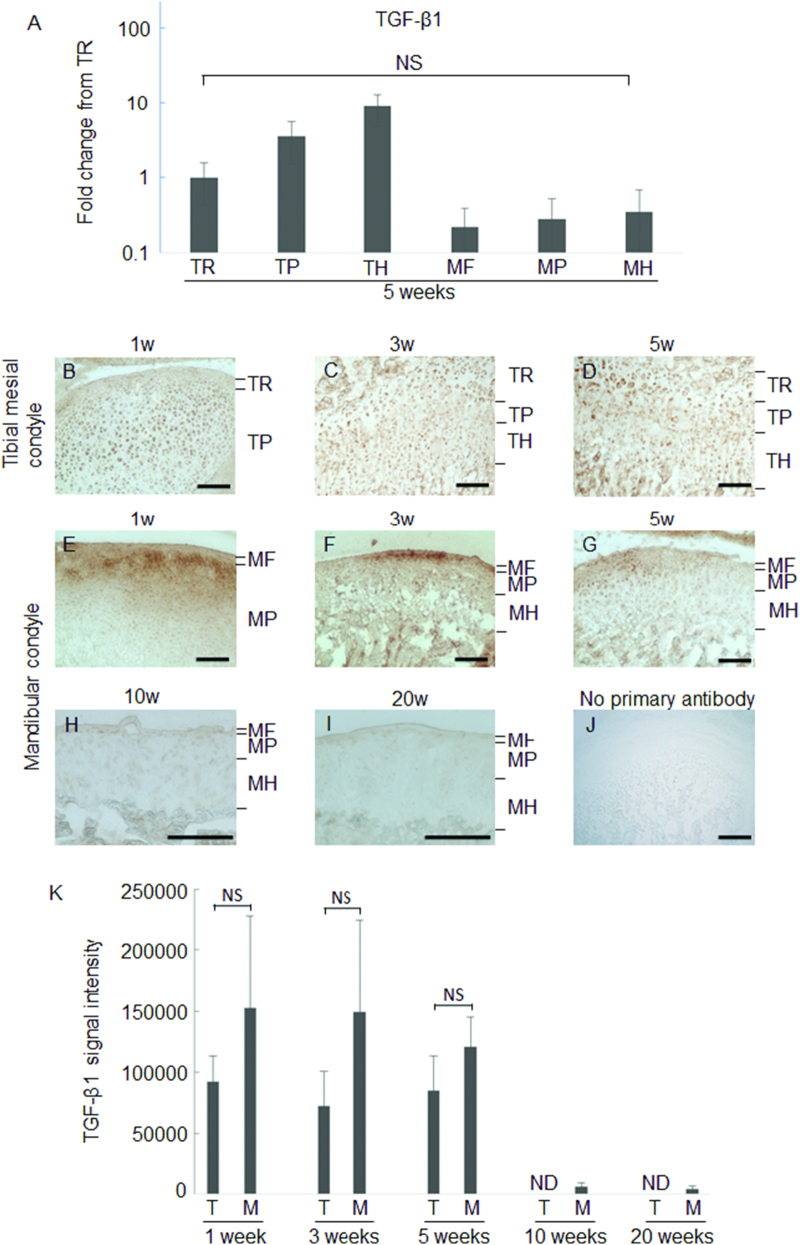
As it was reported that Asporin blocks chondrogenesis and inhibits TGF-β-induced expression of chondrocyte phenotypes (Nakajima et al., 2007, Kou et al., 2010), we next examined the expression of TGF-β at 5 weeks-old by real-time RT-PCR (Fig. 4A). Each layers of MCC expressed TGF-β though the expression was weak as compared to that in tibia.
Fig. 4.
Expression levels of TGF-β1 in tibia and MCC.
Real-time RT-PCR for TGF-β1 (A). NS: Not significant difference among the groups. Immunohistochemical staining for TGF-β1.Representative images of 1 week (B, E), 3 weeks (C, F), 5 weeks (D, G), 10 weeks (H), 20 weeks (I) and negative control of no primary antibody (J) are shown. Signal intensity of immune-reactivity is shown (n = 3). The mean value from three images was calculated using ImageJ (K). ND: not detectable NS: not significant difference bar: 100 μm.
Then we examined protein level expression of TGF-β, and found that TGF-β1 expression was intense at both mandibular and tibial cartilage in the growing phase (Fig. 4B–G). It was decreased in MCC at 10 and 20 weeks old (Fig. 4H and I). The measurement of signal intensity clearly demonstrated that stable TGF-β expression was present until 5 weeks and then reduced with aging (Fig. 4K). These changes in Asporin and TGF-β expression suggest that TGF-β is neutralized after growth, and thereby TGF-β signaling would be relatively weak in adult.
3.5. Asporin and TGF-β1 were strongly co-localized on the fibrous layer of MCC
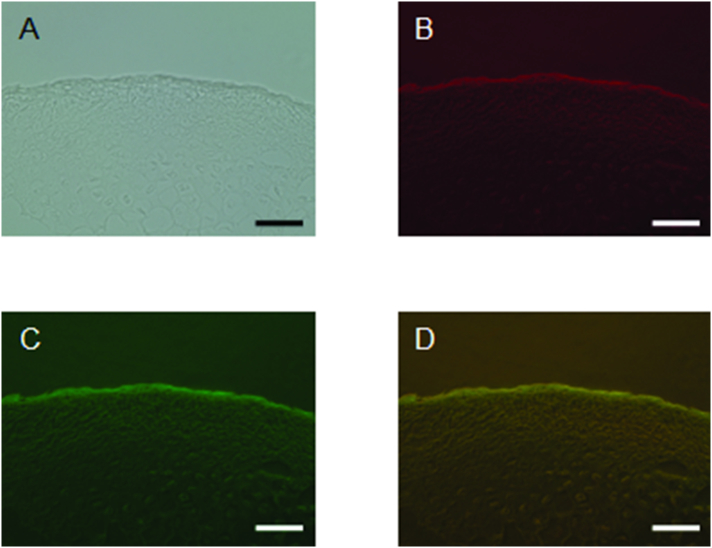
Immuno-fluorescent staining of Asporin and TGF-β were performed using the MCC of 3 weeks-old mice (Fig. 5). Consistent with Fig. 3, Fig. 4, Asporin (red: Fig. 5B) and TGF-β (green: Fig. 5C) were expressed in the fibrous layer of MCC. Merged image of the green and red fluorescence revealed that the co-localization between Asporin and TGF-β in the fibrous layer of MCC (Fig. 5D). These results suggest that the TGF-β binds to Asporin in the fibrous layer of MCC.
Fig. 5.
Immuno-fluorescent staining of Asporin and TGF-β in MCC.
Representative images of bright field (A), immuno-fluorescent staining of Asporin (B) and TGF-β (C), and merged image of green and red fluorescence (D) are shown (Panel D). Bar: 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. TGF-β signaling is attenuated by augmented Asporin in MCC
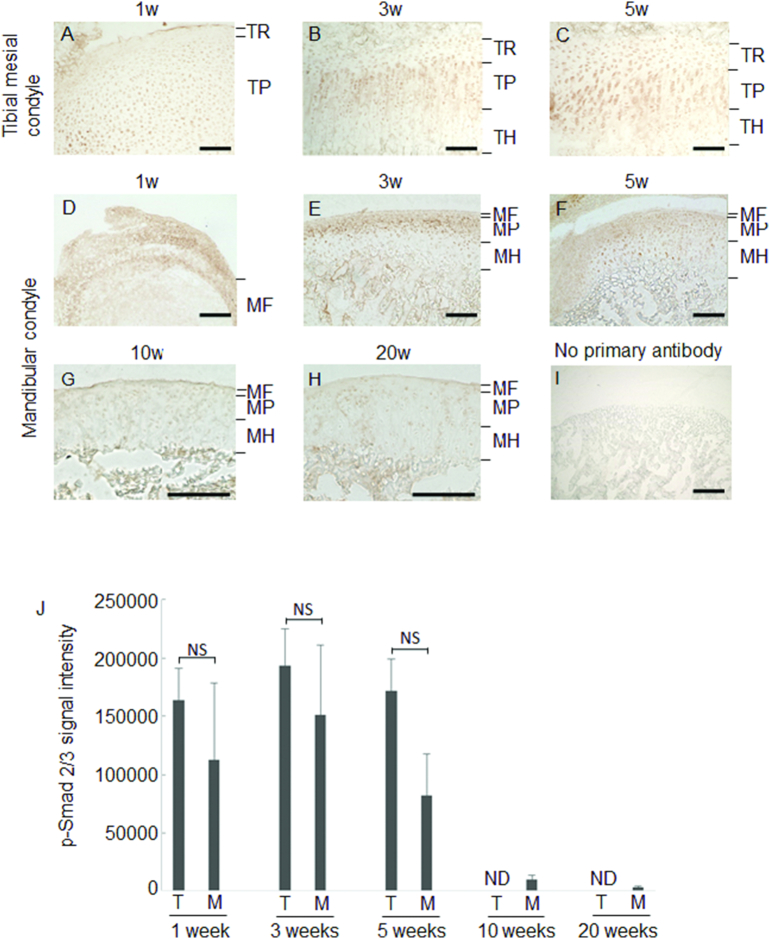
Finally, we observed whether TGF-β signaling is attenuated by augmented Asporin in MCC with immunohistochemical staining for phosphorylated Smad-2/3 (p-Smad-2/3), which is one of the well-known signaling molecule after TGF receptor (Heldin et al., 1997). Immuno-reactivity against p-Smad-2/3 was observed at the entire layers of tibial cartilage (Fig. 6A–C). In MCC, immuno-reactivity against p-Smad-2/3 was intense at deeper layers at 3 and 5 weeks old, while MF layer exhibited no immuno-reactivity against p-Smad-2/3 (Fig. 6E and F). p-Smad-2/3 in the deeper layers of MCC was gradually reduced with age (Fig. 6D–H). These results suggest that TGF-β signaling is suppressed by augmented Asporin and decreased TGF-β production in MCC.
Fig. 6.
Immunohistochemical staining for p-Smad2/3.
Representative images of Tibia (Panels A–C), MCC (Panels D–H), and negative control of no primary antibody (I) are shown. Signal intensity of immune-reactivity is shown (n = 3). The mean value from three images was calculated using ImageJ (J). ND: not detectable NS: not significant difference bar: 100 μm.
4. Discussion
In the present study, we discovered that Asporin, inhibitor of TGF-β signaling (Xu et al., 2015), was expressed in MCC. Asporin was stably expressed at the fibrous layer of MCC at any time points, and the deeper layer of MCC exhibited the gradual augmentation of Asporin with age. To our knowledge, this is the first report which describes temporal and spatial change of Asporin expression in MCC.
Microarray analysis demonstrated the different gene expression pattern between MCC and growth plate of tibia. Asporin was highly expressed in MCC. On the other hand, tibial epiphyseal cartilage exhibited faint Asporin expression, which was consistent with the previous study. These results indicate that MCC is different from the other growth cartilage in the expression of Asporin. Besides Asporin, some genes related to the bone metabolism were upregulated. Among them, Sema3e inhibits osteoblast migration and decrease osteoclast formation (Hughes et al., 2012). Tenascin-N was reported to inhibit proliferation and differentiation of preosteoblasts during endochondral bone formation (Kimura et al., 2007). These also indicate that MCC is different from the other growth cartilage. Further investigation is necessary to clarify the relationship between these unique gene expression and tissue specificity of MCC.
Asporin binds to TGF-β and consequently inhibits the binding of TGF-β to the receptor, which results in the suppression of TGF-β signaling (Nakajima et al., 2007). Nakajima also reported that Asporin has putative binding sites to TGF-β; peptide 159–205 (P159–205), P33–373, P33–167, P48–167 and P279–373. Although each site differs in its binding ability, all of them can impede the dimerization of TGF-β RII to affect the stability of the TGF-β RI and RII complexes, thus hindering TGF-β induced chondrogenesis (Nakajima et al., 2007).
When TGF-β1 binds to its receptor, cytoplasmic Smads, such as Smad-2 and Smad-3 are phosphorylated, which results in the activation of intercellular signal of TGF-β (Heldin et al., 1997). The results in immunohistochemistry revealed that Asporin and TGF-β were strongly co-localized in the fibrous layer of MCC. Furthermore, the immunohistochemistry of p-Smad-2/3 clearly revealed that TGF-β signaling was attenuated in the MF layer where Asporin was highly expressed, as compared to the epiphyseal cartilage in tibia. These indicate that Asporin in MF layer binds to TGF-β and inhibits TGF-β signaling in MF layer.
MCC is classified as a secondary cartilage (Symons, 1965) and is structurally fibrocartilages which is different from both the limb growth plate and the other articular cartilage (Sprinz and Stockwell, 1977). The surface fibrous layer composes a perichondrium in which the cells are relatively undifferentiated and produce type I collagen rather than type II collagen (Silbermann et al., 1987, Mizoguchi et al., 1990). MCC was thick in the developing stage, though the thickness gradually reduced with age (at 10 and 20 weeks old). In addition, fibrocartilage cells in the condylar cartilage secrete various kind of cytokines which regulates chondrocyte proliferation and differentiation (Mizoguchi et al., 1990, Watahiki et al., 2004). Among them, TGF-β is one of important promotors of chondrocyte proliferation and differentiation (Worster et al., 2001). Robust Asporin expression in the fibrous layer of condylar cartilage in this study negatively regulates TGF-β-mediated chondrocyte proliferation and differentiation during growing stage. In this context, Asporin expression in the fibrous layer maintains the function of MCC as the fibrocartilage through the negative regulation of TGF-β.
The high expression of Asporin expands from the surface fibrous layer to the deeper layers of MCC with age, signifying TGF-β signaling is gradually suppressed in the deeper layer of MCC with age. This augmented Asporin in the deeper layers in the aged mice inhibits chondrocyte differentiation and transition to bone formation with age and fine-tune TGF-β-mediated condylar morphogenesis, which consequently regulates the mandibular growth.
On the other hand, a long-term presence of Asporin keeps the cells in undifferentiated state and might reduce the capacity for the adaptation to temporomandibular disorder.
Orthodontic treatment applies mechanical stress on MCC for the control of endochondral growth (Arat et al., 2003), though cartilage tissue in MCC is thought to have resistance to mechanical stress (Copray et al., 1986, Bray et al., 1992). Difference in Asporin expression between MCC and tibia would give the interpretation of the difference in mechano-resistance. Further investigation is necessary to clarify whether mechanical stress changes the expression of Asporin in MCC.
Recently, Asporin expression is considered to be one of the major factors for osteoarthritis. Asporin intensity was significantly associated with the severity of cartilage degeneration in the study on Asporin expression in the knee osteoarthritis patients and the healthy (Sakao et al., 2009). Osteoarthritis in MCC is high prevalence in the elderly, which results in the deformation of mandibular condyle as compared to young people (Akihiro, 2012). Considering the high expression of Asporin in MCC, the long-term presence of Asporin in the MCC might attenuate the protective mechanism against the condylar degeneration. Further study will be necessary to investigate the relationship between Asporin and osteoarthritis in MCC.
5. Conclusion
We discovered that Asporin was expressed in MCC, which controls dual roles of articular cartilage and growth center through regulation of TGF-β signaling.
Acknowledgements
This research was supported in part by the Grants-in-Aid for Scientific Research from JSPS (grant numbers 19592373, 23689081, 25670841, 15K11376, and 16H05552). The authors have no conflict of interest.
References
- Akihiro I. Pathology and treatment of the geriatric patients with temporomandibular disorders. Tokyo Jikei-kai Ika Daigaku Zasshi. 2012;127:41–48. [Google Scholar]
- Arat Z.M., Akcam M.O., Gokalp H. Long-term effects of chin-cap therapy on the temporomandibular joints. Eur. J. Orthod. 2003;25(5):471–475. doi: 10.1093/ejo/25.5.471. [DOI] [PubMed] [Google Scholar]
- Bismar H., Kloppinger T., Schuster E.M., Balbach S., Diel I., Ziegler R., Pfeilschifter J. Transforming growth factor beta (TGF-beta) levels in the conditioned media of human bone cells: relationship to donor age, bone volume, and concentration of TGF-beta in human bone matrix in vivo. Bone. 1999;24(6):565–569. doi: 10.1016/s8756-3282(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Bray R.C., Shrive N.G., Frank C.B., Chimich D.D. The early effects of joint immobilization on medial collateral ligament healing in an ACL-deficient knee: a gross anatomic and biomechanical investigation in the adult rabbit model. J. Orthop. Res. 1992;10(2):157–166. doi: 10.1002/jor.1100100202. [DOI] [PubMed] [Google Scholar]
- Chen P., Carrington J.L., Hammonds R.G., Reddi A.H. Stimulation of chondrogenesis in limb bud mesoderm cells by recombinant human bone morphogenetic protein 2B (BMP-2B) and modulation by transforming growth factor beta 1 and beta 2. Exp. Cell Res. 1991;195(2):509–515. doi: 10.1016/0014-4827(91)90403-h. [DOI] [PubMed] [Google Scholar]
- Chung U.I., Schipani E., McMahon A.P., Kronenberg H.M. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Invest. 2001;107(3):295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray J.C., Jansen H.W., Duterloo H.S. Growth and growth pressure of mandibular condylar and some primary cartilages of the rat in vitro. Am. J. Orthod. Dentofac. Orthop. 1986;90(1):19–28. doi: 10.1016/0889-5406(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Copray J.C., Dibbets J.M., Kantomaa T. The role of condylar cartilage in the development of the temporomandibular joint. Angle Orthod. 1988;58(4):369–380. doi: 10.1043/0003-3219(1988)058<0369:TROCCI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Duval E., Bigot N., Hervieu M., Kou I., Leclercq S., Galera P., Boumediene K., Bauge C. Asporin expression is highly regulated in human chondrocytes. Mol. Med. (Cambridge, Mass.) 2011;17(7–8):816–823. doi: 10.2119/molmed.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390(6659):465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hellstadius A. On the importance of epiphyseal cartilage to growth in length. Acta Orthop. Scand. 1950;20(1):84–88. [PubMed] [Google Scholar]
- Henry S.P., Takanosu M., Boyd T.C., Mayne P.M., Eberspaecher H., Zhou W., de Crombrugghe B., Hook M., Mayne R. Expression pattern and gene characterization of asporin. A newly discovered member of the leucine-rich repeat protein family. J. Biol. Chem. 2001;276(15):12212–12221. doi: 10.1074/jbc.M011290200. [DOI] [PubMed] [Google Scholar]
- Hinton R.J., Jing J., Feng J.Q. Genetic influences on temporomandibular joint development and growth. Curr. Top. Dev. Biol. 2015;115:85–109. doi: 10.1016/bs.ctdb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Hughes A., Kleine-Albers J., Helfrich M.H., Ralston S.H., Rogers M.J. A class III semaphorin (Sema3e) inhibits mouse osteoblast migration and decreases osteoclast formation in vitro. Calcif. Tissue Int. 2012;90(2):151–162. doi: 10.1007/s00223-011-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker E.B., Kapfinger E., Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr. Cartil. 2007;15(4):403–413. doi: 10.1016/j.joca.2006.09.010. OARS, Osteoarthritis Research Society. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Nakata K., Nakahara H., Nakase T., Kimura T., Kimata K., Caplan A.I., Ono K. Transforming growth factor-beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993;132(4):1603–1608. doi: 10.1210/endo.132.4.8462458. [DOI] [PubMed] [Google Scholar]
- Janssens K., ten Dijke P., Janssens S., Van Hul W. Transforming growth factor-beta1 to the bone. Endocr. Rev. 2005;26(6):743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- Jian H., Shen X., Liu I., Semenov M., He X., Wang X.F. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006;20(6):666–674. doi: 10.1101/gad.1388806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T., Shimizu M. A method for preparing whole-body sections suitable for autoradiographic, histological and histochemical studies. Stain. Technol. 1986;61(3):169–183. doi: 10.3109/10520298609110728. [DOI] [PubMed] [Google Scholar]
- Kimura H., Akiyama H., Nakamura T., de Crombrugghe B. Tenascin-W inhibits proliferation and differentiation of preosteoblasts during endochondral bone formation. Biochem. Biophys. Res. Commun. 2007;356(4):935–941. doi: 10.1016/j.bbrc.2007.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizawa H., Kou I., Iida A., Sudo A., Miyamoto Y., Fukuda A., Mabuchi A., Kotani A., Kawakami A., Yamamoto S., Uchida A., Nakamura K., Notoya K., Nakamura Y., Ikegawa S. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat. Genet. 2005;37(2):138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- Kou I., Nakajima M., Ikegawa S. Binding characteristics of the osteoarthritis-associated protein asporin. J. Bone Miner. Metab. 2010;28(4):395–402. doi: 10.1007/s00774-009-0145-8. [DOI] [PubMed] [Google Scholar]
- Li X.B., Zhou Z., Luo S.J. Expressions of IGF-1 and TGF-beta 1 in the condylar cartilages of rapidly growing rats. Chin. J. Dent. Res. 1998;1(2):52–56. [PubMed] [Google Scholar]
- Lorenzo P., Aspberg A., Onnerfjord P., Bayliss M.T., Neame P.J., Heinegard D. Identification and characterization of asporin. A novel member of the leucine-rich repeat protein family closely related to decorin and biglycan. J. Biol. Chem. 2001;276(15):12201–12211. doi: 10.1074/jbc.M010932200. [DOI] [PubMed] [Google Scholar]
- Masoud M.I., Marghalani H.Y., Masoud I.M., Gowharji N.F. Prospective longitudinal evaluation of the relationship between changes in mandibular length and blood-spot IGF-1 measurements. Am. J. Orthod. Dentofac. Orthop. 2012;141(6):694–704. doi: 10.1016/j.ajodo.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Mizoguchi I., Nakamura M., Takahashi I., Kagayama M., Mitani H. An immunohistochemical study of localization of type I and type II collagens in mandibular condylar cartilage compared with tibial growth plate. Histochemistry. 1990;93(6):593–599. doi: 10.1007/BF00272201. [DOI] [PubMed] [Google Scholar]
- Moroco J.R., Hinton R., Buschang P., Milam S.B., Iacopino A.M. Type II collagen and TGF-betas in developing and aging porcine mandibular condylar cartilage: immunohistochemical studies. Cell Tissue Res. 1997;289(1):119–124. doi: 10.1007/s004410050857. [DOI] [PubMed] [Google Scholar]
- Murtaugh L.C., Chyung J.H., Lassar A.B. Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 1999;13(2):225–237. doi: 10.1101/gad.13.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Kizawa H., Saitoh M., Kou I., Miyazono K., Ikegawa S. Mechanisms for asporin function and regulation in articular cartilage. J. Biol. Chem. 2007;282(44):32185–32192. doi: 10.1074/jbc.M700522200. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Nomura Y., Arai C., Noda K., Oikawa T., Kogure K., Kawamoto T., Hanada N. Laser capture microdissection of rat periodontal ligament for gene analysis. Biotech. Histochem. 2007;82(6):295–300. doi: 10.1080/10520290701778372. [DOI] [PubMed] [Google Scholar]
- Oka K., Oka S., Sasaki T., Ito Y., Bringas P., Jr., Nonaka K., Chai Y. The role of TGF-beta signaling in regulating chondrogenesis and osteogenesis during mandibular development. Dev. Biol. 2007;303(1):391–404. doi: 10.1016/j.ydbio.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakao K., Takahashi K.A., Arai Y., Saito M., Honjyo K., Hiraoka N., Kishida T., Mazda O., Imanishi J., Kubo T. Asporin and transforming growth factor-beta gene expression in osteoblasts from subchondral bone and osteophytes in osteoarthritis. J. Orthop. Sci. 2009;14(6):738–747. doi: 10.1007/s00776-009-1401-4. [DOI] [PubMed] [Google Scholar]
- Silbermann M., Reddi A.H., Hand A.R., Leapman R.D., Von der Mark K., Franzen A. Further characterisation of the extracellular matrix in the mandibular condyle in neonatal mice. J. Anat. 1987;151:169–188. [PMC free article] [PubMed] [Google Scholar]
- Sprinz R., Stockwell R.A. Effect of experimental lipoarthrosis on the articular fibrocartilage of the rabbit mandibular condyle. Arch. Oral Biol. 1977;22(5):313–316. doi: 10.1016/0003-9969(77)90028-0. [DOI] [PubMed] [Google Scholar]
- Symons N.B. A histochemical study of the secondary cartilage of the mandibular condyle in the rat. Arch. Oral Biol. 1965;10(4):579–584. doi: 10.1016/0003-9969(65)90003-8. [DOI] [PubMed] [Google Scholar]
- Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., Zhao L., Nagy T.R., Peng X., Hu J., Feng X., Van Hul W., Wan M., Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan N.S., Lash J.W. Proteogylcan heterogeneity in embryonic chick articular and epiphyseal cartilages. Connect. Tissue Res. 1978;6(3):191–199. doi: 10.3109/03008207809152631. [DOI] [PubMed] [Google Scholar]
- Watahiki J., Yamaguchi T., Irie T., Nakano H., Maki K., Tachikawa T. Gene expression profiling of mouse condylar cartilage during mastication by means of laser microdissection and cDNA array. J. Dent. Res. 2004;83(3):245–249. doi: 10.1177/154405910408300312. [DOI] [PubMed] [Google Scholar]
- Worster A.A., Brower-Toland B.D., Fortier L.A., Bent S.J., Williams J., Nixon A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J. Orthop. Res. 2001;19(4):738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Xu L., Li Z., Liu S.Y., Xu S.Y., Ni G.X. Asporin and osteoarthritis. Osteoarthr. Cartil. 2015;23(6):933–939. doi: 10.1016/j.joca.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Yamada S., Murakami S., Matoba R., Ozawa Y., Yokokoji T., Nakahira Y., Ikezawa K., Takayama S., Matsubara K., Okada H. Expression profile of active genes in human periodontal ligament and isolation of PLAP-1, a novel SLRP family gene. Gene. 2001;275(2):279–286. doi: 10.1016/s0378-1119(01)00683-7. [DOI] [PubMed] [Google Scholar]