Abstract
Optimal prophylaxis for prevention of venous thromboembolism (VTE) after total joint arthroplasty (TJA) remains debated. The purpose of this study was to compare postoperative complications in patients receiving different VTE chemoprophylactic regimens.
Using a nationwide healthcare database, 72,670 THA patients without a history of VTE were identified. Study cohorts received VTE prophylaxis within 30 days postoperatively. Odds ratios and 95% confidence intervals were used to assess 30-day and 90-day postoperative complications (hematoma, hemorrhage, transfusion, pulmonary embolism (PE), VTE, prosthetic joint infection (PJI), and incision/drainage (I&D)).
Of the 72,670 THA patients, 25,966 received single medication VTE prophylaxis; 551 (2.12%) aspirin, 6791 (26.15%) enoxaparin, 12,008 (46.25%) warfarin, 5403 (20.81%) rivaroxaban, 876 (3.37%) fondaparinux and 337 (1.30%) apixaban. 30-day complications included; aspirin: I&D; warfarin: I&D, hematoma, hemorrhage, transfusion, PJI, PE and DVT; apixaban: hematoma and hemorrhage. 90-day complications included; aspirin: I&D; warfarin: I&D, hematoma, hemorrhage, transfusion, PJI, PE and DVT.
Warfarin was the only anticoagulant associated with a higher risk for DVT, and the highest risk for 30-day and 90-day complications. Aspirin had the highest risk for I&D. Despite three times increased 30-day risk for bleeding, apixaban was effective in preventing VTE during the high-risk 3-month-period. Enoxaparin had the lowest risk for PE and DVT while rivaroxaban had the lowest risk for PJI, hematoma, I&D, hemorrhage and transfusion.
Keywords: Venous thromboembolism, Total hip arthroplasty, Postoperative complications, Chemoprophylaxis, Low-molecular weight heparin, Total joint arthroplasty
1. Introduction
Venous thromboembolic (VTE) events continue to be a relatively common and sometimes fatal complications following total hip arthroplasty (THA). Despite similar recommendations from the American Academy of Orthopaedic Surgeons (AAOS) and the American College of Chest Physicians (ACCP), debate continues to exist regarding the most optimal VTE prophylactic regimen. ACCP now recommends aspirin and mechanical compression devices as adequate forms of thromboprophylactic agents. This recommendation was based on the Pulmonary Embolism Prevention (PEP) trial that did not include asymptomatic deep venous thrombosis (DVT) through screening with duplex ultrasonography as a thromboembolic event.1 To date there are few large studies comparing outcomes of different VTE prophylactic methods following THA.2, 3 Because pulmonary embolism is an overall rare complication, large studies are needed to potentially demonstrate significant difference in occurrence using different prophylactic regimens. Currently, under the Surgical Care Improvement Project (SCIP) Guidelines, low-molecular weight heparin (LMWH), warfarin, fondaparinux, and rivaroxaban are approved following total hip and knee arthroplasty. Mechanical compression devices alone are acceptable following TKA, while the addition of aspirin is required following THA.4 Ideally, the best prophylactic agent would provide the most effective defense against thromboembolic events while limiting post-operative complications such bleeding and infection. The purpose of this study is to compare post-operative surgical and thromboembolic complications among patients on different chemoprophylactic agents following total hip arthroplasty.
2. Methods
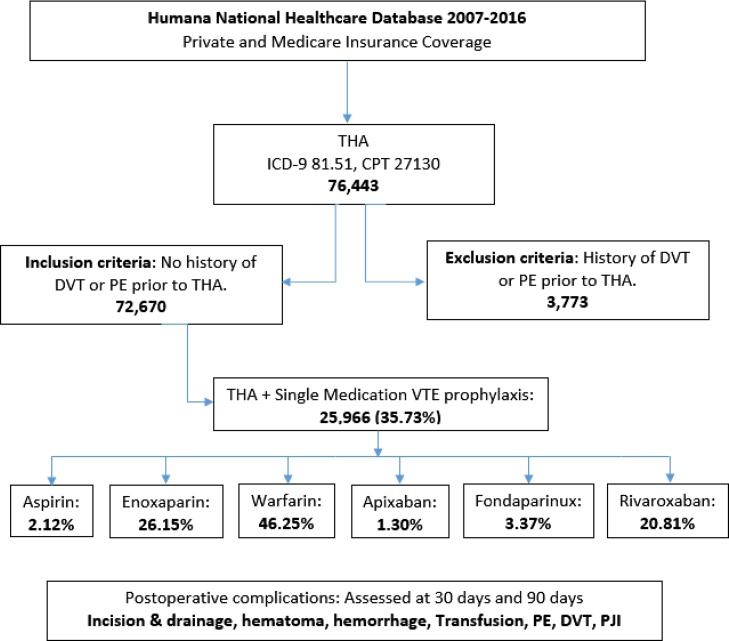
This retrospective review was conducted from January 2007 to April 2016 within a nationwide private and Medicare insurance healthcare database containing healthcare administrative records of over 20 million patients using Pearl Diver technologies (Warsaw, IN). This Humana database study was exempt from institutional review board approval at our institution as deidentified patient data were reviewed. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and Current Procedural Terminology (CPT) codes were used to identify patients who underwent total hip arthroplasty (THA) from 2007 to 2016 within the database. Using the relevant ICD-9-CM diagnosis codes, CPT codes, and the PearlDiver Boolean language commands, patients with a history of venous thromboembolism (VTE) prior to their THA were eliminated from this cohort. The emerging cohort was further subdivided into six postoperative single agent VTE prophylactic sub-cohorts; aspirin, enoxaparin, warfarin, apixaban, fondaparinux, and rivaroxaban. The Boolean commands, ICD-9-CM and CPT codes were used to identify patients within each sub-cohort who received only one type of VTE prophylaxis within 30 days of surgery and postoperative complications were assessed at 30 days and 90 days (Fig. 1).
Fig. 1.
Patient cohort selection flow chart.
THA: Total Hip Arthroplasty, CPT: Current Procedural Terminogy codes, ICD-9: International Classification of Disease, Ninth Revision codes, PE: Pulmonary Embolism, DVT: Deep Venous Thrombosis, PJI: Prosthetic Joint Infection.
ICD-9-CM codes were used to identify postoperative complications within each sub-cohort including; hematoma, hemorrhage, transfusion, pulmonary embolism (PE), deep vein thrombosis (DVT), prosthetic joint infection (PJI) and incision & drainage (I&D). All the ICD-9-CM and CPT codes corresponding to each diagnosis or procedure can be found in the appendix (Appendix A in Supplementary data).
3. Statistical analysis
All data analyses were done using STATA v.13 software (Stata corp., College Station, TX). Categorical variables were summarized as counts (Table 1). Odds ratios (OR) were estimated for the associated postoperative outcome within each sub-cohort with 95% confidence intervals (CI). Generalized linear models with a log link were used to report odds ratios and explore associations between postoperative VTE prophylaxis and the various postoperative complications. A p value of <0.05 was considered statistically significant.
Table 1.
Patient demographics.
| Patient Characteristics | THA + Aspirin (N = 551) | THA + Enoxaparin (N = 6791) | THA + Warfarin (N = 12,008) | THA + Apixaban (N = 337) | THA + Fondaparinux (N = 876) | THA + Rivaroxaban (N = 5403) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 279 | 2931 | 4979 | 125 | 389 | 2175 |
| Female | 272 | 3860 | 6804 | 212 | 487 | 3228 |
| Age | ||||||
| <65 | 370 | 2172 | 3143 | 159 | 325 | 1559 |
| ≥65 | 181 | 4693 | 8865 | 186 | 564 | 3900 |
THA: Total Hip Arthroplasty, N: Number of patients.
4. Results
Of the 72,670 patients that underwent THA, 25,966(35.73%) received a single medication for VTE prophylaxis during the early postoperative period. Table 1 shows the individual medication cohorts with 551(2.12%) receiving aspirin alone, 6791 (26.15%) receiving enoxaparin alone, 12,008(46.25%) receiving warfarin alone, 337(1.30%) receiving apixaban alone, 876(3.37%) receiving fondaparinux alone and 5403(20.81%) receiving rivaroxaban alone during the 30 days following THA.
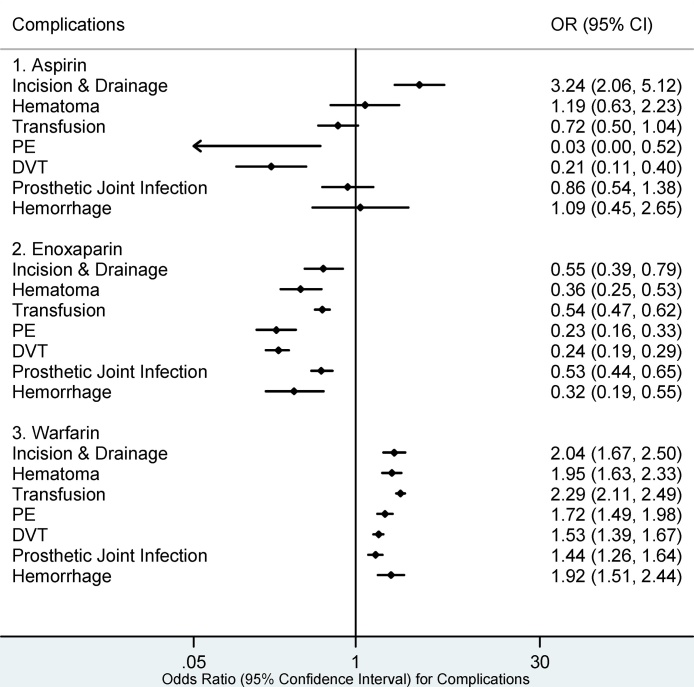
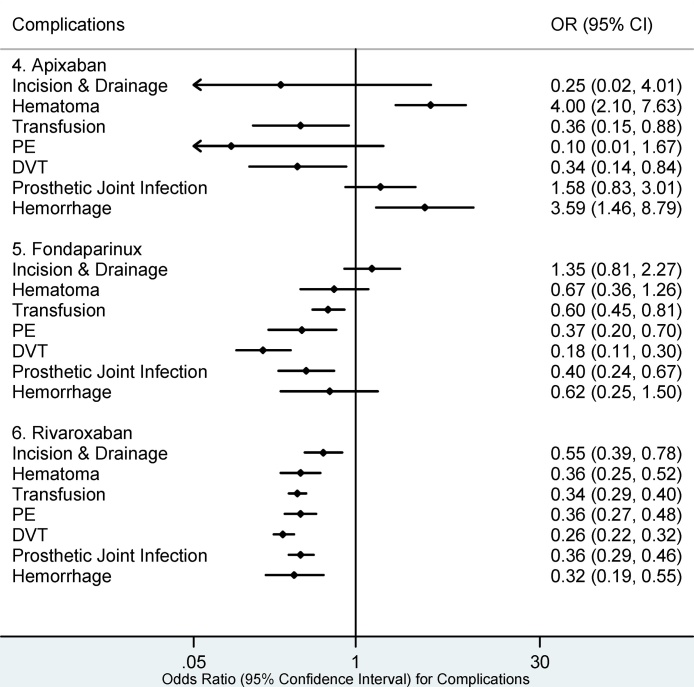
30 day medical complications associated with aspirin (Fig. 1) included I&D (OR 3.24, 95% CI: 2.06–5.12). Warfarin was associated with the highest number of postoperative complications at 30 days following surgery including; I&D (OR 2.04, 95% CI: 1.67–2.50), hematoma (OR 1.95, 95% CI: 1.63–2.33), transfusion (OR 2.29, 95% CI: 2.11–2.49), PE (OR 1.72, 95% CI: 1.49–1.98), DVT (OR 1.53, 95% CI: 1.39–1.67), PJI (OR 1.44, 95% CI: 1.26–1.64), hemorrhage (OR 1.92, 95% CI: 1.51–2.44). Apixaban was associated with hematoma (OR 4.00, 95% CI: 2.10–7.63) and hemorrhage (OR 3.59, 95% CI: 1.46–8.79) during the 30-day period following surgery. There were no statistically significant complications associated with enoxaparin, rivaroxaban, or fondaparinux during the 30-day postoperative period (Fig. 2 and Fig. 3).
Fig. 2.
30 day Complications for Aspirin, Enoxaparin, and Warfarin.
PE: Pulmonary Embolism, DVT: Deep Venous Embolism. Complications whose 95% confidence intervals (CI) completely fall on either side of the vertical line (line of no effect, odds ratio (OR) = 1) indicate statistical significance. Complications in which the 95% CI overlaps the OR of 1 are statistically insignificant.
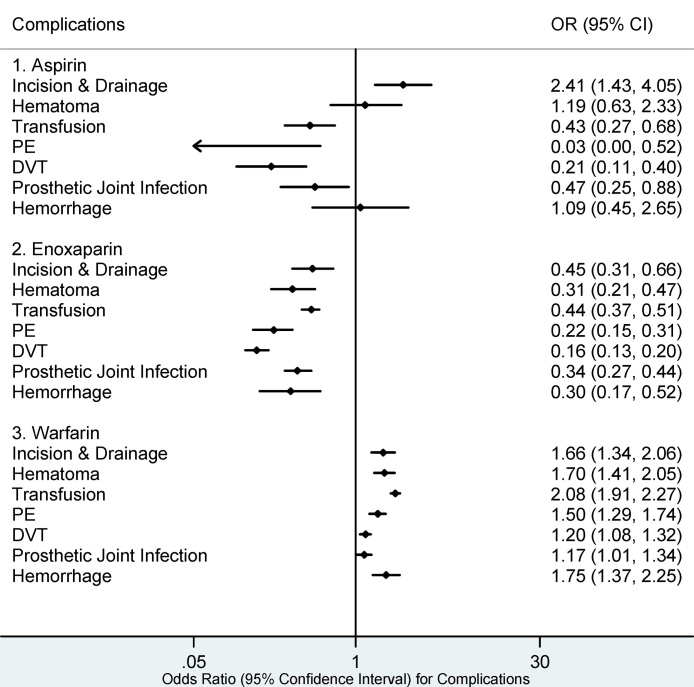
The highest number of 90-day postoperative complications was seen in the warfarin cohort (Fig. 4) including; I&D (OR 1.66, 95% CI: 1.34–2.06), hematoma (OR 1.70, 95% CI: 1.41–2.05), transfusion (OR 2.08, 95% CI: 1.91–2.27), PE (OR 1.50, 95% CI: 1.29–1.74), DVT (OR 1.20, 95% CI: 1.08–1.32), PJI (OR 1.17, 95% CI: 1.01–1.34) and hemorrhage (OR 1.75, 95% CI: 1.37–2.25). I&D (OR 2.41, 95% CI: 1.43–4.05) was the only significant 90-day postoperative complication within the aspirin cohort. No statistically significant complications were associated with enoxaparin, fondaparinux, or rivaroxaban at 90 days postoperatively (Fig. 4, Fig. 5).
Fig. 4.
90 day Complications for Aspirin, Enoxaparin, and Warfarin.
PE: Pulmonary Embolism, DVT: Deep Venous Embolism. Complications whose 95% confidence intervals (CI) completely fall on either side of the vertical line (line of no effect, odds ratio (OR) = 1) indicate statistical significance. Complications in which the 95% CI overlaps the OR of 1 are statistically insignificant.
Fig. 5.
90 day Complications for Apixaban, Fondaparinux, and Rivaroxaban.
PE: Pulmonary Embolism, DVT: Deep Venous Embolism. Complications whose 95% confidence intervals (CI) completely fall on either side of the vertical line (line of no effect, odds ratio (OR) = 1) indicate statistical significance. Complications in which the 95% CI overlaps the OR of 1 are statistically insignificant.
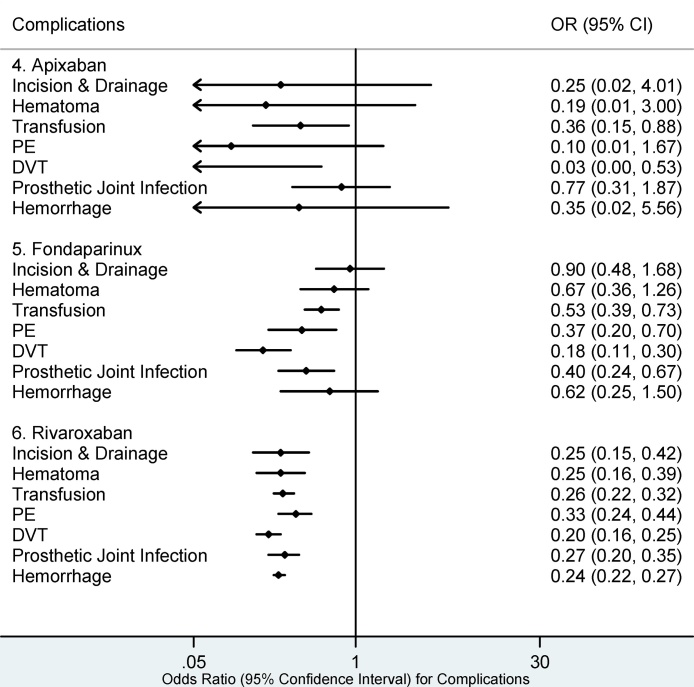
Fig. 2, Fig. 3, Fig. 4, Fig. 5 show that the lowest number of the 30 day and 90 day postoperative complications was seen with enoxaparin and rivaroxaban. Fondaparinux had the lowest risk for DVT (OR 1.18, 95% CI: 0.11–0.30) at both 30 and 90 days. The lowest risk for PE at both 30 and 90 days postoperatively was identified in the enoxaparin cohort; (OR 0.23, 95% CI: 0.16–0.33) and (OR 0.22, 95% CI: 0.15–0.31) respectively.
Fig. 3.
30 day Complications for Apixaban, Fondaparinux, and Rivaroxaban.
PE: Pulmonary Embolism, DVT: Deep Venous Embolism. Complications whose 95% confidence intervals (CI) completely fall on either side of the vertical line (line of no effect, odds ratio (OR) = 1) indicate statistical significance. Complications in which the 95% CI overlaps the OR of 1 are statistically insignificant.
Rivaroxaban (Fig. 4, Fig. 5) had the lowest risk for PJI (OR 0.27, 95% CI: 0.20–0.35), hematoma (OR 0.25, 95% CI: 0.16–0.39), hemorrhage (OR 0.24, 95% CI: 0.22–0.27), I&D (OR 0.25, 95% CI: 0.15–0.42), and transfusion (OR 0.26, 95% CI: 0.22–0.32) at 90 days postoperatively.
5. Discussion
There continues to be controversy regarding the optimal agent for prophylaxis against VTE after total joint arthroplasty.5 For a specific agent to be considered optimal, it would ideally meet the following criteria: high efficacy in preventing VTE, minimal risk of bleeding, cost-effectiveness, ease of administration, and associated with decreased postoperative complications. There have been limited large cohort studies looking at the various postoperative complications among patients receiving different VTE chemoprophylaxis following total joint arthroplasty. In this large cohort study, we evaluated the postoperative complications associated with six commonly used chemoprophylactic agents.
Aspirin is an antiplatelet agent that prevents platelet aggregation by inhibiting thromboxane A2 production.6 The Pulmonary Embolism Prevention (PEP) trial showed no significant difference in the rate of symptomatic DVT among patients who had undergone elective THA between the aspirin and placebo cohorts.1 In contrast, our study shows that aspirin was associated with significantly lower rates for both DVT and PE at both the 30 day and 90 day postoperative period following THA. Several studies have cited a low risk of bleeding among patients receiving aspirin following TJA7, 8 which is in accordance with our findings showing that the bleeding risk within the aspirin cohort was insignificant. Raphael et al.5 reports that the PE incidence was lower in the aspirin cohort compared to the warfarin cohort. This was similar to our findings, showing the aspirin cohort had the lowest risk for PE of all the agents evaluated. Various studies have reported that aspirin is not only safe but also just as or even more effective than other agents in VTE prevention.9, 10, 11, 12, 13, 14 The only postoperative complication seen among these patients was the need for I&D likely due to mild bleeding that may be attributed to patients with a high risk of bleeding. Various studies have shown that aspirin is the most cost-saving agent available and an effective alternative, especially in patients with a moderate to low bleeding risk.15, 16 Despite the limited number of patients who received aspirin alone (2.12%) vs enoxaparin alone (26.15%) for VTE prophylaxis, our study showed that the former had lower risk for DVT within the 30 day postoperative period. Furthermore, as an oral agent without a need to monitor blood levels, aspirin is an easy to use, safe, and effective prophylactic agent.
Enoxaparin is a low molecular weight heparin (LMWH) agent that indirectly inhibits Factor Xa by binding antithrombin (an endogenous anticoagulant glycoprotein produced by the liver) preventing the growth and propagation of already formed thrombi. However, enoxaparin does not break down existing blood clots.17, 18 This agent is administered subcutaneously and does not need serum monitoring. Prior studies comparing LMWH to warfarin have shown equal or higher bleeding rates among patients taking LMWH.19, 20, 21, 22 However, our study shows that enoxaparin is associated with significantly lower bleeding rates and lower overall postoperative complications compared to warfarin after THA. Our results are similar to those reported by Lieberman et al.3 in regards to enoxaparin being more effective in preventing VTE when compared to warfarin. This study shows that enoxaparin was superior to the other agents studied in preventing DVT within 90 days while having the lowest risk for bleeding within the first 30 days following THA.
Warfarin is a vitamin K antagonist that inhibits hepatic synthesis of procoagulant vitamin K dependent factors II, VII, IX, and X as well as anticoagulant proteins C, S, and Z.17, 23 Prior randomized clinical trials have shown no significant differences in symptomatic events including PE between warfarin and LMWH following TJA.19, 21, 24 However, our study shows that warfarin was associated with the highest overall risk for all the postoperative complications evaluated including PE and DVT following THA (Fig. 2, Fig. 4). Warfarin also requires international normalized ratio (INR) monitoring and interacts with certain medications and foods making it a cumbersome choice for VTE prophylaxis.25 Furthermore, warfarin has no effect on previously formed circulating coagulation factors causing a 3–7 day delay in the anticoagulation effect.26 This presents the need to bridge all patients who are on warfarin with another agent for at least 3–5 days during the early postoperative period for optimal prophylaxis. Sachs et al.27 reports double the rate of wound related complications among patients receiving warfarin postoperatively compared to those not receiving any form of thromboprophylaxis. Similarly, our results show a two-fold increased risk for I&D and hematoma formation within the warfarin cohort. For patients on long term warfarin management due to their medical history, we suggest taking a multi-disciplinary approach to optimize patients in order to decrease the risk of postoperative complications after THA given their complex postoperative profile (Fig. 2, Fig. 4).
Fondaparinux is a synthetic pentassacharide and indirect inhibitor of activated Factor Xa.28 It is an effective VTE prophylactic agent with peak levels occurring 2–3 h following subcutaneous administration.29 Prior studies have shown that despite fondaparinux being more effective compared to enoxaparin at preventing asymptomatic blood clot formation, its use is limited in North America due to concerns for bleeding complications.3, 30 In accordance with Turpie et al.31 our study shows that fondaparinux had the lowest risk for DVT during the 30 day postoperative period among all the other agents studied. Additionally, our study shows that fondaparinux was associated with a low risk for PJI, PE, and need for transfusion. Some studies have shown that fondaparinux is more cost-effective in the short term compared to enoxaparin32, 33, 34, 35, 36 while the opposite has been reported in the long term.37 This may explain why some patients are opting for fondaparinux in the early postoperative period. In-depth cost-analysis studies are necessary as fondaparinux is considered a more expensive option currently.
Apixaban is a competitive, selective, and reversible direct Factor Xa inhibitor and does not require blood monitoring for therapeutic dosing. Prior multicenter randomized trials have shown that apixaban, a direct oral anticoagulant, was associated with a significantly lower VTE rate compared to enoxaparin without additional bleeding risk.38, 39, 40 This is in contrast to our findings in which enoxaparin had a lower rate for both VTE and hemorrhage compared to apixaban 30 days after THA. Keating et al.41 reports a bleeding rate of 4.5% per year for atrial fibrillation patients receiving apixaban with a hazard ratio of 1.18 compared to 3.8 for aspirin. Our study shows that apixaban had the highest rate for hemorrhage and hematoma during the 30 day postoperative period compared to any other agent evaluated. However, by 90 days postoperatively, the bleeding risk was insignificant and apixaban had the lowest risk for VTE compared to any other agent (Fig. 2, Fig. 3, Fig. 4, Fig. 5). In this study, apixaban had the smallest cohort size (1.3%) for a single medication VTE chemoprophylaxis and a larger sample size may be needed to fully evaluate the extent of the postoperative complications in patients using this agent alone for prophylaxis after THA.
Rivaroxaban, like apixaban, is a competitive direct factor Xa inhibitor with high selectivity and also does not require monitoring. Phase-III randomized trials have previously shown that rivaroxaban is more effective at VTE prevention after total hip arthroplasty when compared to enoxaparin.42, 43, 44, 45 Our study shows that both rivaroxaban and enoxaparin were associated with an overall lower rate of postoperative complications compared to the other agents evaluated (Fig. 2, Fig. 3, Fig. 4, Fig. 5). In addition, our study shows that enoxaparin had a slightly lower rate for both DVT and PE than rivaroxaban at both 30 and 90 days postoperatively. These differences, however, were not statistically significant. Eriksson et al.42 reports that the bleeding risk was higher in patients receiving rivaroxaban compared to enoxaparin unlike our findings. Our study shows that rivaroxaban had the lowest rate for hemorrhage, hematoma, transfusion, and I&D at 90 days after THA and a similar bleeding rate when compared to enoxaparin at 30 days postoperatively. The differences in the bleeding events between rivaroxaban and enoxaparin were statistically insignificant which is similar to the results reported by Diamantopoulos et al.46 Furthermore, rivaroxaban was associated with the lowest rates for PJI at both the 30 days and 90 days postoperatively.
To our knowledge, this is the first study to use a large nationwide database to compare outcomes between the six most commonly described chemoprophylactic agents after THA. The advantage of using a large database is the ability to obtain a large sample size that is likely to accurately represent this patient population. Limitations of a large, nationwide, multicenter database study include the potential for confounding factors, especially since patients were not randomized to studied cohorts. Under the SCIP guidelines, all patients require appropriate VTE prophylaxis within 24 h before or after surgery. As a result, the cohorts could not be matched to a control group that was not provided VTE prophylaxis. This study did not match patients based on comorbidities across all cohorts. This could have a potentially significant impact on the results. Although there are no current guidelines regarding optimal VTE prophylaxis for certain patient populations, patients who are arbitrarily identified as high risk for VTE are likely prescribed anti-thrombotic agents as opposed to anti-platelet agents. Patients in the aspirin cohort may consist of healthier patients with minimal comorbidities compared to the other cohorts and could be at less risk of post-operative complications. According to the demographic data, the majority of patients in the aspirin cohort were less than 65 years of age as opposed to the other cohorts. Selection bias also exists as patients on two or more chemoprophylactic agents were excluded from this study. This included all patients taking low-dose aspirin in combination with another agents following THA. This excluded group of patients could have been on low-dose aspirin for cardioprotective purposes or using enoxaparin to bridge for warfarin. It can be argued that these patients inherently have more comorbidities biasing selection to include a majority of healthy patients in this study. This can also explain the low percentage (35.73%) of all patients who underwent THA that we included in this study as over 30% of adults over the age of 40 use low-dose aspirin for primary or secondary cardiovascular disease prevention.47
In this study, we did not indicate the specific duration of VTE prophylaxis. Other confounding variables include, but are not limited to, the length of hospital stay, the use of mechanical prophylaxis, dose of chemoprophylactic agent used. This study is based purely on ICD-9 codes, which are inherently subjective based on the accuracy of coding at different institutions.
6. Conclusion
Although our study shows agents such as rivaroxaban and fondaparinux are associated with lower risk of post-operative bleeding and thromboembolic events, they remain relatively expensive choices for VTE prophylaxis. Aspirin has a relatively low bleeding and thromboembolic complication profile and is an inexpensive, easy to administer option for VTE prophylaxis following TJA. Further high-powered prospective randomized studies investigating the efficacy of these agents in higher risk patients with history of VTE, hypercoagulable diseases, and cancer are warranted.
Conflict of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jor.2017.08.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: pulmonary embolism prevention (PEP) trial. Lancet. 2000;355(9212):1295–1302. [PubMed] [Google Scholar]
- 2.Khatod M. Prophylaxis against pulmonary embolism in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1767–1772. doi: 10.2106/JBJS.J.01130. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman J.R., Pensak M.J. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801–1811. doi: 10.2106/JBJS.L.01328. [DOI] [PubMed] [Google Scholar]
- 4.Bratzler D.W., Hunt D.R. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43(3):322–330. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- 5.Raphael I.J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472(2):482–488. doi: 10.1007/s11999-013-3135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catella-Lawson F. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345(25):1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 7.Reitman R.D. A multimodality regimen for deep venous thrombosis prophylaxis in total knee arthroplasty. J Arthroplasty. 2003;18(2):161–168. doi: 10.1054/arth.2003.50026. [DOI] [PubMed] [Google Scholar]
- 8.Lotke P.A., Lonner J.H. The benefit of aspirin chemoprophylaxis for thromboembolism after total knee arthroplasty. Clin Orthop Relat Res. 2006;452:175–180. doi: 10.1097/01.blo.0000238822.78895.95. [DOI] [PubMed] [Google Scholar]
- 9.Berend K.R., Lombardi A.V., Jr. Multimodal venous thromboembolic disease prevention for patients undergoing primary or revision total joint arthroplasty: the role of aspirin. Am J Orthop (Belle Mead NJ) 2006;35(1):24–29. [PubMed] [Google Scholar]
- 10.Boyd H.S. VTE prevention in major orthopedic surgery. Cleve Clin J Med. 2008;75(7):471–472. doi: 10.3949/ccjm.75.7.471-a. author reply 472-3. [DOI] [PubMed] [Google Scholar]
- 11.Bozic K.J. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(7):1053–1060. doi: 10.1016/j.arth.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cusick L.A., Beverland D.E. The incidence of fatal pulmonary embolism after primary hip and knee replacement in a consecutive series of 4253 patients. J Bone Joint Surg Br. 2009;91(5):645–648. doi: 10.1302/0301-620X.91B5.21939. [DOI] [PubMed] [Google Scholar]
- 13.Poultsides L.A. Meta-analysis of cause of death following total joint replacement using different thromboprophylaxis regimens. J Bone Joint Surg Br. 2012;94(1):113–121. doi: 10.1302/0301-620X.94B1.27301. [DOI] [PubMed] [Google Scholar]
- 14.White R.H., Meehan J.P., Romano P.S. Re: does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(4):667. doi: 10.1016/j.arth.2009.10.017. author reply 667-8. [DOI] [PubMed] [Google Scholar]
- 15.Sarasin F.P., Bounameaux H. Out of hospital antithrombotic prophylaxis after total hip replacement: low-molecular-weight heparin, warfarin, aspirin or nothing? A cost-effectiveness analysis. Thromb Haemost. 2002;87(4):586–592. [PubMed] [Google Scholar]
- 16.Kapoor A. Cost effectiveness of venous thromboembolism pharmacological prophylaxis in total hip and knee replacement: a systematic review. Pharmacoeconomics. 2010;28(7):521–538. doi: 10.2165/11535210-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nutescu E.A. Pharmacology of anticoagulants used in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):15–31. doi: 10.1007/s11239-015-1314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holbrook A. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S–e184S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald R.H., Jr. Prevention of venous thromboembolic disease following primary total knee arthroplasty. A randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am. 2001;83-a(6):900–906. [PubMed] [Google Scholar]
- 20.Francis C.W. Prevention of deep-vein thrombosis after total hip arthroplasty: comparison of warfarin and dalteparin. J Bone Joint Surg Am. 1997;79(9):1365–1372. doi: 10.2106/00004623-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Hull R.D. Subcutaneous low-molecular-weight heparin vs warfarin for prophylaxis of deep vein thrombosis after hip or knee implantation. An economic perspective. Arch Intern Med. 1997;157(3):298–303. [PubMed] [Google Scholar]
- 22.Leclerc J.R. Prevention of venous thromboembolism after knee arthroplasty. A randomized: double-blind trial comparing enoxaparin with warfarin. Ann Intern Med. 1996;124(7):619–626. doi: 10.7326/0003-4819-124-7-199604010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Ageno W. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heit J.A. Efficacy and safety of low molecular weight heparin (ardeparin sodium) compared to warfarin for the prevention of venous thromboembolism after total knee replacement surgery: a double-blind, dose-ranging study. Ardeparin arthroplasty study group. Thromb Haemost. 1997;77(1):32–38. [PubMed] [Google Scholar]
- 25.Daly A.K., Aithal G.P. Genetic regulation of warfarin metabolism and response. Semin Vasc Med. 2003;3(3):231–238. doi: 10.1055/s-2003-44458. [DOI] [PubMed] [Google Scholar]
- 26.Stirling Y. Warfarin-induced changes in procoagulant and anticoagulant proteins. Blood Coagul Fibrinolysis. 1995;6(5):361–373. doi: 10.1097/00001721-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Sachs R.A. Does anticoagulation do more harm than good?: a comparison of patients treated without prophylaxis and patients treated with low-dose warfarin after total knee arthroplasty. J Arthroplasty. 2003;18(4):389–395. doi: 10.1016/s0883-5403(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 28.Petitou M. The synthetic pentasaccharide fondaparinux: first in the class of antithrombotic agents that selectively inhibit coagulation factor Xa. Semin Thromb Hemost. 2002;28(4):393–402. doi: 10.1055/s-2002-34309. [DOI] [PubMed] [Google Scholar]
- 29.Donat F. The pharmacokinetics of fondaparinux sodium in healthy volunteers. Clin Pharmacokinet. 2002;41(Suppl. 2):1–9. doi: 10.2165/00003088-200241002-00001. [DOI] [PubMed] [Google Scholar]
- 30.Bauer K.A. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345(18):1305–1310. doi: 10.1056/NEJMoa011099. [DOI] [PubMed] [Google Scholar]
- 31.Turpie A.G. New pentasaccharides for the prophylaxis of venous thromboembolism: clinical studies. Chest. 2003;124(6 Suppl):371s–378s. [PubMed] [Google Scholar]
- 32.Bjorvatn A., Kristiansen F. Fondaparinux sodium compared with enoxaparin sodium: a cost-effectiveness analysis. Am J Cardiovasc Drugs. 2005;5(2):121–130. doi: 10.2165/00129784-200505020-00006. [DOI] [PubMed] [Google Scholar]
- 33.Dranitsaris G. Pharmacoeconomic analysis of fondaparinux versus enoxaparin for the prevention of thromboembolic events in orthopedic surgery patients. Am J Cardiovasc Drugs. 2004;4(5):325–333. doi: 10.2165/00129784-200404050-00005. [DOI] [PubMed] [Google Scholar]
- 34.Gordois A. The cost-effectiveness of fondaparinux compared with enoxaparin as prophylaxis against thromboembolism following major orthopedic surgery. J Thromb Haemost. 2003;1(10):2167–2174. doi: 10.1046/j.1538-7836.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- 35.Spruill W.J., Wade W.E., Leslie R.B. Cost analysis of fondaparinux versus enoxaparin as venous thromboembolism prophylaxis in elective hip replacement surgery. Blood Coagul Fibrinolysis. 2004;15(7):539–543. doi: 10.1097/00001721-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan S.D. A cost-effectiveness analysis of fondaparinux sodium compared with enoxaparin sodium as prophylaxis against venous thromboembolism: use in patients undergoing major orthopaedic surgery. Pharmacoeconomics. 2004;22(9):605–620. doi: 10.2165/00019053-200422090-00005. [DOI] [PubMed] [Google Scholar]
- 37.Annemans L. Cost consequence analysis of fondaparinux versus enoxaparin in the prevention of venous thromboembolism after major orthopaedic surgery in Belgium. Acta Clin Belg. 2004;59(6):346–357. doi: 10.1179/acb.2004.050. [DOI] [PubMed] [Google Scholar]
- 38.Lassen M.R. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 39.Lassen M.R. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 40.Lassen M.R. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 41.Keating G.M. Apixaban: a review of its use for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Drugs. 2013;73(8):825–843. doi: 10.1007/s40265-013-0063-x. [DOI] [PubMed] [Google Scholar]
- 42.Eriksson B.I. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 43.Kakkar A.K. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372(9632):31–39. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 44.Lassen M.R. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776–2786. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 45.Turpie A.G. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373(9676):1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 46.Diamantopoulos A. Cost-effectiveness of rivaroxaban versus enoxaparin for the prevention of postsurgical venous thromboembolism in Canada. Thromb Haemost. 2010;104(4):760–770. doi: 10.1160/TH10-01-0071. [DOI] [PubMed] [Google Scholar]
- 47.Stuntz M., Bernstein B. Recent trends in the prevalence of low-dose aspirin use for primary and secondary prevention of cardiovascular disease in the United States, 2012–2015. Prev Med Rep. 2017;5:183–186. doi: 10.1016/j.pmedr.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.