Abstract
Despite the existence of many promising anti-cancer therapies, not all breast cancers are equally treatable, due partly to the fact that focus has been primarily on a few select breast cancer biomarkers- notably ERα, PR and HER2. In cases like triple negative breast cancer (ERα-, PR-, and HER2-), there is a complete lack of available biomarkers for prognosis and therapeutic purposes. The goal of this review is to determine if other steroid receptors, like ERβ and AR, could play a prognostic and/or therapeutic role. Data from various in vitro, in vivo, and clinical breast cancer studies were examined to analyze the presence and function of ERβ, PR, and AR in the presence and absence of ERα. Additionally, we focused on studies that examined how expression of the various steroid receptor isoforms affects breast cancer progression. Our findings suggest that while we have a solid understanding of how these receptors work individually, how they interact and behave in the presence and absence of other receptors requires further research. Furthermore, there is an incomplete understanding of how the various steroid receptor isoforms interact and impact receptor function and breast cancer progression, partly due to the difficulty in detecting all the various isoforms. More large-scale clinical studies must be made to analyze systematically the expression of steroid hormone receptors and their respective isoforms in breast cancer patients in order to determine how these receptors interact with each other and in turn affect cancer progression.
Keywords: Breast cancer, steroid hormone receptors, prognostic markers, estrogen, progesterone, androgen
Introduction
Though both estrogen and progesterone receptors are commonly used as prognostic markers for breast cancer, current endocrine therapy primarily targets the estrogen receptor, ERα. Unfortunately, for about 10-15% of breast cancer patients [1-3]- like those diagnosed with triple negative breast cancer, defined as breast tumors lacking the expression of estrogen receptor alpha (ERα), progesterone receptor (PR) and human epidermal growth factor receptor type 2 (HER2), ERα-/PR-/HER2- - the established endocrine therapies are ineffective, highlighting an urgent need for additional therapeutic targets in breast cancer. Therefore, the goal of the following review is to examine the role of the steroid hormone receptors- ERα, ERβ, PR, and androgen receptor (AR)- in the progression of breast cancer in order to determine their role and utility as prognostic markers and therapeutic targets.
Steroid receptors: an overview
The steroid hormone receptor subfamily, which includes estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR), and glucocorticoid receptor (GR), is part of the larger superfamily of nuclear hormone receptors [4]. The members of this superfamily function as ligand-gated transcription factors that modulate the expression of genes [5]. While unbound steroid receptors are typically located in the cytosol, ligand binding induces receptor dimerization and conformational changes which in turn exposes the nuclear localization signal, allowing translocation into the nucleus [6]. Once inside the nucleus, the receptor dimer recognizes and binds specific DNA sequences that in turn results in enhancing or silencing the transcription of specific target genes regulated by the receptor [7,8].
As depicted in Figure 1, nuclear hormone receptors share common functional domains, such as a DNA binding domain (DBD), a ligand binding domain (LBD), and two transactivation domains (AF-1 and AF-2) [9-12]. DBDs contain two zinc finger motifs that allow them to recognize and bind to specific DNA sequences- often referred to as hormone response elements (HREs)- within the promoter and/or enhancer regions to regulate transcription. Different steroid hormone receptors bind to different response elements, thus allowing the receptors to regulate subsets of genes that are necessary to elicit a physiological response. The LBD is involved in the binding of specific hormones, which induces dimerization and nuclear translocation [9-12]. The two transactivation domains, AF-1 and AF-2, are important for modulating transcription of the target genes. AF-2 is located within the LBD and is involved in ligand-dependent transactivation, while AF-1 is found in the N-terminal A/B hypervariable domain (NTD) and is responsible for ligand-independent transcriptional activation and mediates protein-protein interaction with other transcription factors [13]. AF-1 is also responsive to phosphorylation by kinases that are activated in various signaling pathways, including the epidermal growth factor receptor (EGFR) pathway.
Figure 1.
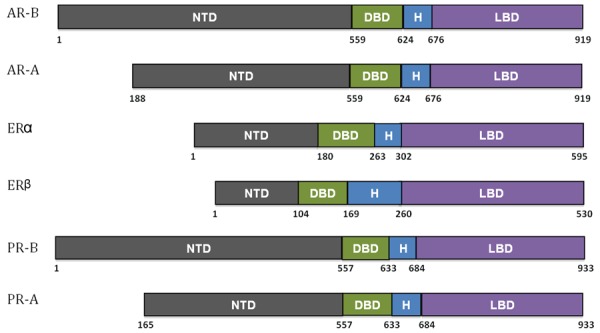
Comparing the functional domains of nuclear hormone receptors. NTD, N-terminal A/B hypervariable domain; DBD, DNA binding domain; H, hinge region; LBD, ligand binding domain. Adapted from [196].
Estrogen receptor
The estrogen receptor (ER) was identified in the 1950s by Dr. Elwood V. Jensen as reviewed in [14,15]. Eventually, it was determined that three forms of ER exist- ERα, ERβ, and GPR-30 [16-19]. All three ERs are encoded by different genes located on different chromosomes [20]. ERα is encoded by the gene ESR1 located on chromosome 6; ERβ is encoded by gene ESR2 on chromosome 14; and GPR-30 is encoded by the GPER gene on chromosome 7. While ERα and ERβ are nuclear hormone receptors, GPR-30 is not a member of the steroid receptor family but is instead a G-protein coupled receptor that has been shown to bind and respond to estrogen [21-23]. Therefore, since our interest is in the role of steroid hormone receptors in breast cancer, GPR-30 will not be discussed further in this review.
Although ERα and ERβ are both expressed in breast tissue and bind to estrogen with similar affinities [24], studies have shown that only ERα is necessary for normal mammary gland development [25,26] leading researchers to question the function of ERβ in normal breast tissue. Multiple studies have also reported that ERβ expression actually represses ERα expression and function [27,28]. In addition to the breast, both nuclear receptors are expressed in many other tissues within the human body, including the endometrium, ovary, testes, cerebral cortex, myocardium, and thyroid [29-32]. However, their expression patterns do differ in certain tissues- for example, ERα is the sole estrogen receptor expressed in the hippocampus, and only ERβ is found in prostate tissue [32].
In addition to full length ERα (as shown in Figure 1), there are 2 truncated splice variants of ERα- 46 kDa estrogen receptor (ER46) [33]and 36 kDa estrogen receptor (ER36) [34,35]. Some of the full length ERα (ER66) along with ER36 and ER46 [36] associate with the plasma membrane and are referred to as mERs due to their ability to translocate to the plasma membrane through palmitoylation and caveolin-1 association, which in turn allows for rapid estrogen receptor signaling. ER46 does not have an AF-1 transactivation domain, which suggests that ER46 does not mediate any nuclear functions. ER36 lacks both AF-1 and AF-2 transactivation domains, and part of the ligand binding domain is replaced by a 27 amino acid sequence in the C terminus [34]. ER36 has been shown to mediate estrogen stimulation via mitogen-activated protein kinase (MAPK) pathway [35].
As with ERα, ERβ also exists as several isoforms- ERβ1 (Figure 1), ERβ1 “short form”, ERβ2/cx, ERβ3, ERβ4, and ERβ5 [37-40]. Most are due to the alternative splicing of exons 7 and 8, although the truncated form of ERβ1 is due to proteolysis at the N-terminus [41,42]. Of all the isoforms, only ERβ1 contains the ligand-binding domain [43]. However, it has been shown that the formation of heterodimers between ERβ1 and the other isoforms increases the transcriptional activity of ERβ1 [37].
Progesterone receptor
The expression of PR is primarily regulated by ERα at the transcriptional level [44,45]. There are two known isoforms of PR, PR-A and PR-B. PR-A is a truncated version of PR-B, lacking 164 amino acids at the N-terminus (Figure 1). The two proteins are transcribed from two different promoters located within the same gene on chromosome 11 [46] and can form homo- or heterodimers. Studies using knockout mice confirmed the functional importance of both PR isoforms [47,48]. Although animals lacking PR-A did not display significant developmental effects in the mammary glands or thymus, they did display severe dysfunctions in their ovaries and uterus resulting in infertility, suggesting that its primary function is maintaining normal ovarian and uterine functions [49-52]. Conversely, PR-B knockout-mice retained normal ovarian, uterine, and thymic functions but exhibited a significant decrease in mammary ductal morphogenesis [49-51,53], indicating that PR-B mediates the proliferative effects of progesterone in the mammary gland.
Structural and functional analyses of each PR isoform suggest that they have different transcription activation properties when bound to progesterone [53,54]. According to Richer et al., approximately 27% of PR-regulated genes are controlled by both PR isoforms. However, this study also indicated that PR-B alone controls the majority of the PR-regulated genes in comparison to PR-A alone (69% versus 4%) [55]which may be in part due to PR-B being intrinsically a stronger transcriptional activator than PR-A. In fact, PR-A has been reported to function as a transcriptional repressor under certain cellular conditions [56]. Curiously however, it is PR-A- not PR-B- that is more frequently over-expressed in breast cancer [57]. Some studies have even indicated that it is not so much the expression of either isoform but rather the ratio of the two isoforms that are important in breast cancer development. For example, a higher ratio of PR-A/B has been associated with poorer prognosis and response to hormone therapy [58].
Androgen receptor
AR is expressed in all tissues, including testis, prostate, foreskin, cervix, vagina, mammary glands, bone, brain, sebaceous and sweat glands of the skin, and breast [59-61]. The gene that codes for the androgen receptor (AR) is located on the X chromosome [60,62]. While AR is closely related to PR and ER, one distinctive feature of the AR protein is the presence of glutamine and glycine repeats in the N-terminal activation domain of the receptor which have been linked to certain cancers and chronic neurological diseases in humans [60,63,64]. In 1994, Wilson and McPhaul discovered two isoforms of AR in human genital skin fibroblasts that are structurally very similar to PR-A and PR-B [65]. Their formation is due to two distinct translation initiation sites which result in the full-length receptor (110 kDa) and an N-terminally truncated form (87 kDa) known as AR-B and AR-A, respectively (Figure 1). Since 1994, several low molecular weight isoforms of AR have been identified, particularly in prostate cancer cell lines and tumors (reviewed in [66]). A few different mechanisms are responsible for these variants, including premature chain termination during translation, proteolysis by calpains, and alternative splicing [66]. Several isoforms, such as AR-V7 (also known as AR3), lack the LBD and are thus capable of transcription activation in the absence of androgen (i.e. androgen-independent transcription) [67,68]. Though originally found in prostate cancer cases that have become androgen deprivation therapy (ADT) or castration resistant [67-70], AR-V7 has likewise been found in a substantial number of primary breast tumors (~50%) and most breast cancer cell lines [71]. Another AR variant, Δ3AR, has been found exclusively in some breast tumors and breast cancer cell lines but not in normal breast tissue [72]. Originally discovered in patients suffering from androgen insensitivity syndrome (AIS), the Δ3AR isoform lacks the second zinc finger in the DBD, which potentially could reduce its ability to inhibit cell growth, at least within an ERα+ setting [72,73].
ERα-positive breast cancer
Prognosis and treatment
In 1896, George Thomas Beatson found that removing the ovaries from patients with advanced stages of breast cancer resulted in significant regression, which later lead to the speculation of estrogen’s stimulating effect on breast cancer. Hence, oophorectomy and/or the use of drugs that target the estrogen receptor have become standard therapies for treating estrogen responsive breast cancer. To date, much of what we know about the relationship between ER and breast cancer centers primarily on one particular receptor- ERα. In fact, it is the presence or absence of ERα that determines whether a patient’s breast cancer can be classified as either estrogen receptor positive or negative, respectively. ERα-negative breast cancers may express other hormone receptors such as PR, AR, and even ERβ, but they are often non-responsive to estrogen. Of all the breast cancer subtypes, ERα-positive breast cancer is the most prevalent, accounting for approximately 75% of breast cancers diagnosed in women [1].
Studies have shown that patients with ERα-positive breast cancers have a better prognosis because these tumors tend to be lower grade and have less aggressive phenotypes. Even patients with metastatic tumors that expressed ERα often had significantly better survival outcomes in comparison to patients with ERα-negative tumors [74], and this is most likely due to the fact that most patients with ERα-positive tumors also had an increase likelihood of responding to the established endocrine therapies [74]. However, not all ER-positive tumors respond to endocrine therapy, and even those that are initially responsive eventually become resistant as the disease progresses.
Drugs that specifically target ERα- estrogen receptor antagonists- can be used to treat or manage the disease. ER antagonists specifically compete with estrogen and block it from binding to the receptor. There are two types of ER antagonists- 1) selective ER modulators (SERMs), also referred to as partial antagonists, and 2) pure or complete antagonists, also referred to as selective ER down-regulators (SERDs) [75]. Among the most common SERMs are the anti-estrogens tamoxifen and raloxifene. Tamoxifen is effective in antagonizing estrogen-dependent cancer cell growth by binding to ERα and promoting the recruitment of co-repressors rather than co-activators in mediating transcriptional repression of ER target genes [11,76]. An example of a pure antagonist or SERD is ICI 182, 780, also referred to as faslodex or fulvestrant, which binds to either ERα or β and promotes receptor degradation [77-79]. Ultimately, ERα is considered a good prognostic marker for breast cancer not only because it is vital in both the development and progression of the disease but also because its presence determines whether the cancer will likely respond to anti-estrogen treatment.
Association with progesterone receptors
Of all the ERα+ mammary tumors, about 50-60% are PR+ [80-82]. Yet while the ER status has been well established as a predictive factor for breast cancer prognosis and cancer treatment, less is known about the significance of PR in the presence of ERα. Studies have shown that tumors expressing both receptors tend to be less aggressive and least likely to metastasize [83,84]. Others have confirmed that the presence of both ERα and PR in tumors often translates to better prognosis [83-86]. Specifically, Dunnwald et al. examined the correlation between PR/ERα expression and mortality risk amongst 155,175 breast cancer patients and found that patients with ERα+/PR+ tumors had lower mortality rates compared to women with ERα+/PR-, ERα-/PR+, and ERα-/PR-. The highest mortality rate was seen in ERα-/PR- patients. A similar study carried out by Salmen et al. determined that patients with ERα-/PR- tumors had worse prognoses than patients with ERα-/PR+ or ERα+/PR+ tumors [87]. Furthermore, tumors that have lost PR expression are often more aggressive and have negative prognoses, signifying that PR is an important indicator of the progression of the disease [82].
Compared to ERα+/PR+ patients, a smaller percentage of ERα+/PR- breast cancer patients respond to tamoxifen treatment [85,88-91], suggesting that PR plays an important role in endocrine therapy response. This could account for the consistent observation that not all ERα+ breast cancer patients respond to endocrine therapies like tamoxifen. As noted earlier, the presence of ERα may not always be sufficient to indicate positive outcome towards endocrine based-therapeutics. This may be due in part to the fact that expression of the ERα protein does not always translate to a functional ERα signaling pathway. Since PR is one of the target genes of ERα, it has long been proposed that the expression of PR may serve as a good indicator of ERα functionality and signaling [92]. Studies have in fact confirmed that the presence PR in ERα+ breast cancer significantly improves the outcome prediction for adjuvant endocrine therapy [85,91,93-96]. Specifically, Ferno et al. found that patients with ERα+/PR+ tumors had significant increase in response to adjuvant tamoxifen therapy compared with patients with ERα+/PR- tumors [94,97]. Other studies have also confirmed that PR status can be a better indicator of tamoxifen response than ERα status alone [96,98]. We speculate that the presence of ERα without PR expression likely suggests a signaling dysfunction in the ERα pathway that reduces its ability to transcriptionally regulate its target genes; therefore, it is not surprising that the presence of both ERα and PR is a better indicator of endocrine therapy responsiveness.
Studies have also demonstrated that PR is associated with overall survival of cancer patients [85,99]. Specifically, patients diagnosed as ERα+/PR+ had less cancer recurrence in comparison to ERα+/PR- cancer patients [85]. We acknowledge that this reported decrease in breast cancer recurrence in ERα+/PR+ patients contradicted earlier studies [86,100], but this may be attributed to the researchers’ use of biochemical assays to measure PR, which lack the sensitivity of other methods such as immunohistochemistry, which is the current practice.
Further complicating the analysis of PR’s role in breast cancer prognosis and therapeutic response is the existence of the two PR isoforms mentioned previously- PR-A and PR-B. The ratio of PR-A to PR-B has been shown to change during the development of breast cancer [55,101], and this may alter prognosis and therapeutic response. Although some breast cancers may express both isoforms, the ratios of the two receptors vary, with PR-A showing a higher expression in most tumors [55,101]. This ratio of PR-A to B can impact the prognosis and staging of the disease, with an equal to low PR-A:PR-B ratio associated with lower tumor grading (G1 and G2) and a high PR-A:PR-B ratio associated with undifferentiated, higher grade tumors (G3) [101]. Recent microarray analysis also suggests that the PR-A:PR-B ratio is a critical determinant of PR target gene selectivity and response to hormonal stimuli [102]. This indicates the importance of evaluating the interplay between PR-A and PR-B when determining the clinical outcomes and responsiveness to endocrine therapy. However, typical methods used to determine the presence of PR in tumors are either ligand binding assays using tumor extracts or antibody-based assays such as immunohistochemistry [103-106], and neither of these assays is capable of differentiating between the two PR isoforms. In some cases, immunohistochemistry may only detect PR-A and not PR-B in formalin fixed tissue, suggesting that conformational differences between the two may interfere with detection [105]. Ultimately, further analysis of the role of PR-A and PR-B in breast cancer is needed to provide a better understanding of PR in prognosis and therapeutic response.
Association with ERβ
ERβ is not commonly used as a prognostic marker for breast cancer, partially because the presence and function of ERβ in ERα-positive tumors are not well understood. As with PR, the existence of several isoforms of ERβ adds to the confusion and has resulted in conflicting data, since the majority of studies that analyze the role of ERβ in ERα+ cells do not differentiate between the various ERβ isoforms. Despite this complexity, some researchers have had the foresight to study the specific ERβ isoforms, and several agree that expression of the ligand-binding isoform ERβ1 in ERα+ cells counteracts ERα activity, thereby suppressing cell proliferation and enhancing apoptosis [39,107,108]. Two other studies carried out by Ogawa et al. and Peng et al. found that overexpression of the isoform ERβ2/cx inhibited the transcriptional activity of ERα [38,109], while this same isoform has been shown to enhance the transcriptional activity of ERβ1 [37]. An in vitro study led by John Hawse concluded that the tamoxifen metabolite endoxifen worked best at inhibiting estrogen-mediated cell proliferation in ERα+ cells if ERβ was also expressed [110]. It was also determined that endoxifen treatment of cells led to ERβ accumulation and subsequent increase in ERα/ERβ heterodimers, providing further evidence that the observed cancer suppressing activity of ERβ is due in part to a direct interaction between ERβ and ERα. However, a clinical study analyzing the effect of a 2-year tamoxifen treatment on 353 patients with stage II primary breast tumors found that patients who were ERα-/ERβ+ had significantly greater distant disease-free survival compared with patients who were ERα+/ERβ+, suggesting a mechanism for ERβ independent of ERα [111]. Since neither the levels of the tamoxifen metabolite endoxifen nor the exact ERβ isoforms were determined in these patients, it is difficult to directly compare this study with that of Hawse’s. Yet these seemingly differing conclusions do point to a rather complex role for ERβ in breast cancer, which clearly involves more than just inactivation of ERα.
Association with androgen receptors
AR expression is most commonly associated with prostate cancer, and prostate tumor progression is as dependent on AR activity as breast tumor progression is on ERα activity. In fact, the treatment of prostate cancers usually involves hindering AR function via ligand depletion, treatment with AR antagonists, or both [112]. The role of AR in breast cancer is not quite as clear. AR is frequently expressed in ductal carcinoma in situ (DCIS) and invasive breast carcinoma [113]. In addition, most ERα+ breast cancers also appear to express AR, as exemplified by a study carried out by Hu et al. which found that 88% of 1,164 ER positive breast cancer cases also expressed AR [114]. Another study by Agrawal et al. analyzed the importance of using AR as a prognostic marker in 488 breast cancer patients who underwent radical mastectomies [115]. Data from this study suggest that the presence of AR increased the success of adjuvant therapy and prognosis in patients. Additionally, they found that 50.7% of breast cancer patients who were AR negative had lower 5-year survival rates, indicating poorer prognosis [115]. In yet another study carried out by Qu and colleagues, 109 breast cancer patients in Shanghai were retrospectively analyzed between 2003 and 2008 [116]. Of the 109 patients, 52 were diagnosed AR+. Overall, there were 13 deaths and 15 recurrences but only two of the deaths and three of the recurrences were from the AR+ group, which again indicates that AR could be a good marker for longer overall survival and lower risk of recurrence. Conversely, mammary tumors that do not express AR have been shown to respond poorly to hormone therapy [117]. The absence of AR has also been correlated with higher levels of Ki-67, a cell proliferation marker associated with cancer progression [118], though the molecular mechanism behind this finding has not been elucidated. Furthermore, androgen-activated AR appears to directly bind to the ERβ promoter and enhance transcription of ERβ in both ERα-positive and -negative breast cancer cells [119], suggesting that blocking AR function may subsequently decrease ERβ-dependent gene expression.
Although many studies appear to support the hypothesis that AR helps counteract the tumorigenic effects of ERα, others- such as one carried out by Paliouras and Diamandis- report a synergistic mechanism between the two receptors, resulting in an increase in breast cancer progression [120]. Specifically, the ER-dependent expression of a group of cancer biomarkers called kallikrein (KLKs) genes was shown to be enhanced significantly by the binding of androgen to AR. One noteworthy limitation of this study is that it focused on just one breast cancer cell line (BT474) and therefore may not be indicative of most breast cancer cases. Another study by Liao et al. showed that simultaneous treatment with androgen and estrogen stimulated mammary gland carcinomas in 100% of the Noble rats tested [121]. The researchers concluded that high levels of androgen and estrogen together may be an important risk factor for breast cancer and that direct binding of androgens to either AR or PR were involved in this carcinogenic process. However, although the researchers analyzed various isoforms of ER and PR, they only concentrated on the two AR isoforms, AR-A and AR-B, and used only male rats in their study. Additionally-and perhaps most importantly- the authors did not sufficiently rule out the possibility that the results were due to aromatization of androgen to estrogen. A more recent study carried out by Richer and colleagues did determine that treatment of AR+/ERα+ breast cancer cell lines with the AR inhibitor enzalutamide- a drug currently used to treat metastatic prostate cancer- effectively inhibited cell proliferation both in vitro and in animal models, suggesting that AR activity may indeed promote cancer progression in the presence of ERα [122]. Yet again, isoforms of AR were neither analyzed nor even acknowledged in this study.
In contrast to the above studies, several clinical studies have indicated that administration of normal, physiological levels of androgen to women receiving estrogen therapy actually decreases breast cancer risk (reviewed in [59]), but the mechanism behind this- particularly in regards to AR signaling- is not clear. Agrawal and colleagues reported that only in the absence of estrogen will androgens directly bind to ERα and stimulate the proliferation of cancerous cells [115]. Finally, in an excellent, in-depth review of AR in breast cancer, K. M. McNamara and colleagues acknowledge that the role of AR in breast cancer risk and progression depends greatly on the specific disease subtype and the presence or absence of the other steroid receptors, such as ERα [123]. The authors further assert that in the presence of ERα, AR activation appears to counteract disease progression in most breast cancer subtypes. However, a further complication involves the ratio of AR to ER, which also appears to affect progression and efficacy of endocrine treatment. A clinical analysis of 192 ERα+ breast cancer patients carried out by Richer and colleagues revealed that a high AR to ERα ratio correlated positively with increased incidence of tamoxifen failure [122]. Despite all the confusing and contradictory findings, it does appear evident that AR could serve as an important prognostic indicator as long as AR isoforms, ERα status and estrogen levels are also taken into account.
ERα-negative breast cancer
ERβ-positive breast cancer
Although studies have found that both mRNA and protein levels of ERβ are significantly lower in breast tumors compared to normal breast tissue [124,125], approximately 17% of primary breast cancers are ERα-negative/ERβ-positive [126,127]. In addition, 47-60% of all ERα-negative tumors have been reported to be ERβ-positive [42,126]. Most studies agree that the presence of ERβ in these tumors is correlated with a positive prognosis, as the absence of ERβ in ERα-negative patients (ERα-/ERβ-) is associated with early relapse [111,128-130]. It has also been reported that as breast tumors become more malignant, ERβ expression decreases (reviewed in [131]). However, one recent study carried out by Chen et al. contradicts these findings [132]. The researchers found that expression of ERβ actually enhanced cancer progression by inducing the expression of IL-8, which is known to play a role in angiogenesis and metastasis [132,133].
Predictably, most of the aforementioned studies failed to differentiate between the various isoforms of ERβ, which once again could be responsible for these conflicting results. The small handful of studies that actually have analyzed the expression of specific ERβ isoforms have found that ERβ1, -2/cx, -3, and -5 are expressed at varying degrees in breast tumors and breast tumor cell lines [42,43,134,135]. ERβ2/cx (also designated simply as ERβ2) is the main isoform expressed in the hormone-sensitive breast tumor cell line T47D, whereas ERβ5 is the major isoform in the hormone-insensitive BT20 breast tumor cell line [134]. Increased expression of both ERβ2/cx and ERβ5 relative to the full-length isoform ERβ1 appears to correlate with increased breast cancer progression [135], and yet increased expression of ERβ5 has also been positively correlated with breast cancer survival [136,137]. A recent clinical study of 95 patients with ERα-negative invasive breast carcinomas demonstrated a correlation between prognosis and the ERβ1 to ERβ2 ratio, associating higher levels of ERβ2 with tumor relapse [138]. Yet despite their apparent statistical significance, the differences between ERβ1, ERβ2 and tumor relapse appeared fairly modest, which could be due to the relatively modest sample size or to the researchers’ sole use of immunohistochemistry to detect the individual isoforms. Though the clinical significance of this particular study is somewhat questionable, an earlier prostate cancer study carried out by Leung et al. did reveal a correlation between the increased expression of ERβ2 and ERβ5 and enhanced metastasis and poor prognosis [139]. Clearly more studies are needed to better understand the prognostic value of the various ERβ isoforms in breast cancer.
Regardless, the overwhelming consensus that ERβ is a positive prognostic marker is due largely to the fact that a significant number of ERβ+ patients respond well to endocrine therapy, such as tamoxifen. Several clinical studies have shown that patients who are ERα-negative or even triple negative (ERα-/PR-/HER2-) but express high levels of ERβ respond well to tamoxifen [111,128,130], whereas low levels of ERβ correlate with resistance to tamoxifen treatment [140,141]. In addition, other chemotherapeutic agents such as doxorubicin and cisplatin have been found to be more effective on breast cancer cell lines that express ERβ5 [142]. Aside from drug response, modification of ERβ has recently been shown to indicate good prognosis as well. Specifically, a clinical study led by Valerie Speirs found that breast cancer patients expressing ERβ phosphorylated at serine 105 had more favorable prognosis [143]. Though the mechanism behind this finding is unknown, it adds yet another level of complexity to an already complicated ERβ narrative.
PR-positive breast cancer
ERα-/PR+ breast cancers only account for about 2-7% of total breast cancer cases [81,144-146], although there is controversy over whether or not such cancers actually exist. While many studies have supported the claim that ERα-/PR+ is a distinct class of breast tumors [81,94,144-152], skeptics contend that since PR is an ER target gene, ER expression is a prerequisite for the expression of PR [153-155]. They maintain that the PR positivity in ERα-negative tumors may simply reflect methodological errors in detecting PR and/or ERα, resulting in either a false-negative ERα result or a false-positive PR result [153,154]. In fact, it is possible that tumors denoted as ERα-/PR+ may actually have fairly low levels of ERα- far below the sensitivity of the current assays [153]. Furthermore, results from Iwase et al. have indicated the possibility that the presence of ERα variants may exist in ERα-/PR+ tumors, specifically a variant with a deletion of exon 5 [156]. Though the presence of this variant in human breast tumors was confirmed in three other studies [157-159], this and other ERα variants may be difficult to detect via the traditional ERα assays used in immunohistochemistry, thus complicating the ERα status of certain mammary tumors. However, ERα aside, the PR promoter has been shown to be regulated by other transcription factors such as AP-1 and SP-1 [160-162]. In fact, an earlier study by Encarnación et al. indicated that while most of the ERα+ tumors converted to an ERα- phenotype, the PR status remained unchanged, further supporting the existence of an ERα-/PR+ clinical subtype and the possibility that PR can be regulated by signaling pathways other than ERα [163].
Although the expression of PR in breast cancer cells has been linked to both positive endocrine response and clinical outcome [82], it is unclear how PR affects the outcome in ERα-negative breast cancers. Multiple reports have indicated that ERα-/PR+ breast tumors constitute a distinct clinicopathological group of cancers that results in outcomes worse than those that are ERα+/PR+ or ERα+/PR-, yet results in a better prognosis than double negative tumors (ERα-/PR-) [144,152,164-166]. It is questionable whether this clinical subtype of tumor is responsive to endocrine therapies like tamoxifen. As noted earlier, PR has been shown to be an indicator of responsiveness to endocrine therapy in the presence of ERα, and thus it is conceivable that ERα-/PR+ tumors may also respond to tamoxifen. On the other hand, since current endocrine therapies are believed to target the ERα signaling pathway, it seems counterintuitive to attempt such therapeutic options on ERα-/PR+ cancer patients, particularly since the presence of PR in ERα+ tissues merely indicates a functional ERα, the presence of which is believed necessary for tamoxifen to have any effect. Yet a recent study by Yang et al. found that patients with low-grade ERα-/PR+ tumors did experience an overall survival benefit from adjuvant hormone therapy using tamoxifen; and conversely, no benefit was observed in patients with high-grade ERα-/PR+ tumors [96]. Unfortunately, the scarcity of studies on this particular clinical subtype limits our ability to do a complete evaluation of the effectiveness of tamoxifen.
Theoretically, tumors that are ERα-/PR+ should be treatable with selective progesterone receptor modulators (SPRM), also referred to as anti-progestins. Anti-progestins such as mifepristone (RU-486) have been proposed as a new form of endocrine treatment or as an adjunct to the anti-estrogenic treatments for breast cancer. Multiple in vitro studies using cancer cell lines have indicated that low doses of anti-progestins can inhibit PR- and estrogen-mediated cell proliferation [167-169]. Contrary to these observations, other studies have demonstrated that at higher concentrations, mifepristone and other anti-progestins can actually stimulate proliferation of the ER+/PR+ breast cancer cells, T47D and MCF7 [170-172], indicating that the effect of anti-progestins on proliferation is dose-dependent and perhaps even dependent on the level of functional ERα. The few clinical studies that have tested the effectiveness of anti-progestins show limited to minimal efficacy in treating PR+ breast cancer [173-175]. Yet there is evidence that anti-progestins can augment the effects of anti-estrogens like tamoxifen [176-178]. Clearly, further clinical studies are necessary to determine if PR is a viable therapeutic target in ERα- breast cancer.
AR-positive breast cancer
AR has been found in a significant number of ERα-negative tumors as well, with 22 to 49% of ERα-negative breast tumors expressing AR, depending on the clinical study [114,118,179,180]. The presence of AR in ERα- breast cancer is often associated with lower tumor grade, smaller tumor size, and significant increase in survival rates Results inconsistent with these clinical data have been reported by some researchers who have found that androgen-enhanced expression of AR in the ER-/PR-/HER2+ breast cancer cell line MDA-MB-453 increases cell proliferation [181,182], which can be inhibited by the AR antagonist bicalutamide [182]. Additionally, Richer et al. found that the AR inhibitor enzalutamide inhibited cell proliferation in ERα-/AR+ breast cancer cell lines [122]. Given that isoforms of AR were not taken into consideration in any of these studies, such contradictory findings between clinical and in vitro studies are not that surprising.
A fraction of triple-negative breast cancer cases (13-35%) appear to express AR [180,183,184]. Although some in vitro studies on triple-negative/AR+ cell lines have demonstrated an androgen-induced increase in cell proliferation [182,185], most clinical studies find that the presence of AR in triple-negative tumors is correlated with a lower recurrence rate, fewer positive lymph node and distant metastases, lower histological grade, and higher overall survival rate compared to triple-negative tumors that are AR-negative [180,183,184,186]. These data support the notion that AR can be used as a positive prognostic factor, not only in ERα-negative but also triple-negative breast cancers.
In addition to its prospective use as a prognostic biomarker for breast cancer, additional studies have indicated that AR may also serve as a potential therapeutic target for those breast cancer patients who traditionally have had very few treatment options, such as those whose tumors are ER-/PR-/AR+. Hardin and colleagues tested the effectiveness of the androgen dehydroepiandrosterone sulfate (DHEAS) on ER-/PR-/AR+ breast cancer cell lines and found that DHEAS stimulation of AR hampered cell growth by enhancing apoptosis [187]. Conflicting studies by Ni et al. and Arce-Salinas et al. showed that inhibiting AR with the antagonist bicalutamide led to growth inhibition and enhanced cell death [182,188]. Interestingly, AR expression seems to be correlated with the over-expression of the oncogene HER2 via a complicated signaling cascade that involves upregulation of HER2 by AR and transcription factor β-catenin [182]. In addition, expression of the constitutively active AR variant AR-V7 in both ERα- breast cancer cell lines (e.g. MDA-MB-453) and ERα- primary tissues is actually enhanced by the AR antagonist enzalutamide, which in turn increases cell growth and, as with more advanced stages of prostate cancer, could result in ADT resistance [71]. Clearly the use of AR as a therapeutic target is wrought with complications, as both agonists and antagonists of AR have succeeded in either suppressing or promoting tumor progression, and success appears to be highly dependent on AR isoform expression and ERα levels.
Discussion and conclusion
Triple negative breast cancer (i.e. ER-/PR-/HER2-) is virtually impossible to treat with the established endocrine-based therapies, as such therapies were originally intended for cancers that are ERα-positive. However, our review illustrates how confining the triple negative designation can be, potentially preventing clinicians from considering other factors that play a role in breast cancer progression. Data obtained from basic and translational research studies indicate that there are indeed many other proteins involved in breast cancer development and progression; but despite these findings, most clinical pathologists continue to follow the standard practice of categorizing breast tumors using only the three established markers- ERα, PR, and HER2. This apparent disconnect between the bench and the clinic is alarming when one considers that 10-15% of breast cancers are diagnosed as triple negative [2,3] and that even patients diagnosed as ERα-positive do not all respond equally well to anti-estrogen treatment, which further demonstrates the complexity of breast cancer and emphasizes the need for more therapeutic targets.
The goal of this review was to determine if other steroid hormone receptors- most notably ERβ and AR- should also be routinely analyzed and used as additional targets for breast cancer treatment and prognosis. Although a significant number of studies exist describing the presence of ERβ, AR, and PR in breast cancer cells or tissues, obtaining truly accurate expression levels from the literature proved difficult as all three of these receptors exist in at least two isoform states. Most studies did not account for this variability. Therefore, it is probable that many isoforms went undetected, which in turn could have led to an underestimate of a receptor’s actual expression level. Yet, quantitative inaccuracies aside, we still found very strong evidence that each of these receptors influences tumor progression, either negatively or positively, depending upon which isoforms are present and the level of ERα expression. For example, ERα+/PR+ breast tumors are generally found to be more responsive to endocrine treatment than ERα+/PR- tumors [85,91,93-96], and yet a higher expression level of the PR-A isoform compared to PR-B has been associated with anti-estrogen resistance and subsequently poorer prognosis [102]. The isoforms of ERβ are capable of forming heterodimers with ERα, inhibiting ERα activity and consequently resulting in tumor suppression. However, other studies have shown a positive correlation between tumor progression and increased levels of ERβ2/cx and ERβ5 isoforms relative to ERβ1 [135,138,139]. The expression of certain isoforms, such as ERβ5, has also been correlated with positive drug response [142]. Unfortunately, due to the relative scarcity of studies analyzing AR in breast tumors, few clinically significant associations were found between a specific AR isoform and breast tumor progression. However, certain splice variants such as the ligand-independent isoform AR-V7 have been shown to impact tumor development and progression in prostate cancer [67,68], and a recent study by the Tilley lab strongly suggest that it may have the same impact on breast cancer progression [71]. Additionally, the available data do show that the presence of AR is correlated with good prognosis for both ERα-positive and ERα-negative breast cancer, indicating that anti-androgen therapy might be a viable option in certain breast cancers as it is in prostate cancer.
We acknowledge that more basic research is necessary to elucidate the molecular mechanisms behind the steroid hormone receptors’ effects on breast cancer development and progression. In particular, more studies are needed to better understand how ERβ, PR, and AR influence cell proliferation, apoptosis, and metastasis. However, in the interest of saving lives, we feel that the existing data justify collecting more clinical data on a more massive scale. We contend that all breast tumor biopsies should be analyzed not only for ERα, HER2, and overall PR expression but also for each isoform of ERα, PR, ERβ, and AR. Obviously this is an ambitious undertaking that would require developing new techniques and improving current detection methods for each isoform. In addition, subsequent large-scale analysis of the data will be necessary to determine if consistent patterns emerge linking the various expression levels of these markers and their isoforms with particular grades and stages of breast cancer.
More and more clinical labs are trending towards molecular diagnostic procedures such as next generation sequencing (NGS), RNA sequencing, and high-throughput qRT-PCR, all of which will allow scientists to analyze more genetic markers in a relatively short period of time. Additionally, the isolation and analysis of circulating cell-free DNA (ccfDNA) could allow for initial prognosis using very little sample. In fact, several clinical and translational studies have already shown how NGS and ccfDNA analysis can aid in breast cancer prognosis and tumor classification [189-193]. However, techniques such as NGS are best suited for patients who may have a genetic predisposition for a particular cancer (e.g. BRCA1 and BRCA2 in some familial breast cancers); and though RNA sequencing and qRT-PCR do give information regarding transcriptional expression, it is at the protein level that phenotypes are often determined.
Therefore, although the utilization of molecular techniques will enable clinicians to obtain more information about a patient’s tumor, these techniques should always be used in conjunction with protein expression analyses- particularly since not all of the steroid receptor isoforms are due to splicing variations but instead are generated post-translationally. In addition, post-translational modifications such as phosphorylation and glycosylation cannot be detected at the DNA or RNA level but only by analyzing the protein expression profiles of tumors. Finally, the heterogeneity of the cell population within an individual tumor must be taken into account when analyzing all the data this new technology will generate. To date, the most common procedures for studying protein expression are immunohistochemistry and western blotting. Though immunohistochemistry provides an important visual of protein expression variations between tissues and even between cells within the tumor, and western blotting is a more stringent and exact method for detecting isoforms of varying molecular weights, both depend on antibody-binding, which is an indirect method of protein detection. Recent advances in multiplexed protein analysis include mass spectrometry immunohistochemistry (MSIHC), in which metal tags of varying masses are used to label antibodies, thus allowing for the simultaneous detection of up to 100 different protein targets [194]. Such a technique has been used successfully in analyzing various markers such as ERα, PR, and HER2 in breast tumor samples [195]. Again, however, such a technique still relies on antibody recognition and binding. Another technique that involves rapid protein isolation and sequencing on a large-scale would need to be developed in future to directly identify the various steroid receptor isoforms expressed in a given tumor. Ultimately, we envision a multipronged approach to breast cancer analysis that will allow detection of a variety of markers, including the many isoforms of ERα, ERβ, PR, and AR, coupled with the pathology of the tumor. The overall goal would be to provide each patient with a more personalized and accurate prognosis and more effective treatment options, and maybe even render obsolete the term “triple negative breast cancer”.
Acknowledgements
We thank Esmeralda Ponce, Mariah Alejo, and Rubi Garcia for their support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of conflict of interest
None.
Abbreviations
- ER
estrogen receptor
- PR
progesterone receptor
- AR
androgen receptor
- HER2
human epidermal growth factor receptor type 2
- GR
glucocorticoid receptor
- GPR-30
G-protein coupled receptor-30
- DBD
DNA binding domain
- LBD
ligand binding domain
- HRE
hormone response element
- NTD
N-terminal domain
- SERMs
selective ER modulators
- SERDs
selective ER down-regulators
References
- 1.American Cancer Society. Cancer facts & figures 2016. Atlanta American Cancer Society; 2016. [Google Scholar]
- 2.Dawood S. Triple-negative breast cancer: epidemiology and management options. Drugs. 2010;70:2247–2258. doi: 10.2165/11538150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triplenegative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 5.MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev. 1998;50:151–196. [PubMed] [Google Scholar]
- 6.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: a G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265-266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stossi F, Madak-Erdogan Z, Katzenellenbogen BS. Estrogen receptor alpha represses transcription of early target genes via p300 and CtBP1. Mol Cell Biol. 2009;29:1749–1759. doi: 10.1128/MCB.01476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 9.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Li X, Yi P, Hilf R, Bambara RA, Muyan M. Targeting estrogen responsive elements (EREs): design of potent transactivators for ERE-containing genes. Mol Cell Endocrinol. 2004;218:65–78. doi: 10.1016/j.mce.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–272. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 13.Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Malley BW, Khan S. Elwood V. Jensen (1920-2012): father of the nuclear receptors. Proc Natl Acad Sci U S A. 2013;110:3707–3708. doi: 10.1073/pnas.1301566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- 16.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a Gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 17.Filardo EJ, Quinn JA, Frackelton AR, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 18.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt SC, Korach KS. Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord. 2002;3:193–200. doi: 10.1023/a:1020068224909. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 21.Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz ER, Cappello AR, Dolce V, Abonante S, Pezzi V, Maggiolini M. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ Health Perspect. 2008;116:1648–1655. doi: 10.1289/ehp.11297. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 23.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speirs V, Skliris GP, Burdall SE, Carder PJ. Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol. 2002;55:371–374. doi: 10.1136/jcp.55.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta RG, Hawthorne M, Mehta RR, Torres KE, Peng X, McCormick DL, Kopelovich L. Differential roles of ERalpha and ERbeta in normal and neoplastic development in the mouse mammary gland. PLoS One. 2014;9:e113175. doi: 10.1371/journal.pone.0113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci U S A. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stope MB, Popp SL, Knabbe C, Buck MB. Estrogen receptor alpha attenuates transforming growth factor-beta signaling in breast cancer cells independent from agonistic and antagonistic ligands. Breast Cancer Res Treat. 2010;120:357–367. doi: 10.1007/s10549-009-0393-2. [DOI] [PubMed] [Google Scholar]
- 29.Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002;53:709–719. doi: 10.1016/s0008-6363(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 30.Halon A, Materna V, Drag-Zalesinska M, Nowak-Markwitz E, Gansukh T, Donizy P, Spaczynski M, Zabel M, Dietel M, Lage H, Surowiak P. Estrogen receptor alpha expression in ovarian cancer predicts longer overall survival. Pathol Oncol Res. 2011;17:511–518. doi: 10.1007/s12253-010-9340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mylonas I, Jeschke U, Shabani N, Kuhn C, Balle A, Kriegel S, Kupka MS, Friese K. Immunohistochemical analysis of estrogen receptor alpha, estrogen receptor beta and progesterone receptor in normal human endometrium. Acta Histochemica. 2004;106:245–252. doi: 10.1016/j.acthis.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 33.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membraneinitiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin AH, Li RW, Ho EY, Leung GP, Leung SW, Vanhoutte PM, Man RY. Differential ligand binding affinities of human estrogen receptoralpha isoforms. PLoS One. 2013;8:e63199. doi: 10.1371/journal.pone.0063199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-β isoforms: a key to understanding ER-β signaling. Proc Natl Acad Sci U S A. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998;26:3505–3512. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S. Estrogen receptor (ER) beta1 and ERbetacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene. 2003;22:5011–5020. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- 40.Poola I, Abraham J, Baldwin K, Saunders A, Bhatnagar R. Estrogen receptors beta4 and beta5 are full length functionally distinct ERbeta isoforms: cloning from human ovary and functional characterization. Endocrine. 2005;27:227–238. doi: 10.1385/ENDO:27:3:227. [DOI] [PubMed] [Google Scholar]
- 41.Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 42.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-β in oestrogen receptor-α negative human breast tumours. Br J Cancer. 2006;95:616–626. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leygue E, Murphy LC. A bi-faceted role of estrogen receptor beta in breast cancer. Endocr Relat Cancer. 2013;20:R127–139. doi: 10.1530/ERC-12-0389. [DOI] [PubMed] [Google Scholar]
- 44.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 45.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 46.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conneely OM, Lydon JP. Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids. 2000;65:571–577. doi: 10.1016/s0039-128x(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 48.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 49.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 50.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science (New York, N.Y. ) 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 52.Soyal S, Ismail PM, Li J, Mulac-Jericevic B, Conneely OM, Lydon JP. Progesterone’s role in mammary gland development and tumorigenesis as disclosed by experimental mouse genetics. Breast Cancer Res. 2002;4:191–196. doi: 10.1186/bcr451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humphreys RC, Lydon JP, O’Malley BW, Rosen JM. Use of PRKO mice to study the role of progesterone in mammary gland development. J Mammary Gland Biol Neoplasia. 1997;2:343–354. doi: 10.1023/a:1026343212187. [DOI] [PubMed] [Google Scholar]
- 54.Humphreys RC, Lydon J, O’Malley BW, Rosen JM. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol Endocrinol. 1997;11:801–811. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- 55.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 56.Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- 57.Graham JD, Yager ML, Hill HD, Byth K, O’Neill GM, Clarke CL. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endocrinol. 2005;19:2713–2735. doi: 10.1210/me.2005-0126. [DOI] [PubMed] [Google Scholar]
- 58.Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;10:2751–2760. doi: 10.1158/1078-0432.ccr-03-0141. [DOI] [PubMed] [Google Scholar]
- 59.Dimitrakakis C, Bondy C. Androgens and the breast. Breast Cancer Res. 2009;11:212. doi: 10.1186/bcr2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelmann EP. Molecular biology of the androgen receptor. J. Clin. Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Ruizeveld de Winter JA, Trapman J, Vermey M, Mulder E, Zegers ND, van der Kwast TH. Androgen receptor expression in human tissues: an immunohistochemical study. J Histochem Cytochem. 1991;39:927–936. doi: 10.1177/39.7.1865110. [DOI] [PubMed] [Google Scholar]
- 62.Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 63.Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- 64.Poletti A. The polyglutamine tract of androgen receptor: from functions to dysfunctions in motor neurons. Front Neuroendocrinol. 2004;25:1–26. doi: 10.1016/j.yfrne.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are present in human genital skin fibroblasts. Proc Natl Acad Sci U S A. 1994;91:1234–1238. doi: 10.1073/pnas.91.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mudryj M, Tepper CG. On the origins of the androgen receptor low molecular weight species. Horm Cancer. 2013;4:259–269. doi: 10.1007/s12672-013-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormonerefractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hickey TE, Irvine CM, Dvinge H, Tarulli GA, Hanson AR, Ryan NK, Pickering MA, Birrell SN, Hu DG, Mackenzie PI, Russell R, Caldas C, Raj GV, Dehm SM, Plymate SR, Bradley RK, Tilley WD, Selth LA. Expression of androgen receptor splice variants in clinical breast cancers. Oncotarget. 2015;6:44728–44744. doi: 10.18632/oncotarget.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu X, Daffada AA, Chan CM, Dowsett M. Identification of an exon 3 deletion splice variant androgen receptor mRNA in human breast cancer. Int J Cancer. 1997;72:574–580. doi: 10.1002/(sici)1097-0215(19970807)72:4<574::aid-ijc4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 73.Quigley CA, Evans BA, Simental JA, Marschke KB, Sar M, Lubahn DB, Davies P, Hughes IA, Wilson EM, French FS. Complete androgen insensitivity due to deletion of exon C of the androgen receptor gene highlights the functional importance of the second zinc finger of the androgen receptor in vivo. Mol Endocrinol. 1992;6:1103–1112. doi: 10.1210/mend.6.7.1508223. [DOI] [PubMed] [Google Scholar]
- 74.Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5:271–281. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- 75.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 76.Heldring N, Pawson T, McDonnell D, Treuter E, Gustafsson JA, Pike AC. Structural insights into corepressor recognition by antagonistbound estrogen receptors. J Biol Chem. 2007;282:10449–10455. doi: 10.1074/jbc.M611424200. [DOI] [PubMed] [Google Scholar]
- 77.Yeh WL, Shioda K, Coser KR, Rivizzigno D, McSweeney KR, Shioda T. Fulvestrant-induced cell death and proteasomal degradation of estrogen receptor alpha protein in MCF-7 cells require the CSK c-Src tyrosine kinase. PLoS One. 2013;8:e60889. doi: 10.1371/journal.pone.0060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Long X, Nephew KP. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J Biol Chem. 2006;281:9607–9615. doi: 10.1074/jbc.M510809200. [DOI] [PubMed] [Google Scholar]
- 79.Kocanova S, Mazaheri M, Caze-Subra S, Bystricky K. Ligands specify estrogen receptor alpha nuclear localization and degradation. BMC Cell Biol. 2010;11:98. doi: 10.1186/1471-2121-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arpino G, Weiss H, Lee AV, Schiff R, Placido SD, Osborne CK, Elledge RM. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 81.Chu KC, Anderson WF, Fritz A, Ries LA, Brawley OW. Frequency distributions of breast cancer characteristics classified by estrogen receptor and progesterone receptor status for eight racial/ethnic groups. Cancer. 2001;92:37–45. doi: 10.1002/1097-0142(20010701)92:1<37::aid-cncr1289>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 82.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J. Clin. Oncol. 2005;23:7721–7735. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50:7057–7061. [PubMed] [Google Scholar]
- 85.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J. Clin. Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 86.Pichon MF, Pallud C, Brunet M, Milgrom E. Relationship of presence of progesterone receptors to prognosis in early breast cancer. Cancer Res. 1980;40:3357–3360. [PubMed] [Google Scholar]
- 87.Salmen J, Neugebauer J, Fasching PA, Haeberle L, Huober J, Wockel A, Rauh C, Schuetz F, Weissenbacher T, Kost B, Stickeler E, Klar M, Orlowska-Volk M, Windfuhr-Blum M, Heil J, Rom J, Sohn C, Fehm T, Mohrmann S, Loehberg CR, Hein A, Schulz-Wendtland R, Hartkopf AD, Brucker SY, Wallwiener D, Friese K, Hartmann A, Beckmann MW, Janni W, Rack B. Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res Treat. 2014;148:143–151. doi: 10.1007/s10549-014-3130-4. [DOI] [PubMed] [Google Scholar]
- 88.Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, Martino S, Osborne CK. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89:111–117. [PubMed] [Google Scholar]
- 89.McGuire WL. Steroid receptors in human breast cancer. Cancer Res. 1978;38:4289–4291. [PubMed] [Google Scholar]
- 90.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Osborne CK, Yochmowitz MG, Knight WA, McGuire WL. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980;46:2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 92.Horwitz KB, Costlow ME, McGuire WL. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975;26:785–795. doi: 10.1016/0039-128x(75)90110-5. [DOI] [PubMed] [Google Scholar]
- 93.Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, Kim SW, Lee JE, Nam SJ. Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15:138. doi: 10.1186/s12885-015-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferno M, Stal O, Baldetorp B, Hatschek T, Kallstrom AC, Malmstrom P, Nordenskjold B, Ryden S. Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. South Sweden Breast Cancer Group, and South-East Sweden Breast Cancer Group. Breast Cancer Res Treat. 2000;59:69–76. doi: 10.1023/a:1006332423620. [DOI] [PubMed] [Google Scholar]
- 95.Purdie CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, Thompson AM. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer. 2014;110:565–572. doi: 10.1038/bjc.2013.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang LH, Tseng HS, Lin C, Chen LS, Chen ST, Kuo SJ, Chen DR. Survival benefit of tamoxifen in estrogen receptor-negative and progesterone receptor-positive low grade breast cancer patients. J Breast Cancer. 2012;15:288–295. doi: 10.4048/jbc.2012.15.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 98.Stendahl M, Ryden L, Nordenskjold B, Jonsson PE, Landberg G, Jirstrom K. High progesterone receptor expression correlates to the effect of adjuvant tamoxifen in premenopausal breast cancer patients. Clin Cancer Res. 2006;12:4614–4618. doi: 10.1158/1078-0432.CCR-06-0248. [DOI] [PubMed] [Google Scholar]
- 99.Clark GM, McGuire WL, Hubay CA, Pearson OH, Marshall JS. Progesterone receptors as a prognostic factor in Stage II breast cancer. N Engl J Med. 1983;309:1343–1347. doi: 10.1056/nejm198312013092240. [DOI] [PubMed] [Google Scholar]
- 100.Rutqvist LE, Cedermark B, Fornander T, Glas U, Johansson H, Nordenskjöld B, Rotstein S, Skoog L, Somell A, Theve T, et al. The relationship between hormone receptor content and the effect of adjuvant tamoxifen in operable breast cancer. J. Clin. Oncol. 1989;7:1474–1484. doi: 10.1200/JCO.1989.7.10.1474. [DOI] [PubMed] [Google Scholar]
- 101.Bamberger AM, Milde-Langosch K, Schulte HM, Löning T. Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res. 2000;54:32–37. doi: 10.1159/000063434. [DOI] [PubMed] [Google Scholar]
- 102.Khan JA, Bellance C, Guiochon-Mantel A, Lombès M, Loosfelt H. Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line. PLoS One. 2012;7:e45993. doi: 10.1371/journal.pone.0045993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 104.Gasparini G, Pozza F, Dittadi R, Meli S, Cazzavillan S, Bevilacqua P. Progesterone receptor determined by immunocytochemical and biochemical methods in human breast cancer. J Cancer Res Clin Oncol. 1992;118:557–563. doi: 10.1007/BF01225273. [DOI] [PubMed] [Google Scholar]
- 105.Mote PA, Johnston JF, Manninen T, Tuohimaa P, Clarke CL. Detection of progesterone receptor forms A and B by immunohistochemical analysis. J Clin Pathol. 2001;54:624–630. doi: 10.1136/jcp.54.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seymour L, Meyer K, Esser J, MacPhail AP, Behr A, Bezwoda WR. Estimation of PR and ER by immunocytochemistry in breast cancer. Comparison with radioligand binding methods. Am J Clin Pathol. 1990;94:S35–40. [PubMed] [Google Scholar]
- 107.Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Estrogen receptors alfa (ERalpha) and beta (ERbeta) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- 108.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng B, Lu B, Leygue E, Murphy LC. Putative functional characteristics of human estrogen receptor-beta isoforms. J Mol Endocrinol. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- 110.Wu X, Subramaniam M, Grygo SB, Sun Z, Negron V, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011;13:R27. doi: 10.1186/bcr2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A, Ferno M. Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res. 2007;13:1987–1994. doi: 10.1158/1078-0432.CCR-06-1823. [DOI] [PubMed] [Google Scholar]
- 112.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703–711. doi: 10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 114.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA, Tamimi RM. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17:1867–1874. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agrawal AK, Jelen M, Grzebieniak Z, Zukrowski P, Rudnicki J, Nienartowicz E. Androgen receptors as a prognostic and predictive factor in breast cancer. Folia Histochem Cytobiol. 2008;46:269–276. doi: 10.2478/v10042-008-0039-y. [DOI] [PubMed] [Google Scholar]
- 116.Qu Q, Mao Y, Fei XC, Shen KW. The impact of androgen receptor expression on breast cancer survival: a retrospective study and meta-analysis. PLoS One. 2013;8:e82650. doi: 10.1371/journal.pone.0082650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bryan RM, Mercer RJ, Bennett RC, Rennie GC, Lie TH, Morgan FJ. Androgen receptors in breast cancer. Cancer. 1984;54:2436–2440. doi: 10.1002/1097-0142(19841201)54:11<2436::aid-cncr2820541121>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 118.Higa GM, Fell RG. Sex hormone receptor repertoire in breast cancer. Int J Breast Cancer. 2013;2013:284036. doi: 10.1155/2013/284036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rizza P, Barone I, Zito D, Giordano F, Lanzino M, De Amicis F, Mauro L, Sisci D, Catalano S, Dahlman Wright K, Gustafsson JA, Ando S. Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res. 2014;16:R21. doi: 10.1186/bcr3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paliouras M, Diamandis EP. Androgens act synergistically to enhance estrogen-induced upregulation of human tissue kallikreins 10, 11, and 14 in breast cancer cells via a membrane bound androgen receptor. Mol Oncol. 2008;1:413–424. doi: 10.1016/j.molonc.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liao DZ, Pantazis CG, Hou X, Li SA. Promotion of estrogen-induced mammary gland carcinogenesis by androgen in the male Noble rat: probable mediation by steroid receptors. Carcinogenesis. 1998;19:2173–2180. doi: 10.1093/carcin/19.12.2173. [DOI] [PubMed] [Google Scholar]
- 122.Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gomez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16:R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer. 2014;21:T161–181. doi: 10.1530/ERC-14-0243. [DOI] [PubMed] [Google Scholar]
- 124.Girault I, Andrieu C, Tozlu S, Spyratos F, Bieche I, Lidereau R. Altered expression pattern of alternatively spliced estrogen receptor beta transcripts in breast carcinoma. Cancer Lett. 2004;215:101–112. doi: 10.1016/j.canlet.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 125.Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, Foster CS. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27:1502–1512. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 126.Mann S, Laucirica R, Carlson N, Younes PS, Ali N, Younes A, Li Y, Younes M. Estrogen receptor beta expression in invasive breast cancer. Hum Pathol. 2001;32:113–118. doi: 10.1053/hupa.2001.21506. [DOI] [PubMed] [Google Scholar]
- 127.Murphy L, Cherlet T, Lewis A, Banu Y, Watson P. New insights into estrogen receptor function in human breast cancer. Ann Med. 2003;35:614–631. doi: 10.1080/07853890310014579. [DOI] [PubMed] [Google Scholar]
- 128.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J. Clin. Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 129.Nakopoulou L, Lazaris AC, Panayotopoulou EG, Giannopoulou I, Givalos N, Markaki S, Keramopoulos A. The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol. 2004;57:523–528. doi: 10.1136/jcp.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan Y, Li X, Blanchard A, Bramwell VH, Pritchard KI, Tu D, Shepherd L, Myal Y, Penner C, Watson PH, Leygue E, Murphy LC. Expression of both estrogen receptor-beta 1 (ER-beta1) and its co-regulator steroid receptor RNA activator protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with estrogen receptor-alpha (ER-alpha)-negative early breast cancer (EBC) Ann Oncol. 2013;24:1986–1993. doi: 10.1093/annonc/mdt132. [DOI] [PubMed] [Google Scholar]
- 131.Murphy LC, Leygue E. The role of estrogen receptor-beta in breast cancer. Semin Reprod Med. 2012;30:5–13. doi: 10.1055/s-0031-1299592. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y, Chen L, Li JY, Mukaida N, Wang Q, Yang C, Yin WJ, Zeng XH, Jin W, Shao ZM. ERbeta and PEA3 co-activate IL-8 expression and promote the invasion of breast cancer cells. Cancer Biol Ther. 2011;11:497–511. doi: 10.4161/cbt.11.5.14667. [DOI] [PubMed] [Google Scholar]
- 133.Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, Huang RP. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109:507–515. doi: 10.1002/ijc.11724. [DOI] [PubMed] [Google Scholar]
- 134.Cappelletti V, Miodini P, Di Fronzo G, Daidone MG. Modulation of estrogen receptor-beta isoforms by phytoestrogens in breast cancer cells. Int J Oncol. 2006;28:1185–1191. [PubMed] [Google Scholar]
- 135.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Expression of estrogen receptor beta1, beta2, and beta5 messenger RNAs in human breast tissue. Cancer Res. 1999;59:1175–1179. [PubMed] [Google Scholar]
- 136.Davies MP, O’Neill PA, Innes H, Sibson DR, Prime W, Holcombe C, Foster CS. Correlation of mRNA for oestrogen receptor beta splice variants ERbeta1, ERbeta2/ERbetacx and ERbeta5 with outcome in endocrine-treated breast cancer. J Mol Endocrinol. 2004;33:773–782. doi: 10.1677/jme.1.01574. [DOI] [PubMed] [Google Scholar]
- 137.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, Groome NP, Speirs V. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 138.Chantzi NI, Tiniakos DG, Palaiologou M, Goutas N, Filippidis T, Vassilaros SD, Dhimolea E, Mitsiou DJ, Alexis MN. Estrogen receptor beta 2 is associated with poor prognosis in estrogen receptor alpha-negative breast carcinoma. J Cancer Res Clin Oncol. 2013;139:1489–1498. doi: 10.1007/s00432-013-1467-4. [DOI] [PubMed] [Google Scholar]
- 139.Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, Wu CL, Ho SM. Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer. 2010;17:675–689. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, Lavaill R, Mollevi C, Warner M, Gustafsson JA, Rochefort H. Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res. 2004;10:5769–5776. doi: 10.1158/1078-0432.CCR-04-0389. [DOI] [PubMed] [Google Scholar]
- 141.Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA. Low levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res. 2004;10:7490–7499. doi: 10.1158/1078-0432.CCR-04-1114. [DOI] [PubMed] [Google Scholar]
- 142.Lee MT, Ho SM, Tarapore P, Chung I, Leung YK. Estrogen receptor beta isoform 5 confers sensitivity of breast cancer cell lines to chemotherapeutic agent-induced apoptosis through interaction with Bcl2L12. Neoplasia. 2013;15:1262–1271. doi: 10.1593/neo.131184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hamilton-Burke W, Coleman L, Cummings M, Green CA, Holliday DL, Horgan K, Maraqa L, Peter MB, Pollock S, Shaaban AM, Smith L, Speirs V. Phosphorylation of estrogen receptor beta at serine 105 is associated with good prognosis in breast cancer. Am J Pathol. 2010;177:1079–1086. doi: 10.2353/ajpath.2010.090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bernoux A, de Cremoux P, Lainé-Bidron C, Martin EC, Asselain B, Magdelénat H. Estrogen receptor negative and progesterone receptor positive primary breast cancer: pathological characteristics and clinical outcome. Institut curie breast cancer study group. Breast Cancer Res Treat. 1998;49:219–225. doi: 10.1023/a:1006011328655. [DOI] [PubMed] [Google Scholar]
- 145.Nagai R, Kataoka M, Kobayashi S, Ishihara K, Tobioka N, Nakashima K, Naruse M, Saito K, Sakuma S. Estrogen and progesterone receptors in human breast cancer with concomitant assay of plasma 17beta-estradiol, progesterone, and prolactin levels. Cancer Res. 1979;39:1834–1840. [PubMed] [Google Scholar]
- 146.Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, Nicholson RI, Lee AH, Robertson JF, Ellis IO. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J. Clin. Oncol. 2007;25:4772–4778. doi: 10.1200/JCO.2007.12.2747. [DOI] [PubMed] [Google Scholar]
- 147.Allan GF, Sui Z. Non-steroidal progesterone receptor specific ligands. Mini Rev Med Chem. 2005;5:701–708. doi: 10.2174/1389557054553794. [DOI] [PubMed] [Google Scholar]
- 148.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 149.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 150.Dowsett M, Dixon JM, Horgan K, Salter J, Hills M, Harvey E. Antiproliferative effects of idoxifene in a placebo-controlled trial in primary human breast cancer. Clin Cancer Res. 2000;6:2260–2267. [PubMed] [Google Scholar]
- 151.Johnston SR, Saccani-Jotti G, Smith IE, Salter J, Newby J, Coppen M, Ebbs SR, Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res. 1995;55:3331–3338. [PubMed] [Google Scholar]
- 152.Mason BH, Holdaway IM, Mullins PR, Yee LH, Kay RG. Progesterone and estrogen receptors as prognostic variables in breast cancer. Cancer Res. 1983;43:2985–2990. [PubMed] [Google Scholar]
- 153.De Maeyer L, Van Limbergen E, De Nys K, Moerman P, Pochet N, Hendrickx W, Wildiers H, Paridaens R, Smeets A, Christiaens MR, Vergote I, Leunen K, Amant F, Neven P. Does estrogen receptor negative/progesterone receptor positive breast carcinoma exist? J. Clin. Oncol. 2008;26:335–336. doi: 10.1200/JCO.2007.14.8411. author reply 336-338. [DOI] [PubMed] [Google Scholar]
- 154.Hefti MM, Hu R, Knoblauch NW, Collins LC, Haibe-Kains B, Tamimi RM, Beck AH. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013;15:R68. doi: 10.1186/bcr3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123:21–27. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 156.Iwase H, Greenman JM, Barnes DM, Hodgson S, Bobrow L, Mathew CG. Sequence variants of the estrogen receptor (ER) gene found in breast cancer patients with ER negative and progesterone receptor positive tumors. Cancer Lett. 1996;108:179–184. doi: 10.1016/s0304-3835(96)04406-0. [DOI] [PubMed] [Google Scholar]
- 157.Auchus RJ, Fuqua SA. Prognostic factors and variant estrogen receptor RNAs in clinical breast cancer. Nucl Med Biol. 1994;21:449–454. doi: 10.1016/0969-8051(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 158.Daffada AA, Johnston SR, Smith IE, Detre S, King N, Dowsett M. Exon 5 deletion variant estrogen receptor messenger RNA expression in relation to tamoxifen resistance and progesterone receptor/pS2 status in human breast cancer. Cancer Res. 1995;55:288–293. [PubMed] [Google Scholar]
- 159.Fuqua SA, Allred DC, Auchus RJ. Expression of estrogen receptor variants. J Cell Biochem Suppl. 1993;17G:194–197. doi: 10.1002/jcb.240531135. [DOI] [PubMed] [Google Scholar]
- 160.Schultz JR, Petz LN, Nardulli AM. Estrogen receptor alpha and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol. 2003;201:165–175. doi: 10.1016/s0303-7207(02)00415-x. [DOI] [PubMed] [Google Scholar]
- 161.Rickard DJ, Waters KM, Ruesink TJ, Khosla S, Katzenellenbogen JA, Katzenellenbogen BS, Riggs BL, Spelsberg TC. Estrogen receptor isoform-specific induction of progesterone receptors in human osteoblasts. J Bone Miner Res. 2002;17:580–592. doi: 10.1359/jbmr.2002.17.4.580. [DOI] [PubMed] [Google Scholar]
- 162.Shen Q, Zhang Y, Uray IP, Hill JL, Kim HT, Lu C, Young MR, Gunther EJ, Hilsenbeck SG, Chodosh LA, Colburn NH, Brown PH. The AP-1 transcription factor regulates postnatal mammary gland development. Dev Biol. 2006;295:589–603. doi: 10.1016/j.ydbio.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 163.Encarnación CA, Ciocca DR, McGuire WL, Clark GM, Fuqua SA, Osborne CK. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat. 1993;26:237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- 164.Colomer R, Beltran M, Dorcas J, Cortes-Funes H, Hornedo J, Valentin V, Vargas C, Mendiola C, Ciruelos E. It is not time to stop progesterone receptor testing in breast cancer. J. Clin. Oncol. 2005;23:3868–3869. doi: 10.1200/JCO.2005.05.203. author reply 3869-3870. [DOI] [PubMed] [Google Scholar]
- 165.Keshgegian AA, Cnaan A. Estrogen receptor-negative, progesterone receptor-positive breast carcinoma: poor clinical outcome. Arch Pathol Lab Med. 1996;120:970–973. [PubMed] [Google Scholar]
- 166.Ng CH, Pathy NB, Taib NA, Mun KS, Rhodes A, Yip CH. The estrogen receptor negativeprogesterone receptor positive breast carcinoma is a biological entity and not a technical artifact. Asian Pac J Cancer Prev. 2012;13:1111–1113. doi: 10.7314/apjcp.2012.13.4.1111. [DOI] [PubMed] [Google Scholar]
- 167.Bardon S, Vignon F, Montcourrier P, Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res. 1987;47:1441–1448. [PubMed] [Google Scholar]
- 168.Bardon S, Vignon F, Chalbos D, Rochefort H. RU486, a progestin and glucocorticoid antagonist, inhibits the growth of breast cancer cells via the progesterone receptor. J Clin Endocrinol Metab. 1985;60:692–697. doi: 10.1210/jcem-60-4-692. [DOI] [PubMed] [Google Scholar]
- 169.Bray JD, Zhang Z, Winneker RC, Lyttle CR. Regulation of gene expression by PRA-910, a novel progesterone receptor modulator, in T47D cells. Steroids. 2003;68:995–1003. doi: 10.1016/s0039-128x(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 170.Bowden RT, Hissom JR, Moore MR. Growth stimulation of T47D human breast cancer cells by the anti-progestin RU486. Endocrinology. 1989;124:2642–2644. doi: 10.1210/endo-124-5-2642. [DOI] [PubMed] [Google Scholar]
- 171.Hissom JR, Moore MR. Progestin effects on growth in the human breast cancer cell line T-47D--possible therapeutic implications. Biochem Biophys Res Commun. 1987;145:706–711. doi: 10.1016/0006-291x(87)91022-9. [DOI] [PubMed] [Google Scholar]
- 172.Jeng MH, Langan-Fahey SM, Jordan VC. Estrogenic actions of RU486 in hormone-responsive MCF-7 human breast cancer cells. Endocrinology. 1993;132:2622–2630. doi: 10.1210/endo.132.6.8504763. [DOI] [PubMed] [Google Scholar]
- 173.Jonat W, Bachelot T, Ruhstaller T, Kuss I, Reimann U, Robertson JFR. Randomized phase II study of lonaprisan as second-line therapy for progesterone receptor-positive breast cancer. Ann Oncol. 2013;24:2543–2548. doi: 10.1093/annonc/mdt216. [DOI] [PubMed] [Google Scholar]
- 174.Klijn JG, de Jong FH, Bakker GH, Lamberts SW, Rodenburg CJ, Alexieva-Figusch J. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res. 1989;49:2851–2856. [PubMed] [Google Scholar]
- 175.Perrault D, Eisenhauer EA, Pritchard KI, Panasci L, Norris B, Vandenberg T, Fisher B. Phase II study of the progesterone antagonist mifepristone in patients with untreated metastatic breast carcinoma: a National Cancer Institute of Canada Clinical Trials Group study. J. Clin. Oncol. 1996;14:2709–2712. doi: 10.1200/JCO.1996.14.10.2709. [DOI] [PubMed] [Google Scholar]
- 176.El Etreby MF, Liang Y, Wrenn RW, Schoenlein PV. Additive effect of mifepristone and tamoxifen on apoptotic pathways in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1998;51:149–168. doi: 10.1023/a:1006078032287. [DOI] [PubMed] [Google Scholar]
- 177.Lee JY, Shin JY, Kim HS, Heo JI, Kho YJ, Kang HJ, Park SH, Lee JY. Effect of combined treatment with progesterone and tamoxifen on the growth and apoptosis of human ovarian cancer cells. Oncol Rep. 2012;27:87–93. doi: 10.3892/or.2011.1460. [DOI] [PubMed] [Google Scholar]
- 178.Nishino T, Ishibashi K, Hirtreiter C, Nishino Y. Potentiation of the antitumor effect of tamoxifen by combination with the antiprogestin onapristone. J Steroid Biochem Mol Biol. 2009;116:187–190. doi: 10.1016/j.jsbmb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 179.Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120:725–731. doi: 10.1309/42F0-0D0D-JD0J-5EDT. [DOI] [PubMed] [Google Scholar]
- 180.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21:488–492. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 181.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25:3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 182.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. Targeting androgen receptor in estrogen receptornegative breast cancer. Cancer Cell. 2011;20:119–131. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.He J, Peng R, Yuan Z, Wang S, Peng J, Lin G, Jiang X, Qin T. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol. 2012;29:406–410. doi: 10.1007/s12032-011-9832-0. [DOI] [PubMed] [Google Scholar]
- 184.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 185.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tang D, Xu S, Zhang Q, Zhao W. The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol. 2012;29:526–533. doi: 10.1007/s12032-011-9948-2. [DOI] [PubMed] [Google Scholar]
- 187.Hardin C, Pommier R, Calhoun K, Muller P, Jackson T, Pommier S. A new hormonal therapy for estrogen receptor-negative breast cancer. World J Surg. 2007;31:1041–1046. doi: 10.1007/s00268-007-0694-8. [DOI] [PubMed] [Google Scholar]
- 188.Arce-Salinas C, Riesco-Martinez MC, Hanna W, Bedard P, Warner E. Complete response of metastatic androgen receptor-positive breast cancer to bicalutamide: case report and review of the literature. J. Clin. Oncol. 2016;34:e21–4. doi: 10.1200/JCO.2013.49.8899. [DOI] [PubMed] [Google Scholar]
- 189.Desmedt C, Voet T, Sotiriou C, Campbell PJ. Next-generation sequencing in breast cancer: first take home messages. Curr Opin Oncol. 2012;24:597–604. doi: 10.1097/CCO.0b013e328359554e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Elshimali YI, Khaddour H, Sarkissyan M, Wu Y, Vadgama JV. The clinical utilization of circulating cell free DNA (CCFDNA) in blood of cancer patients. Int J Mol Sci. 2013;14:18925–18958. doi: 10.3390/ijms140918925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mirza S, Sharma G, Parshad R, Srivastava A, Gupta SD, Ralhan R. Clinical significance of promoter hypermethylation of ERbeta and RARbeta2 in tumor and serum DNA in Indian breast cancer patients. Ann Surg Oncol. 2012;19:3107–3115. doi: 10.1245/s10434-012-2323-5. [DOI] [PubMed] [Google Scholar]
- 192.Russnes HG, Navin N, Hicks J, Borresen-Dale AL. Insight into the heterogeneity of breast cancer through next-generation sequencing. J Clin Invest. 2011;121:3810–3818. doi: 10.1172/JCI57088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tung N, Battelli C, Allen B, Kaldate R, Bhatnagar S, Bowles K, Timms K, Garber JE, Herold C, Ellisen L, Krejdovsky J, DeLeonardis K, Sedgwick K, Soltis K, Roa B, Wenstrup RJ, Hartman AR. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121:25–33. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- 194.Levenson RM, Borowsky AD, Angelo M. Immunohistochemistry and mass spectrometry for highly multiplexed cellular molecular imaging. Lab Invest. 2015;95:397–405. doi: 10.1038/labinvest.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, Levenson RM, Lowe JB, Liu SD, Zhao S, Natkunam Y, Nolan GP. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Trevino LS, Weigel NL. Phosphorylation: a fundamental regulator of steroid receptor action. Trends Endocrinol Metab. 2013;24:515–524. doi: 10.1016/j.tem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


