Abstract
CS1 (also known as CD319, CRACC and SLAMF7) was identified as an NK cell receptor regulating immune functions. It is also expressed on B cells, T cells, Dendritic cells, NK-T cells, and monocytes. CS1 is overexpressed in multiple myeloma and makes it a target for immunotherapy. A humanized anti-CS1 antibody, Elotuzumab or Empliciti has shown promising results in clinical studies. This review focuses on the biology of CS1 in NK and other hematopoietic cells and multiple myeloma. Anti-CS1 mAb can activate natural cytotoxicity of NK cells as well as enhance ADCC (antibody-dependent cell-mediated cytotoxicity) and thus makes an effective target for immunotherapy of MM.
Keywords: CS1, SLAMF7, CRACC, elotuzumab, antibody-based immunotherapy, multiple myeloma
Introduction
Multiple myeloma (MM) is a hematologic cancer characterized by malignant plasma cells in bone marrow along with monoclonal protein found in the serum and/or urine. MM can cause anemia, renal failure, hypercalcemia, or bone destruction [1]. According to American Cancer Society, it is estimated that in 2017, there will be 30,280 new cases of MM and 12,590 deaths in the United States. Although MM is considered incurable, the 5-year relative survival rate has increased from 29% in 1987-1989 to 49% in 2005-2011, potentially due to recent advances in conventional treatment and the availability of novel therapies [2]. The genetic background of MM is complex and result in the heterogeneous biology and clinical outcome and thus an obstacle to therapies targeting MM signaling pathways [3,4]. The expression of several surface molecules on MM make it potentially a good target for immunotherapy [5].
Natural killer cell function and role in cancer immunotherapy
Natural killer (NK) cells are bone-marrow derived lymphocytes that play a vital role in the innate immune response against cancer and infection. NK cells recognize and destroy target cells via natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) [6]. NK cells also regulate other immune cells though the secretion of cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor-a (TNF-α) [7]. NK cell function is regulated through a delicate balance of signals received from activating and inhibitory receptors [8,9]. Early research was devoted to identifying MHC-recognizing receptors [10]; however, members of the CD2 subset of receptors, such as CS1 and 2B4 (CD244), do not recognize MHC but play a major role in NK cell function [11,12]. It has been recently show that there is not a singly dominant activating NK cell receptor but rather that activating receptors work in a combinatorial manner to reach the threshold required for cytokine release or natural cytotoxicity. Due to the synergism of activating receptors, there has not been great success in the creation of a single therapeutic agent [13]. Studies over the last decade that revealed molecular mechanisms of NK cell activation and recognition has opened novel strategies for NK cell based immunotherapy [14].
CS1 (CRACC, CD319, SLAMF7)
We have cloned and characterized CS1 (CD2 subset-1) as a human NK cell receptor [15]. CS1 is a member of the Signaling Lymphocyte Activation Molecule Family (SLAMF7) and is expressed on NK cells, CD8+ T lymphocytes, B lymphocytes, and mature dendritic cells [15,16]. CS1 is a homophilic receptor, and the CS1-CS1 interaction leads to activation of NK cell natural cytotoxicity [17]. The human CS1 gene is located on the long arm of chromosome 1 at 1q23-24 between CD48 and CD229 [11]. Human NK cells express two splice variants of CS1; CS1-S which lack the intracellular domain for activation, and the CS1-L which contain the intracellular domain and is thus capable of activating NK cytotoxicity [18]. Both the isoforms of CS1 are membrane bound forms and are expressed in NK cells. However, only the CS1-L isoform is expressed in B cells and signaling through CS1 induce B cell proliferation and autocrine secretion [19].
CS1 is upregulated in MM and this has been implicated in the uncontrolled proliferation of MM cells [20,21]. However, the mechanism of CS1 upregulation in MM is not known. We have analyzed the transcriptional regulation of CS1 in NK and B lymphocytes. CS1 transcription is regulated by YY1 (Ying Yang 1) in mice and Blimp-1 (B lymphocyte-induced maturation protein-1) in humans [22,23]. Blimp-1, encoded by the gene positive regulatory domain zinc finger protein 1 (PRDM1), regulates gene transcription in macrophage, NK cells, B cells, T cells, and skin epithelia [23,24]. Originally thought to be a transcriptional repressor, Blimp-1/PRDM1 was recently shown to positively regulate the human CS1 (hCS1) gene in NK and B cells. While Blimp-1/PRDM1 is not required for basal level of hCS1 expression, Blimp-1/PRDM1 binding leads to increased transcription and expression of hCS1 [23]. Interestingly, while Blimp-1/PRDM1 is required for the specific stages of B and T cell differentiation, Blimp-1/PRDM1 is induced in immature NK cells and expression is maintained and increased in mature NK subsets [25-27]. Blimp-1/PRDM1 expression and function suggests fundamental differences in transcriptional regulation of CS1 in NK cells compared to other lymphocyte subsets.
CS1 activates NK cell cytotoxicity
CS1 is a self-ligand and homophilic interaction of CS1 activates NK cell function [17]. CS1 contains two signaling motifs called immunoreceptor tyrosine-based switch motif (ITSM) found on the cytoplasmic tail which mediates interaction with Ewing’s sarcoma-associated transcript 2 (EAT-2) [18]. Both isoforms of CS1 (CS1-L and CS1-S) are expressed on human NK cells, but only CS1-L mediates signals upon ligation with anti-CS1 mAb [18]. By generating CS1-deficient mice, Cruz-Munoz and colleagues showed that CS1 may function as inhibitory or activating receptor depending on cellular context and availability of effector proteins [28]. Thus, in presence of EAT-2, CS1 acts as an activating receptor and in its absence as an inhibitory receptor for NK cells [28]. The humanized anti-CS1 mAb Elotuzumab (Empliciti) also activates natural cytotoxic function of NK cells against MM cells [29].
Role of CS1 in other immune cells
Naïve B cells only produce the CS1-L variant and expression is upregulated upon activation [18,19]. In B cells, CS1 signaling leads to proliferation and leads to production of autocrine cytokines such as IL-14 and does not lead to immunoglobulin (Ig) production [19]. Human B cells stimulated with anti-CD40 mAb, and IL-4, crosslinking surface CS1 with anti-CS1 mAb increased proliferation as well as cytokine with growth promoting activity [19]. These results suggest that CS1 may have growth promoting activity in B cells. In contrast, T cells express CS1 but lack the adaptor protein EAT-2; therefore, CS1 functions as an inhibitory receptor [28]. Our study on the function of CS1 in human monocytes showed an inhibitory role to control proinflammatory immune responses [30]. Crosslinking CS1 in human monocytes cultured in LPS reduced the production of proinflammatory cytokines TNF-alpha and IL-12p70 [30]. CS1 is also expressed in NKT cells and some DCs, but its functional role yet to be investigated.
Targeting multiple myeloma with anti-CS1 mAb
CS1 is highly expressed in MM cell lines and patient MM cells, but not found on healthy tissue, primary tumor tissues, or hematologic and nonhematologic cancer cell lines [21,31]. Moreover, there was a correlation between soluble CS1 in the patient sera and the disease stage [20]. This indicate that soluble CS1 may be a useful biomarker for MM disease progression. The high expression of CS1 on MM cells make it an attractive target for treatment of this disease. It has been reported that CS1 may contribute to tumor promoting activity of MM cells [21]. A humanized mAb, Elotuzumab or Empliciti was used in preclinical studies and lead to phase 1 and phase 2 studies of MM. In phase 1 studies, treatment with Elotuzumab alone did not show much progress, whereas Elotuzumab in combination with bortezomib or lenalidomide was active against MM [32,33]. Elotuzumab could activate NK cells in two different ways (Figure 1). Elotuzumab binds to CS1 on MM cells and NK cells recognize the Fc region of antibody by CD16 and thus mediate ADCC (Antibody-dependent cell-mediated cytotoxicity) against MM cells. In addition, binding of Elotuzumab on CS1 on the NK cells activate natural cytotoxicity of NK cells [17]. Activation of NK cells induce the secretion of IFN-γ and this could play a role regulating the immune function of other cells. Moreover, CS1 is also expressed on DCs and monocytes. Binding of anti-CS1 to monocytes inhibits the production of proinflammatory cytokines [27]. This could affect the bone marrow microenvironment. Elotuzumab could also prevent the adhesion of MM cells to bone marrow stromal cells. Thus, unlike other mAbs, elotuzumab because of its multiple effects on NK cells and other immune cell function, is more effective in targeting MM cells. Recent study revealed a critical role for CS1 in phagocytosis of hematopoietic tumor cells by macrophages [34]. Phase 3 clinical trial of elotuzumab in combination with lenalidomide/dexamethasone showed clinically relevant improvement in efficacy, with minimal incremental toxicity [35].
Figure 1.
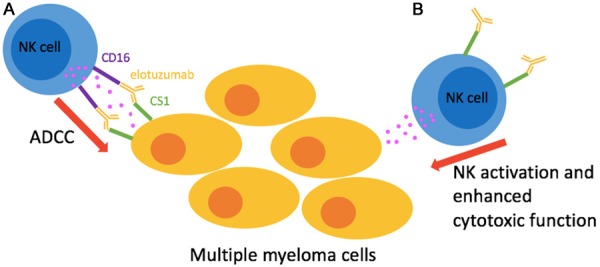
Mechanism of action of anti-CS1 antibody (elotuzumab) against multiple myeloma (MM). A: Activation of antibody-dependent cell-mediated cytotoxicity (ADCC) when NK cell receptor CD16 (FCγRIII) binds to the Fc region of elotuzumab bound to CS1 on MM cells; B: Direct activation of NK cell through CS1-elotuzumab interaction.
The overexpression of CS1 on MM cells makes it an effective immunotherapeutic target. The mechanism by which elotuzumab control MM is not fully understood. Binding of elotuzumab to CS1 can play an inhibitory role on other immune cells, such as macrophages. While, elotuzumab has shown great success in clinical studies in MM patients, its role in normal hematopoietic cells needs to be evaluated more closely.
Disclosure of conflict of interest
None.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Lohr JG, Stojanov P, Carter SL, Cruz-Gordillo P, Lawrence MS, Auclair D, Sougnez C, Knoechel B, Gould J, Saksena G, Cibulskis K, McKenna A, Chapman MA, Straussman R, Levy J, Perkins LM, Keats JJ, Schumacher SE, Rosenberg M Multiple Myeloma Research Consortium. Getz G, Golub TR. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25:91–101. doi: 10.1016/j.ccr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolli N, Avet-Loiseau H, Wedge DC, Van Loo P, Alexandrov LB, Martincorena I, Dawson KJ, Iorio F, Nik-Zainal S, Bignell GR, Hinton JW, Li Y, Tubio JM, McLaren S, O’Meara S, Butler AP, Teague JW, Mudie L, Anderson E, Rashid N, Tai YT, Shammas MA, Sperling AS, Fulciniti M, Richardson PG, Parmigiani G, Magrangeas F, Minvielle S, Moreau P, Attal M, Facon T, Futreal PA, Anderson KC, Campbell PJ, Munshi NC. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun. 2014;5:2997. doi: 10.1038/ncomms3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasche L, Weinhold N, Morgan GJ, van Rhee F, Davies FE. Immunologic approaches for the treatment of multiple myeloma. Cancer Treat Rev. 2017;55:190–199. doi: 10.1016/j.ctrv.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 7.Paolini R, Bernardini G, Molfetta R, Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26:113–120. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long EO, Rajagopalan S. HLA class I recognition by killer cell Ig-like receptors. Semin Immunol. 2000;12:101–108. doi: 10.1006/smim.2000.0212. [DOI] [PubMed] [Google Scholar]
- 11.Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol Rev. 2001;181:234–249. doi: 10.1034/j.1600-065x.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- 12.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat Rev Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 13.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domogala A, Madrigal JA, Saudemont A. Natural killer cell immunotherapy: from bench to bedside. Front Immunol. 2015;6:264. doi: 10.3389/fimmu.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boles KS, Mathew PA. Molecular cloning of CS1, a novel human natural killer cell receptor belonging to the CD2 subset of the immunoglobulin superfamily. Immunogenetics. 2001;52:302–307. doi: 10.1007/s002510000274. [DOI] [PubMed] [Google Scholar]
- 16.Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J Immunol. 2001;167:5517–5521. doi: 10.4049/jimmunol.167.10.5517. [DOI] [PubMed] [Google Scholar]
- 17.Kumaresan PR, Lai WC, Chuang SS, Bennett M, Mathew PA. CS1, a novel member of the CD2 family, is homophilic and regulates NK cell function. Mol Immunol. 2002;39:1–8. doi: 10.1016/s0161-5890(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee JK, Boles KS, Mathew PA. Molecular and functional characterization of a CS1 (CRACC) splice variant expressed in human NK cells that does not contain immunoreceptor tyrosine-based switch motifs. Eur J Immunol. 2004;34:2791–2799. doi: 10.1002/eji.200424917. [DOI] [PubMed] [Google Scholar]
- 19.Lee JK, Mathew SO, Vaidya SV, Kumaresan PR, Mathew PA. CS1 (CRACC, CD319) induces proliferation and autocrine cytokine expression on human B lymphocytes. J Immunol. 2007;179:4672–4678. doi: 10.4049/jimmunol.179.7.4672. [DOI] [PubMed] [Google Scholar]
- 20.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weller E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE, Anderson KC. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai YT, Soydan E, Song W, Fulciniti M, Kim K, Hong F, Li XF, Burger P, Rumizen MJ, Nahar S, Podar K, Hideshima T, Munshi NC, Tonon G, Carrasco RD, Afar DE, Anderson KC. CS1 promotes multiple myeloma cell adhesion, clonogenic growth, and tumorigenicity via c-mafmediated interactions with bone marrow stromal cells. Blood. 2009;113:4309–4318. doi: 10.1182/blood-2008-10-183772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Dongre P, Mathew S, Akopova I, Gryczynski I, Mathew P. YY1 and a unique DNA repeat element regulates the transcription of mouse CS1 (CD319, SLAMF7) gene. Mol Immunol. 2013;54:254–263. doi: 10.1016/j.molimm.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Kim JR, Mathew SO, Mathew PA. Blimp-1/PRDM1 regulates the transcription of human CS1 (SLAMF7) gene in NK and B cells. Immunobiology. 2016;221:31–9. doi: 10.1016/j.imbio.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Bikoff EK, Morgan MA, Robertson EJ. An expanding job description for Blimp-1/PRDM1. Curr Opin Genet Dev. 2009;19:379–385. doi: 10.1016/j.gde.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Savitsky D, Calame K. B-1 B lymphocytes require Blimp-1 for immunoglobulin secretion. J Exp Med. 2006;203:2305–2314. doi: 10.1084/jem.20060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kallies A, Carotta S, Huntington ND, Bernard NJ, Tarlinton DM, Smyth MJ, Nutt SL. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117:1869–1879. doi: 10.1182/blood-2010-08-303123. [DOI] [PubMed] [Google Scholar]
- 27.Kim JR, Horton NC, Mathew SO, Mathew PA. CS1 (SLAMF7) inhibits production of proinflammatory cytokines by activated monocytes. Inflamm Res. 2013;62:765–72. doi: 10.1007/s00011-013-0632-1. [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Munoz ME, Dong Z, Shi X, Zhang S, Veillette A. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol. 2009;10:297–305. doi: 10.1038/ni.1693. [DOI] [PubMed] [Google Scholar]
- 29.Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, Starling GC, Ciarlariello D, Bhaskar S, Briercheck EL, Hughes T, Yu J, Rice A, Benson DM Jr. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol Immunother. 2013;62:1841–1849. doi: 10.1007/s00262-013-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JR, Horton NC, Mathew SO, Mathew PA. CS1 (SLAMF7) inhibits production of proinflammatory cytokines by activated monocytes. Inflamm Res. 2013;62:765–772. doi: 10.1007/s00011-013-0632-1. [DOI] [PubMed] [Google Scholar]
- 31.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, Rice AG, van Abbema A, Wong M, Liu G, Zhan F, Dillon M, Chen S, Rhodes S, Fuh F, Tsurushita N, Kumar S, Vexler V, Shaughnessy JD Jr, Barlogie B, van Rhee F, Hussein M, Afar DE, Williams MB. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, Richardson PG, Afar DE, Singhal AK, Anderson KC. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J. Clin. Oncol. 2012;30:1960–1965. doi: 10.1200/JCO.2011.37.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Fry J, Afar DE, Singhal AK. A phase 1, multicenter, openlabel, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120:552–559. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Zhong MC, Guo H, Davidson D, Mishel S, Lu Y, Rhee I, Perez-Quintero LA, Zhang S, Cruz-Munoz ME, Wu N, Vinh DC, Sinha M, Calderon V, Lowell CA, Danska JS, Veillette A. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544:493–497. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimopoulos MA, Lonial S, White D, Moreau P, Palumbo A, San-Miguel J, Shpilberg O, Anderson K, Grosicki S, Spicka I, Walter-Croneck A, Magen H, Mateos MV, Belch A, Reece D, Beksac M, Bleickardt E, Poulart V, Sheng J, Sy O, Katz J, Singhal A, Richardson P. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. Br J Haematol. 2017 doi: 10.1111/bjh.14787. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


