Abstract
Neuroinflammation can be monitored using fluorine-19 (19F)-containing nanoparticles and 19F MRI. Previously we studied neuroinflammation in experimental autoimmune encephalomyelitis (EAE) using room temperature (RT) 19F radiofrequency (RF) coils and low spatial resolution 19F MRI to overcome constraints in signal-to-noise ratio (SNR). This yielded an approximate localization of inflammatory lesions. Here we used a new 19F transceive cryogenic quadrature RF probe (19 F-CRP) that provides the SNR necessary to acquire superior spatially-resolved 19F MRI. First we characterized the signal-transmission profile of the 19 F-CRP. The 19 F-CRP was then benchmarked against a RT 19F/1H RF coil. For SNR comparison we used reference compounds including 19F-nanoparticles and ex vivo brains from EAE mice administered with 19F-nanoparticles. The transmit/receive profile of the 19 F-CRP diminished with increasing distance from the surface. This was counterbalanced by a substantial SNR gain compared to the RT coil. Intraparenchymal inflammation in the ex vivo EAE brains was more sharply defined when using 150 μm isotropic resolution with the 19 F-CRP, and reflected the known distribution of EAE histopathology. At this spatial resolution, most 19F signals were undetectable using the RT coil. The 19 F-CRP is a valuable tool that will allow us to study neuroinflammation with greater detail in future in vivo studies.
Introduction
Central nervous system (CNS) inflammation, as occurs in multiple sclerosis (MS), involves immune cell recruitment from the periphery into the CNS, resulting in tissue destruction and neurodegeneration1. During active disease, a massive infiltration of immune cells is predominant, particularly around white matter lesions. T cells find their way into the white matter via a disruption of the blood brain barrier2. In MS, T cells may also enter the CNS grey matter such as the cerebral cortex via the meninges3, 4. Even in the cerebellum, extensive grey matter pathology in secondary progressive MS is linked to inflammation of the subarachnoid space5. Studies of the animal model of MS, experimental autoimmune encephalomyelitis (EAE), have helped identify mechanisms of cell migration between the periphery, CNS and lymphatic system during neuroinflammation6–8. This is a topic of active interest, with divergent views regarding immune cell entry and exit in the CNS (inside-out versus outside-in hypotheses) in MS9, 10. Therefore there is an acute need for more precise and non-invasive methods that support longitudinal studies of inflammatory cell migration during disease progression to resolve some of the discrepancies in the literature.
Previously we studied immune cell infiltration in EAE brains using fluorine-19 (19F)-loaded nanoparticles (NPs) and a room temperature (RT) dual-tuned 19F/1H radio frequency (RF) volume resonator11. Intravenously administered NPs are taken up by inflammatory cells during their migration from the systemic circulation into the inflamed organ11–17. Although tracking of inflammation following intravenous 19F-NP administration is one application for 19F MRI, several other state-of-the-art applications for 19F imaging exist. These include in vivo tracking of cell therapies labeled in culture with 19F-NPs prior to their adoptive transfer18–20 and intracellular oximetry using 19F-NP emulsions21 to study changes in pO2 in tumor cells during therapy22.
One major limitation of 19F MRI is the low signal-to-noise ratio (SNR). The acquisition method is one aspect of 19F MRI that influences SNR. SNR efficiency of the most commonly used acquisition methods — RARE (Rapid Acquisition with Relaxation Enhancement), UTE (Ultra-short Echo Time), and bSSFP (Balanced Steady-State Free Precession) — depends on the T 1 and T 2 values of the particular 19F compound studied23. For most T 1 and T 2 combinations, especially those pertaining to intracellular 19F-NPs, bSSFP and 3D RARE sequences have the highest SNR sensitivity. However, while bSSFP often has a higher SNR efficiency, it is not always the method of choice due to the high RF energy deposition associated with longer acquisition times, and pronounced banding artifacts. The SNR and the sensitivity of the radio frequency (RF) probe used are main determinants that dictate the level of spatial resolution. Factors to be kept in mind when designing a probe include the geometry, the filling factor and the homogeneity of the B 1 + transmit field.
The SNR constraint limited spatial resolution to approximately 600 μm when detailing the dynamics of inflammation during EAE11. Given this limited precision, the location of inflammatory cells within the brain was not sharply defined. To overcome the sensitivity constraints in 19F MR and improve detail of inflammatory cell location, we applied the concept of cryogenically-cooling RF coil hardware to improve SNR by reducing thermal noise. Until now this technology has been available only for 1H, 13C and 31P small animal MRI. Here we made use of the first 19F transceive cryogenically-cooled RF probe (19 F-CRP) to substantially boost SNR beyond that of available RT coils, thus facilitating the acquisition of better spatially-resolved images. In this study we evaluated the advantages and disadvantages of the 19 F-CRP for imaging neuroinflammation.
Methods
Radio frequency coils
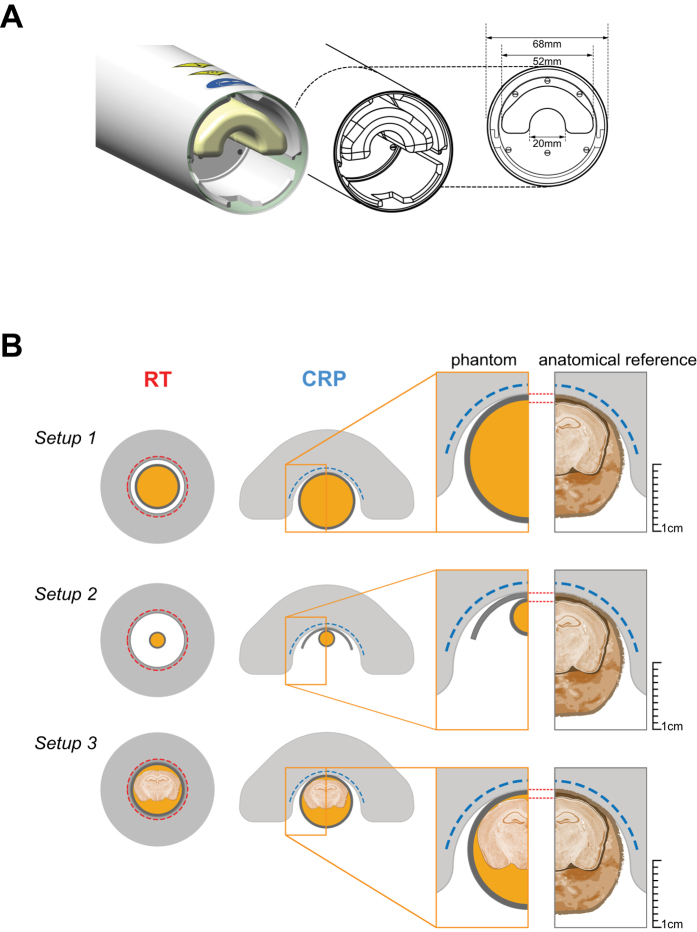
The performance of a novel transceive 19F cryogenic quadrature RF surface probe at 9.4T (19 F-CRP, f ~ 376 MHz) was compared to a dual-tunable 19F/1H volume resonator (ϕin = 18.4 mm, ltotal = 39 mm), previously developed for imaging mouse brain inflammation11. The 19 F-CRP has a similar geometry to the existing Bruker 1H quadrature CryoProbes24. The rectangular transceive copper coil elements are overlapping side-by-side on a cylindrical surface (r ~ 11 mm, axis parallel to the main magnetic field direction). The outer dimensions (O.D.) of one coil element are: 16 × 20 mm2 [arc length (ϕ × z)] and the total O.D. are: 27 × 20 mm2 [ϕ × z]. The 19 F-CRP operates at ~28 K with a dual cooled preamplifier at the base running at ~77 K. Constant cooling is ensured by a closed loop system connected to a remote cryo-cooler. The RF coil is thermally insulated by a vacuum separating it from the surrounding ceramic finger (Fig. 1A). The outer surface of the RF finger is equipped with a temperature sensor and kept at a temperature of choice (35 °C) using a resistive heater. The SNR gain of this CRP relative to a RT coil with similar geometry is expected to be comparable to existing 400 MHz proton CryoProbes24, 25.
Figure 1.
19F Cryogenic Radiofrequency Probe design and experimental setup. (A) Side view of the 19 F-CRP showing its geometry including external protective cylinder and an inner ceramic probe head that encloses the loop coil elements (not shown). The inner diameter dimension for the inner ceramic structure is shown in the cross-sectional view (right). (B) Three different experimental setups that were used to assess the 19 F-CRP quality. Shown are Setup 1 for the high concentration 19F phantom (upper panel), Setup 2 for the 19F nanoparticle phantoms (middle panel) and Setup 3 for the mouse brain phantom (lower panel). The dimension of the phantom setups are to scale with the dimensions of both 19 F-CRP and RT coil and an anatomic reference is shown on the right for comparison. The nanoparticles used in this study had the following physical characteristics: Z-average diameter = 164 nm, PdI = 0.06, z-Potential = 0.19 mV.
Experimental setup
To evaluate the 19 F-CRP performance, three different phantom-setups were prepared (Fig. 1B):
Setup 1 (high concentration 19F): a 10 ml syringe (inner/outer diameter = 17.0 mm/15.5 mm) for the 19 F-CRP and a 5 ml syringe (I.D./O.D. = 13.5/12.0 mm) for the 19 F/1 H RT-coil, both containing the same 19F reference compound to study and compare spatial SNR. The reference compound was 33% v/v 2,2,2-Trifluoroethanol (TFE, Sigma-Aldrich, Germany) in water.
Setup 2 (19F nanoparticles): NMR tubes (I.D./O.D. = 4.0/5.0 mm) containing different concentrations of perfluoro-15-crown-5-ether (PFCE) loaded nanoparticles to compare 19F signal sensitivity as a function of the number of 19F atoms. Nanoparticles were prepared by emulsifying 1200 mM PFCE (Fluorochem, UK) with Pluronic F-68 (Sigma-Aldrich, Germany) using a titanium sonotrode (Sonopuls GM70, Bandelin, Germany) as previously described26. The PFCE nanoparticle stock was then diluted to 25 mM, 50 mM, 100 mM, 200 mM, 400 mM and 600 mM nanoparticle suspensions. NMR tubes containing different nanoparticle concentrations were placed below the CRP using a spacer of 0.75 mm thickness to mimic the distance of the mouse brain from the CRP surface in in vivo applications.
Setup 3 (mouse brain): Ex vivo tissues from fixed EAE mice embedded in 15-ml tubes, for comparing 19F signal sensitivity and anatomical detail. All experiments were conducted in accordance with procedures approved by the Animal Welfare Department of the State Office of Health and Social Affairs Berlin (LAGeSo), and conformed to national and international guidelines to minimize discomfort to animals (86/609/EEC). EAE was induced as described previously11 in SJL/J mice (n = 6, female, 6–8 weeks old). Five days following EAE induction, mice were administered nanoparticles (10µmol PFCE) intravenously each day for 5 d as described previously11. EAE mice were transcardially perfused with 20 ml PBS followed by 20 ml 4% paraformaldehyde (PFA) following terminal anesthesia. Mice were cleared from external pelt, extremities, and abdominal tissues. Brain, spinal cord and neck lymphoid organs were preserved in situ within the skull and vertebral column. The tissues were transferred into a 15 ml tube filled with 4% PFA and stored at 4 °C.
MRI Methods and Data Analysis
All experiments were carried out on a 9.4 T small animal MR system (BioSpec 94/20, Bruker BioSpin MRI, Ettlingen, Germany) operating at 400 MHz (1H) and 376 MHz (19F).
Transmit Field Characteristics
Using a 15 ml tube containing 33% TFE in water (Setup 1), we acquired 2D-FLASH images (TR = 20 s, TE = 4.9 ms, FOV = (20 × 20) mm2, matrix = 256 × 256, 1 slice of 4 mm thickness, averages = 1, TA = 1 h 25 min) with nominal excitation flip angles α = 60° and 2α = 120° and calculated the actual flip angles (FA) using the double-angle method27, 28:
| 1 |
with SIα and SI2α being the signal intensities obtained with α and 2α. FA maps were normalized to a nominal angle of 90° by multiplying by the factor 90°/α.
SNR assessment in phantoms
To measure the spatial distribution of SNR at increasing distances from the 19 F-CRP surface, a high-concentration 19F phantom (Setup 1) and an axial 2D-RARE scan (TR = 10 s, TE = 6.2 ms, ETL = 256, FOV = (25.6 × 25.6) mm2, matrix = 256 × 256, averages = 100, TA = 17 m) was used. To quantify and compare SNR in a way more relevant for brain inflammation, we measured SNR as a function of the number of 19F atoms using phantoms containing different concentrations of 19F nanoparticles (Setup 2, Fig. 1B). Measurements involved 2D-RARE scans (TR = 3000 ms, TE = 10.8 ms, ETL = 8, FOV = (10 × 10)mm2, matrix = 96 × 96, averages = 1, TA = 36 s) with varying slice thicknesses: 0.4/1.0/1.2/2.0/3.6/4.7/6.0 mm to measure SNR as a function of the number of 19F atoms.
SNR was calculated by dividing signal S m from magnitude images by background standard deviation σ m, and corrected to compensate for the non-Gaussian distribution29. For single channel RF coils, intensity values of MR images follow a Rician distribution30, 31. For a two-receiver, quadrature system (19 F-CRP), they follow a non-central chi distribution32. We estimated the true SNR from the S m and background σ m using
| 2 |
where c σ is 0.655 (Rician) and 0.687 (chi), and the correction function f S is derived from the respective distribution’s mean30, 32. For Setup 2, a single SNR value was determined from the mean signal intensity over a central circular region-of-interest covering ~90% of pixels. The number of atoms per image pixel was estimated from nanoparticle concentration and voxel size.
Ex vivo mouse brain 19F and 1H MRI (Setup 3)
19F MR images of the EAE mouse brain were acquired using 3D-RARE: TR = 800 ms, TE = 5.1 ms, ETL=33, FOV=(30 × 20 × 20) mm3, matrix = 195 × 65 × 65 zero-filled to 195 × 130 × 130, averages = 384, TA = 11 h. 1H MR images were acquired using 3D-FLASH (TR = 50 ms, TE = 12.5 ms, FOV = (30 × 20 × 20) mm3, matrix = 384 × 256 × 284 zero-filled to 768 × 512 × 512, averages = 2, TA = 6 h 3 min). 19F MR images from the 19 F-CRP were registered with those from the 19 F/1 H RT-coil. Since the 19 F-CRP has no 19 F/1 H dual resonant capacity, we registered the CRP 19F images onto the RT 19F images in order for both 19F images (RT and CRP) to be spatially aligned with the RT 1H images. For this, three repetitions of the RT 19F scan were averaged to achieve sufficient 19F signal with the RT-coil and an effective registration. Co-registration was applied using affine diffeomorphic image registration (12 degrees of freedom) by explicit B-spline regularization33, which is part of the Advanced Normalisation Tool (ANTs)34. Registration of the Allen brain atlas35 to the 1H image was achieved as follows: (1) 1H image and atlas template were segmented in grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) probability maps with SPMMouse (http://www.spmmouse.org/)36, (2) two synthetic images were generated with signal intensity in each voxel I(x,y,z) = 1.0 × GM (x y, z) + 2.0 × WM + 4.0 × CSF, i.e. one registered with the 1H image and one registered with the atlas, (3) both synthetic images were warped to the 1H image using nonlinear B-spline registration in ELASTIX (http://elastix.isi.uu.nl/)37. Raw 1H MRI files were converted to NIFTI-format and brains segmented with ITK-SNAP version 3.4.038. For 2D representation of 19F/1H MRI we performed overlays of the raw 19F MR data with SNR-based scaling using Matlab. For 3D representation we used ImageJ (National Institutes of Health, USA, http://imagej.nih.gov/ij).
Results
Transmit field characteristics of the 19F-CRP
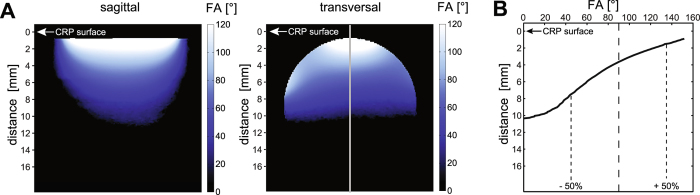
Since transceive surface coils do not achieve a spatially uniform excitation like volume resonators24, we assessed the B 1 + characteristics of the 19 F-CRP (Fig. 2A) and quantified changes in FA. A profile plot of the FA along the vertical axis (Fig. 2B) reveals a strong FA decrease with increasing distance from the CRP surface. Across a distance of 10.4 mm the measured FA varies between 152° and 0°. From the nominal FA of 90° the actual FA deviates up to 50% within a range of 6.0 mm (1.5–7.5 mm from CRP surface).
Figure 2.
Transmission B 1 + Field (B 1 +) for the 19 F-CRP. (A) Flip angle maps acquired in vertical and transversal orientation using a high concentration 19F phantom (Setup 1). (B) Profile plot of the FA along the vertical axis that depicts the change in FA with increasing distance to the CRP surface.
SNR assessment in phantoms
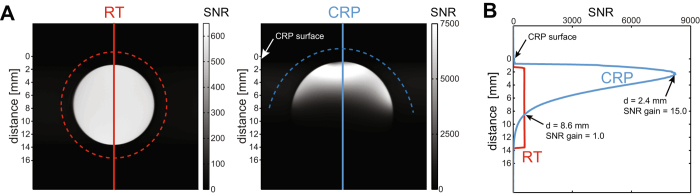
To study the SNR performance of the 19 F-CRP, we first used a high 19F concentration (33% TFE solution) (Fig. 3A). The transversal spin-echo 19F MR images demonstrate a homogenous SNR for the RT coil and a spatially varying SNR for the CRP (Fig. 3A). We adjusted the reference pulse power in order to avoid substantial signal loss at the dorsal side of the brain. Using this reference pulse power, the SNR reached its peak at a distance of 2.4 mm, where it was ~15-fold higher than the SNR of the RT coil (Fig. 3B). The SNR of both RF coils are approximately equal at a distance of 8.6 mm from the CRP.
Figure 3.
Comparison of SNR between the 19 F-CRP and 19 F/1 H RT-coil. (A) Cross-sectional spin-echo 19F MR images of a TFE phantom acquired with the RT RF coil (left) and the CRP (right). The CRP showed a spatially varying sensitivity that is typical for transceive surface coils. (B) Plots of the SNR profile along the vertical axis at the center of the phantom. For the RT volume resonator (red curve) the SNR was very uniform within the phantom. In contrast, for the CRP the SNR drops rapidly with increasing distance to the RF coil. For this particular reference pulse power, SNR reached its maximum at 2.4 mm from the CRP surface, where it is 15-fold higher than the SNR of the RT coil. Beyond a distance of 8.1 mm the 19 F-CRP did not provide any SNR gain with regard to the 19 F/1 H RT-coil.
We next investigated the detection limits for both coils by measuring 19F nanoparticles, as a biologically relevant preparation. We employed concentrations of PFCE (25 mM–200 mM) yielding a range of 1015–1018 19F atoms per voxel (Fig. 4A). Qualitatively, we reached a detection limit in the order of 1015 fluorine atoms using the 19 F-CRP, compared to 1016 fluorine atoms with the 19 F RT-coil. Specifically, an SNR of 3.0 was achieved with (0.1 × 0.1 × 0.4) mm³ voxels of a 25 mM PFCE concentration (equating to 5.2 × 1015 fluorine atoms) when using the 19 F-CRP. In contrast an SNR of 2.4 was achieved with (0.1 × 0.1 × 1.2) mm³ voxels of a 100 mM PFCE concentration (equating to 6.2 × 1016 fluorine atoms) when using the 19 F- RT-coil. In both cases the measurement time was 36 s. MR images with an SNR value below 2 were not sharply defined. To estimate SNR provided by the 19 F-CRP compared to the 19 F/1 H RT-coil, we used SNR = 2 as a cutoff equating to ~5 × 1016 (RT) and ~4 × 1015 (CRP) fluorine atoms per voxel. Next we prepared higher concentrations of 19F nanoparticles (200 mM to 1200 mM) to achieve SNR values well above 2, spanning a range of 1017–1019 atoms per voxel. From these experiments we calculated an SNR gain of ~16 for the 19 F-CRP when compared to the 19 F/1 H RT-coil (Fig. 4B).
Figure 4.
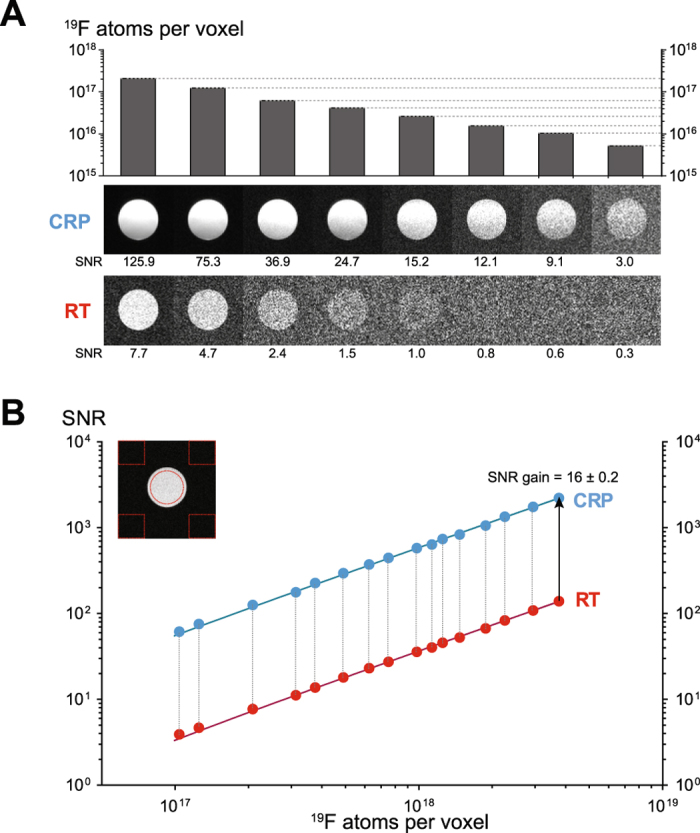
Comparison of 19F signal sensitivity between 19 F-CRP and 19 F/1 H RT-coil as a function of the number of 19F atoms. (A) Cross-sectional spin-echo 19F MR images of 19F nanoparticle phantoms acquired for both CRP (middle panel) and RT coil (lower panel). Each 19F MR image indicates an MR scan with a defined number of 19F atoms per voxel (upper panel) achieved with different concentrations of PFCE (ranging from 25 mM to 200 mM) and slice thicknesses varying from 0.4 to 2.0 mm. (B) Estimation of SNR gain provided by the 19 F-CRP compared to the 19 F/1 H RT-coil using high PFCE concentrations (200 mM to 1200 mM) and slice thicknesses varying from 1.0 to 6.0 mm. Shown is a log-log plot of SNR versus 19F atoms per voxel including a linear fit for both CRP (y = 5e−16x) and RT coil (y = 4e−17x).
High spatially-resolved 19F MRI
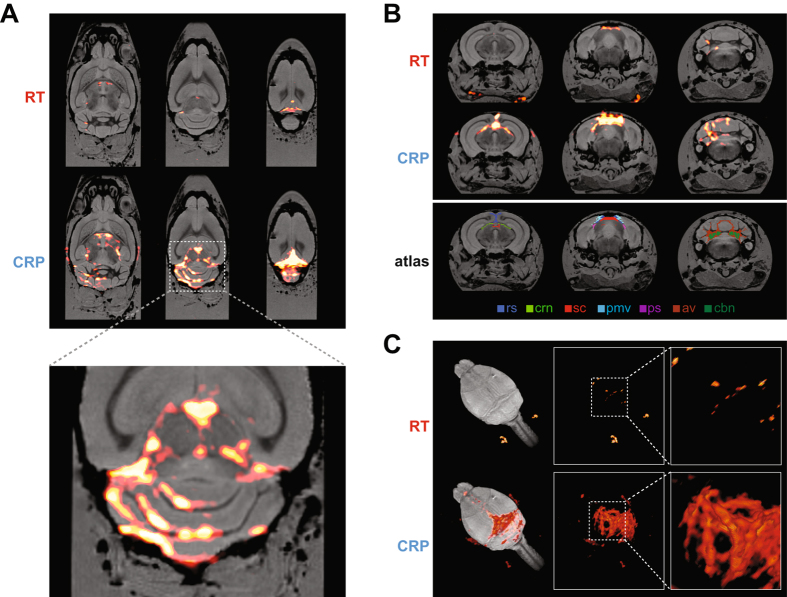
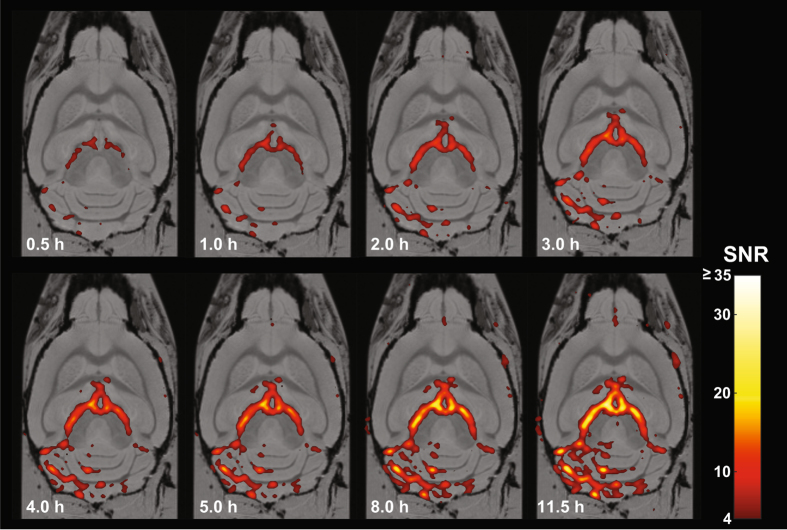
An important utilization of the SNR gain is to localize cell infiltrates in the brain with more detail. Previously areas of inflammation were detected using spatial resolutions greater than 600 μm11. Here we exploited the superior SNR of the 19 F-CRP, and used an isotropic spatial resolution of 150 μm. Ex vivo MR images obtained with the 19 F-CRP from an exemplary EAE mouse (day 10 following EAE induction, score = 1.25) show a more precise distribution of intraparenchymal inflammation. At this spatial resolution, the majority of the 19F signals obtained by the 19 F-CRP were not detected with the RT coil (Fig. 5A–C). In addition we show similar inflammatory patterns in a pre-symptomatic mouse, also sacrificed on day 10 following EAE induction (Supplementary Figure). Within the cerebellum, inflammatory infiltrates were mostly localized within the white matter of the arbor vitae, particularly near deep cerebellar nuclei (Fig. 5B). Clearly delineated inflammatory areas were found in grey matter regions running adjacent to white matter tracts in the cerebellum (Fig. 5A). This is consistent with the expected patterns of inflammation in the EAE model39, 40, also as observed in our own prior studies11, 41, 42. Using the 19 F-CRP, we also observed strong 19F signals in the cerebrum emanating from the striatum and pallidum appearing continuous with 19F signals from the third ventricle (Fig. 5A). Additionally, clear extraparenchymal meningeal inflammation could be seen, consistent with recent reports43–45. Especially strong inflammatory signals were observed along the dorsal surface of the brain, including meningeal regions lining fissures between the cerebellar lobules. These inflammatory regions extended ventrally to the prepyramidal fissure, parafloccular sulcus and lateral recess of the fourth ventricle. A dominant 19F signal was observed around the meninges lining the ventral part of the retrosplenial area of the cerebral cortex (Fig. 5B), spreading caudally towards the cerebellum, running in parallel to the superior sagittal sinus, and eventually the retroglenoid vein (Fig. 5C). In these experiments we focused on highly resolved inflammation imaging in the EAE brain, employing long acquisition times in order to compensate for the considerably lower 19F signal sensitivity of the 19 F/1 H RT-coil. Since these acquisition times (11 h) are not applicable for in vivo studies, we performed further experiments in which we reduced the scan time. Upon reducing the scan time from 11 h to 0.5 h we could still detect 19F signals with the 19 F-CRP (Fig. 6). Despite the clear differences we were nevertheless still able to detect a considerable 19F signal, even with a scan of only 2 h, which is amenable for in vivo MRI.
Figure 5.
High spatial resolution 19F MR image of an ex vivo brain from an EAE mouse showing clinical disease. With both 19 F-CRP and 19 F/1 H RT-coil, 19F MR images were acquired using a 3D-RARE sequence. 19F MR images (shown in red) were combined with 1H MR images (shown in grayscale). 1H MR images were acquired using a 3D-FLASH sequence and the 19 F/1 H RT-coil. (A) Three exemplary slices from horizontal views of combined 19F/1H MR images for both 19 F/1 H RT-coil (upper panel) and 19 F-CRP (middle panel), in the lower panel a 300% zoom of the 19F/1H MR images acquired with the CRP. (B) Three exemplary slices from coronal views of combined 19F/1H MR images for both RT coil (upper panel) and CRP (middle panel). Registration of the Allen brain atlas to the 1H image (lower panel) shows following labelled brain regions: rs: retrosplenial area; crn: cranial nerves; sc: superior colliculus (sensory related); pmv: posteromedial visual area; ps: postsubiculum; av: arbor vitae; cbn: cerebellar nuclei. (C) 3-D rendering of the combined 19F/1H MR images for both 19 F/1 H RT-coil (upper panel) and 19 F-CRP (lower panel).
Figure 6.
High spatial resolution 19F MRI using acquisition times feasible for in vivo imaging. 19F MR images were acquired with the 19 F-CRP using acquisition times between 30 min and 11 h. The 19F images were scaled to units of SNR, thresholded at SNR = 4, and overlayed onto the 1H MR images using a pseudocolor scale.
Discussion
In this study we show first 19F MR images obtained with a 19 F-CRP driven in quadrature mode. Compared to the 19 F/1 H RT-coil we previously developed11, we show that the 19 F-CRP facilitates superior ex vivo images of brain inflammation in an animal model of MS. At the current stage of development the 19 F-CRP cannot yet be employed for in vivo imaging due to incompatibilities with conventional 1H RT coils, as discussed later. Nevertheless the results are encouraging, and offer proof-of-concept demonstration of the potential for this technology.
After introducing the concept of cryogenically-cooled RF coil hardware to reduce thermal noise and thus increase SNR46, CRP technologies were developed for small animal MRI, particularly for anatomical 1H MRI of mouse brain41, 47–50. Introducing a quadrature CRP design, enabled further SNR gains (~2.5) at 400 MHz24, 25 compared to RT coils with similar geometries. The SNR gain prediction for the 19 F-CRP is expected to be equivalent due to the close Larmor frequency (376 MHz at 9.4T).
The potential applications of 19F MR methods to image inflammation have long been recognized11–17. For several years, neuroinflammation has been studied using gadolinium-based contrast agents. However, gadolinium-enhancing lesions are diffuse, and lack spatial precision. Improvements have been realized with the use of alternative contrast agents, such iron oxide nanoparticles, although their effects on magnetic susceptibility limit their discrimination from endogenous confounding artifacts. 19F MR methods abrogate this, since 19F signals derive exclusively from exogenously applied 19F nanoparticles. Efforts have been made to boost 19F signal e.g. by promoting 19F nanoparticle cellular uptake20. Nevertheless, major challenges of signal sensitivity constraints remain. Improving 19F sensitivity with the 19 F-CRP will be essential to realizing the full potential of 19F MR.
Our motivation to investigate the 19 F-CRP was to increase the sensitivity to detect neuroinflammation. Considering the geometrical differences between both coils, it was imperative to measure SNR at locations below the CRP that correspond to the mouse brain, using phantoms spanning the entire coronal view, as a basis for future in vivo studies. We performed SNR measurements for both 19 F-CRP and control 19 F/1 H RT-coil using a spin echo sequence (RARE), commonly used for 19F MRI due to its high SNR per unit time compared to spoiled gradient echo sequences.
The sensitivity of the 19 F-CRP is spatially dependent. Given that the CRP is a transceive quadrature surface coil array, both transmit field (B 1 +) and receive sensitivity (B 1 −) diminish with increasing distance from the RF coil – a factor that must be accounted for in quantitative imaging by measuring the actual B 1 and correcting the signal intensities using the signal equation of the employed pulse sequence. This is absolutely essential when signal quantification is necessary in order to ascertain the level of inflammation over the entire region of the brain during EAE. Nevertheless, this characteristic is shared by all transceive surface coils. This adverse effect is counterbalanced by an SNR gain, up to ~15-fold in the practical comparison made within this study. This SNR gain can be attributed to factors including cooling (in the range of 2–3 for 1H24, 25), differences in RF coil design (birdcage vs. surface coil; quadrature versus linear), RF coil sample loading, and the specific RF pulse power adjustments. Here, pulse power was adjusted in order to avoid substantial signal loss at the dorsal part of the brain, which is observed when using a RARE sequence with excessive RF power. Predicting the sensitivity and detection limits of 19F measurements for specific hardware setups51 will help facilitate further 19 F-CRP studies with other fluorinated compounds.
An SNR gain of 15 can be exploited in several ways — by reducing scan time by a factor 225 (e.g. from 1 h to ~15 s), or doubling 3D spatial resolution (e.g. from 600 µm to 300 µm) while still gaining SNR (~2.5). In this study we made use of the superior SNR, employing isotropic spatial resolutions of 150 μm to study neuroinflammation. Using the 19 F-CRP at this resolution, we gained more precise information regarding inflammatory cell localization in the brain, compared to our previous study11. The 19F MR images with the CRP showed excellent correspondence with the typical pattern of histopathology39, 40. A robust accumulation of inflammatory lesions, especially in the white matter tracts of the cerebellum, is a hallmark of EAE in SJL mice, which we also observed in our prior studies using high resolution 1H MR41, 42 and low resolution 19F MR11. The pathology also extends into the cerebrum, as shown both prior to the occurrence of clinical symptoms (Supplementary Figure) and also during ongoing clinical disease (Figs 5 and 6). The 19 F-CRP MR images also enabled discrimination of extraparenchymal meningeal inflammation, consistent with recent reports highlighting the relevance of inflammatory cell trafficking via the blood meningeal barrier43, 44 and extravasation via leptomeningeal microvessels into the subarachnoid space45. This also reflects the situation in MS3–5. Recent studies have argued for the presence of a lymphatic circulation in the meninges in association with these vessels, capable of draining immune cells from meningeal spaces8 and brain parenchyma7 into deep cervical lymph nodes. Therefore, the capacity to perform non-invasive longitudinal investigations with fidelity 19F MRI to monitor the dynamics and distribution of infiltrating immune cells will be directly relevant for experimental neuroimmunologists.
The gradient in the B 1 field of the 19 F-CRP leads to a gradual decline in 19F MR signal with increasing distance from the probe head. This results in reduced signal in ventral regions. Studies of the EAE model are, in general, more focused on imaging of the CNS, and less so on imaging of the superficial lymph nodes. When imaging of the lymph nodes in the ventral regions is necessary, one could consider measuring the mouse brain in the supine and prone positions, in order to ensure coverage of the dorsal sides comprising the whole brain as well as ventral sides to include the draining lymph nodes. Other possible workarounds include adding an anterior 19F RT RF coil to the mouse bed or combining 19F images from RT and CRP. These approaches could help to overcome this inherent limitation of the 19 F-CRP, while still utilizing its superior SNR. While the spatial dependency poses a constraint for studies investigating the involvement of the draining lymph nodes, the translational applications of the 19 F-CRP are not limited to EAE. The 19 F-CRP will also be useful for studying brain inflammation in animal models of tumour growth (especially those tumours implanted in the cortex or striatum), and studies on the middle cerebral artery occlusion model of stroke. Inflammation in these preclinical models could readily be imaged, since the focus of pathology in these models is located in regions where the 19 F-CRP clearly outperforms the 19 F/1 H RT-coil.
In vivo 19F MRI studies require acquisition of anatomical 1H MR images within a reasonable time frame. A dual-tunable RF probe would be most ideal, in order to avoid inaccurate co-registration of both signals52. Despite the clear improvement in SNR of the 19 F-CRP, the quadrature design prohibits the presence of a dual resonant MR signal that would be needed for anatomical 1H MRI. Furthermore conventional 1H RF resonators cannot be used in combination with the 19 F-CRP due to coupling between both RF coils. To avoid this, the 19 F-CRP would need to be removed while the in vivo 1H images are acquired. This would cause changes in the alignment of the mouse within the scanner during in vivo measurements that are serious enough to constitute a major hindrance. Even with the use of reference markers, any slight shift in the position of the markers with respect to the mouse during the procedure will result in an incorrect registration between 19F and 1H images. The current procedure of registering the 19F images of the CRP with those of the RT RF coil is complicated and time consuming, requires sufficient SNR and is an impediment for in vivo experiments. A proposed solution to this limitation could be to construct an anterior 1H RT RF coil, specifically designed to be added to the mouse bed while the 19 F-CRP remains installed, in order to provide anatomical guidance. A dual-tunable 1H/19F RT RF coil would also take into account the above approach (implementation of a 19F RF-coil below the mouse head).
This study presents the first demonstration of the performance of a quadrature 19 F-CRP tailored for small rodents, showing superior SNR and 19F MR image quality. The logical extension of this work will be to translate these results into in vivo studies, such as those studying pathological changes during neuroinflammatory disease. While the results of the current study are highly encouraging, a challenging road still lies ahead for the application of the 19 F-CRP in in vivo studies. Previous studies using 19F MR have been seriously hampered by the low SNR, and compensating for this limitation by using low spatial resolution has generally yielded images with rather poor definition, and therefore limited scientific utility. The current study aims to improve this situation, bringing 19F MR imaging a step closer to the objective of ‘microscopic MRI’. Our results showed a remarkable SNR and detail of neuroinflammation, compared to conventional 19F MRI, heralding a bright potential for the application of 19 F-CRP for non-invasive MRI in vivo.
Acknowledgements
This study was funded by the Deutsche Forschungsgemeinschaft to S.W. (DFG WA2804) and A.P. (DFG PO1869). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Stefanie Kox for preparation of nanoparticles, Marco Küng for building and optimizing the RF coil and associated circuitry, and Marco Sacher for contributions to mechanical design.
Author Contributions
S.W., T.N., and A.P. conceived the development of the 19F-C R P and designed the study S.W., J.M.M, P.R.D., C.P., D.W., R.W. and A.P. carried out the experiments and measurements. S.W., L.S., P.R.D., T.H., S.P.K., P.B.S., H.W. and A.P. performed the analyses. D.M. developed the RF-Probe. S.W., J.M.M., T.N. and A.P. wrote the manuscript with the assistance of all other co-authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stadelmann C, Wegner C, Bruck W. Inflammation, demyelination, and degeneration-recent insights from MS pathology. Biochim.Biophys.Acta. 2011;1812:275–282. doi: 10.1016/j.bbadis.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann.Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::AID-ANA3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Gilmore CP, et al. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J. Neurol. Neurosurg. Psychiatry. 2009;80:182–187. doi: 10.1136/jnnp.2008.148767. [DOI] [PubMed] [Google Scholar]
- 4.Howell OW, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134:2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- 5.Howell OW, et al. Extensive grey matter pathology in the cerebellum in multiple sclerosis is linked to inflammation in the subarachnoid space. Neuropathol. Appl. Neurobiol. 2015;41:798–813. doi: 10.1111/nan.12199. [DOI] [PubMed] [Google Scholar]
- 6.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 7.Aspelund A, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212 doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt B, et al. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta. Neuropathologica. 2016;132:317–338. doi: 10.1007/s00401-016-1606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waiczies H, et al. Visualizing brain inflammation with a shingled-leg radio-frequency head probe for 19F/1H MRI. Sci. Rep. 2013;3 doi: 10.1038/srep01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flögel U, et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahrens ET, Young WB, Xu H, Pusateri LK. Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. Biotechniques. 2011;50:229–234. doi: 10.2144/000113652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flogel U, et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003717. [DOI] [PubMed] [Google Scholar]
- 15.Temme S, Bonner F, Schrader J, Flogel U. 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4:329–343. doi: 10.1002/wnan.1163. [DOI] [PubMed] [Google Scholar]
- 16.Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 2013;26:860–871. doi: 10.1002/nbm.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby C, et al. Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014;27:261–271. doi: 10.1002/nbm.3059. [DOI] [PubMed] [Google Scholar]
- 18.Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat. Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 19.Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine‐19 MRI. Magn. Reson. Med. 2014;72:1696–701. doi: 10.1002/mrm.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waiczies S, et al. Anchoring dipalmitoyl phosphoethanolamine to nanoparticles boosts cellular uptake and fluorine-19 magnetic resonance signal. Sci. Rep. 2015;5 doi: 10.1038/srep08427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dardzinski BJ, Sotak CH. Magnetic resonance in medicine. 1994. Rapid tissue oxygen tension mapping using 19F inversion‐recovery echo‐planar imaging of P erfluoro‐15‐crown‐5‐ether; pp. 88–97. [DOI] [PubMed] [Google Scholar]
- 22.Kadayakkara DK, Janjic JM, Pusateri LK, Young WB, Ahrens ET. In vivo observation of intracellular oximetry in perfluorocarbon‐labeled glioma cells and chemotherapeutic response in the CNS using fluorine‐19 MRI. Magn. Reson. Med. 2010;64:1252–1259. doi: 10.1002/mrm.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faber, C. & Schmid, F. Pulse Sequence Considerations and Schemes in Fluorine Magnetic Resonance Imaging (ed. Flögel, U. and Ahrens, E.) 1–28 (Pan Stanford Publishing (2016).
- 24.Baltes C, Radzwill N, Bosshard S, Marek D, Rudin M. Micro MRI of the mouse brain using a novel 400 MHz cryogenic quadrature RF probe. NMR Biomed. 2009;22:834–842. doi: 10.1002/nbm.1396. [DOI] [PubMed] [Google Scholar]
- 25.Junge, S. Cryogenic and Superconducting Coils for MRI in eMagRes (ed. Wasylishen, R.) 505–514 (John Wiley & Sons, Ltd (2012).
- 26.Waiczies H, et al. Perfluorocarbon particle size influences magnetic resonance signal and immunological properties of dendritic cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akoka S, Franconi F, Seguin F, Le Pape A. Radiofrequency map of an NMR coil by imaging. Magn. Reson. Imaging. 1993;11:437–441. doi: 10.1016/0730-725X(93)90078-R. [DOI] [PubMed] [Google Scholar]
- 28.Insko EK, Bolinger L. Mapping of the Radiofrequency Field. J. Magn. Reson., Series A. 1993;103:82–85. doi: 10.1006/jmra.1993.1133. [DOI] [Google Scholar]
- 29.NEMA. Determination of signal-to-noise ratio (SNR) in diagnostic magnetic resonance imaging. NEMA Standards Publication MS, 1–2008 (2008).
- 30.Henkelman RM. Measurement of signal intensities in the presence of noise in MR images. Med. Phys. 1985;12:232–233. doi: 10.1118/1.595711. [DOI] [PubMed] [Google Scholar]
- 31.Gudbjartsson H, Patz S. The Rician Distribution of Noisy MRI Data. Magn. Reson. Med. 1995;34:910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Constantinides CD, Atalar E, McVeigh ER. Signal-to-noise measurements in magnitude images from NMR phased arrays. Magn. Reson. Med. 1997;38:852–857. doi: 10.1002/mrm.1910380524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tustison, N. & Avants, B. Explicit B-spline regularization in diffeomorphic image registration. Front. Neuroinform. 7, doi:10.3389/fninf.2013.00039 (2013). [DOI] [PMC free article] [PubMed]
- 34.Avants BB, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 36.Sawiak S, Wood N, Williams G, Morton A, Carpenter T. Use of magnetic resonance imaging for anatomical phenotyping of the R6/2 mouse model of Huntington’s disease. Neurobiol. Dis. 2009;33:12–19. doi: 10.1016/j.nbd.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Shamonin DP, et al. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front. Neuroinform. 2014;7 doi: 10.3389/fninf.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yushkevich PA, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 40.Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J. Comp. Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- 41.Waiczies H, et al. Identification of Cellular Infiltrates during Early Stages of Brain Inflammation with Magnetic Resonance Microscopy. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lepore S, et al. Enlargement of cerebral ventricles as an early indicator of encephalomyelitis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartholomaus I, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 44.Mues M, et al. Real-time in vivo analysis of T cell activation in the central nervous system using a genetically encoded calcium indicator. Nat. Med. 2013;19:778–783. doi: 10.1038/nm.3180. [DOI] [PubMed] [Google Scholar]
- 45.Schläger C, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 46.Hoult DI, Richards RE. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson.(1969) 1976;24:71–85. doi: 10.1016/0022-2364(76)90233-X. [DOI] [PubMed] [Google Scholar]
- 47.Ratering D, Baltes C, Nordmeyer-Massner J, Marek D, Rudin M. Performance of a 200-MHz cryogenic RF probe designed for MRI and MRS of the murine brain. Magn. Reson. Med. 2008;59:1440–1447. doi: 10.1002/mrm.21629. [DOI] [PubMed] [Google Scholar]
- 48.Nouls JC, Izenson MG, Greeley HP, Johnson GA. Design of a superconducting volume coil for magnetic resonance microscopy of the mouse brain. J. Magn. Reson. 2008;191:231–238. doi: 10.1016/j.jmr.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagenhaus B, et al. Functional and morphological cardiac magnetic resonance imaging of mice using a cryogenic quadrature radiofrequency coil. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niendorf T, et al. Advancing Cardiovascular, Neurovascular and Renal Magnetic Resonance Imaging in Small Rodents Using Cryogenic Radiofrequency Coil Technology . Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor AJ, et al. Probe-Specific Procedure to Estimate Sensitivity and Detection Limits for 19F Magnetic Resonance Imaging. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomanek B, Volotovskyy V, Gruwel MLH, McKenzie E, King SB. Double-frequency birdcage volume coils for 4.7T and 7T. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2005;26B:16–22. doi: 10.1002/cmr.b.20038. [DOI] [Google Scholar]