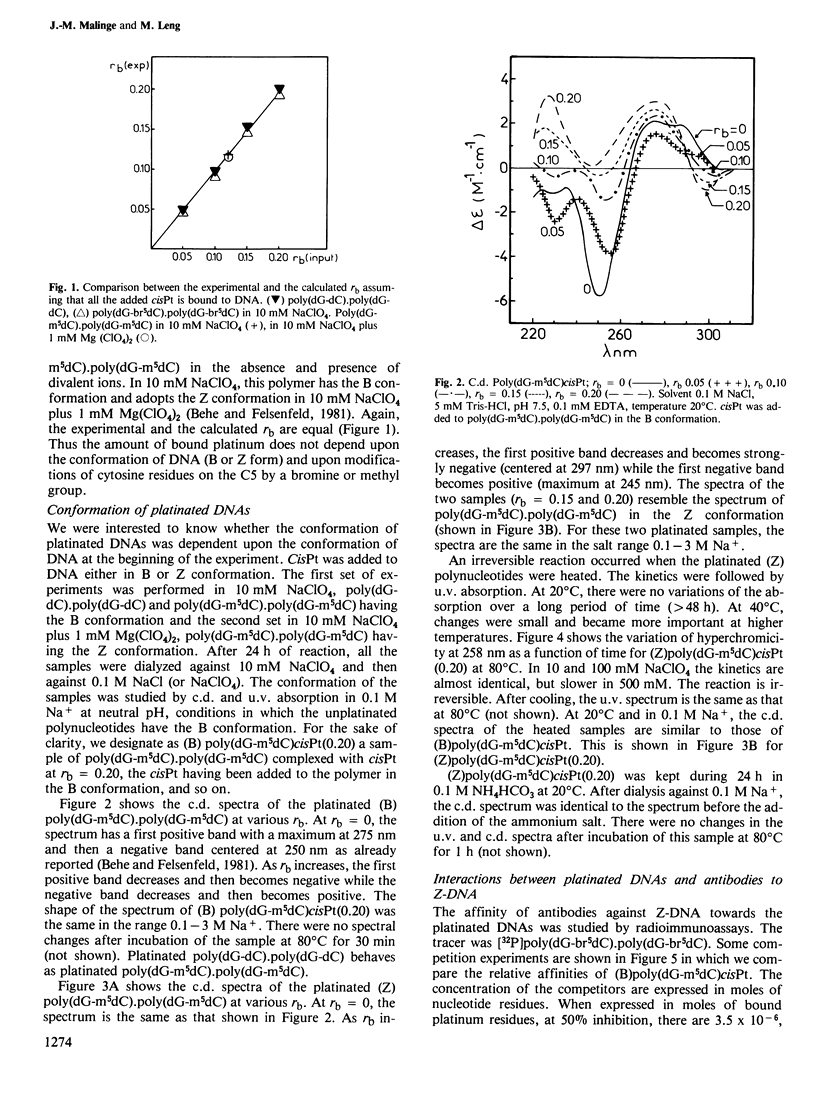
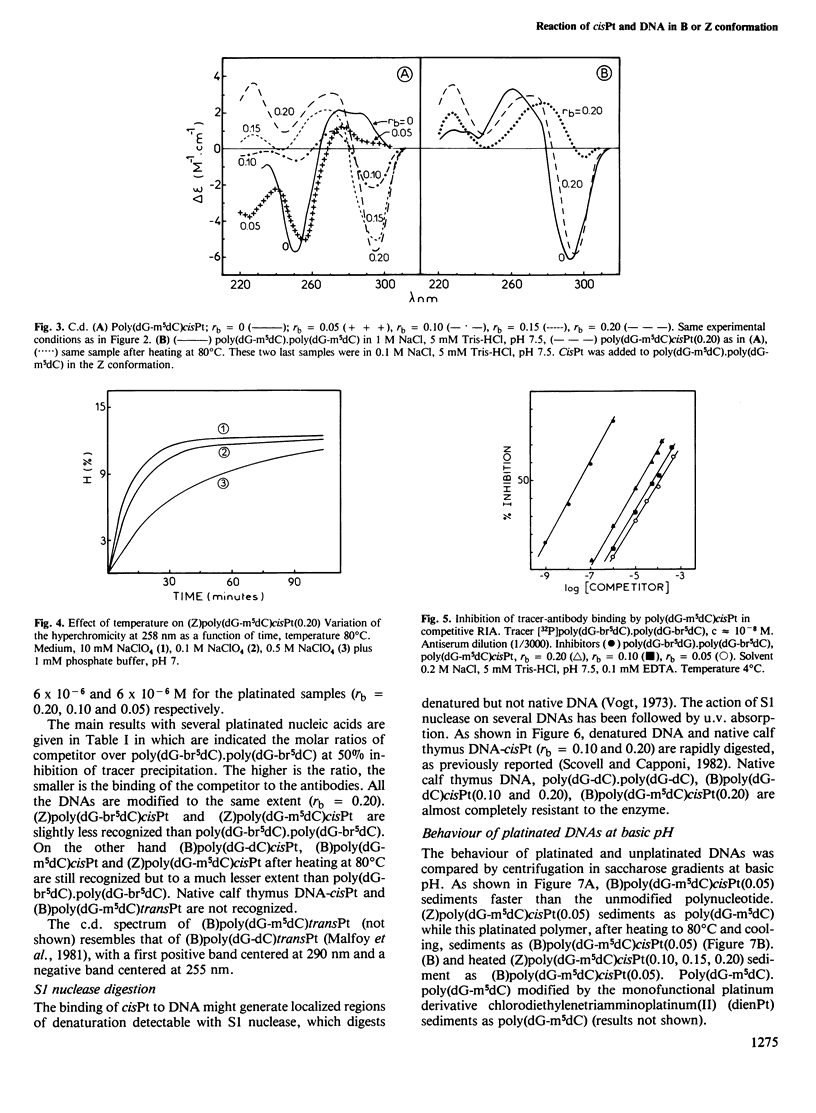
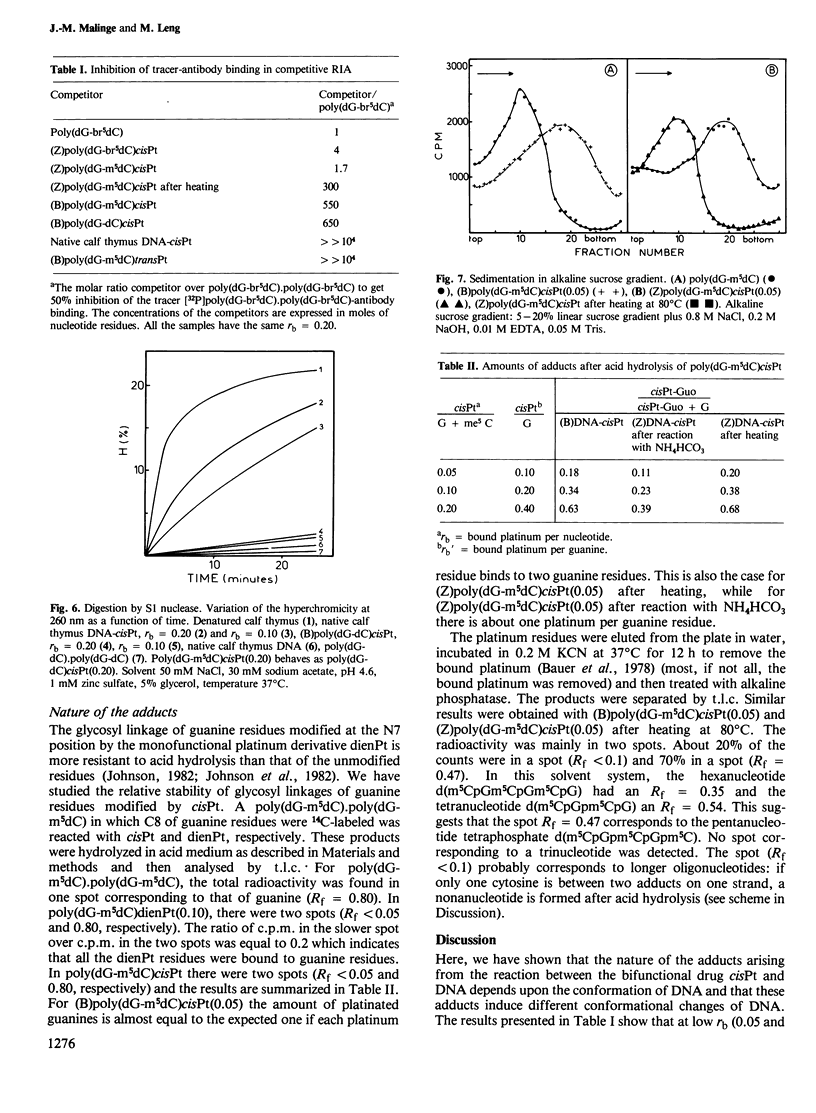
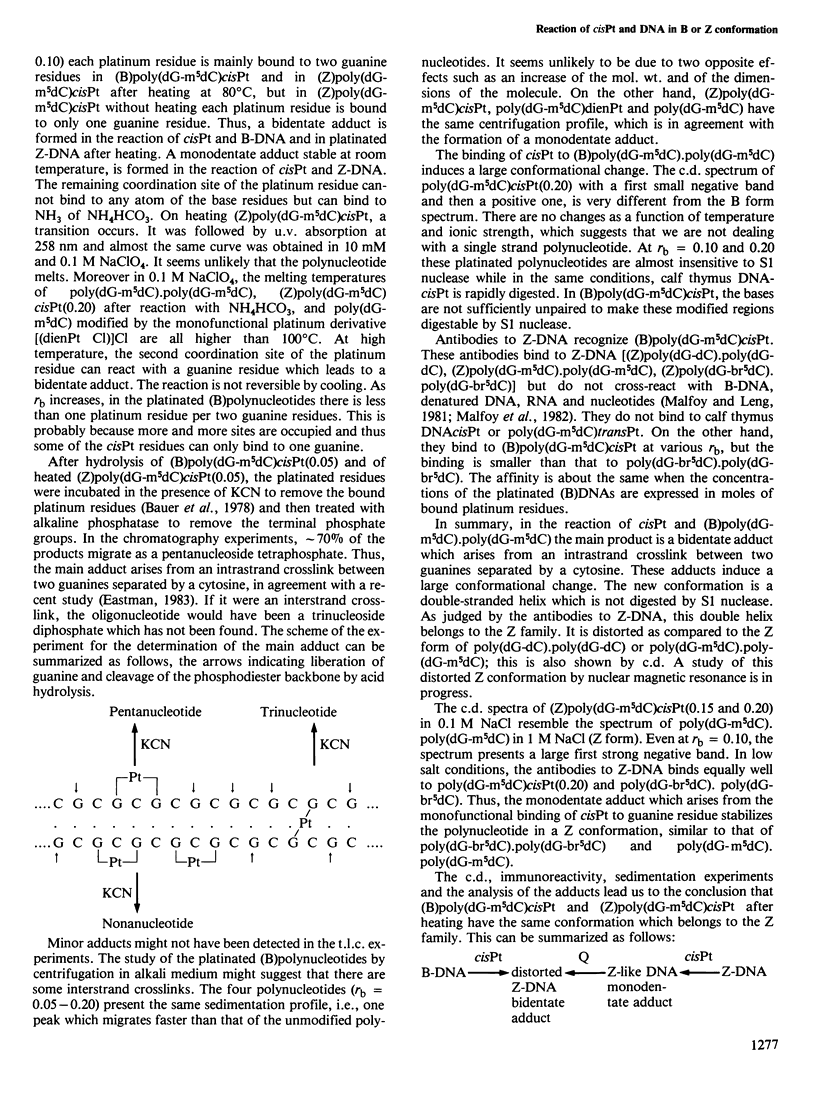
Abstract
The nature of the adducts and the conformational changes produced in poly(dG-m5dC).poly(dG-m5dC) by cis-diamminedichloroplatinum(II) (cisPt) have been studied. In the reaction of cisPt and B-DNA, the main adduct is bidentate and arises from an intrastrand cross-link between two guanine residues separated by a cytosine. This was deduced from the study of the compounds by t.l.c. after acid hydrolysis of the polymer. The platinated polymer is not digested by S1 nuclease. The antibodies to Z-DNA bind to the platinated polymer with a smaller affinity than to poly (dG-br5dC).poly(dG-br5dC). The c.d. spectrum differs from that of poly(dG-br5dC).poly(dG-br5dC) or poly(dG-m5dC).poly-(dG-m5dC) in Z conformation. It is concluded that the bidentate adduct induces a conformational change from the B form towards a distorted Z form. In the reaction of cisPt and Z-DNA, a monodentate adduct is formed. This adduct stabilizes the Z conformation as shown by c.d. and binding to the anti-Z-DNA antibodies. At room temperature, the second function of the drug can still react with small ligands such as NH4HCO3. By heating, the second function reacts with a guanine residue. A bidentate adduct is formed as in the reaction of cisPt and B-DNA and it induces a transition from the Z form to the distorted Z form.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Robert-Nicoud M., Zarling D. A., Greider C., Weimer E., Jovin T. M. Left-handed Z-DNA in bands of acid-fixed polytene chromosomes. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4344–4348. doi: 10.1073/pnas.80.14.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W., Gonias S. L., Kam S. K., Wu K. C., Lippard S. J. Binding of the antitumor drug platinum uracil blue to closed and nicked circular duplex DNAs. Biochemistry. 1978 Mar 21;17(6):1060–1068. doi: 10.1021/bi00599a019. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer J., van de Putte P., Fichtinger-Schepman A. M., Reedijk J. Base-pair substitution hotspots in GAG and GCG nucleotide sequences in Escherichia coli K-12 induced by cis-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1981 Nov;78(11):7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., Lohman P. H., Reedijk J. Detection and quantification of adducts formed upon interaction of diamminedichloroplatinum (II) with DNA, by anion-exchange chromatography after enzymatic degradation. Nucleic Acids Res. 1982 Sep 11;10(17):5345–5356. doi: 10.1093/nar/10.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli P. K., Theophanides T. Preferential interstrand cross-linking of DNA rich in guanine and cytosine by cis-dichlorodiammineplatinum(II). Eur J Biochem. 1979 Nov;101(2):377–383. doi: 10.1111/j.1432-1033.1979.tb19729.x. [DOI] [PubMed] [Google Scholar]
- Girault J. P., Chottard G., Lallemand J. Y., Chottard J. C. Interaction of cis-[Pt(NH3)2(H2O)2](NO3)2 with ribose deoxyribose diguanosine phosphates. Biochemistry. 1982 Mar 16;21(6):1352–1356. doi: 10.1021/bi00535a038. [DOI] [PubMed] [Google Scholar]
- Harder H. C., Lee C. C. Coordination of interstrand cross-links between polydeoxyguanylic acid and polydeoxycytidylic acid by cis-diamminedichloroplatinum (II). Cancer Res. 1983 Oct;43(10):4799–4804. [PubMed] [Google Scholar]
- Harder H. C. Renaturation effects of cis and trans platinum II and IV compounds on calf thymus deoxyribonucleic acid. Chem Biol Interact. 1975 Jan;10(1):27–39. doi: 10.1016/0009-2797(75)90044-7. [DOI] [PubMed] [Google Scholar]
- Johnson N. P., Macquet J. P., Wiebers J. L., Monsarrat B. Structures of the adducts formed between [Pt(dien)Cl]Cl and DNA in vitro. Nucleic Acids Res. 1982 Sep 11;10(17):5255–5271. doi: 10.1093/nar/10.17.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N. P. Preliminary characterization of the adducts formed between the antitumor compounds cis-Pt(NH3)2Cl2 and DNA. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1394–1400. doi: 10.1016/0006-291x(82)91404-8. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng M., Hartmann B., Malfoy B., Pilet J., Ramstein J., Sage E. Interactions between nucleic acids and antibodies to Z-DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):163–170. doi: 10.1101/sqb.1983.047.01.021. [DOI] [PubMed] [Google Scholar]
- Lippard S. J. New chemistry of an old molecule: cis-[Pt(NH3)2Cl2]. Science. 1982 Dec 10;218(4577):1075–1082. doi: 10.1126/science.6890712. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Nordheim A., Lafer E. M., Ammermann D., Stollar B. D., Rich A. Antibodies against Z DNA react with the macronucleus but not the micronucleus of the hypotrichous ciliate stylonychia mytilus. Cell. 1983 Feb;32(2):435–441. doi: 10.1016/0092-8674(83)90463-4. [DOI] [PubMed] [Google Scholar]
- Macquet J. P., Jankowski K., Butour J. L. Mass spectrometry study of DNA-cisplatin complexes: perturbation of guanine-cytosine base-pairs. Biochem Biophys Res Commun. 1980 Jan 15;92(1):68–74. doi: 10.1016/0006-291x(80)91520-x. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Rousseau N., Leng M. Interaction between antibodies to Z-form deoxyribonucleic acid and double-stranded polynucleotides. Biochemistry. 1982 Oct 26;21(22):5463–5467. doi: 10.1021/bi00265a013. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelis A. T., den Hartog J. H., van der Marel G. A., Wille G., Reedijk J. Interaction of platinum compounds with short oligodeoxynucleotides containing guanine and cytosine. Eur J Biochem. 1983 Sep 15;135(2):343–349. doi: 10.1111/j.1432-1033.1983.tb07660.x. [DOI] [PubMed] [Google Scholar]
- Morgenegg G., Celio M. R., Malfoy B., Leng M., Kuenzle C. C. Z-DNA immunoreactivity in rat tissues. Nature. 1983 Jun 9;303(5917):540–543. doi: 10.1038/303540a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969 Apr 26;222(5191):385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. R., Sabat M., Sundaralingam M. Similar binding of the carcinostatic drugs cis-[Pt(NH3)2Cl2] and [Ru(NH3)5Cl] Cl2 to tRNAphe and a comparison with the binding of the inactive trans-[Pt(NH3)2Cl2] complex - reluctance in binding to Watson-Crick base pairs within double helix. Nucleic Acids Res. 1983 Sep 24;11(18):6571–6586. doi: 10.1093/nar/11.18.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scovell W. M., Capponi V. J. Cis-diamminedichloroplatinum(II) modified DNA stimulates far greater levels of S1 nuclease sensitive regions than does the modification produced by the trans- isomer. Biochem Biophys Res Commun. 1982 Aug;107(3):1138–1143. doi: 10.1016/0006-291x(82)90640-4. [DOI] [PubMed] [Google Scholar]
- Viegas-Péquignot E., Derbin C., Malfoy B., Taillandier E., Leng M., Dutrillaux B. Z-DNA immunoreactivity in fixed metaphase chromosomes of primates. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5890–5894. doi: 10.1073/pnas.80.19.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- den Hartog J. H., Altona C., van Boom J. H., Marcelis A. T., van der Marel G. A., Rinkel L. J., Wille-Hazeleger G., Reedijk J. cis-Platinum induced distortions in DNA. Conformational analysis of d(GpCpG) and cis-pt(NH3)2[d(GpCpG)], studied by 500-MHz NMR. Eur J Biochem. 1983 Aug 15;134(3):485–495. doi: 10.1111/j.1432-1033.1983.tb07593.x. [DOI] [PubMed] [Google Scholar]


