Abstract
A recent genome-wide association study showed that a genetic variant within the FER gene is associated with survival in patients with sepsis due to pneumonia. Because severe pneumonia is the main cause of acute respiratory distress syndrome (ARDS), we aimed to investigate the effect of the FER polymorphism rs4957796 on the 90-day survival in patients with ARDS due to pneumonia. An assessment of a prospectively collected cohort of 441 patients with ARDS admitted to three intensive care units at the University Medical Centre identified 274 patients with ARDS due to pneumonia. The 90-day mortality risk was recorded as the primary outcome parameter. Sepsis-related organ failure assessment (SOFA) scores and organ support-free days were used as the secondary variables. FER rs4957796 TT-homozygous patients were compared with C-allele carriers. The survival analysis revealed a higher 90-day mortality risk among T homozygotes than among C-allele carriers (p = 0.0144) exclusively in patients with severe ARDS due to pneumonia. The FER rs4957796 TT genotype remained a significant covariate for the 90-day mortality risk in the multivariate analysis (hazard ratio, 4.62; 95% CI, 1.58–13.50; p = 0.0050). In conclusion, FER rs4957796 might act as a prognostic variable for survival in patients with severe ARDS due to pneumonia.
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by excessive and protracted pulmonary inflammation with increased permeability of pulmonary capillary and alveolar epithelial cells, leading to hypoxemia that is refractory to the usual oxygen therapy1, 2. ARDS represents a common clinical problem in ICU patients and is associated with a short-term mortality of up to 45%3, 4 and significant long-term morbidity5.
Attempts to reduce the mortality in ARDS patients by decreasing the overwhelming pulmonary inflammation have proven mostly disappointing6–8, most likely because these interventions have usually been applied unselectively to heterogeneous groups of patients, without considering the potential influence of host genetic diversity on the response to treatment. Genomics has the potential to substantially advance our understanding of the key biological pathways implicated in human disease and to suggest new targets for treatment or prevention9. Additionally, the characterization of genetic variants associated with the outcome of sepsis could enable identification of those at high risk who might benefit from more aggressive interventions or from specific, individually targeted, early, or pre-emptive measures.
A recent genome-wide association study has shown that a genetic variant within the intronic region of the FER gene, rs4957796, is associated with survival in patients with sepsis due to pneumonia. The FER gene encodes a non-receptor protein tyrosine kinase that acts downstream of cell-surface receptors for growth factors and is ubiquitously expressed10. FER is known to play a role in the regulation of the actin cytoskeleton, cell adhesion, migration and invasion, and chemotaxis11–14. FER impacts leucocyte recruitment and intestinal barrier dysfunction in response to bacterial lipopolysaccharides15, 16, findings relevant to the potential mechanisms through which variants in this gene could influence sepsis survival. Furthermore, studies in mice targeted with an FER kinase-inactivating mutation have shown that FER can inhibit neutrophil chemotaxis17. Neutrophil recruitment to the site of infection is essential in innate immune defense, and changes in relevant signaling pathways could lead to a failure to clear bacterial infections or the promotion of further tissue damage18.
Because the most frequent lung condition leading to ARDS is sepsis due to pneumonia19, 20, this study aimed to investigate the effect of the FER rs4957796 variant on the 90-day survival in patients with ARDS due to pneumonia according to the severity of ARDS.
Results
Baseline characteristics
A total of 274 adult Caucasian patients with ARDS due to pneumonia were enrolled in this study. The patients’ ages ranged from 18 to 90 years (median, 62 years) (Table 1). Thirty-one percent of the patients were women, and 69% were men. The genotype distribution of FER rs4957796 was 10:79:185 (CC:CT:TT), which is consistent with Hardy-Weinberg equilibrium (p = 0.9111). The minor allele frequency was 18%. The FER rs4957796 CC and CT genotypes were pooled to explore the clinical effect of the TT genotype compared to that of C-allele carriers in accordance with our a priori hypothesis (Table 1). The distribution values of sepsis/severe sepsis and septic shock were 21% and 79%, respectively. At baseline, the mean SOFA and APACHE II morbidity scores were 9.4 ± 3.3 and 21.8 ± 6.3, respectively (Table 1). The frequencies of organ support therapies including mechanical ventilation, vasopressor therapy and renal replacement therapy at sepsis onset were 93%, 65% and 7%, respectively (Table 1).
Table 1.
Patients’ baseline characteristics.
| Parameter | All (n = 274) | ARDS (all patients) | p value | |
|---|---|---|---|---|
| T/C+C/C | T/T | |||
| (n=89) | (n=185) | |||
| Age, years | 61 ± 15 | 62 ± 16 | 60 ± 15 | 0.0967 |
| Male [%] | 69 | 66 | 70 | 0.5638 |
| Body mass index | 28 ± 7 | 27 ± 5 | 28 ± 8 | 0.6523 |
| Severity of sepsis | ||||
| Sepsis/severe sepsis, % | 21 | 27 | 18 | 0.1032 |
| Septic shock, % | 79 | 73 | 82 | 0.1032 |
| Sequential Organ Failure Assessment (SOFA) score | 9.4 ± 3.3 | 9.5 ± 3.1 | 9.3 ± 3.4 | 0.7312 |
| Acute Physiology and Chronic Health Evaluation (APACHE II) score | 21.8 ± 6.3 | 22.0 ± 6.2 | 21.6 ± 6.3 | 0.6409 |
| Comorbidities [%] | ||||
| Hypertension | 54 | 57 | 53 | 0.5003 |
| History of myocardial infarction | 7 | 7 | 6 | 0.9356 |
| Chronic obstructive pulmonary disease | 18 | 22 | 15 | 0.1346 |
| Renal dysfunction | 9 | 10 | 9 | 0.8071 |
| Noninsulin-dependent diabetes mellitus | 9 | 8 | 10 | 0.6157 |
| Insulin-dependent diabetes mellitus | 9 | 8 | 10 | 0.6157 |
| Chronic liver disease | 4 | 2 | 4 | 0.3905 |
| History of cancer | 15 | 15 | 15 | 0.9086 |
| History of stroke | 6 | 9 | 4 | 0.1231 |
| Recent surgical history [%] | 0.9169 | |||
| Elective surgery | 25 | 24 | 25 | |
| Emergency surgery | 54 | 56 | 54 | |
| No history of surgery | 21 | 20 | 21 | |
| Organ support [%] | ||||
| Used during observation period | ||||
| Mechanical ventilation | 99 | 100 | 99 | 0.3249 |
| Use of vasopressor | 79 | 73 | 82 | 0.1032 |
| Renal replacement therapy | 19 | 17 | 20 | 0.5340 |
| Used on sepsis onset | ||||
| Mechanical ventilation | 93 | 96 | 92 | 0.2702 |
| Use of vasopressor | 65 | 63 | 66 | 0.5615 |
| Renal replacement therapy | 7 | 7 | 7 | 0.9306 |
| Use of statins | 29 | 38 | 24 | 0.0176 |
Outcomes
Mortality
To detect the effect of FER rs4957796 on the 90-day outcome of ARDS and the dependence on ARDS severity (mild, moderate, and severe), Kaplan-Meier survival analysis was performed for the 90-day survival in these three groups of patients (Fig. 1); FER rs4957796 affected the 90-day survival exclusively among patients with severe ARDS; patients with severe ARDS who were C-allele carriers showed a lower 90-day mortality rate than TT-homozygous patients (p = 0.0144) (Fig. 1).
Figure 1.
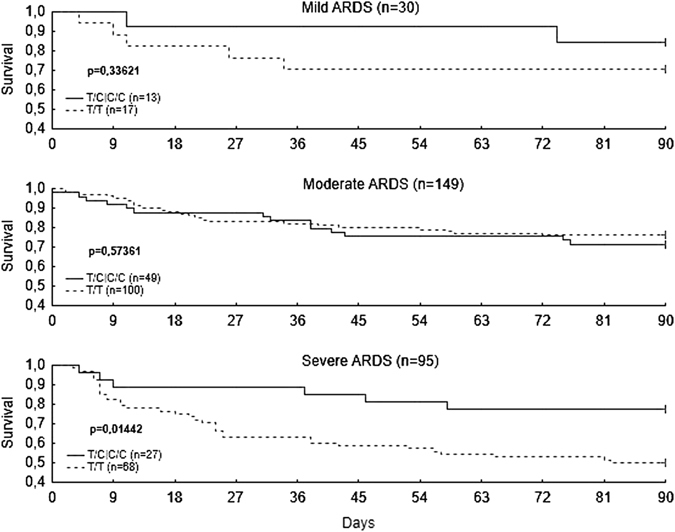
Kaplan-Meier survival analysis. Kaplan-Meier survival analysis of 90-day survival according to FER rs4957796 for the three acute respiratory distress syndrome (ARDS) groups. FER polymorphism rs4957796 is associated with higher 90-day mortality exclusively in patients with severe ARDS due to pneumonia (p = 0.0144, log-rank test).
Multivariate analysis
To exclude the effects of potential confounders and different baseline variables on the 90-day mortality among patients with severe ARDS, the baseline patient characteristics in the severe ARDS group were analyzed according to the FER rs4957796 genotype (Table 2). A multivariate Cox regression model including different baseline variables and relevant confounders revealed the highest hazard ratio for patients with the FER rs4957796 TT genotype (hazard ratio, 4.62; 95% CI, 1.58–13.50; p = 0.0050) (Table 3), followed by lack of statin use (hazard ratio, 2.31; 95% CI, 0.85–6.30; p = 0.1024) and a poor SOFA score (hazard ratio, 1.09; 95% CI, 0.96–1.24; p = 0.1784) (Table 3). This finding indicates that despite potential baseline confounders (age, gender, initial APACHE II and SOFA scores, and history of stroke and statin therapy), the FER rs4957796 TT genotype is an independent prognostic variable for outcome and exhibits the most significant effect on the 90-day mortality (Table 3).
Table 2.
Severe acute respiratory distress syndrome patients’ baseline characteristics.
| Parameter | All (n = 95) | Severe ARDS | p value | |
|---|---|---|---|---|
| T/C+C/C | T/T | |||
| (n = 27) | (n = 68) | |||
| Age, years | 58 ± 15 | 59 ± 15 | 58 ± 15 | 0.6430 |
| Male [%] | 68 | 70 | 68 | 0.7967 |
| Body mass index | 29 ± 8 | 27 ± 4 | 30 ± 9 | 0.5966 |
| Severity of sepsis | ||||
| Sepsis/severe sepsis, % | 9 | 19 | 6 | 0.0578 |
| Septic shock, % | 91 | 81 | 94 | |
| Sequential Organ Failure Assessment (SOFA) score | 23.5 ± 6.2 | 23.4 ± 7.1 | 23.6 ± 5.9 | 0.8794 |
| Acute Physiology and Chronic Health Evaluation (APACHE II) score | 10.8 ± 3.2 | 10.6 ± 3 | 10.9 ± 3.3 | 0.7336 |
| Comorbidities [%] | ||||
| Hypertension | 56 | 52 | 57 | 0.6262 |
| History of myocardial infarction | 3 | 4 | 3 | 0.8479 |
| Chronic obstructive pulmonary disease | 18 | 15 | 19 | 0.6216 |
| Renal dysfunction | 6 | 11 | 4 | 0.2259 |
| Noninsulin-dependent diabetes mellitus | 9 | 7 | 10 | 0.6647 |
| Insulin-dependent diabetes mellitus | 8 | 7 | 9 | 0.8226 |
| Chronic liver disease | 2 | 4 | 1 | 0.4940 |
| History of cancer | 20 | 22 | 19 | 0.7329 |
| History of stroke | 7 | 15 | 4 | 0.0800 |
| Recent surgical history [%] | 0.1643 | |||
| Elective surgery | 25 | 37 | 21 | |
| Emergency surgery | 45 | 44 | 46 | |
| No history of surgery | 29 | 19 | 34 | |
| Organ support [%] | ||||
| Used during observation period | ||||
| Mechanical ventilation | 100 | 100 | 100 | |
| Use of vasopressor | 91 | 81 | 94 | 0.0578 |
| Renal replacement therapy | 29 | 26 | 31 | 0.6327 |
| Used on sepsis onset | ||||
| Mechanical ventilation | 98 | 96 | 99 | 0.4941 |
| Use of vasopressor | 77 | 74 | 78 | 0.6869 |
| Renal replacement therapy | 7 | 11 | 6 | 0.3789 |
| Use of statins | 23 | 37 | 18 | 0.0433 |
Table 3.
Cox regression analysis of severe acute respiratory distress syndrome patients.
| Variable | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Age | 1.00 | 0.98–1.02 | 0.9720 |
| Male gender | 1.01 | 0.49–2.07 | 0.9724 |
| Body mass index | 0.98 | 0.94–1.02 | 0.3415 |
| Sequential Organ Failure Assessment (SOFA) score | 1.09 | 0.96–1.24 | 0.1784 |
| Acute Physiology and Chronic Health Evaluation (APACHE II) score | 1.05 | 0.99–1.12 | 0.0873 |
| History of stroke | 0.41 | 0.09–1.86 | 0.2500 |
| No statin therapy | 2.31 | 0.85–6.30 | 0.1024 |
| T/T genotype | 4.62 | 1.58–13.50 | 0.0050 |
Disease severity
To assess the effect of FER rs4957796 on disease severity and organ dysfunction over the first 28 days in the ICU, organ-specific SOFA score analysis was conducted; no differences were found in the organ-specific SOFA scores between the genotypes of FER rs4957796 (Table 4). Similarly, the requirement of organ support as measured by organ support-free days did not differ between the two FER rs4957796 groups. Adjustment for potential confounder and different baseline characteristic revealed no significant differences in organ-specific SOFA scores and the need of organ support (Supplementary information).
Table 4.
Disease severity among patients with severe acute respiratory distress syndrome.
| Variable | All | Severe ARDS | p Value | |
|---|---|---|---|---|
| (n = 95) | T/C|C/C | T/T | ||
| (n = 27) | (n = 68) | |||
| SOFA | 8.6 ± 3.7 | 7.8 ± 2.9 | 8.9 ± 3.9 | 0.3102 |
| SOFA-Respiratory score | 2.6 ± 0.5 | 2.5 ± 0.6 | 2.6 ± 0.5 | 0.1682 |
| SOFA-Cardiovascular score | 1.8 ± 1 | 1.7 ± 0.9 | 1.9 ± 1.1 | 0.8141 |
| SOFA-Central nervous system score | 2.4 ± 1 | 2.1 ± 1 | 2.5 ± 0.9 | 0.0571 |
| SOFA-Renal score | 0.8 ± 1 | 0.8 ± 0.8 | 0.8 ± 1.1 | 0.6986 |
| SOFA-Coagulation score | 0.4 ± 0.6 | 0.2 ± 0.4 | 0.5 ± 0.7 | 0.1340 |
| SOFA-Hepatic score | 0.3 ± 0.7 | 0.4 ± 0.6 | 0.3 ± 0.7 | 0.7590 |
| Length of stay in ICU, days | 21 ± 16 | 19 ± 12 | 22 ± 18 | 0.4523 |
| Organ support-free days | ||||
| Ventilator-free days | 2 ± 3 | 3 ± 2 | 2 ± 3 | 0.0628 |
| Dialysis-free days | 15 ± 8 | 15 ± 7 | 16 ± 8 | 0.6981 |
| Vasopressor-free days | 10 ± 7 | 10 ± 5 | 10 ± 7 | 0.8851 |
| ECMO-free days | 16 ± 9 | 16 ± 8 | 15 ± 9 | 0.5541 |
| Inflammatory values | ||||
| Leucocytes (1000/µl) | 13 ± 4 | 12 ± 3 | 13 ± 4 | 0.2853 |
| CRP (mg/l) | 164 ± 107 (35) | 113 ± 86 (8) | 179 ± 110 (27) | 0.1882 |
| Procalcitonin (ng/dl) | 4.4 ± 10.9 (90) | 3.2 ± 7.6 (24) | 4.9 ± 11.9 (66) | 0.4434 |
| Kidney values | ||||
| Urine output (ml/day) | 2955 ± 1264 | 3388 ± 1267 | 2783 ± 1231 | 0.1342 |
| Urine output (ml/kg/h) | 1.4 ± 0.7 | 1.7 ± 0.7 | 1.3 ± 0.6 | 0.0165 |
| Creatinine (mg/dl) | 1.2 ± 0.7 | 1.3 ± 0.7 | 1.2 ± 0.7 | 0.4552 |
| Liver values | ||||
| AST (GOT) (IU/l) | 426 ± 1430 (60) | 296 ± 588 (14) | 465 ± 1605 (46) | 0.2451 |
| ALT (GPT) (IU/l) | 124 ± 243 (93) | 157 ± 329 | 111 ± 199 (66) | 0.8689 |
| Bilirubin (mg/dl) | 1.4 ± 2.9 | 1.1 ± 1.4 | 1.6 ± 3.3 | 0.7917 |
Discussion
This observational study addresses the question of whether the FER polymorphism rs4957796 is associated with 90-day survival in patients with sepsis-associated ARDS due to pneumonia and its association with the severity of ARDS (mild, moderate, and severe). The main finding of this investigation was that FER rs4957796 TT-homozygous patients with severe ARDS have a significantly higher mortality risk than C-allele carriers.
Our finding of a deleterious effect of the FER rs4957796 TT genotype on survival in patients with severe ARDS due to pneumonia is consistent with the results of a recent GWA showing that the T-allele of FER rs4957796 was associated with increased mortality in patients with sepsis due to pneumonia in four independent European cohorts21.
FER rs4957796 polymorphism is a non-coding variant localized in intron 19 of the gene. Until now there are no records regarding this polymorphism in the eQTL data bases (http://genenetwork.nl/bloodeqtlbrowser/, status 16–6–2017) or in ExSNP (http://www.exsnp.org/ status 16–6–2017). In addition, there are no indications for special regulatory features in close proximity based on DNase hypersensitivity or specific histone acetylation patterns (Supplementary Fig. 1). However, rs4957796 is in a linkage disequilibrium with variants spanning several coding regions22 and thus may be only a marker for a linked functional variant. Our study confirms the association of rs4957796 polymorphism with sepsis and underlines the necessity of functional analyses to reveal the biological mechanism behind these associations. According to a recent study by Dolgachev, et al.23 showing that FER gene delivery affects survival in a mouse model of combined lung contusion and pneumonia and improves the efficiency of bacterial clearance within contused lungs, we hypothesize that the T-allele might be associated with lower FER gene expression, causing the observed poor outcome among ARDS patients.
The observed effect of FER rs4957796 on the 90-day survival exclusively in patients with severe sepsis-associated ARDS due to pneumonia is consistent with several investigations showing that ARDS subgroups (mild, moderate, and severe) are associated with clearly definable distinct histopathological features that may affect the clinical course and outcome of ARDS patients24. Studies aiming to investigate the effects of patient characteristics on the outcome of ARDS should follow a differentiated approach to assess the three ARDS subgroups separately, e.g., according to our own prospective investigation, statin therapy affects mortality nearly exclusively in patients with severe ARDS, with no significant effect in either the mild or moderate ARDS groups25.
Because the clinical course and mortality in patients with sepsis and ARDS are affected by several factors, e.g., comorbidities26, 27, comedications, and treatment modalities, the detected independent strong effect of the rs4957796 TT genotype on mortality risk indicates that this polymorphism should be considered in the clinical routine to identify patients at risk and in future studies investigating the outcome of patients with sepsis and ARDS caused by pneumonia.
The lack of an effect of the unfavorable genotype rs4957796 TT on disease severity (secondary variables) within 28 days in the ICU, as measured by SOFA sub-score analysis and organ support-free days, may be due to the possibility that the FER protein may exert deleterious effects on the lungs of ARDS patients and on other organ systems that cannot yet be detected using standard clinical assessment tools, e.g., SOFA pulmonary score or ventilator-free days.
This study has some limitations. Because the effect of the polymorphism on the 90-day mortality in patients with sepsis-associated ARDS due to pneumonia according to disease severity was unknown, we could not conduct power calculations at the beginning of the investigation to determine a sample size with adequate power. However, an ad hoc power analysis revealed a power of 0.83 in the severe ARDS group, considering the observed mortalities of 22% and 50% among the TT-homozygous and C-allele carriers, respectively. Thus, our sample size was sufficiently large to adequately address the objective. Another potential limitation is that this study was conducted in a single center. Thus, our findings must be further validated in independent cohorts from other centers to assess their generalizability. Although one of the major strengths of our investigation is that we have assessed a well-defined homogeneous cohort of patients with sepsis-associated ARDS caused by pneumonia, it has become evident that assessing such homogeneous cohorts from a single center is particularly advantageous because it is the best way to control confounding evoked by potential inter-center heterogeneity.
To the best of our knowledge, this is the first investigation to assess the effect of FER rs4957796 on mortality among patients with sepsis-associated ARDS caused by pneumonia. The observed independent increased risk for the 90-day mortality in patients with severe ARDS may help identify patients at risk in clinical settings.
Methods
Patients
The patients were recruited through the GENOSEP database of the Department of Anaesthesiology at the University Medical Centre, Goettingen, Germany. This database comprises a prospectively collected cohort of patients with sepsis. As described previously, consecutive adult patients admitted to the three surgical ICUs of the University Medical Centre between April 2012 and May 2015 were screened daily according to the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) criteria for sepsis, severe sepsis, or septic shock28, 29. Patients with sepsis were screened daily according to the Berlin definition of ARDS to identify those with sepsis-associated ARDS24, 25, 28. According to the PaO2/FiO2 ratio, patients with ARDS were classified into three categories: mild ARDS, 201–300 mmHg (≤39.9 kPa); moderate ARDS, 101–200 mmHg (≤26.6 kPa); and severe ARDS, ≤ 100 mmHg (≤13.3 kPa). Finally, patients with sepsis-associated ARDS caused by pneumonia were included in this study.
As described previously, the exclusion criteria were as follows: (1) age younger than 18 years; (2) pregnancy or breastfeeding; (3) immunosuppressive therapy; (4) acute myocardial infarction within the past 6 weeks; (5) congestive heart failure, classified as New York Heart Association functional class IV; (6) HIV infection; (7) a do not resuscitate or do not treat order; (8) very likely to die within 28 days due to end-stage uncorrectable disease; (9) a persistent vegetative state; (10) participation in an interventional trial; and (11) study-site employee or a family member of a study-site employee. This investigation was approved by the institutional ethics committee of the University of Goettingen in Goettingen, Germany, and was performed in accordance with the provisions of the Declaration of Helsinki. The study was performed in accordance with relevant guidelines and regulations. The methods were carried out in accordance with the approved guidelines. Written informed consent was obtained either from the patient or their legal representative.
Data collection and clinical endpoints
The baseline characteristics of the patients were recorded upon enrolment and included all relevant comorbid conditions, type of sepsis, recent surgical history, and organ support. The patients were followed up for 90 days, and the mortality risk within this period was assessed as the primary outcome variable. The Sequential Organ Failure Assessment (SOFA)30 and Acute Physiology and Chronic Health Evaluation (APACHE) II31 scores were evaluated at sepsis onset. Morbidity was assessed over 28 days in the ICU via recording of the organ-specific SOFA scores and the need for organ support (mechanical ventilation, vasopressor therapy, and renal replacement therapy) as the secondary outcome variable. Given that the majority of patients leave the ICU within 28 days (survive or decease), our study focused on the clinical progression within the first 28 days in the ICU. Clinical data were obtained from the electronic patient record system (IntelliSpace Critical Care and Anaesthesia (ICCA); Philips Healthcare, Andover, MA, USA).
FER rs4957796 genotyping
DNA was extracted by automated solid-phase extraction from 350 µl of EDTA whole blood using an EZ1® DNA Blood Kit in BioRobot EZ1® or from PBMCs using an AllPrep DNA Mini Kit according to the manufacturer’s instructions (all from Qiagen, Hilden, Germany). The DNA quantity and quality were determined spectrophotometrically. Genotyping was performed using the pre-designed TaqMan® SNP genotyping assay C__28002866_10 according to the manufacturer’s instructions (Life Technology, Darmstadt, Germany).
A total of 15% of the samples were genotyped in duplicate, yielding results that showed complete concordance. The observed genotypes were in Hardy-Weinberg equilibrium. The identity of the DNA samples was controlled by sex-typing and showed 100% concordance between the initially documented and the genetically determined sex32.
Statistical analyses
Statistical analyses were performed using Statistica software (version 12; StatSoft, Tulsa, Oklahoma, USA). The significance of categorical variables was calculated using two-sided Fisher’s exact or chi-squared tests, as appropriate. Two continuous variables were compared using the Mann-Whitney test. Time-to-event data were compared using the log-rank test from the Statistica package for Kaplan-Meier survival analysis. To exclude the effects of potential confounders (age, gender and body mass index (BMI), and morbidity scores (SOFA and APACHE II), statin therapy25 and covariates that varied at baseline (differently distributed comorbidities with p-values < 0.1) on survival, we performed a multivariate Cox regression analysis to examine the survival time. A power calculation was conducted using the Statistica package for power analysis. A p value < 0.05 was considered statistically significant
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors thank the staff of the ICUs of the Department of Anaesthesiology and Department of General and Visceral Surgery, all of whom were involved in patient care and monitoring. The authors also thank Benjamin Liese, Simon Wilmers, Yvonne Klee, Sebastian Gerber, and Chang Ho Hong for their help with data acquisition. This study was supported by the German Research Foundation (DFG) and the Open Access Publication Funds of Göttingen University.
Author Contributions
All authors contributed to the study design, data acquisition, and data analysis and interpretation. Specifically, F.K., B.B. and M.S. performed the clinical data collection. A.F.P., M.G., M.T. and T.B. participated in the study design and clinical data monitoring or interpretation. T.B. contributed to the study design and conception, performed the bioinformatics analyses, and performed and approved the statistical analyses. J.H., B.B., I.B. and A.M. designed the study, performed the statistical analyses, and drafted the manuscript. All authors were involved in either the drafting or revision of the manuscript. All authors approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
José Hinz, Benedikt Büttner, Ingo Bergmann and Ashham Mansur contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08540-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ARDS Definition Task Force et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.Phua J, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. American journal of respiratory and critical care medicine. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 4.Villar J, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive care medicine. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS. Recovery and long-term outcome in acute respiratory distress syndrome. Crit Care Clin. 2011;27:685–704. doi: 10.1016/j.ccc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 6.National Heart, Lung. et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. The New England journal of medicine. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong B, et al. Statins for the prevention and treatment of acute lung injury and acute respiratory distress syndrome: A systematic review and meta-analysis. Respirology (Carlton, Vic.) 2016;21:1026–1033. doi: 10.1111/resp.12820. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg KP, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. The New England journal of medicine. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 9.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. American journal of human genetics. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao QL, Ferris DK, White G, Heisterkamp N, Groffen J. Nuclear and cytoplasmic location of the FER tyrosine kinase. Molecular and cellular biology. 1991;11:1180–1183. doi: 10.1128/MCB.11.2.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim L, Wong TW. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. The Journal of biological chemistry. 1998;273:23542–23548. doi: 10.1074/jbc.273.36.23542. [DOI] [PubMed] [Google Scholar]
- 12.Xu G, et al. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. Journal of cell science. 2004;117:3207–3219. doi: 10.1242/jcs.01174. [DOI] [PubMed] [Google Scholar]
- 13.Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Molecular and cellular biology. 2007;27:6140–6152. doi: 10.1128/MCB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig AW, Greer PA. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Molecular and cellular biology. 2002;22:6363–6374. doi: 10.1128/MCB.22.18.6363-6374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCafferty DM, Craig AW, Senis YA, Greer PA. Journal of immunology (Baltimore, Md.: 1950) 2002. Absence of Fer protein-tyrosine kinase exacerbates leukocyte recruitment in response to endotoxin; pp. 4930–4935. [DOI] [PubMed] [Google Scholar]
- 16.Qi W, Ebbert KV, Craig AW, Greer PA, McCafferty DM. Absence of Fer protein tyrosine kinase exacerbates endotoxin induced intestinal epithelial barrier dysfunction in vivo. Gut. 2005;54:1091–1097. doi: 10.1136/gut.2004.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khajah M, et al. Fer kinase limits neutrophil chemotaxis toward end target chemoattractants. Journal of immunology (Baltimore, Md.: 1950) 2013;190:2208–2216. doi: 10.4049/jimmunol.1200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Current opinion in infectious diseases. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 19.Sloane PJ, et al. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. The American review of respiratory disease. 1992;146:419–426. doi: 10.1164/ajrccm/146.2.419. [DOI] [PubMed] [Google Scholar]
- 20.Estenssoro E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Critical care medicine. 2002;30:2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Rautanen A, et al. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir Med. 2015;3:53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rautanen A, et al. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. The Lancet Respiratory Medicine. 2015;3:53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolgachev VA, et al. Electroporation-mediated delivery of the FER gene in the resolution of trauma-related fatal pneumonia. Gene therapy. 2016;23:785–796. doi: 10.1038/gt.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thille AW, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. American journal of respiratory and critical care medicine. 2013;187:761–767. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 25.Mansur A, et al. Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: a prospective observational cohort study. BMC medicine. 2015;13:128. doi: 10.1186/s12916-015-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansur A, et al. Chronic kidney disease is associated with a higher 90-day mortality than other chronic medical conditions in patients with sepsis. Scientific reports. 2015;5:10539. doi: 10.1038/srep10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansur A, et al. Primary bacteraemia is associated with a higher mortality risk compared with pulmonary and intra-abdominal infections in patients with sepsis: a prospective observational cohort study. BMJ open. 2015;5:e006616. doi: 10.1136/bmjopen-2014-006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bone RC, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 2009;136:e28. doi: 10.1378/chest.09-2267. [DOI] [Google Scholar]
- 29.Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive care medicine. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 30.Vincent JL, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Critical care medicine. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Tzvetkov MV, Meineke I, Sehrt D, Vormfelde SV, Brockmoller J. Amelogenin-based sex identification as a strategy to control the identity of DNA samples in genetic association studies. Pharmacogenomics. 2010;11:449–457. doi: 10.2217/pgs.10.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


