Abstract
The relationship between a potential requirement for cell DNA synthesis and the expression of differentiated muscle cell functions was investigated using primary chicken embryo myoblasts infected with a temperature-sensitive mutant of Rous sarcoma virus (RSV). Under optimized conditions, transformed myoblasts growing at the permissive temperature could differentiate into multinucleated myotubes, express muscle-specific myosin, desmin and acetylcholine receptors in the absence of DNA synthesis and cell division following a shift to the non-permissive temperature. Furthermore, the experiments demonstrate that individual RSV-infected myoblasts have two options: either to divide and express the transformed phenotype or to withdraw from the cell cycle and differentiate into myotubes. The choice between these options appears to depend on the protein-kinase activity of pp60src, the src gene product.
Full text
PDF

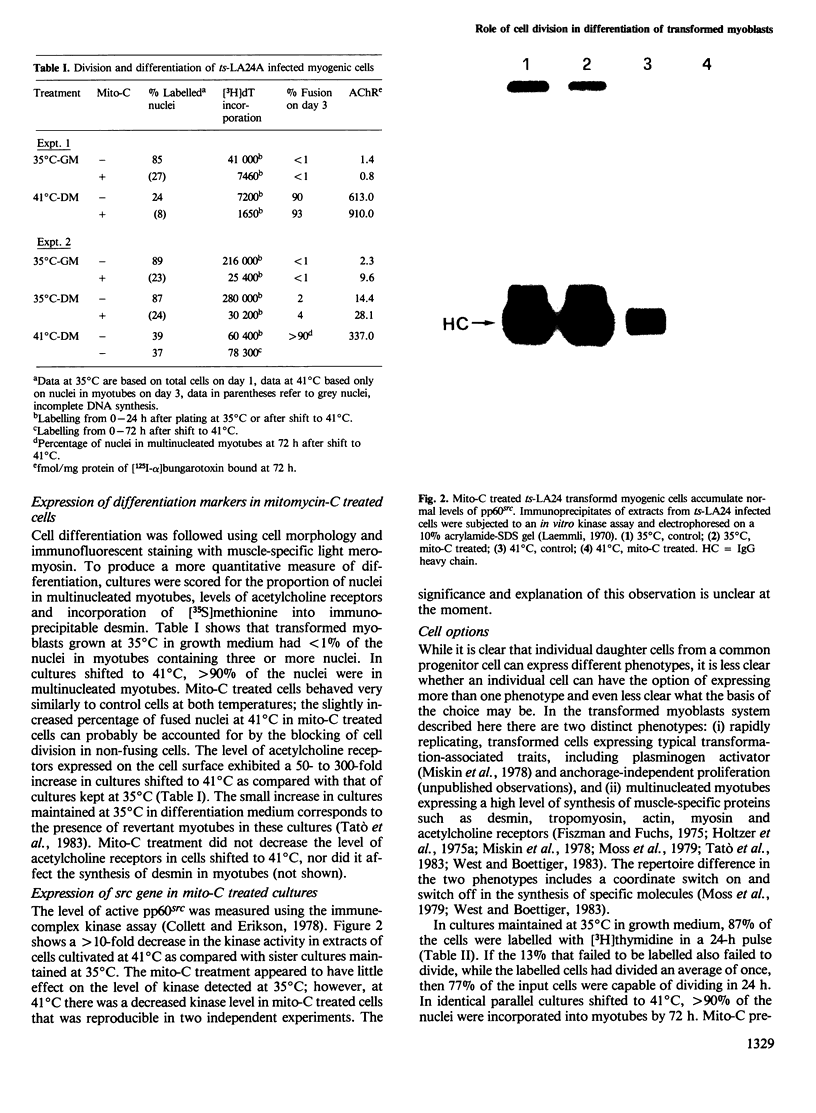


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignami M., Dogliotti E., Benigni R., Branca M., Tatò F., Alemà S. Expression of transformation-associated traits in the myogenic cell lines L6 and L8. Exp Cell Res. 1982 Feb;137(2):239–248. doi: 10.1016/0014-4827(82)90024-6. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Brunk C. F. Quantal division and a postmitotic state in myoblast differentiation. Differentiation. 1979;14(1-2):95–99. doi: 10.1111/j.1432-0436.1979.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Quade K., Wyke J. A. Expression of the ASV src gene in hybrids between normal and virally transformed cells: specific suppression occurs in some hybrids but not others. Cell. 1982 Sep;30(2):491–498. doi: 10.1016/0092-8674(82)90246-x. [DOI] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Friedlander M., Beyer E. C., Fischman D. A. Muscle development in vitro following X irradiation. Dev Biol. 1978 Oct;66(2):457–469. doi: 10.1016/0012-1606(78)90251-8. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Rubinstein N., Fellini S., Yeoh G., Chi J., Birnbaum J., Okayama M. Lineages, quantal cell cycles, and the generation of cell diversity. Q Rev Biophys. 1975 Nov;8(4):523–557. doi: 10.1017/s0033583500001980. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Maelicke A., Reich E. Metabolism of acetylcholine receptor in chick embryo muscle cells: effects of RSV and PMA. Cell. 1978 Dec;15(4):1287–1300. doi: 10.1016/0092-8674(78)90054-5. [DOI] [PubMed] [Google Scholar]
- Moss P. S., Honeycutt N., Pawson T., Martin G. S. Viral transformation of chick myogenic cells. The relationship between differentiation and the expression of the SRC gene. Exp Cell Res. 1979 Oct 1;123(1):95–105. doi: 10.1016/0014-4827(79)90425-7. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Tatò F., Alemà S., Dlugosz A., Boettiger D., Holtzer H., Cossu G., Pacifici M. Development of 'revertant' myotubes in cultures of Rous sarcoma virus transformed avian myogenic cells. Differentiation. 1983;24(2):131–139. doi: 10.1111/j.1432-0436.1983.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Turner D. C. Differentiation in cultures derived from embryonic chicken muscle: the postmitotic, fusion-capable myoblast as a distinct cell type. Differentiation. 1978 Mar 13;10(2):81–93. doi: 10.1111/j.1432-0436.1978.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., Boettiger D. Selective effect of Rous sarcoma virus src gene expression on contractile protein synthesis in chick embryo myotubes. Cancer Res. 1983 May;43(5):2042–2046. [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]
- Yeoh G. C., Holtzer H. The effect of cell density, conditioned medium and cytosine arabinoside on myogenesis in primary and secondary cultures. Exp Cell Res. 1977 Jan;104(1):63–78. doi: 10.1016/0014-4827(77)90069-6. [DOI] [PubMed] [Google Scholar]




