Abstract
Object: MicroRNAs (miRNAs) play key roles in progression of cervical cancer. In the present study, we investigated the role of miR-214 in the process of migration, invasion and drug sensitivity to cisplatin in cervical cancer. Methods: We detected the differential expression of miR-214 in 19 cases cervical cancer tissues and normal tissues as well as 4 cervical cancer cells and one normal cervical cells by Real-time PCR. Then, wound healing assay, transwell invasion assay and MTT were used to detect the effects of migration, invasion and sensitivity to cisplatin of cervical cancer when miR-214 was overexpressed. Western blot, immunofluorescence and Flow Cytometry were used to detect the mechanism of migration, invasion and sensitivity to cisplatin. Next, bioinformatics analysis was used to find the target of miR-214. Through the luciferase reporter assay, Real-time PCR and western blot, we confirmed the binding relationship of miR-214 and FOXM1. In cervical cancer tissues, the expression of FOXM1 was detected by western blot and Immunohistochemistry. We also knocked down FOXM1 in cervical cancer cells, wound healing assay, transwell invasion assay and MTT were performed to detect the migration, invasion and sensitivity to cisplatin abilities of FOXM1. Western blot and Flow Cytometry were used to detect the mechanism of migration, invasion and sensitivity to cisplatin by FOXM1. Finally, we performed rescue expriments to confirm the function relationship between miR-214 and FOXM1. Results: 1. Our results showed that miR-214 was frequently downregulated in tumor tissues and cancer cells especially in CIN III and cervical cancer stages. 2. Overexpression of miR-214 significantly inhibited migration and invasion of cervical cancer cells and prompted the sensitivity to cisplatin. 3. FOXM1 was identified as a target of miR-214 and down-regulated by miR-214. 4. Knocking down FOXM1 could inhibited migration and invasion of cervical cancer cells and prompted the sensitivity to cisplatin. 5. FOXM1 was upregulated in tumor tissues. 6. The mechanism of migration, invasion and sensitivity to cisplatin were the resluts of changes of EMT and apoptosis. 7. The restoration of FOXM1 expression can counteract the effect of miR-214 on cell migration, invasion and sensitivity to cisplatin of cervical cancer cells. Conclusions: These findings indicate that miR-214 acts as a tumor suppressor during the process of migration, invasion and drug sensitivity through targeting FOXM1, suggesting miR-214 as a potential new diagnostic and therapeutic target for the treatment of cervical cancer.
Keywords: miR-214, cervical cancer, EMT, migration and invasion, drug sensitivity, FOXM1
Introduction
Cervical cancer is one of the most common malignant tumors in the female reproductive system, and the incidence rate ranks second after breast cancer in the world [1]. Globally, there are about more than half million new cases and 40% of the cervical cancer patients dead every year [2]. Annual new cases of cervical cancer are about 135,000 in China, accounting for about 30% in the world. The incidence of cervical cancer has become the highest in malignant tumor in women [3]. Currently, the treatments of cervical cancer are mainly therapy surgery, radiotherapy, chemotherapy and other comprehensive treatments [2]. Like other malignant tumors, poor prognosis as well as tumor invasion and metastasis in advanced stage greatly impacts on the survival rate of patients [3]. Therefore, exploring the mechanism of the occurrence and development of cervical cancer has great significance to explore the effective method for the diagnosis and treatment of cervical cancer.
The dysfunction of cervical cancer cells is possibly caused by carcinogenic factors, such as the human papillomavirus (HPV), certain cytokines and growth factors through different mechanisms [4-6]. In recent study, it has been found that the abnormal expression of miRNA as a post-transcriptional regulatory factor is closely associated with the occurrence and development of cancer [7]. microRNA (miRNA) is a class of about 22 nucleotides non-encoding RNA and mainly plays a regulatory role in the expression of target gene by targeting mRNA cleavage or translational repression at the post-transcriptional level [8]. Climmino et al found that expression of BCL-2 can negatively regulated by miR-15a and miR-16 simultaneously. BCL-2 genes can make cells apoptosis in prostate cancer, leukemia and lymphoma diseases, which plays a reinforcing role of oncogenes [9]. Hayashita et al also demonstrated that miR-17-92 gene cluster may be a group of tumor-related gene [10]. miR-214 is a member of the miRNA family. It has been reported that miR-214 can promote melanoma cell movement and survival in malignant melanoma so as to promote their transference to the distal end. Such biological behavior that miR-214 promotes tumor malignant phenotype is achieved by inhibiting the expression of tumor suppressor genes such TFAP2C [11]. In ovarian cancer, cell cycle arrest can be induced at G1 phase by miR-214 as tumor suppressor gene, which is achieved by upregulating the expression of PTEN [12]. However, it has rarely been reported that how miR-214 plays the role and its mechanism of action in cervical cancer. In current research, we investigated the role of miR-214 metastasis, invasion as well as the drug sensitivity of cervical cancer.
FOMX1, a member of transcription factors in the Fox family, is contributed with the process of G1/S and G2/M conversion, the stimulation of mitosis, DNA repair, chromatin assembly and protein synthesis through a wide range of gene transcription regulation [12]. Plenty of researches suggest that FOXM1 is associated with the occurrence and development of many types of cancer. For example, in lung cancer or colon cancer, the expression of FOXM1 is increased, which will induce the growth, metastasis and angiogenesis of tumor cells as well as the change of sensitivity to chemotherapeutic drugs [13,14]. In ovarian cancer, FOXM1 inhibitor thiostrepton can inhibit ovarian cancer cell proliferation, invasion and migration [15]. What’s more, FOXM1 expression is primarily associated with the tumor staging and the prognosis of cancer patients [16]. What’s more, FOXM1 is involved in many oncogenic pathways [17]. As we have learned that FOXM1 plays a very important role in a wide variety of cancers, but its role in cervical cancer still needs further research.
In this study, we found miR-214 was frequently downregulated in tumor tissues and cancer cells especially in CIN III and cervical cancer stages. miR-214 can inhibit cell migration and invasion of cervical cancer cells and prompted the sensitivity to cisplatin. FOXM1 was identified as a target of miR-214 and down-regulated by miR-214. FOXM1 knockdown alone can inhibit the migration and invasion of cervical cancer cells and prompte the sensitivity to cisplatin, FOXM1 was upregulated in tumor tissues. The mechanism of migration, invasion and sensitivity to cisplatin were the resluts of changes of EMT and apoptosis. The restoration of FOXM1 expression can counteract the effect of miR-214 on cell migration, invasion and sensitivity to cisplatin of cervical cancer cells. Taken together, our results indicate that miR-214 may function as an tumor suppressor gene through targeting FOXM1 in human cervical cancer.
Materials and methods
Tissue samples of cervical cancer
Nineteen pairs of cervical cancer tissues and adjacent non-tumor tissues were collected from the department of gynecology in the second hospital of Tianjin Medical University. All patients were pathologically diagnosed and not associated with other inflammatory diseases or immune-related diseases. 169 specimens of normal tissues, cervical intraepithelial neoplasia (CIN) tissues and cervical carcinoma tissues from our hospital, of which were 29 cases of normal cervical tissues, 31 cases of CIN I stage, 26 cases of CIN II phase, 29 cases of CIN III stage and 31 cases of cervical cancer tissues. According to the scope of atypical cells accounted for cervical epithelial layer, CIN is divided into three levels-----Level I: atypical cells are confined to the 1/3 lower epithelial layer, Level II: atypical cells were confined within the 1/3-2/3 epithelial layer, Level III: atypical cells spend to all or almost all epithelial layer, that is severe atypical hyperplasia and cervical carcinoma in situ. All tissue samples were maintained in 30 min built-in liquid nitrogen tank. All operations are to be approved by the ethics committee.
Cell lines
Cervical cancer cells CaSki, SiHa, C33A and Hela were all provided by our laboratory and cultured in RPMI 1640 that consisted of 10% fetal bovine serum or 20% fetal bovine serum, 100 μg/ml penicillin, 100 μg/ml streptomycin with 5% CO2 at 37°C. After being digested by digestive solution containing 0.02% EDTA and 0.05% trypsin every 48-72 hours, cells were passaged with routine.
Transfection
Cervical cancer cells were seeded on 6-well plates at the density 1×106 a well overnight. And then we followed the manufacturer’s instructions to transfect the cells with miR-214 mimic (GenePharma) or si-FOXM1 (GenePharma) as well as those in control groups using Lipofectamine 2000 (produced by Invitrogen) with liposome method. Six hours after transfecting, cells were transferred into normal culture medium, and detected 48 hours later.
RNA extraction and real-time PCR
Total RNA in cells and tissues was extracted according to the instructions of mirVANA RNA isolation Kit (purchased from Ambion, Austin, Tex). The concentration was measured with NanoDrop spectrophotometer (produced by Nanodrop, Wilmington, Del), and the extractive was preserved at -80°C. When Real-time PCR was operated, we first reversly transcribed RNA into cDNA using reverse transcriptase M-MLV and nucleic acid enzyme inhibitor RiboLock (from Applied Biosystems, Foster City, Calif). Next, we used SYBR (purchased from GenePharma, Shanghai, China) to conduct the real-time quantitative PCR reaction with IQ-5 (purchased from Bio-Rad, USA). The procedure was performed for 10 minutes at 95°C, 94°C 15 sec, 55°C 30 sec, 70°C 30 sec, cycled 50 times successively. 2-ΔΔt method was used for quantization.
Drug sensitivity determined with MTT assay
24 hours after the cells were transfected with miR-214-mimics or miR-214-mimics-control, cells were counted and seeded on 12-well plates. Cell inhibition rate was in accordance with the in vitro sensitivity standard of solid tumors. It was considered as sensitive, when inhibition rate was greater than 30%, less than 30% was regarded as drug resistance. It was regarded as highly sensitive in vitro, when the inhibition rate was 70%. 50% inhibition rate was less than 70% for moderate sensitivity, 30% inhibition rate was less than 50% for low sensitivity. Inhibition rate = [(average OD value of control group-mean OD value of experimental group)/(average OD value of control group-mean OD value of blank group)]×100%.
Western blot
Well-grown cells were lysed in lysis buffer with RIPA method, 50 ug of which was taken to conduct electrophoresis with 10% SDS-PAGE. And the protein on the gel was electrically transferred onto PVDF membrane by wet transferring, blocked with 5% bloto, added primary antibody, which was rabbit anti human polyclonal antibody 1:500, and overnight at 4°C. After washing the membrane, secondary antibody was added, which was goat anti rabbit 1:1000. When performing film exposure, the membrane was first soaked in Western Lightning™ Chemiluminescence Reagent for two minutes, then placed in exposure box. The photographic film was exposed in the darkroom for a minute, then developed and fixed. We used LabWorks™ gel imaging and analysis system to photograph and analyze the luminance value of target band strip in each group.
Luciferase reporter assay
3×104 cells were seeded on 12-well plates. About 24 hours later, cervical cancer cells were co-transfected with 200 ng miR-214 and 50 ng FOMX1-3’UTR plasmids. 36 hours after transfecting, the cells were lysed and the fluorescence activity was measured with dual luciferase system (purchased from Promega).
Flow cytometry
Hela cells and SiHa cells were transfected respectively. Cell apoptosis was measured by flow cytometry 48 hours after transfecting. The cells were washed twice with PBS, trypsinized with 0.2% EDTA-free parenzyme, and collected with 1 ml PBS under the blowing. Cells were centrifuged for 5 min at 2000 rpm at room temperature, the supernatant was removed. And then the cells were resuspended with PBS once more, centrifuged supernatant obtained. According to Apoptosis Detection Kit (keyGEN, Nanjing), we performed the following operations, we added 300 ul 1× buffer to resuspend the cells, and then added 5 ul AnnexinV-FITC to proceed for ten minutes lucifugally, subsequently we added PI to proceed for 5 minutes lucifugally, finally mixed with 200 ul 1× buffer, flow cytometry analysis was performed within an hour after mixing.
Wounding healing assay
In vitro wounding healing assay was used to detect the capability of cell migration. When cells were transfected and fused in 6-well plates, we scratched the surface of the cell layer with a sterile plastic pipette tip. And then the cells were cultured in the double nutrient culture medium. Wound healing was observed under a microscope 48 h later.
Transwell invasion experiment
The aperture of bottom membrane of Transwell chambers or wells (from Corning, NY) was 8 um. The chambers were coated with matrigel produced by Sigma, which were used for detecting cell invasion ability. The under layer was 600 ul RPMI-1640 nutrient solution which contained 10% FBS. The volume of upper layer was 200 ul inoculated with 5×105 Hela or SiHa cells. Cells were cultured in incubator at 37°C with 5% CO2 for 48 hours, then the wells were removed and fixed in stationary liquid, which consisted of methanol and glacial acetic acid with proportional 3:1, for 30 minutes. Then the wells were washed with PBS and stained with 0.1% crystal violet and finally mounted. 5 wells were randomly selected, cells were observed and counted under a microscope.
Immunohistochemistry
The sections were pretreated using microwave irradiation, then were blocked and incubated with polyclonal rabbit anti-human FOXM1 antibodies. The staining intensity was then assessed.
Immunofluorescence
Immunofluorescence staining was performed with 5 μm paraffin cross-sections from the femoral artery. After deparaffinized with xylene and rehydrated, the slides were pre-incubated with 10% normal goat serum and then incubated with primary antibodies anti-N-Cadherin and anti-E-Cadherin. Secondary antibodies were fluorescein labeled antibody to rabbit IgG and rhodamine labeled antibody to mouse IgG. In each experiment, DAPI was used for nuclear counter staining. Images were captured by confocal microscopy and processed by LAS AF software.
Statistical analysis
Statistical analyses were performed using SPSS22 statistical software, date are expressed as the mean ± standard division (X±S). Statistical analyses were performed with a t-test. *P<0.05 or **P<0.01 was considered statistically difference.
Results
Expression level of miR-214 in human cervical cancer tissues and cells
To explore the role of miR-214 in the progress of cervical cancer. Firstly, we detected the expression level of miR-214 in 19 pairs of cervical cancer and normal tissues by Real-time PCR. The results were as shown Figure 1A that compared with normal cervical tissues, the expression of miR-214 was reduced in cervical cancer tissues. Secondly, we detected the expressions of miR-214 in human normal endometrial epithelial cells (ECS) and four kinds of cervical cancer cells (CaSki, SiHa, C33A and Hela) using Real-time PCR. The result was shown that the expression of miR-214 in human cervical cancer cell lines (CaSki, SiHa, C33A and Hela) w significantly decreased compared with ECS and in Hela was the most significant (Figure 1B). What’s more, we collected another 29 cases of normal cervical tissues, 32 cases of CIN I phase, 26 cases of CIN II phase, 29 cases of CIN III phase, 31 cases of cervical cancer tissues. The expressions of miR-214 in these tissues were detected respectively by Real-time PCR. The results were shown in Figure 1C that the expression of miR-214 in the samples of CIN I and CINII stage did not change significantly compared with that in normal cervical tissues, whereas the expression of miR-214 in the samples of CIN III stage decreased slightly, and decreased significantly in cervical cancer tissues. These results suggested that miR-214 expression was reduced in the tissues of cervical severe atypical hyperplasia, cervical carcinoma in situ and cervical carcinoma and the low expression of miR-214 may be related to the malignant process of cervical cancer.
Figure 1.

The miR-214 expression level in cervical cancer tissues and cells. (A-C) Real-time PCR, western blot were performed to detect the miRNA-214 level in cervical cancer tissues (A), cells (B), normal tissues, different stage cervical tissues and cervical cancer tissues (C).
miR-214 promoted migration and invasion of human cervical cancer cells by the promoting of epithelial mesenchymal transition (EMT)
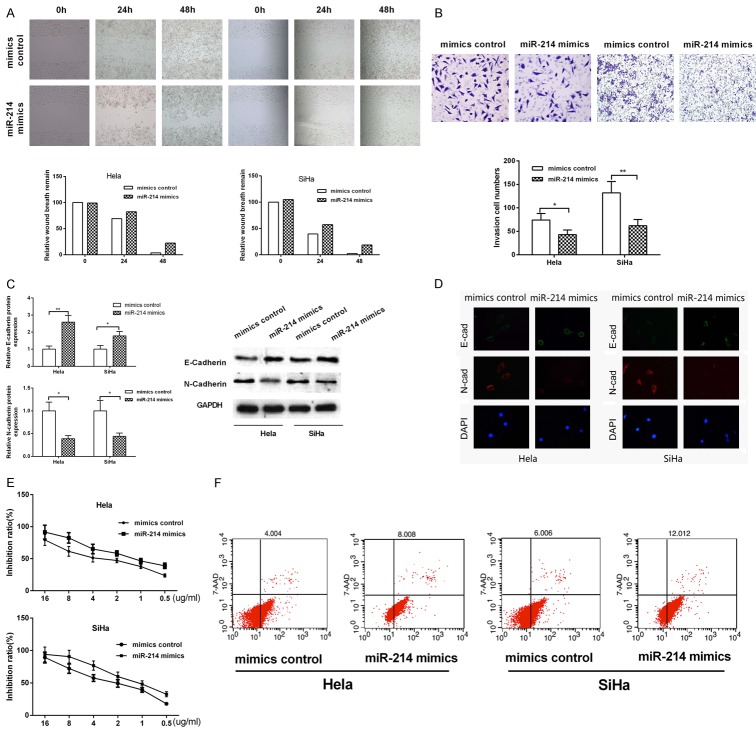
Based on the low expression of miR-214 in cervical cancer tissues and cells, we next went on to explore the effects of miR-214 on the migration and invasion of human cervical cancers. Wounding healing assay was used to detect the effect of miR-214 on the migration in Hela and SiHa cells. The results showed, when miR-214 expression increased at 24 h and 48 h, the wound distance was significantly larger than that of the control group in Hela and SiHa cells, suggesting that miR-214 could inhibit the migration of cervical cancer cells (Figure 2A). In order to detect the effect of miR-214 on the invasion of cervical cancer cells, we performed Transwell Invasion Assay and found that the number of invasion cell was signified decreased (~50% or 60%) in Hela and SiHa cells when miR-214 was overexpressed. These results indicated that miR-214 could significantly inhibit the invasion ability of cerrical cancer cells (Figure 2B).
Figure 2.
The effect of miRNA-214 on migration, invasion and drug sensitivity. A. Migration ability was detected in Hela and SiHa cells by wound healing assay. B. The invasion ability was analyzed by transwell invasion assay in Hela and SiHa cells. C. Western blot was used to test the E-Cadherin and N-Cadherin protien level. D. Immunofluorescence was used to test the morphology changes of cervical cancer cells. E. Drug sensitivity was detected in Hela and SiHa cells by MTT assay. F. The apoptosis ability was analyzed by flow cytometry in Hela and SiHa cells.
Epithelial-mesenchymal transition (EMT) is an mechanism for tumor to obtain the migration and invasion ability. The morphological change is generally accompanied by a change of a number of molecular markers of tumor cells, such as, the epithelial cell markers (E-Cadherin), phenotype markers (vimentin and N-Cadherin). Taking account of the phenotypes that miR-214 played an inhibitory role in the metastasis and invasion of cervical cancer cells, we detected the expression changes of the markers in the process of EMT by knocking down the expression of miR-214 using western blot. The results were shown in Figure 2C that when the expression of miR-214 increased, the expression level of E-Cadherin was increased, whereas the expression of N-Cadherin decreased both in Hela and SiHa cells. Immunofluorescence was also used to detect the morphological changes of cells when miR-214 overexpressioned, the results showed that miR-214 can inhibit the cells convert from cobblestones to the morphology of the fibroblast like cells (Figure 2D). These results indicated that miR-214 could inhibit the EMT process of cervical cancer cells, thus making the cell migration and invasion ability decreased.
miR-214 promoted the drug sensitivity in Hela and SiHa cells
In addition to the migration and invasion capabilities, drug sensitivity is another malignant behavior of cervical cancer cells. We used MTT assay to detect the effect of miR-214 on the drug sensitivity of Hela and SiHa cells. The results displayed that overexpression of miR-214 in Hela and SiHa cells can make the promotion rate of different concentrations of cisplatin (Figure 2E). In order to explore the mechanism of miR-214 on regulating the drug sensitivity to cisplatin, we next investigated whether miR-214 is involved in the regulation of apoptosis. In Hela cells, overexpression of miR-214 promoted the proportion of apoptotic cells compared with control groups (Figure 2F). In SiHa cells, proportion of apoptotic cells decreased significantly compared with control groups when miR-214 was overexpressed (Figure 2F). These results indicated that miR-214 promoted the sensitivity of cervical cancer cells to cisplatin by increasing apoptotic cells.
miR-214 targets FOXM1 mRNA 3’UTR
The biological funtion of miRNA depends on its specific target. Thus, we used miRNA target gene prediction software to analyze the potential target genes of miR-214. Depending on bioinformatic and funtional analyses, we found that miR-214 might target FOXM1 3’UTR of mRNA (Figure 3A). To further identify whether miR-214 directly targeted of FOXM1 3’UTR, we performed the luciferase reporter assay. We cloned the FOXM1 3’UTR fragments containing presumed target sites. Subsequently, we constructed an additional luciferase reporter vector containing the 3’UTR of FOXM1 with the potential mutant target sites of miR-214 seed sequence (Figure 3A). Co-transfection was performed with FOXM1 3’UTR wt or 3’UTR mut and with miR-214 mimics in HeLa and SiHa cells. The results were as shown in Figure 3B that in Hela cells, that miR-214 mimics were co-transfected with FOXM1 3’UTR wt could suppress the activity of luciferase, whereas the co-transfection of miR-214 mimics and FOXM1 3’UTR mut can’t suppress the activity of luciferase. Similar results were obtained in SiHa cells (Figure 3B). In addition, we also examined the effect of miR-214 on the expression level of FOXM1 by qRT-PCR and western blot. The results shown that miR-214 could negatively regulate the protein expression level of FOXM1, while no significant effect on the mRNA expression level (Figure 3C and 3D). We also detected the protein expression level of FOXM1 in 15 pairs of cervical cancer and normal tissues. The results indicated that compared with normal tissues, the expression level of FOXM1 was significantly increased in cancer tissues (Figure 4A and 4B). Meanwhile, we also detected the expression of FOXM1 in normal cervical tissues, CIN I phase, CIN II phase, CIN III phase, well-differentiated adenocarcinoma and well-differentiated squamous cell carcinoma by Immunohistochemistry and found that FOXM1 expression was higher in CIN II phase, CIN III phase, well-differentiated adenocarcinoma and well-differentiated squamous cell carcinoma (Figure 4C). In summary, these results indicated that miR-214 negatively regulated FOXM1 protein expression by identifying FOXM1 3’UTR.
Figure 3.

FOXM1 is a target gene of miR-214. A. The binding relationship of miR-214 and FOXM1. B. Fluorescence report analysis was used to detect the relationship of miR-214 and FOXM1 3’UTR. C. Real-time PCR was performed to analyze the relationship of miR-214 and FOXM1 mRNA. D. Western blot was performed to analyze the relationship of miR-214 and FOXM1 protein.
Figure 4.
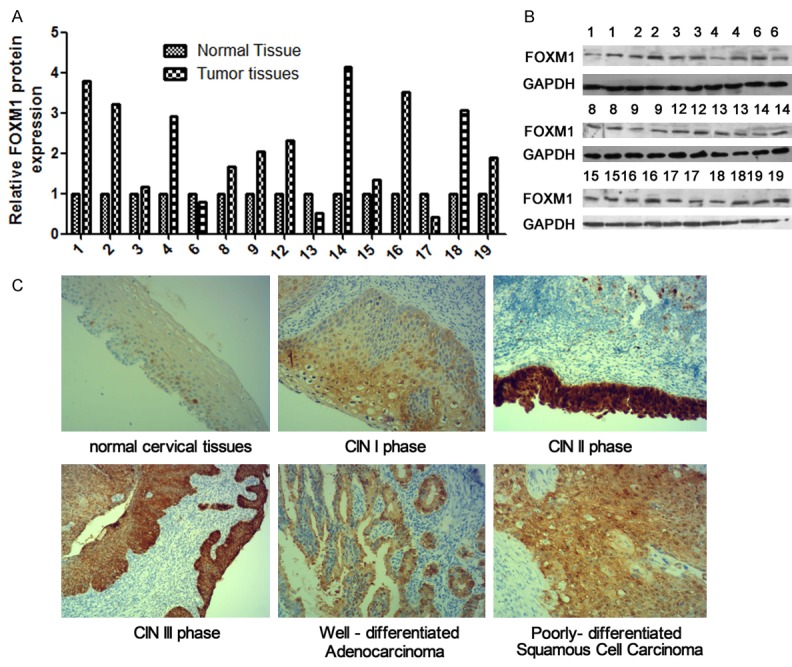
The expression of FOXM1 in tissues. A, B. Western blot was performed to detect the FOXM1 expression in cervical cancer tissues. C. Immunohistochemistry was used to detect FOXM1 expression in normal cervical tissues and different stage cervical caner tissues.
FOXM1 promoted metastasis, invasion and inhibited sensitivity to cisplatin in cervical cancer
Based on the inhibition of metastasis, invasion, the promotion of drug sensitivity by miR-214 and the regulationship between miR-214 and FOXM1. Next, we explored the effect of FOXM1 on malignant behavior of cervical cancer. Hela and SiHa cells were transfected with specific small interfering RNA (siRNA) targeting FOXM1. Initially, we demonstrated the effectiveness of siRNA-FOXM1 by Real-time PCR and western blot. The results were shown in Figure 5A and 5B that FOXM1 siRNA decreased the mRNA and protein expression level of intracellular content of FOXM1. Subsequently, wound healing assay and Transwell Invasion experiments were used to detect the effects of FOXM1 on migration and invasion in of cervical cancer cells. The results were shown that the wound healing rates and cell number of invasion was significantly suppressed after transfecting with FOXM1 siRNA compared with siRNA-control group in HeLa and SiHa cells (Figure 5C and 5D). These results indicated that knocked down of FOXM1 could inhibit metastasis and invasion in cervical cancer cells. What’s more, western blot was used to detect the role of FOXM1 on EMT. The result shown that, when knocking down FOXM1, the expression of mRNA and protein in the E-cadherin was increased, and the expression of N-cadherin was inhibited compared with control groups in Hela and SiHa cells (Figure 5E). Finally, we examined the drug sensitivity of cervical cancer cells to cisplatin by MTT. It was shown as in Figure 6A and 6B that knocked down of FOXM1 increased the inhibitory rate of cisplatin on cervical cancer cells. We also used Annexin-V/7-AAD flow cytometry to detect cell apoptosis and found that knockdown FOXM1 enhanced apoptosis induced by cisplatin in cervical cancer (Figure 6C). These results suggested that FOXM1 could promote the migration and invasion of cervical cancer cells and inhibit the sensitivity to cisplatin.
Figure 5.
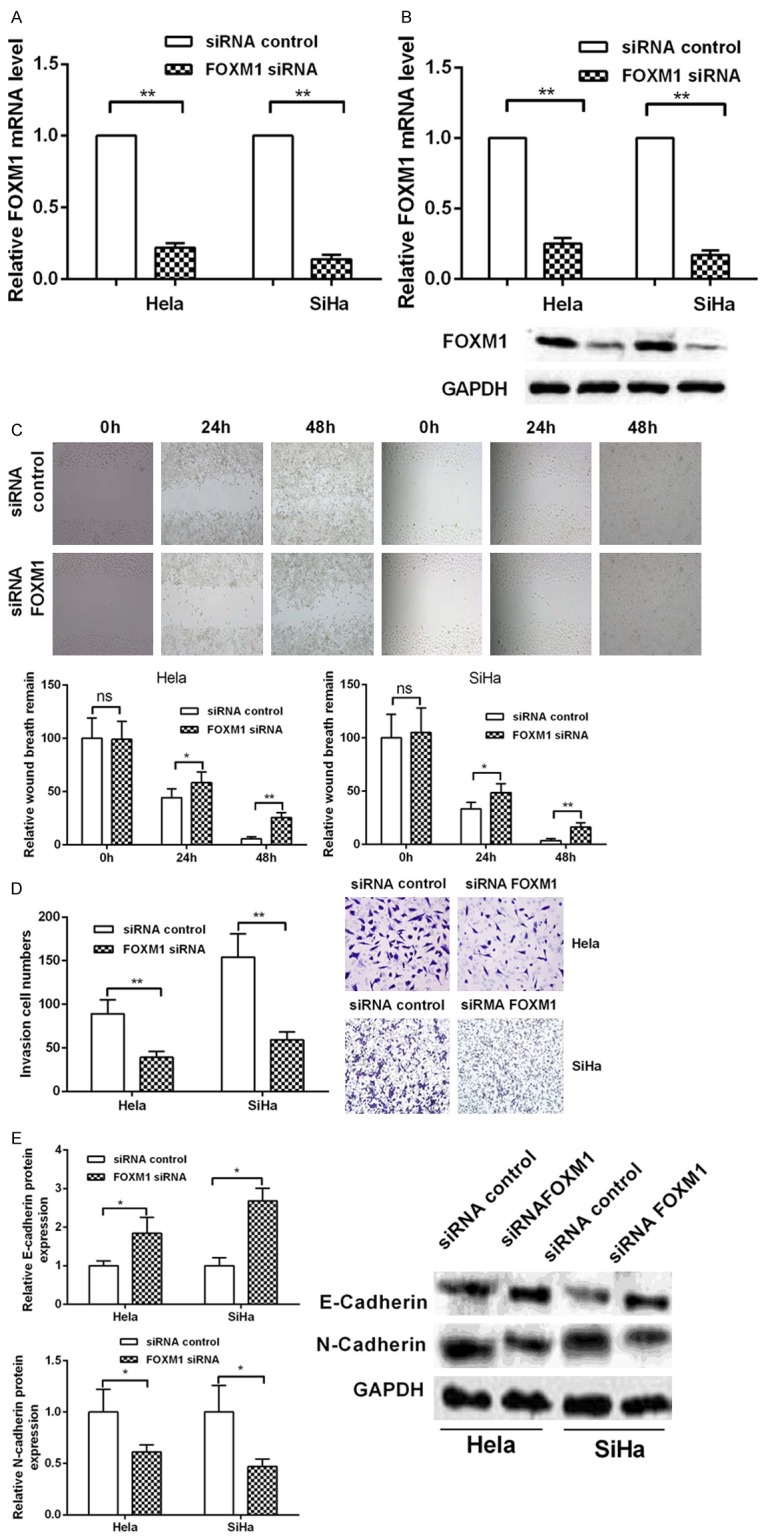
The effect of FOXM1 on migration and invasion in cervical caner cells. (A, B) The siRNA FOXM1 effectiveness was detected via Real-time PCR (A) and western blot (B). (C) Migration ability was detected in Hela and SiHa cells by wound healing assay. (D) The invasion ability was analyzed by transwell invasion assay in Hela and SiHa cells. (E) Western blot analyzed the EMT protein makers at mRNA and protein level.
Figure 6.
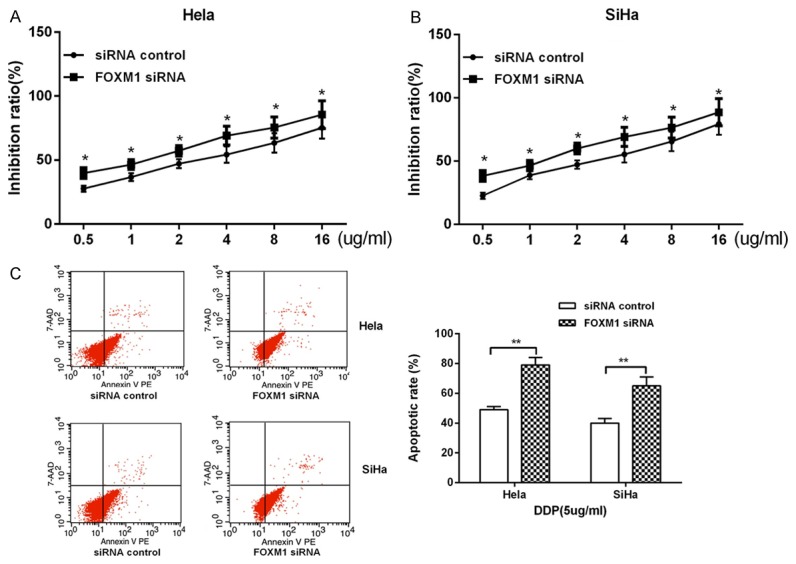
The effect of FOXM1 on drug sensitivity. (A, B) MTT was performed to detect the effect of FOXM1 on drug sensitivity in Hela (A) and SiHa (B) cells. (C) The apoptosis ability was analyzed by flow cytometry in Hela and SiHa cells.
FOXM1 overexpression counteracts miR-214 in cervical cancer
To further investigate the relationship between FOXM1 and miR-214-mediated metastasis, invasion promotion and sensitivity to cisplatin inhibiiton in cervical cancer, we overexpressed miR-214 with FOXM1 together in HeLa and SiHa cells. FOXM1 protein level was measured using western blot. We found that overexpression of FOXM1 reversed the inhibition of FOXM1 protein level by miR-214 (Figure 7A). Overexpression of FOXM1 reversed the inhibition in cell invasion caused by miR-214 as well as the process of EMT (Figure 7B-D). Meanwhile, overexpression of FOXM1 also neutralized the promotion of drug sensitivity to cisplatin that was induced by miR-214 (Figure 7E-G).
Figure 7.
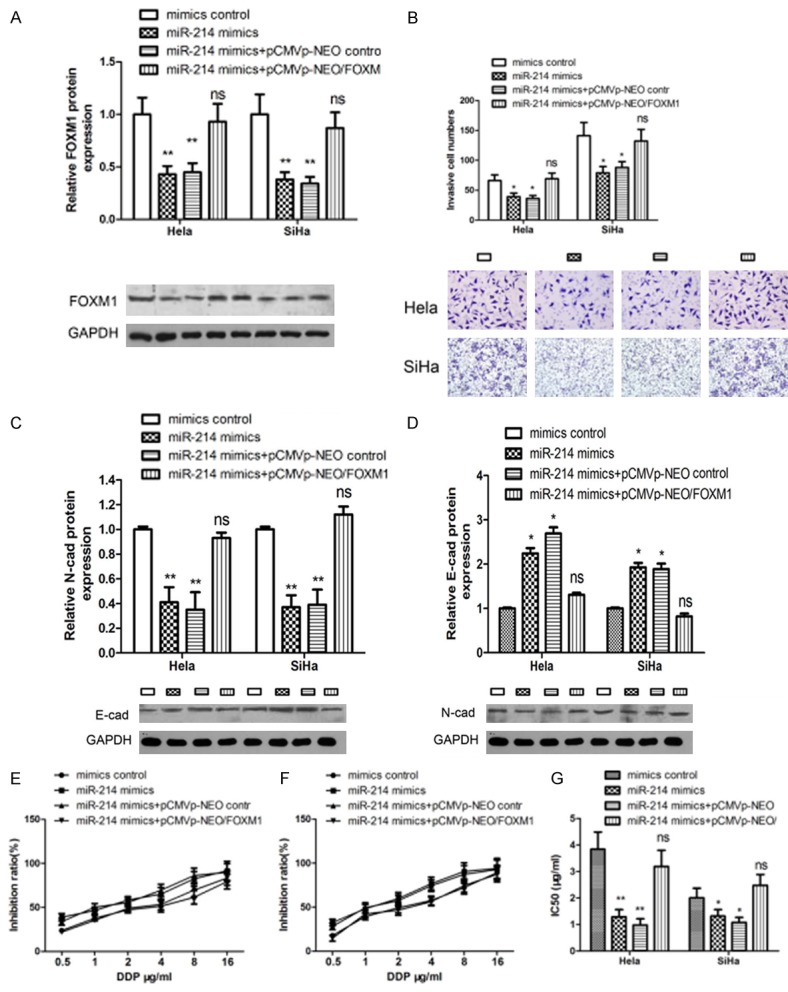
FOXM1 rescues miR-214-induced cellular phenotypes in cervical cancer cells. (A) Western blot was used to detect FOXM1 expression in HeLa and SiHa cells. (B) Transwell invasion assay was used to detect the rescue ability by FOXM1. (C, D) Western blot was used to detect N-Cadherin (C) and E-Cadherin (D) expression in HeLa cells. (E-G) Drug sensitivity was detected in Hela (E) and SiHa (F) cells by MTT assay.
Discussion
Recent studies have confirmed that miRNAs can inhibit the expression of the specific target gene, which leads to the occurrence of tumor. miRNAs may serve as a biomarker for predicting the prognosis of cancer, including cervical cancer [18]. Therefore, to find a specific miRNAs and their target genes involved in tumorigenesis will provide important clues to the diagnosis and treatment of malignant tumor. The role of miRNA in cervical cancer has been extensively studied in recent years. miR-21 can regulate CCL20 that is involved in tumor differentiation and nodular metastasis [19]. miR-10a enhances the growth, invasion and metastasis of tumor cells by inhibiting the expression of CHL1 [20]. Overexpression of miR-19a and miR-19b can promote the progress of cervical cancer [21]. In current study, we detected the expression level of miR-214 in 19 pairs of cervical cancer tissues and in normal adjacent tissues or in four cervical cancer cells and cervical cells by Real-time PCR. It was found that the expression levels of miR-214 in cervical cancer tissues and cervical cancer cell lines were significantly decreased compared with those in normal tissues or cells. What’s more, expression of miR-214 in the samples of CIN III stage decreased slightly, and decreased significantly in cervical cancer tissues, which may predicted the inhibition role of miR-214 in progress of cervical cancer.
Wound healing assay and transwell invasion assay were used to detect the effects of miR-214 on cell migration and invasion of cervical cancer and found that miR-214 could significantly inhibit the migration and invasion of cervical cancer cells. The processes of tumor cell migration anf invasion depend on the interaction between the specific cell-cell and the cell-extracellular matrix. In many studies, EMT can partially explain the migration and invasion of tumor cells. EMT is a process by which original normal epithelial cells transited to cells with mesenchymal characters in some physical and chemical conditions. In 2002, Theiry and Weinberg presented their findings in which it is suggested that EMT in malignant tumor cells occurs before metastasis. In the process, mesenchymal cells gain their migratory and motility traits, and further metastasis of tumor cells includes lymphatic vessels and blood tube metastasis [22-24]. This point of view has been widely recognized that the morphological evolution of malignant cells may well explain the mechanism of tumor cell metastasis. EMT mainly affects the connection among cells, which is a mainly a process of cell phenotype transformation without interconversion of cell types. Connections between epithelial cells are generally more, in close and neat arrangement, whereas there are so less connections between mesenchymal cells that they are in scattered arrangement. As cells are in EMT process, their morphology changes. The morphology changes of EMT is from a regular pattern of cubic, rectangular, or flat like cells to transformed into a star, spindle and other irregular cobblestone-like morphology, which is bound to affect the distribution and arrangement of the cytoskeleton, leading to changes in cell polarity [25]. Cell phenotype changes are accompanied by changes in phenotype associated molecules. So EMT are closely related with the decreased expression levels of epithelial phenotype markers, such as E-cadherin, and the increased expression levels of mesenchymal phenotype associated molecular markers, such as Vimentin, α-smooth muscle actin, etc. Researchers investigated the universal and specific molecular markers that occur in the EMT process. E-Cadherin, N-Cadherin and Vimentin are generally recognized as EMT molecular markers. In current study, we judged the change of EMT-associated molecular markers by means of overexpression of miR-214 to understand the mechanism of the inhibition of migration and metastasis in cervical cancer cells. As the expression of miR-214 increased, the expression levels of epithelial cell marker E-Cadherin increased, whereas mesenchymal cell marker N-Cadherin decreased. In conclusion, miR-214 can suppress the EMT process, so as to reduce the migration and invasion capacity of cervical cancer cells.
Drug resistance is the bottleneck in the treatment of almost all the malignant tumors. One of the urgent problems to be solved in clinical practice is that tumor cells are resistant to cisplatin, which seriously affects its treatment effect. Tumor cells not only built up drug resistance to the antitumor drug, but may develop cross-resistance to a variety of antitumor drugs. The sensitivity to chemotherapeutic drugs is a major problem in the treatment of cancer. miRNA also plays an important role in drug resistance. Phuah et al found that there are as many as 25 kinds of miRNAs including miR-138, miR-210 and miR-744 that can change the sensitivity to chemotherapeutic agents ACA and cisplatin [26]. Lei et al found that miR-155 can negatively regulate the EGF-induced EMT process, suppress cell proliferation, migration and invasion and enhance the sensitivity to chemotherapeutic agent cisplatin [27]. miR-214 can upregulate the expression of Bax, Cas-9, Cas-8 and Cas-3, enhance cell apoptosis, inhibit cell proliferation and increase the sensitivity to cisplatin by silencing the expression of Bcl-2 protein [28,29]. In cervical cancer cells, miR-218 induce apoptosis, suppress tumor growth, and increase the sensitivity to cisplatin through the AKT-mTOR signaling pathway [30,31]. In order to understand the characteristics of miR-214 on the sensitivity to cisplatin in cervical cancer, in our study MTT was used to study the effect of miR-214 on the sensitivity to cisplatin in cervical cancer. We found that overexpression of miR-214 can inhibit the sensitivity of cervical cancer cells to cisplatin. Similarly, we used flow cytometry to detect the effect of miR-214 on the apoptosis of cervical cancer cells treated with cisplatin. The results suggested that miR-214 can reduce cell apoptosis to inhibit the sensitivity of cervical cancer cells to cisplatin after cisplatin treatmen. Our study confirmed that miR-214 inhibits the drug resistance of cancer cells to cisplatin in cervical cancer.
In the study of the role of miRNA, it was found that miRNA participates in various life activities by targeting its target genes. In the previous studies, miR-214 has been reported with a plurality of different target genes. In breast cancer, miR-214 can regulate cell invasion by targeting p53 [32]. In bladder cancer, miR-214 can target PDRG1 and regulate cell proliferation, migration and apoptosis [33]. In cervical cancer, miR-214 may regulate cell proliferation and migration by TFAM. In cervical cancer, it has currently been found that plexin-B1 [34], GALNT7 [35], Bcl2l2 [28] which can affect cell cycle, migration and apoptosis are the target genes of miR-214 in cervical cancer. In osteosarcoma, miR-214 can promote cell proliferation and migration by LZTS1, promoting the growth of tumor in nude mice [36].
Here, we used bioinformatics analyse to find the target gene of miR-214 and found that FOXM1 might be the target of miR-214. We found that miR-214 might be able to identify FOXM1 mRNA 3’UTR. We constructed wild-type and mutant plasmids containing FOXM1 3’UTR and applied fluorescence reporter assay to detect the directly target of miR-214 and FOXM1 3’UTR. The results indicated that miR-214 was able to inhibit the expression of the wild-type FOXM1 3’UTR luciferase plasmid, but cannot suppress the relative expression activity of mutant FOXM1 3’UTR luciferase plasmid, which is proved that miR-214 can target FOXM1 3’UTR. Meanwhile, western blot and Real-time PCR were used to showed that overexpression of miR-214 could inhibit the protein and mRNA of FOXM1. Typical miRNA takes effects in two ways: When the miRNA sequence and the target gene mRNA are completely complementary, miRNA can cut off the mRNA, which leads to the degradation of mRNA and makes it impossible to translate the protein [37], whereas, when the miRNA sequence and the target gene mRNA are not completely complementary, miRNA can suppress mRNA translation, but does not affect its stability [38]. Thus, miR-214 can identify and combine FOXM1 mRNA 3’UTR, but cannot affect its stability. What’s more, miR-214 played a regulatory role by inhibiting protein translation. Finally, we also found that FOXM1 expression was higher in CIN II phase, CIN III phase, well-differentiated adenocarcinoma and well-differentiated squamous cell carcinoma. Thus, these results indicated that miR-214 can regulate the expression of FOXM1 in cervical cancer.
It has been proved that FOXM1 affects cell proliferation, migration and invasion. We used siRNA interference method to detect its role in cervical cancer cells. We found that in two kinds of cervical cancer cells, knockdown of FOXM1 inhibited migration, invasion and sensitivity to cisplatin compared with the control group. As we know that highly expressed FOXM1 is significantly associated with the metastasis of the tumor. It was initially found by Bao B that in pancreatic cancer cells, the overexpression of FOXM1, which regulated by miR-200c can promote the metastasis of pancreatic cancer cells as well as EMT [39]. Yu C et al demonstrated that in nasopharyngeal carcinoma, treatment of cells with FOXM1 inhibitor can inhibit the proliferation, migration and invasion of nasopharyngeal carcinoma cells, and inhibit the survival of NPC cells. It was proved that the growth and metastasis of nasopharyngeal carcinoma in vivo can be suppressed after knocking down of FOXM1 in vivo [36]. Meng FD et al found that FOXM1 can bind to the promoter SNAI1 and regulate its expression [40]. In cervical cancer, it has been demonstrated that AMPK activator can inhibit the growth of cervical cancer, which is achieved by inhibiting the expression of FOXM1 mRNA and protein. Cisplatin can enhance the effect on the cell by inhibiting the promoter of FOXM1 that can enhance the sensitivity to cisplatin when knocked down FOXM1 [41]. In summary, FOXM1 is a transcriptional regulator, which participates the progress of tumor.
In conclusion, this study indicates that miR-214 inhibits migration, invasion and promotes drug sensitivity in cervical cancer cells by targeting FOXM1, suggesting miR-214 may be a potential target for the treatment of cervical cancer, and providing a new strategy for the prevention and treatment of cervical cancer.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–8. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa H. [Progress in the World and challenges in Japan on HPV vaccination for cervical cancer prevention] . Gan To Kagaku Ryoho. 2010;37:971–5. [PubMed] [Google Scholar]
- 4.Pushparaj PN, Aarthi JJ, Manikandan J, Kumar SD. siRNA, miRNA, and shRNA: in vivo applications. J Dent Res. 2008;87:992–1003. doi: 10.1177/154405910808701109. [DOI] [PubMed] [Google Scholar]
- 5.Golden DE, Gerbasi VR, Sontheimer EJ. An inside job for siRNAs. Mol Cell. 2008;31:309–12. doi: 10.1016/j.molcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–32. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 11.Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J. 2011;30:1990–2007. doi: 10.1038/emboj.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–33. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 13.Ma RY, Tong TH, Cheung AM, Tsang AC, Leung WY, Yao KM. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J Cell Sci. 2005;118:795–806. doi: 10.1242/jcs.01657. [DOI] [PubMed] [Google Scholar]
- 14.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66:2153–61. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida Y, Wang IC, Yoder HM, Davidson NO, Costa RH. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 2007;132:1420–31. doi: 10.1053/j.gastro.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW, Cheung AN. Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis. J Pathol. 2008;215:245–52. doi: 10.1002/path.2355. [DOI] [PubMed] [Google Scholar]
- 17.Chan DW, Hui WW, Cai PC, Liu MX, Yung MM, Mak CS. Targeting GRB7/ERK/FOXM1 signaling pathway impairs aggressiveness of ovarian cancer cells. PLoS One. 2012;7:e52578. doi: 10.1371/journal.pone.0052578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang N, Zhan T, Ke T, Huang X, Ke D, Wang Q. Increased expression of RRM2 by human papillomavirus E7 oncoprotein promotes angiogenesis in cervical cancer. Br J Cancer. 2014;110:1034–44. doi: 10.1038/bjc.2013.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao T, Lin Z. MiR-21 is involved in cervical squamous cell tumorigenesis and regulates CCL20. Biochim Biophys Acta. 2012;1822:248–60. doi: 10.1016/j.bbadis.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Long MJ, Wu FX, Li P, Liu M, Li X, Tang H. MicroRNA-10a targets CHL1 and promotes cell growth, migration and invasion in human cervical cancer cells. Cancer Lett. 2012;324:186–96. doi: 10.1016/j.canlet.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Xu XM, Wang XB, Chen MM, Liu T, Li YX, Jia WH. MicroRNA-19a and -19b regulate cervical carcinoma cell proliferation and invasion by targeting CUL5. Cancer Lett. 2012;322:148–58. doi: 10.1016/j.canlet.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 22.Birchmeier W, Birchmeier C. Epithelial-mesenchymal transitions in development and tumor progression. EXS. 1995;74:1–15. doi: 10.1007/978-3-0348-9070-0_1. [DOI] [PubMed] [Google Scholar]
- 23.Bernards R, Weinberg RA. A progression puzzle. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- 24.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phuah NH, In LL, Azmi MN, Ibrahim H, Awang K, Nagoor NH. Alterations of microRNA expression patterns in human cervical carcinoma cells (Ca Ski) toward 1’S-1’-acetoxychavicol acetate and cisplatin. Reprod Sci. 2013;20:567–78. doi: 10.1177/1933719112459220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei C, Wang Y, Huang Y, Yu H, Huang Y, Wu L. Up-regulated miR155 reverses the epithelialmesenchymal transition induced by EGF and increases chemo-sensitivity to cisplatin in human Caski cervical cancer cells. PLoS One. 2012;7:e52310. doi: 10.1371/journal.pone.0052310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Liu M, Li X, Tang H. MiR-214 reduces cell survival and enhances cisplatin-induced cytotoxicity via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett. 2013;587:488–95. doi: 10.1016/j.febslet.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Wen Z, Lei Z, Jin-An M, Xue-Zhen L, Xing-Nan Z, Xiu-Wen D. The inhibitory role of miR-214 in cervical cancer cells through directly targeting mitochondrial transcription factor A (TFAM) Eur J Gynaecol Oncol. 2014;35:676–82. [PubMed] [Google Scholar]
- 30.Li J, Ping Z, Ning H. MiR-218 impairs tumor growth and increases chemo-sensitivity to cisplatin in cervical cancer. Int J Mol Sci. 2012;13:16053–64. doi: 10.3390/ijms131216053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You W, Wang Y, Zheng J. Plasma miR-127 and miR-218 might serve as potential biomarkers for cervical cancer. Reprod Sci. 2015;22:1037–41. doi: 10.1177/1933719115570902. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Lv P, Liu X, Zhu M, Qiu X. microRNA-214 enhances the invasion ability of breast cancer cells by targeting p53. Int J Mol Med. 2015;35:1395–402. doi: 10.3892/ijmm.2015.2123. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Zhang X, Wang L, Yang Y, Dong Z, Wang H. MicroRNA-214 suppresses oncogenesis and exerts impact on prognosis by targeting PDRG1 in bladder cancer. PLoS One. 2015;10:e0118086. doi: 10.1371/journal.pone.0118086. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Qiang R, Wang F, Shi LY, Liu M, Chen S, Wan HY. Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int J Biochem Cell Biol. 2011;43:632–41. doi: 10.1016/j.biocel.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Peng RQ, Wan HY, Li HF, Liu M, Li X, Tang H. MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase 7. J Biol Chem. 2012;287:14301–9. doi: 10.1074/jbc.M111.337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu C, Chen L, Yie L, Wei L, Wen T, Liu Y. Targeting FoxM1 inhibits proliferation, invasion and migration of nasopharyngeal carcinoma through the epithelialto-mesenchymal transition pathway. Oncol Rep. 2015;33:2402–10. doi: 10.3892/or.2015.3834. [DOI] [PubMed] [Google Scholar]
- 37.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103:4034–9. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thermann R, Hentze MW. Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature. 2007;447:875–8. doi: 10.1038/nature05878. [DOI] [PubMed] [Google Scholar]
- 39.Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Meng FD, Wei JC, Qu K, Wang ZX, Wu QF, Tai MH. FoxM1 overexpression promotes epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:196–213. doi: 10.3748/wjg.v21.i1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yung MM, Chan DW, Liu VW, Yao KM, Ngan HY. Activation of AMPK inhibits cervical cancer cell growth through AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 2013;13:327. doi: 10.1186/1471-2407-13-327. [DOI] [PMC free article] [PubMed] [Google Scholar]