Abstract
Incidence of nasopharyngeal carcinoma (NPC) has remained high worldwide, posing a serious health problem. MicroRNAs (miRNAs) are a family of about 20-23 nucleotides small non-coding molecules, which play a significant role in NPC. In this study, we explored the molecular mechanisms of miR-130a-3p in inhibiting viability, proliferation, migration and invasion of NPC cells by suppressing CXCL12. The relative expression of miR-130a-3p and CXCL12 mRNA expression in tissues and cells was measured by qRT-PCR. NPC cell line CNE-2Z was transfected with miR-130a-3p mimics, CXCL12 siRNA, cDNA-CXCL12 and negative control. Western Blot was performed to detect CXCL12 expression. The MTT assay was performed to study cell viability. The colony formation assay was done to test cell growth. Flow cytometry was conducted to analyze cell cycle and apoptosis. The Transwell assay was used to investigate cell migration and invasion. The results found that the up-regulation of miR-130a-3p or down-regulation of CXCL12 could inhibit viability, proliferation, migration and invasion of CNE-2Z cells. Luciferase-reporting system assay was performed to investigate miR-130a-3p could bind to the 3’UTR region of CXCL12 and the overexpression of miR-130a-3p could suppress CXCL12 expression. Collectively, our finding suggested demonstrated that miR-130a-3p could prohibit the progression of NPC by suppressing CXCL12, which might serve as potential therapeutic targets for NPC.
Keywords: Nasopharyngeal carcinoma, miR-130a-3p, CXCL12, proliferation, migration, invasion
Introduction
Nasopharyngeal carcinoma (NPC) was a head and neck cancer which particularly prevalent in China and Southeast Asia, and its estimated incidence was high between 20/100,000 and 50/100,000 per year [1]. Radiotherapy has been considered as the primary treatment for newly developed NPC. After treatment with intensity-modulated radiotherapy, the 5-year local relapse-free survival of early-stage NPC was over 90% [2]. However, the prognosis for advanced NPC was unpleasant with a 5-year survival rate ranging from 50% to 70%, which was primarily due to the radio-resistance of NPC and high metastasis to distant organs [3,4]. Unfortunately, as high as 80-90% of NPC patients were confirmed with NPC until the advanced stage, since the anatomical sites and the early symptoms of NPC were not obvious [1]. Therefore, profound researches into the molecular regulation tumorigenesis and progression of NPC may provide new sight for novel therapeutic treatment for NPC.
MicroRNAs (miRNAs) were small functional non-coding RNAs with conserved sequences. By binding to the 3’-untranslated region (3’UTR) of corresponding mRNAs, miRNAs could inhibit gene expression, and thus modulate various critical cellular processes, including cell differentiation, cell proliferation, cell migration, and cell apoptosis [5]. Ectopic expression of miRNAs can act as either tumor suppressors or oncogenes by abnormally regulating gene expression [6], and the potential of miRNA profiling in cancer diagnosis was highlighted [7,8]. Besides, multiple miRNAs have been identified to suppress the growth, proliferation, invasion and metastasis of NPC cells, such as miR-34b, miR-156a and miR-506 [9-11].
In previous studies, down-regulation of miR-130a-3p was found within ovarian cancer cells [12], non-small cell lung cancer cells [13] and prostate cancer cells [14]. Consistently, a recent study has confirmed that the overexpression of miR-130a-3p could suppress the differentiation, proliferation, migration and invasion of hepatocellular carcinoma cells [15]. Nevertheless, there has been little evidence supporting that miR-130a-3p can retard the growth of NPC, and the molecular mechanism remains unsearchable. Hence, further investigation into miR-130a-3p was necessary, and the identification of the target gene of miRNA-130a-3p was critical.
C-X-C motif chemokine ligand 12 (CXCL12), also known as stromal cell-derived factor-1 or pre-B-cell-growth stimulating factor, has been predicted as the target of miR-130-3p. CXCL12 belonged to the CXC chemokine family, and was overexpressed in various tumors, such as prostate cancer, breast cancer and ovarian cancer [16-18]. Within these tumors, by binding to its receptor CXCR4, CXCL12 could lead to activation of the Src, PI3K/Akt, ERK and JNK pathways, so as to facilitate cellular migration and invasion [19].
In our study, we creatively investigated the role of miR-130a-3p in NPC carcinogenesis and progression by suppressing CXCL10, which might help us better understand the mechanism of miR-130a-3p in NPC and provide some evidences for developing new treatment targets for NPC.
Methods and materials
Clinical samples
Forty-three normal nasopharyngeal epithelial tissues and 35 NPC tissues were collected from the Second Hospital of Jilin University. AllNPC biopsy samples were obtained before patients receiving chemotherapy or radiotherapy. All the protocols in this study were approved by the Ethics Committee of the Second Hospital of Jilin University. All the patients have signed the consent form.
Cell culture
We chose human NPC cell lines CNE-2Z, HONE-1, C666-1 and normal nasopharyngeal epithelial cell line NP69 (BeNaCulture Collection, Suzhou, China). Cells were cultured in 90% Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10% (2% for NP69 cells) fetal bovine serum (FBS) at 37°C in 5% CO2.
Transfection
Cells were seeded in 6-well plates 1 day before transfection. When cells confluence reached 70%-80%, transfection was conducted using LipofectamineTM2000 (Invitrogen). The medium was replaced 4 h after transfection and the cells were incubated in a cell incubator. CNE-2Z cells were transfected with miR-130a-3p mimics or control oligonucleotides (Ribobio, Guangzhou, China). CXCL12 siRNA (20 nmol) was transfected to CNE-2Z to knock down CXCL12. pcDNA3.1-CXCL12 (1 ng) was transfected to CNE-2Z to overexpress CXCL12 protein. pcDNA3.1 empty plasmid was as negative control (pcDNA3.1-NC), and miR-130a-3p mimics+pcDNA3.1-CXCL12 (miR-mimics+pcDNA3.1-CXCL12) was as test group. The pcDNA3.1-CXCL12 was purchased from GenePharma, China. The cells were harvested 48 h and 72 h after transfection.
Dual-luciferase reporter gene assay
The pGL4.13-CXCL12 3’UTR (wt-3’UTR) and pGL4.13-CXCL12 3’UTR-mut (mut-3’UTR) plasmids were constructed using pGL4.13 vector (Promega, Madison, WI, USA). For luciferase assay, CNE-2Z cells were transfected with wt-3’UTR or mut-3’UTR vectors by LipofectamineTM2000. About 48 h after transfection, the luciferase activities were measured with the Dual-Luciferase Reporter System. Four different transfected groups were analyzed as follows: wt-3’UTR+NC group, wt-3’UTR+miR-130a-3p mimics group, mut-3’UTR+NC group and mut-3’UTR+miR-130a-3p mimics group.
qRT-PCR
The total RNA was collected using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcription was performed to obtain cDNA through a RT kit (Fermentas, Maryland, USA). Then the miRNA or mRNA expression levels were analyzed according to the manufacturers’ protocols. The U6 or GAPDH were used as the endogenous control of miRNA and mRNA, respectively. Finally, the RNA relative expression levels were calculated with 2-ΔΔCt method. The primer sequences for PCR were given in Table 1.
Table 1.
The primers for conduction of qRT-PCR
| Forward primers | Reverse primers | |
|---|---|---|
| GAPDH | 5’-AACGGATTTGGTCGTATTG-3’ | 5’-GGAAGATGGTGATGGGATT-3’ |
| U6 | 5’-CTCGCTTCGGCAGCACA-3’ | 5’-AACGCTTCACGAATTTGCGT-3’ |
| miR-130a-3p | 5’-GGCAGTGCAATGTTAAAAG-3’ | 5’-CAGTGCGTGTCGTGGAGT-3’ |
| CXCL12 | 5’-GCCATGAACGCCAAGGTC-3’ | 5’-CGAGTGGGTCTAGCGGAAAG-3’ |
Western blot
Total proteins were extracted by RIPA lysis buffer and protein concentrations was measured using bicinchoninic acid (BCA) assay. About 30 µg proteins were separated by 10% SDS-PAGE electrophoresis. The proteins were then transferred to the polyvinylidenefluoride (PVDF) membrane. The membranes were blocked by 5% skimmed-milk in TBST buffer for 1 h, then incubated with rabbit-anti-human CXCL12 polyclonal antibody (1:1000, Abcam, Cambridge, MA, USA) or with mouse-anti-human β-actin monoclonal antibody (1:10000, Abcam) overnight at 4°C. The membranes were washed with TBST for 3 times and incubated with horseradish peroxidase conjugated with anti-rabbit or anti-mouse secondary antibody (Abcam) for 1 h at room temperature. The film was developed and fixed using ECL solution.
MTT assay
CNE-2Z cells at logarithmic growth phase were seeded into 96-well plates at a density of 1000 cells per well, and each group had 7 duplicates. We analyzed cell viability at different time points (24 h, 48 h, 72 h and 96 h after seeding). Briefly, 20 µl MTT solution (5 mg/ml) was added to each well, then the cells were incubated at a cell incubator for another 4 h. Then 150 µl DMSO was added and finally the absorbance was acquired at 570 nm using a spectrophotometer. The average optical density (OD) was plotted against the time series.
Colony formation assay
CNE-2Z cells were seeded into 6-well plates at a density of 2000 cells per well. After incubation for 24 h, the non-adherent cells were removed, and the remaining cells continued to be cultured for 1 week. The colonies were fixed with 4% paraformaldehyde, stained with 1% crystal violet and countered under a microscope. Each colony should contain no less than 50 cells.
Cell apoptosis analysis
The transfected CNE-2Z cells were digested by 0.25% trypsin to get single cell suspension, and were centrifuged at 1000 rpm to collect cells. After being resuspended with 500 µl binding buffer, they were transferred to 5 ml flow tubes. Then they were stained with Annexin V plus PI or each alone for 10-20 min at room temperature. The data were acquired on a flow cytometer.
Transwell assay
CNE-2Z cells were firstly starved using serum-free media for 24 h, and were then digested with 0.25% trypsin. Then 500 µl cell suspension containing 50000 cells were added to the upper chambers coated with Matrigelgel, and 600 µl complete media were added to the lower chambers. The cells were incubated for 12 h, and then were fixed with 4% paraformaldehyde for 20 min. Later, they were stained with 1% crystal violet for 10 min. The cells that crossed the Matrigel gel membrane were counted under a microscope.
Scratch-wound healing assay
CNE-2Z cell monolayers were wounded by scratching the surface on the 6-well plate as uniformly as possible with a pipette tip. The scratched wells were washed several times with PBS to remove detached debris after scratching. Then RPMI 1640 containing lipofectamine reagent and siRNA (20 nM) and pcDNA3.1 plasmids (1 μg) were added to the scratched wells. The cells were then incubated for 24 h at 37°C. The initial wounding and the movement of the cells in the scratched area were photographically monitored and imaged using an Olympus CKX41 inverted microscope coupled with a digital imaging system at 0 h, 24 h.
Statistical analysis
The numerical data were presented as mean ± SD. Two-tailed Student’s t-tests were used to analyze the differences between two groups. One-way analysis of variance (ANOVA) was performed for analysis of multiple groups. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 21.0 software (SPSS, Chicago, IL, USA).
Results
Down-regulated miR-130a-3p and up-regulated CXCL12 in NPC
The expression level of miR-130a-3p was significantly lower in NPC tissues than normal nasopharyngeal epithelial tissues (Figure 1A), whereas the expression of CXCL12 showed the opposite results (Figure 1B). We also found the similar outcome appeared in 3 different NPC cell lines (CNE-2Z, HONE-1, C666-1), which had down-regulated miR-130a-3p expression (Figure 1C) and up-regulated CXCL12 expression (Figure 1D) compared with the normal nasopharyngeal epithelial cell line (NP96). CNE-2Z cell line had the lowest miR-130a-3p and the highest CXCL12 expression, which was chose for the following researches.
Figure 1.
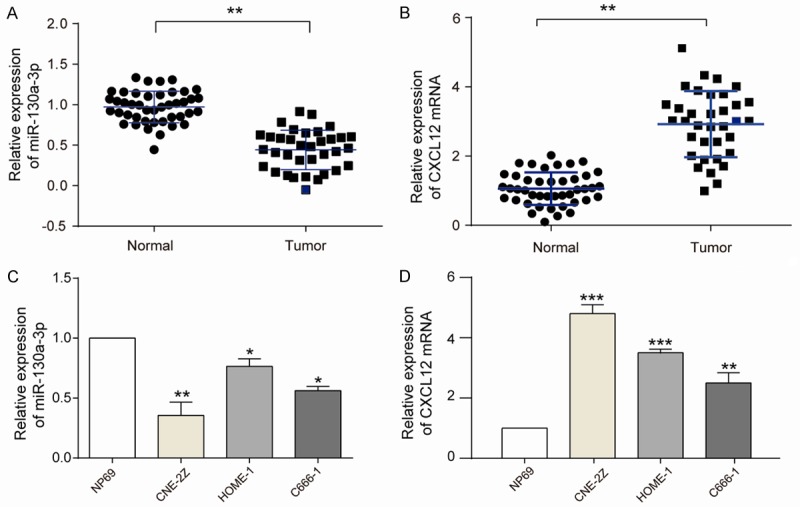
MiR-130a-3p and CXCL12 expression in NPC tissues and cell lines. A, B: miR-130a-3p and CXCL12 expression in 43 normal samples and 35 NPC samples, respectively, assessed by RT-qPCR. MiR-130a-3p expression is high in normal and low in NPC, whereas CXCL12 expression is high in NPC and low in normal (student t-test, *P<0.05, **P<0.01). C, D: qRT-PCR results showed that miR-130a-3p was significantly decreased in NPC cell lines (CNE-2Z, HONE-1, C666-1) when compared with normal nasopharyngeal epithelial cell line NP69. Adverse results appeared on CXCL12 which was significantly increased in NPC cell lines (CNE-2Z, HONE-1, C666-1) when compared with NP69 (student t-test, **P<0.01, ***P<0.001). The biggest expression disparity was found in CNE-2Z cell group. Each assay was conducted in triplicate and repeated three times. The data show the mean of triplicate repeats ± s.e.m.
Overexpression of miR-130a-3p suppresses CNE-2Z cell viability, proliferation, migration and invasion
The RT-qPCR results showed that miR-130a-3p expression was higher in miR-130a-3p mimics group than control (empty) and NC (negative control) groups (Figure 2A) (P<0.01). The MTT assay results suggested that the relative cell viability of cells in miR-mimics group was much less active than other two groups (Figure 2B). The colony formation assay results showed that the CNE-2Z cells proliferation rate substantially dropped in miR-130a-3p mimics group (Figure 2C). Scratch-wound healing assay manifest that overexpressed miR-130a-3p had a lower migratory vitality (Figure 2D). Transwell assay was performed to analyze the cell invasion (Figure 2E). The result showed that the invasive ability of miR-130a-3p mimics group (60.2±5.8%) was much weaker than other two groups.
Figure 2.
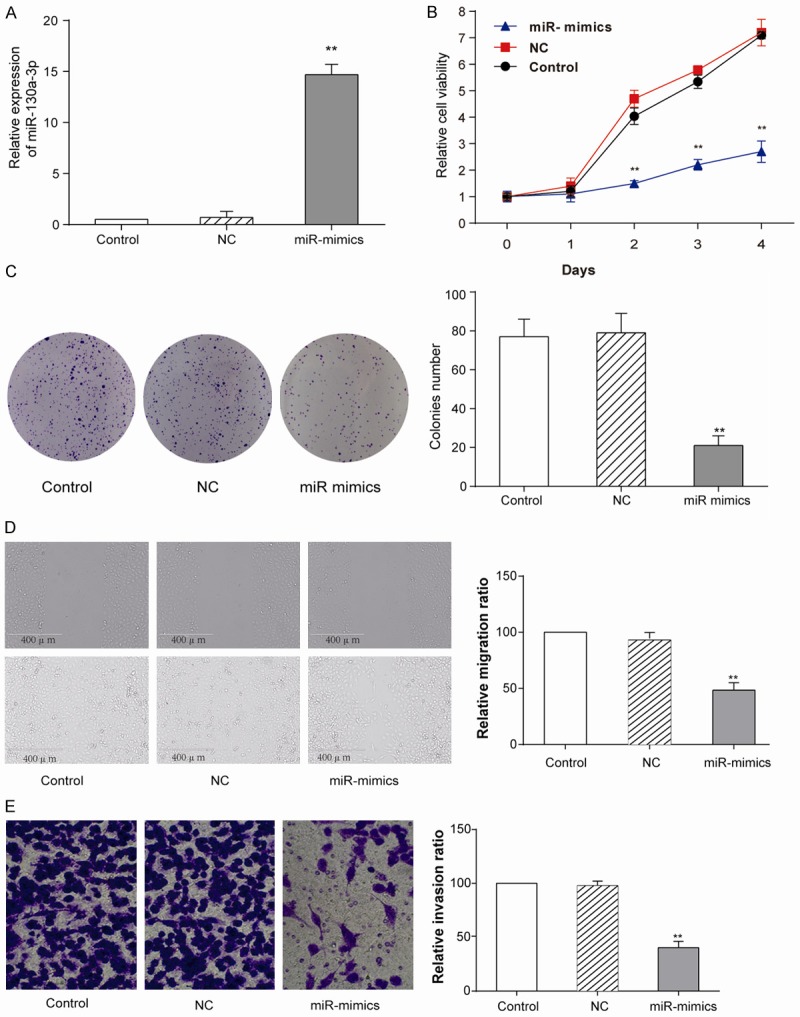
Overexpression of miR-130a-3p prohibited CNE-2Z cells. A: qRT-PCR experiments showed that miR-130a-3p was successfully transfected into CNE-2Z cells (student t-test, **P<0.01). Control means empty transfection and NC as a transfected control. B: MTT assay was performed to analyze CNE-2Z cell viability at different time points (1-4 days), miR-130a-3p transfected group had a comparatively low cell viability (student t-test, **P<0.01). C: Colony formation assay was also done to measure CNE-2Z cell proliferation rate. The advent of miR-mimics group was lower than control (student t-test, **P<0.01). D: Scratch-wound healing assay was applied to analyze cell migration ability. Migration ability in miR-mimics group is lower than control (student t-test, **P<0.01). E: Transwell assay was performed to analyze cell invasion ability. Invasion ability in miR-mimics group is lower (student t-test, **P<0.01). Each assay was conducted in triplicate and repeated three times. The data show the mean of triplicate repeats ± s.e.m.
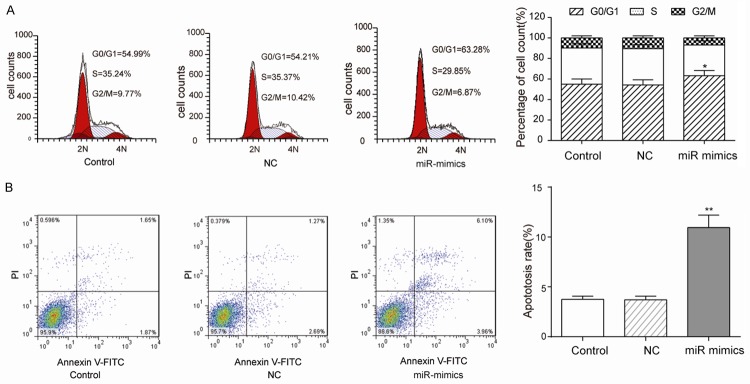
MiR-130a-3p arrests CNE-2Z cells at G0/G1 stage and promotes apoptosis
CNE-2Z cells transfected with miR-130a-3p mimics [(60.16±1.29)%] had a larger number at the G0/G1 stage than the NC [(55.39±1.07)%], whereas cells in the G2 or S stages were no significant discrepancy (Figure 3A). Besides, the miR-130a-3p mimics group had a significantly higher apoptosis rate than control and NC groups, which indicated that the overexpression of miR-130a-3p could promote apoptosis of CNE-2Z cells (Figure 3B).
Figure 3.
Overexpression of miR-130a-3p arresting CNE-2Z cells at G0/G1 stage and promoted apoptosis. A: CNE-2Z cell cycle was analyzed by flow cytometer (PI staining) 48 h post transfection. In the group of miR-mimics (overexpressed miR-130a-3p), the proportion of cells at G0/G1 stage was much higher than control and NC groups (student t-test, *P<0.05). B: Annexin V and PI staining detected CNE-2Z cells apoptosis and miR-mimics group had higher ratio (student t-test, **P<0.01). Each assay was conducted in triplicate and repeated three times. The data show the mean of triplicate repeats ± s.e.m.
MiR-130a-3p binding to 3’UTR region of CXCL12 and down-regulating CXCL12 expression
According to the prediction by TargetScan database (http://www.targetscan.org), CXCL12 could be one of the target genes of miR-130a-3p (Figure 4A). In the dual-luciferase reporter assay, only the cells in the wt-3’UTR+miR-130a-3p mimics group had a remarkably lower luciferase activity than other groups, suggesting that miR-130a-3p could bind to 3’UTR of CXCL12 to restrain translation (Figure 4B). RT-qPCR and Western Blot were performed to quantify CXCL12 mRNA and protein levels, respectively. Consistent results were showed out that si-CXCL12 could apparently restrain CXCL12 expression in CNE-2Z cells, pcDNA3.1 CXCL12 group successfully higher expressed CXCL12 in CNE-2Z cells and miR-mimics prominently decreased CXCL12 mRNAs and proteins expression (Figure 4C, 4D).
Figure 4.
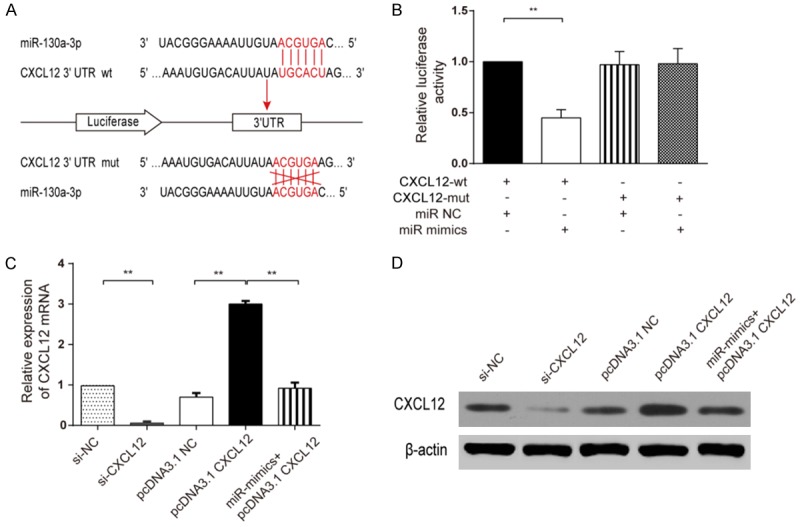
MiR-130a-3p inhibiting CXCL12 expression. (A) Binding sites between miR-130a-3p and CXCL12 3’UTR and 6 mutant sites in CXCL12 3’UTR-mut would not complementary pairing to miR-130a-3p which be used as control. (B) Dual-luciferase assay showed that wt-3’UTR+miR-130a-3p mimics had a remarkably lower luciferase activity when compared with other three groups (student t-test, **P<0.01). (C) qRT-PCR and (D) Western Blot demonstrated that CXCL12 level was remarkably decreased when miR-130a-3p was overexpressed (student t-test, **P<0.01). Each assay was conducted in triplicate and repeated three times. The data show the mean of triplicate repeats ± s.e.m.
MiR-130a-3p suppresses CNE-2Z cell migration, viability, proliferation and invasion by targeting CXCL12
Scratch-wound assay was performed to evaluate CNE-2Z cells migration function, the results manifest that CXCL12 might have a meaning effect on NE-2Z cells migration (i.e. si-CXCL12), overexpressed CXCL12 could notably increase migratory ratio (i.e. pcDNA3.1 CXCL12) and miR-130a-3p mimics could prominently restrain this function (i.e. miR-mimics+pcDNA3.1 CXCL12) (Figure 5A, 5B). MTT assay was performed to assess the viability of cells. Compared with NC group, the CNE-2Z cell OD value sharply dropped when CXCL12 was knocked down (i.e. si-CXCL12). Compared with pcDNA3.1 NC, the proliferation of CNE-2Z significantly increased when CXCL12 was over-expressed (i.e. pcDNA3.1 CXCL12) (Figure 5C). MiR-130a-3p mimics could prominently counteract this function likewise (i.e. miR-mimics+pcDNA3.1 CXCL12). The colony formation assay was performed to observe influence on CNE-2Z cell proliferation. The results showed that CXCL12 had an effect on CNE-2Z cells proliferation (i.e. si-CXCL12 and pcDNA3.1 CXCL12), overexpressed CXCL12 could strengthen proliferation of CNE-2Z cells (i.e. pcDNA3.1 CXCL12) and miR-130a-3p mimics could restrain proliferation (i.e. miR-mimics+pcDNA3.1 CXCL12) (Figure 5D). Transwell assay was performed to assess the cell invasion rate (Figure 5E). The invasion rate of CNE-2Z cells markedly dropped when CXCL12 was knocked down (i.e. si-CXCL12), which meant that the inhibition of CXCL12 could suppress CNE-2Z cell invading. Compared with pcDNA3.1 CXCL12 group (143.7±3.26%), CNE-2Z cells in miR-mimics+pcDNA3.1-CXCL12 (97.27±5.45%) group showed the offsetting effect of invasion ability (97.3±4.2%), which demonstrated that miR-130a-3p could inhibit CNE-2Z cell invasion rate via down-regulating CXCL12.
Figure 5.
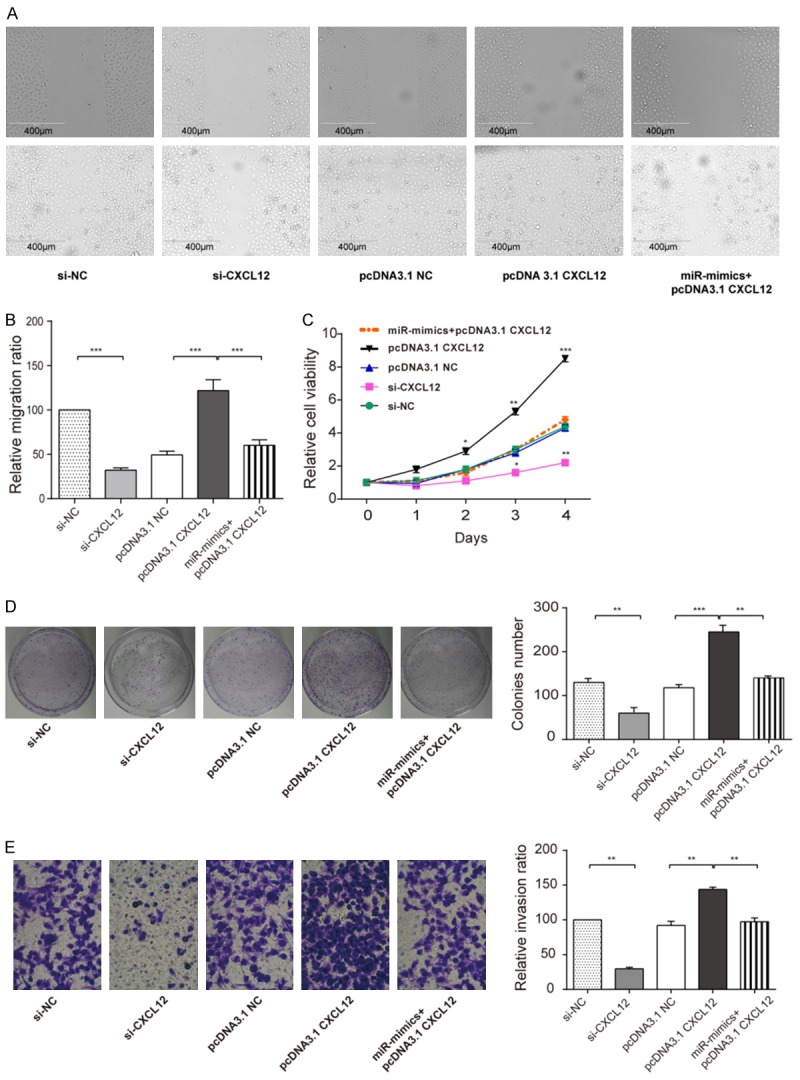
MiR-130a-3p target CXCL12 resulted in suppressed CNE-2Z cell migration, viability, proliferation and invasion. (A) Scratch assay was done to analyze cell migration ability. (B) The cylindrical analysis diagram of relative migration ratio for picture A. (C) MTT assay was performed to analyze CNE-2Z cell viability at different time points (1-4 days), (D) Colony formation assay was also done to measure CNE-2Z cell proliferation rate. (E) Transwell assay was performed to analyze cell invasion ability, All the results showed a similar change that knockdown (si-CXCL12) the CXCL12 had lower relative migration ratio, lower relative cell viability, lower colonies number, lower relative invasion ratio, whereas overexpression (pcDNA3.1-CXCL12) all in higher level. MiR-130a-3p decreased the over-expressed higher level (miR-mimics+pcDNA3.1-CXCL12). (student t-test *P<0.05, **P<0.01, ***P<0.001). Each assay was conducted in triplicate and repeated three times. The data show the mean of triplicaterepeats ± s.e.m.
MiR-130a-3p inhibiting CXCL12 to affect CNE-2Z cell cycle and apoptosis
The effects of CXCL12 on cell cycle and apoptosis were measured by a flow cytometer (Figure 6A). The results testified to knockdown CXCL12 (i.e. si-CXCL12) blocked cell cycle to stay G0/G1 stage, which cells could not effectively differentiated and proliferated, overexpressing CXCL12 (i.e. pcDNA3.1-CXCL12) made more cells stay S stage. Similarly, miR-mimics+pcDNA3.1-CXCL12 group compared to pcDNA3.1-CXCL12 group had a higher proportion at G0/G1 [(56.83±4.14)%>(40.9±2.17)%] and a lower proportion at S stage [(32.07±3.51)%<(37.12±3.04)%]. si-CXCL12 group had a higher apoptosis rate [(23.3±2.84)%] compared with NC group [(4.5±2.56)%]. Overexpress CXCL12 (pcDNA3.1-CXCL12), the apoptosis rate would decrease [(1.2±2.9)%], nevertheless miR-mimics+pcDNA3.1-CXCL12 group reversed this effect [(5.5±3.01)%] (Figure 6B).
Figure 6.
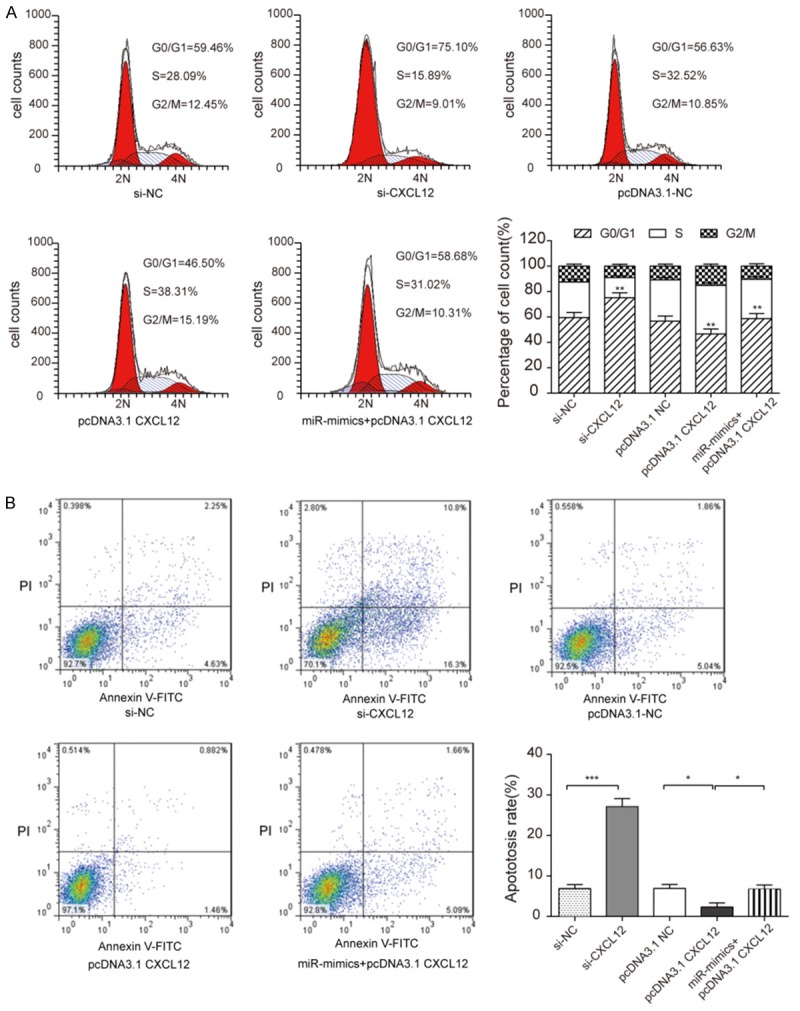
MiR-130a-3p inhibiting CXCL12 affected CNE-2Z cell cycle and promoted cell apoptosis. A: Flow cytometry (PI staining) results showed that si-CXCL12 group had the highest cells at G0/G1 stage, pcDNA3.1-CXCL12 had the lowest and miR-mimics+pcDNA3.1-CXCL12 had the middle cell count (student t-test, **P<0.01). B: Annexin V and PI staining detected CNE-2Z cells apoptosis. Si-CXCL12 group had the highest ratio, while pcDNA3.1-CXCL12 had the lowest ratio. MiR-mimics+pcDNA3.1-CXCL12 had a higher than pcDNA3.1-CXCL12 but lower than si-CXCL12 (student t-test, *P<0.05, ***P<0.001). Each assay was conducted in triplicate and repeated three times. The data show the mean of triplicate repeats ± s.e.m.
Discussion
MiRNAs have been demonstrated to be dysregulated in NPC tissues based on previous studies. For instance, miR-34a-5p, miR-449a and miR-183 were reported to affect the proliferation, migration and invasion of NPC cells [20-23]. Consistent with our in vitro studies that proliferation, migration and invasion of NPC cells could be suppressed by over-expressed miR-130a-3p, which indicated that miR-130a-3p might play a role in the development and progression of NPC. MiR-130a-3p was firstly reported to participate in the activation of platelet physiology by targeting the transcription factor MAFB [24]. Later studies suggested that miR-130a-3p could affect a cluster of cancers, including hepatoma, triple-negative breast cancer and urinary bladder cancer [15,25,26]. In our study, miR-130a-3p was suggested as a NPC suppressor that could negatively influence proliferation and invasion of NPC cells.
CXCL12 was indicated to play an essential role in hematopoiesis in previous studies [27-29]. To be specific, Arabanian et al. suggested that CXCL12 appeared to trigger the entry of more mature hematopoietic stem and progenitor cells into the cell cycle [30]. CXCL12 could counteract cell apoptosis and recruit progenitor cells by combining with its receptor CXCR4 [31]. CXCL12 also had a suppressing effect on papillary thyroid carcinoma and to be look as a powerful prognostic biomarker in prostate cancer [32,33]. Studies also confirmed that CXCR4/CXCR7-CXCL12 axis could exacerbate NPC cell migration and invasion [34], which is consistent with our study. The cancer facilitator role of CXCL12 was further confirmed in the present study.
Oncogenes had been identified and confirmed to be targets of miR-130a-3p. For instance, Smad4 could be regulated by miR-130a-3p in gemcitabine-resistant hepatoma cells [15]. CXCL12 was predicted to be a potential target of miR-130a-3p by TargetScan, and this target relationship was confirmed by dual luciferase reporter gene assay. RT-qPCR and western blot, the abundance of CXCL12 mRNA and protein in CNE-2Z cells was reduced after transfected with miR-130a-3p. Thus in this study, we validated the cancer suppressor role of miR-130a-3p by possibly binding to the oncogene CXCL12 mRNA.
The limitation of our study was that the complicated regulatory network of CXCL12 and miRNAs were not entirely investigated. Various factors involved in the regulation of CXCL12, and miR-130a-3p also had multiple targets other than CXCL12 such as oncogene MDM4, but our study merely contributed to mapping one part of the whole regulatory network in NPC pathogenesis. Besides, both miR-130a-3p and CXCL12 had been proved to have close correlations with hematopoiesis [27-29], but whether the hematopoietic function participated in the NPC progression remained vague to us. Thus it would be deserved to study if hematopoiesis took part in the process of NPC inhibition in further.
In conclusion, our study further confirmed the role of CXCL12 and miR-130a-3p in modifying NPC cells. The targeting relationship between them provided novel insights into the mechanisms of the caner suppressive features of miR-130a-3p in NPC, indicating that miR-130a-3p and CXCL12 might serve as potential therapeutic targets for NPC.
Disclosure of conflict of interest
None.
Abbreviations
- NPC
Nasopharyngeal carcinoma
- miRNAs
MicroRNAs
- 3’UTR
3’-untranslated region
- CXCL12
C-X-C motif chemokine ligand 12
- NC
negative control
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Colaco RJ, Betts G, Donne A, Swindell R, Yap BK, Sykes AJ, Slevin NJ, Homer JJ, Lee LW. Nasopharyngeal carcinoma: a retrospective review of demographics, treatment and patient outcome in a single centre. Clin Oncol (R Coll Radiol) 2013;25:171–177. doi: 10.1016/j.clon.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Jia WH, Huang QH, Liao J, Ye W, Shugart YY, Liu Q, Chen LZ, Li YH, Lin X, Wen FL, Adami HO, Zeng Y, Zeng YX. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20-25 year period (1978/1983-2002) in Sihui and Cangwu counties in southern China. BMC Cancer. 2006;6:178. doi: 10.1186/1471-2407-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 8.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao J, Li Y, Zhang W, Jiang Y, Du B, Tan Y. miR-34b inhibits nasopharyngeal carcinoma cell proliferation by targeting ubiquitin-specific peptidase 22. Onco Targets Ther. 2016;9:1525–1534. doi: 10.2147/OTT.S98378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Y, Cai L, Tian Y, Tu Y, Qiu H, Xie G, Huang D, Zheng R, Zhang W. miR156a mimic represses the epithelial-mesenchymal transition of human nasopharyngeal cancer cells by targeting junctional adhesion molecule A. PLoS One. 2016;11:e0157686. doi: 10.1371/journal.pone.0157686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Ma J, Luan G, Kang L, Su Y, He Y, Luan F. MiR-506 suppresses tumor proliferation and invasion by targeting FOXQ1 in nasopharyngeal carcinoma. PLoS One. 2015;10:e0122851. doi: 10.1371/journal.pone.0122851. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhang X, Huang L, Zhao Y, Tan W. Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly. Acta Biochim Biophys Sin (Shanghai) 2013;45:995–1001. doi: 10.1093/abbs/gmt113. [DOI] [PubMed] [Google Scholar]
- 13.Zhou YM, Liu J, Sun W. MiR-130a overcomes gefitinib resistance by targeting met in non-small cell lung cancer cell lines. Asian Pac J Cancer Prev. 2014;15:1391–1396. doi: 10.7314/apjcp.2014.15.3.1391. [DOI] [PubMed] [Google Scholar]
- 14.Fujita Y, Kojima T, Kawakami K, Mizutani K, Kato T, Deguchi T, Ito M. miR-130a activates apoptotic signaling through activation of caspase-8 in taxane-resistant prostate cancer cells. Prostate. 2015;75:1568–1578. doi: 10.1002/pros.23031. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Li Y, Wang R, Qin S, Liu J, Su F, Yang Y, Zhao F, Wang Z, Wu Q. MiR-130a-3p regulates cell migration and invasion via inhibition of Smad4 in gemcitabine resistant hepatoma cells. J Exp Clin Cancer Res. 2016;35:19. doi: 10.1186/s13046-016-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Secchiero P, Celeghini C, Cutroneo G, Di Baldassarre A, Rana R, Zauli G. Differential effects of stromal derived factor-1 alpha (SDF-1 alpha) on early and late stages of human megakaryocytic development. Anat Rec. 2000;260:141–147. doi: 10.1002/1097-0185(20001001)260:2<141::AID-AR40>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Kinose Y, Sawada K, Nakamura K, Kimura T. The role of microRNAs in ovarian cancer. Biomed Res Int. 2014;2014:249393. doi: 10.1155/2014/249393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao YN, Chen GS, Hong SJ. Circulating MicroRNAs in gynecological malignancies: from detection to prediction. Exp Hematol Oncol. 2014;3:14. doi: 10.1186/2162-3619-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagasawa T. CXCL12/SDF-1 and CXCR4. Front Immunol. 2015;6:301. doi: 10.3389/fimmu.2015.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Lu J, Peng X, Wang J, Liu X, Chen X, Jiang Y, Li X, Zhang B. Integrated analysis of microRNA regulatory network in nasopharyngeal carcinoma with deep sequencing. J Exp Clin Cancer Res. 2016;35:17. doi: 10.1186/s13046-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song P, Ye LF, Zhang C, Peng T, Zhou XH. Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene. 2016;592:8–14. doi: 10.1016/j.gene.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Li X, Ge X, Jia L, Zhang Z, Fang R, Yang J, Liu J, Peng S, Zhou M, Xiang J, Zeng Z, Zhou W, Xiong W, Xiao G, Fang L, Li GY, Li Z. MiR-34b-3 and miR-449a inhibit malignant progression of nasopharyngeal carcinoma by targeting lactate dehydrogenase A. Oncotarget. 2016;7:54838–54851. doi: 10.18632/oncotarget.10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung CC, Lun SW, Chung GT, Chow C, Lo C, Choy KW, Lo KW. MicroRNA-183 suppresses cancer stem-like cell properties in EBV-associated nasopharyngeal carcinoma. BMC Cancer. 2016;16:495. doi: 10.1186/s12885-016-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garzon R, Pichiorri F, Palumbo T, Iuliano R, Cimmino A, Aqeilan R, Volinia S, Bhatt D, Alder H, Marcucci G, Calin GA, Liu CG, Bloomfield CD, Andreeff M, Croce CM. MicroRNA fingerprints during human megakaryocytopoiesis. Proc Natl Acad Sci U S A. 2006;103:5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W, Chang G, Li X, Li Q, Wang S, Wang W. MicroRNA profiling implies new markers of chemoresistance of triple-negative breast cancer. PLoS One. 2014;9:e96228. doi: 10.1371/journal.pone.0096228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segersten U, Spector Y, Goren Y, Tabak S, Malmstrom PU. The role of microRNA profiling in prognosticating progression in Ta and T1 urinary bladder cancer. Urol Oncol. 2014;32:613–618. doi: 10.1016/j.urolonc.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, Ledergor G, Jung Y, Milo I, Poulos MG, Kalinkovich A, Ludin A, Kollet O, Shakhar G, Butler JM, Rafii S, Adams RH, Scadden DT, Lin CP, Lapidot T. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532:323–328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voermans C, van Hennik PB, van der Schoot CE. Homing of human hematopoietic stem and progenitor cells: new insights, new challenges? J Hematother Stem Cell Res. 2001;10:725–738. doi: 10.1089/152581601317210827. [DOI] [PubMed] [Google Scholar]
- 29.Mendt M, Cardier JE. Role of SDF-1 (CXCL12) in regulating hematopoietic stem and progenitor cells traffic into the liver during extramedullary hematopoiesis induced by G-CSF, AMD3100 and PHZ. Cytokine. 2015;76:214–221. doi: 10.1016/j.cyto.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Arabanian LS, Fierro FA, Stolzel F, Heder C, Poitz DM, Strasser RH, Wobus M, Borhauser M, Ferrer RA, Platzbecker U, Schieker M, Docheva D, Ehninger G, Illmer T. MicroRNA-23a mediates post-transcriptional regulation of CXCL12 in bone marrow stromal cells. Haematologica. 2014;99:997–1005. doi: 10.3324/haematol.2013.097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 32.Dong S, Jin M, Li Y, Ren P, Liu J. MiR-137 acts as a tumor suppressor in papillary thyroid carcinoma by targeting CXCL12. Oncol Rep. 2016;35:2151–2158. doi: 10.3892/or.2016.4604. [DOI] [PubMed] [Google Scholar]
- 33.Goltz D, Holmes EE, Gevensleben H, Sailer V, Dietrich J, Jung M, Rohler M, Meller S, Ellinger J, Kristiansen G, Dietrich D. CXCL12 promoter methylation and PD-L1 expression as prognostic biomarkers in prostate cancer patients. Oncotarget. 2016;7:53309–53320. doi: 10.18632/oncotarget.10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao N, Wang L, Wang T, Li H. Inflammatory CXCL12-CXCR4/CXCR7 axis mediates G-protein signaling pathway to influence the invasion and migration of nasopharyngeal carcinoma cells. Tumour Biol. 2016;37:8169–8179. doi: 10.1007/s13277-015-4686-2. [DOI] [PubMed] [Google Scholar]