Abstract
Human non-small cell lung cancer (NSCLC) is the most common cause of cancer-related death in men. Signal transducers and activators of transcription 3 (STAT3) is a potential molecular target in angiogenesis-mediated cancer therapy. In this study, we subcutaneously injected athymic nude mice with NCI-H460 cells to induce ectopic xenograft model, and treated the animals with curcumin (100 mg/kg) or vehicle by oral gavage. Tumor size and tumor weight were significantly reduced by curcumin treatment. Besides, curcumin significantly decreased hemoglobin content and mRNA expression of CD31 and CD105 in tumor tissue, suggesting that curcumin could inhibit angiogenesis in NSCLC xenograft. Similarly, we intrathoracally injected athymic nude mice with H1975 cells to induce orthotopic xenograft model, in which curcumin significantly reduced tumor weight as well as improved the survival rate of mice. STAT3 pathway was involved in curcumin-induced tumor inhibition, in which phosphorylation of STAT3 and JAK in ectopic xenograft were both declined after curcumin treatment, and the STAT3-regulated promoter activation of VEGF, Bcl-xL, Cyclin D1 was also significantly reduced after treatment. In in vitro assays, curcumin significantly inhibited cell migration and tube formation of NCI-H460 cells, but transfection with pMXs-Stat3C, a dominant active mutant, could abolish the inhibitory effects of curcumin on the cells, suggesting curcumin inhibited tumor angiogenesis of NCI-H460 cells through the inactivation of STAT3. All data showed that curcumin could be a potential drug targeting STAT3 to treat NSCLC.
Keywords: Curcumin, non-small cell lung cancer, STAT3, angiogenesis, xenograft
Introduction
Lung cancer is the most common cause of cancer-related death in men and second most common in women after breast cancer all over the world [1], and is mainly caused by smoking, air pollution and genetics [2]. In China, the prevalence of lung cancer is increasing, most probably due to haze more frequently occurred in Northern China [3]. About 85% to 90% of lung cancers are non-small cell lung cancer (NSCLC), with a 5-year survival rate of only 16% [4]. The therapy for NSCLC usually involves a combination of surgery, chemotherapy and radiotherapy, however, the side effect of these treatments is also a concern [5,6].
Signal transducers and activators of transcription 3 (STAT3) is one of the most vital transcription factors (TF) which can be activated by cytokines or growth factors [7,8]. The abnormal activation of STAT3 can cause unrestricted cell proliferation and malignant transformation [7]. STAT3 is also constitutively activated in about 50% of NSCLC primary tumors and lung cancer-derived cell lines [9-11]. Constitutively activated STAT3 up-regulated VEGF expression and promoted tumor angiogenesis [12]. On the contrary, inhibition of STAT3 could regress growth of NSCLC xenograft in mice [13]. Therefore, STAT3 is a potential molecular target of angiogenesis-mediated cancer therapy, and has been paid more attention in drug discovery [14].
Curcumin, also named as diferuloyl methane, is a natural polyphenol obtained from turmeric, the rhizome of Curcuma longa (L.), and has been used in traditional Indian Ayurvedic medicine for centuries [15,16]. Curcumin has anti-viral, anti-bacterial, anti-oxidant, anti-inflammatory, and anti-proliferative activities [15,17,18]. It was reported that curcumin could inhibit growth of human NSCLC xenografts [19,20], but the involved molecular mechanism was still unknown. In this work, we aimed to prove inhibitory effects of curcumin in ectopic and orthotopic mouse xenograft model of human NSCLC, and investigate possible role of STAT3 in anti-angiogenic mechanism using cell-based assays.
Materials and methods
Chemicals
Curcumin (purity of over 99.5%) was purchased from Sigma (St. Louis, MO). For in vitro study, curcumin was prepared with dimethyl sulfoxide (DMSO) at a concentration of 10 mM, stored as small aliquots at -20°C, and thawed and diluted as needed in cell culture medium. For in vivo study, curcumin was prepared with corn oil daily at a concentration of 20 mg/mL.
Cell culture
Human NSCLC cell line NCI-H460 and H1975 were purchased from American Type Culture Collection (ATCC), and were grown and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Gibco, Grand Island, NY) at 37°C under a humidified atmosphere of 5% CO2 in air.
Transfection
pMXs-Stat3C (dominant active mutant) was a kind gift from Dr. Toshio Kitamura of Tokyo University (Kitamura et al., 2003). The pMXs vector containing the Stat3C cDNA or its control vector was transfected into NCI-H460 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and incubated for 48 hours in culture medium.
Cell migration
NCI-H460 cells were seeded into the insert of Transwell at a density of 1 × 105 cells/well, then cultured in serum-free culture media. Curcumin (30 µmol/L) or vehicle was added to the lower reservoirs. Cells were subsequently allowed to migrate across a collagen I-coated polycarbonate filter for 12 h at 37°C. Non-migrated cells were removed from the top side of the filter by scraping. Migrated cells on the bottom side of the filter were subsequently fixed with 4% paraformaldehyde for 30 min and stained by hematoxylin solution (Beyotime, Shanghai, China) for 5 min. Cells in five random fields of each migration well were counted to determine the average number of migrated cells.
Tube formation
24-well plates were coated with 300 μL Matrigel (BD, San Jose, CA) and incubated at 37°C for 20 min to allow the Matrigel to solidify. NCI-H460 cells were plated at a density of 1 × 105 cells/well and incubated with curcumin (30 µmol/L) or vehicle at 37°C for 6 h. The cells were then photographed using a Zeiss digital camera. Tube formation was quantified by measuring the length of capillary structures using the software ImageJ. Five randomly selected fields of view were photographed per well. The average value of the five fields was taken as the value for each sample.
Animals and treatment
Athymic nude mice (4- to 6-week-old) were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in a temperature-controlled room (22°C) with 12-h-light/12-h-dark cycling under pathogen-free conditions, and had free access to food and water. All experimental procedures related to the animals complied with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH) of the United States and were approved by Institutional Animal Care and Use Committee of Zhejiang Cancer Hospital.
The same protocol of treatment was used in both ectopic and orthoptopic xenograft models. In each experiment, all rats were randomly divided into two groups. One of the groups was treated with curcumin (100 mg/kg) by oral gavage, the other was treated with vehicle (corn oil). The treatment begun 7 days prior to cell implantation and lasted until the end of experiment.
Ectopic xenograft model
Subconfluent NCI-H460 cells were harvested by trypsin/EDTA treatment and washed with cold PBS by centrifugation, then resuspended in PBS and kept on ice before used. Tumor cells (1 × 106 cells in 0.2 mL PBS) were injected subcutaneously into the mice. Tumor size was measured every four days by caliper, and tumor volume was calculated by the formula: 0.5 × (larger diameter) × (smaller diameter)2. At the end of the experiment, the animals were sacrificed by CO2 euthanasia and their tumor tissues were harvested and weighted, then stored in -80°C for further analysis.
Orthotopic xenograft model
Subconfluent H1975 cells were harvested by trypsin/EDTA treatment and washed with cold PBS by centrifugation, then resuspended in cold growth factor reduced-Matrigel, which could fix and prevent tumor cells from diffusion into the lung. Tumor cells (1 × 106 cells in 0.2 mL PBS-diluted Matrigel) were injected intrathoracally into the mice. The experiment was terminated when the mice in the vehicle group become moribund, and all death dates were recorded. At the end of the experiment, the animals were sacrificed by CO2 euthanasia and their tumor tissues were harvested and weighted.
Hemoglobin assay
Concentration of hemoglobin in tumor tissue was determined using a Hemoglobin Assay Kit (Sigma) according to the manufacturer’s instructions.
Real-time PCR
mRNA was extracted from tumor tissue and reverse-transcribed into cDNA using PrimeScript RT-PCR-Kit (TAKARA, Dalian, China). The primers were listed in Table 1 [21].
Table 1.
Primers for real-time PCR
| CD31 | 5’-TATCCAAGGTCAGCAGCATCGTGG-3’ |
| 5’-GGGTTGTCTTTGAATACCGCAG-3’ | |
| CD105 | 5’-CCTTTGGTGCCTTCCTGATTG-3’ |
| 5’-TGTTTGGTTCCTGG-GACAAGTTC-3’ | |
| 18S | 5’-GATGGGCGGCGGAAAATAG-3’ |
| 5’-GCGTGGATTCTGCATAATGGT-3’ |
Western blot
Tumor tissue was lysed with Protein Extraction Reagent (Beyotime), and protein concentration was determined by BCA reagent (Beyotime). About 20 μg of protein was separated in 10% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinyl difluoride (PVDF, Millipore, Billerica, MA) membrane. After blocking with TBST containing 5% milk for 1 hour, the membrane was incubated with antibodies against STAT3, p-STAT3, JAK, p-JAK and GAPDH (Cell Signaling, Danvers, MA) overnight at 4°C. After incubation in horseradish peroxidase-conjugated secondary antibody for 1 hour, the membrane was exposed to Immobilon solution (Millipore) for band detection.
Chromatin immunoprecipitation (ChIP)
An Agarose ChIP Kit (Pierce, Rockford, IL) was used to prepare nuclear extracts from tumor tissue homogenate and perform ChIP according to the manufacturer’s instructions. A ChIP-grade primary antibody against STAT3 was purchased from Cell Signaling. Immunoprecipitated DNA was purified with DNA Clean-Up Column (Beyotime) and then quantitated by real-time PCR using PrimeScript RT-PCR-Kit. The primers were listed in Table 2 [22].
Table 2.
Primers for real-time PCR
| VEGF | 5’-CTGGCCTGCAGACATCAAAGTGAG-3’ |
| 5’-CTTCCCGTTCTCAGCTCCACAAAC-3’ | |
| Bcl-xL | 5’-CTGGGTTCCCTTTCCTTCCA-3’ |
| 5’-TCCCAAGCAGCCTGAATCC-3’ | |
| Cyclin D1 | 5’-GTTGACTTCCAGGCACGGTT-3’ |
| 5’-GATCCTCCAATAGCAGCAAACAAT-3’ |
Statistical analysis
Data were presented as mean ± standard deviation (SD). Significance of difference between groups was analyzed by performing two-way RM ANOVA for time course study, or one-way ANOVA with Dunnett’s multiple comparison test or unpaired Student’s t test for other studies. Survival studies were assessed using Kaplan-Meier survival curves and analyzed with the Mantel-Cox log-rank test. P value less than 0.05 was considered statistically significant. Data were analyzed and graphed by Prism 6.0 (GraphPad Software, La Jolla, CA).
Results
Curcumin inhibited human NSCLC ectopic xenograft
NCI-H460 cells were injected subcutaneously into athymic nude mice to induce ectopic xenograft model. Treatment of curcumin (100 mg/kg) could significantly decrease the tumor size from day 20 post-inoculation (Figure 1A). At the termination, tumor size in treatment group was 200.6 ± 24.9 mm3, while that in vehicle group was 405.0 ± 66.6 mm3 (P < 0.001). Likewise, tumor weight in treatment group was also significantly lower than that in vehicle group (Figure 1B, 247.3 ± 42.1 mg vs. 520.0 ± 49.8 mg, P < 0.001).
Figure 1.
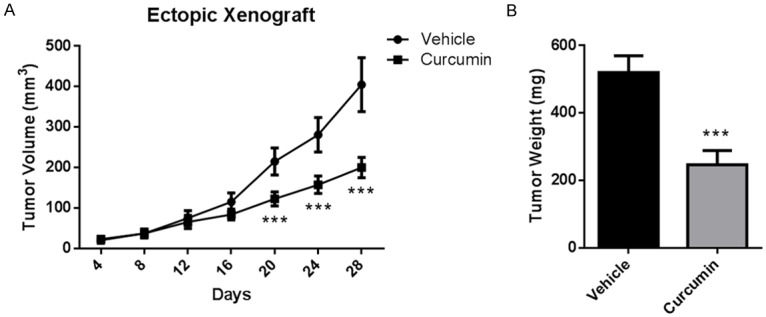
Curcumin inhibited human NSCLC ectopic xenograft. Athymic nude mice were injected subcutaneously with NCI-H460 cells (1 × 106 cells in 0.2 mL PBS), and treated with vehicle (corn oil) or curcumin (100 mg/kg) by oral gavage. The treatment begun 7 days prior to cell implantation and lasted until the end of experiment. A. Tumor volume. B. Tumor weight. Data were presented as Mean ± SD. N=6. ***P < 0.001 vs. vehicle group.
Curcumin inhibited human NSCLC orthotopic xenograft
H1975 cells were injected intrathoracally into athymic nude mice to induce orthotopic xenograft model. Treatment of curcumin (100 mg/kg) could significantly improve the survival rate of mice (Figure 2A, P=0.0354). At the termination, tumor weight in treatment group was significantly lower than that in vehicle group (Figure 2B, 500.5 ± 53.7 mg vs. 714.8 ± 75.4 mg, P < 0.001). These data were consistent with that from ectopic xenograft model.
Figure 2.

Curcumin inhibited human NSCLC orthotopic xenograft. Athymic nude mice were injected intrathoracally with H1975 cells (1 × 106 cells in 0.2 mL PBS-diluted Matrigel), and treated with vehicle (corn oil) or curcumin (100 mg/kg) by oral gavage. The treatment begun 7 days prior to cell implantation and lasted until the end of experiment. A. Percent survival. Survival studies were assessed using Kaplan-Meier survival curves and analyzed with the Mantel-Cox log-rank test. B. Tumor weight. Data were presented as Mean ± SD. N=8. ***P < 0.001 vs. vehicle group.
Curcumin inhibited angiogenesis in tumor tissue
We evaluated angiogenesis in ectopic xenograft by hemoglobin assay and found that curcumin treatment could significantly decreased hemoglobin content from 18.26 ± 0.90 μg/mg to 12.44 ± 0.68 μg/mg (Figure 3A, P < 0.001). Next, we determined mRNA expression level of CD31 and CD105, biomarkers of endothelium, in tumor tissue. Likewise, both of them were also declined after curcumin treatment (Figure 3B, P < 0.001 for CD31, P < 0.01 for CD105). All data showed that curcumin inhibited tumor growth by targeting angiogenesis.
Figure 3.
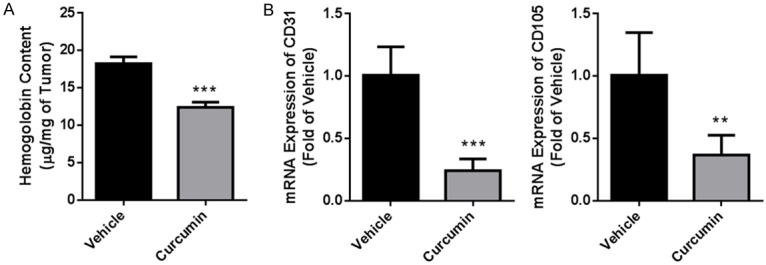
Curcumin inhibited angiogenesis in tumor tissue. At the termination of treatment, tumor tissue from ectopic xenograft was isolated and homogenized for angiogenesis analysis. A. Hemoglobin content. B. mRNA expression of CD31 and CD105 determined by real-time PCR. Data were presented as Mean ± SD. N=6. ***P < 0.001 vs. vehicle group, **P < 0.01 vs. vehicle group.
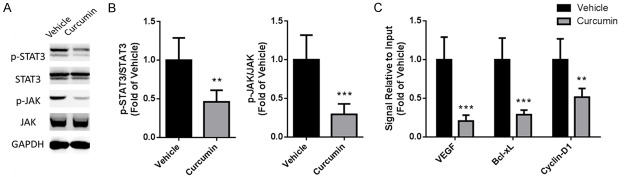
STAT3 pathway was involved in curcumin-induced tumor inhibition
Since STAT3 is a potential molecular target of angiogenesis-mediated cancer therapy, in next work we tried to test whether activation of STAT3 could be changed by curcumin treatment. Using Western blot we found that protein expression of phosphorylated STAT3 and JAK in ectopic xenograft were both declined after treatment (Figure 4A, 4B, P < 0.01 for STAT3, P < 0.001 for JAK). Furthermore, ChIP was performed to investigate changes of gene promoters regulated by STAT3 in tumor tissue. The promoter activation of VEGF, Bcl-xL, Cyclin D1 was significantly reduced after curcumin treatment (Figure 4C, P < 0.001 for VEGF, P < 0.001 for Bcl-xL, P < 0.01 for Cyclin D1).
Figure 4.
Curcumin inhibited activation of STAT3 in tumor tissue. At the termination of treatment, tumor tissue from ectopic xenograft was isolated and homogenized for mechanism research. A. Phosphorylation and total JAK/STAT3 detected by Western blot. GAPDH was used as a loading control. B. Statistical analysis of Western blot data. C. Changes of gene promoters regulated by STAT3. The nuclear extract in tumor tissue was isolated and used to perform chromatin immunoprecipitation with an STAT3 antibody. The change of downstream promoters was analyzed by real-time PCR. Data were presented as Mean ± SD. N=6. ***P < 0.001 vs. vehicle group, **P < 0.01 vs. vehicle group.
Next, we transfected NCI-H460 cells with pMXs-Stat3C, a dominant active mutant which could express consistently activated STAT3, to investigate whether STAT3 was critical in curcumin-induced inhibition of angiogenesis in vitro. In Transwell assay, migration of cells transfected by control vector could be significantly inhibited by curcumin treatment, while there was no significant change in those transfected by pMXs-Stat3C (Figure 5). Likewise, in Matrigel assay, tube formation of cells transfected by pMXs-Stat3C also could not be significantly but slightly changed by curcumin treatment (Figure 6). These data showed that curcumin inhibited tumor angiogenesis of NCI-H460 cells through the inactivation of STAT3.
Figure 5.
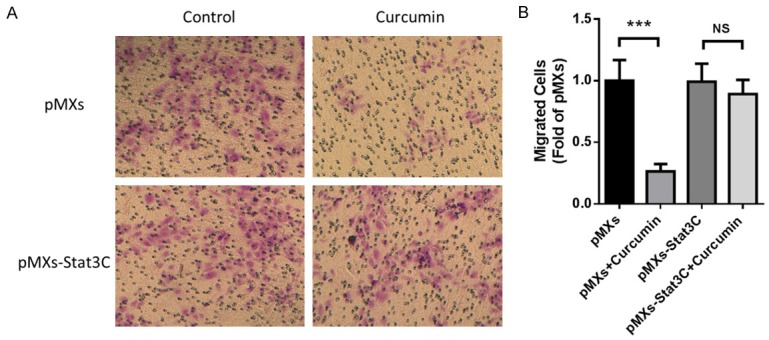
Curcumin inhibited cell migration through the inactivation of STAT3. NCI-H460 cells were transfected with pMXs-Stat3C or control plasmid for 48 hours before seeded in Transwell and treated with curcumin (30 µmol/L) or vehicle for 12 hours. A. Representative photos of each groups. B. Statistical analysis of migrated cells. Data were presented as Mean ± SD. ***P < 0.001 vs. vehicle group, NS, no significant. All experiments were repeated at least three times.
Figure 6.
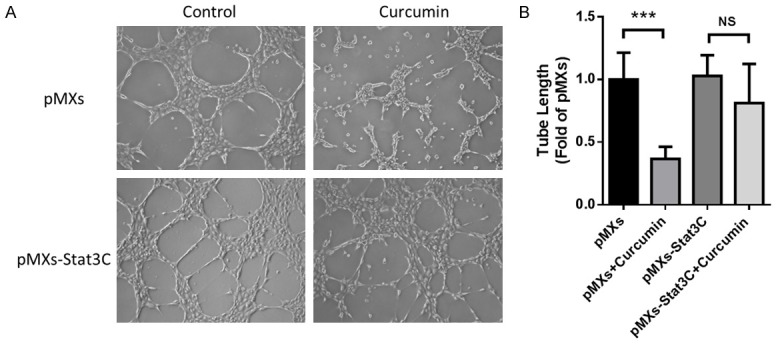
Curcumin inhibited tube formation through the inactivation of STAT3. NCI-H460 cells were transfected with pMXs-Stat3C or control plasmid for 48 hours before seeded in Matrigel and treated with curcumin (30 µmol/L) or vehicle for 6 hours. A. Representative photos of each groups. B. Statistical analysis of tube lengths. Data were presented as Mean ± SD. ***P < 0.001 vs. vehicle group, NS, no significant. All experiments were repeated at least three times.
Discussion
Curcumin has been used in traditional Indian Ayurvedic medicine for centuries, and in recent decades, it was been proved to have inhibitory effects on diverse cancers, such as pancreatic cancer [23], lung cancer [19,20], brain cancer [21], ovarian cancer [24] and breast cancer [25]. In xenograft rodent models, curcumin showed anti-tumor effects in nude mice bearing A549, LL/2, NCI-H460 and H1975 NSCLC cells [19,20,26,27]. Lung cancer induced by tracheal instillation of LLC cells or aspiration of TNFα could also be inhibited by curcumin [28,29]. Besides, using VEGF-overexpressing transgenic mice, Tung reported that curcumin decreased pulmonary function damage and inflammation so that to regress cancer development [30].
Targeting potential molecules to inhibit angiogenesis is a new direction of cancer therapy [31], in which STAT3 is a candidate molecular target in angiogenesis-mediated therapy [32]. It has been reported that VEGF expression is positively related with STAT3 activity in variety of human cancer cell lines, and furthermore, Stat3C, a constantly activated STAT3 mutant, could up-regulate VEGF expression and stimulate angiogenesis in tumor tissue [33]. In addition, targeting STAT3 could block expression of both HIF-1 and VEGF induced by multiple oncogenic growth signaling pathways, and then hinder tumor angiogenesis [34]. In this study, we disclosed that curcumin inhibited tumor growth of NSCLC by targeting angiogenesis, in which phosphorylated STAT3 was also reduced.
In order to investigate whether curcumin inhibited angiogenesis through targeting STAT3 pathway, we transfected NCI-H460 cells with pMXs-Stat3C, a dominant active mutant which could express consistently activated STAT3 [35]. It was reported that the substitution of two cysteine residues within the C-terminal loop of the SH2 domain of STAT3 produced a molecule that could dimerize spontaneously to bind DNA, and consistently activate transcription [7]. In our work, after transfection with the plasmid, high migration and tube formation abilities of NCI-H460 cells induced by pMXs-Stat3C could not be abolished by curcumin treatment. These data showed that inactivation of STAT3 was critical in curcumin-induced inhibition of angiogenesis, suggesting STAT3 could be a potent molecular target for angiogenesis-mediated NSCLC therapy.
In this study, we demonstrated that curcumin reduced STAT3 phosphorylation, followed by inhibiting the expression of STAT3 downstream genes, including VEGF, BclxL and Cyclin D1. VEGF is a key regulator in angiogenesis and it is mainly secreted by tumor cells and targets VEGF receptor on endothelial cells to promote angiogenesis [36]. Beside, VEGF-mediated autocrine loop in endothelial cell is also an important component in this process [37]. BclxL belongs to Bcl-2 family, and acts as an anti-apoptotic protein by preventing the release of mitochondrial contents such as cytochrome c, which leads to caspase activation and programmed cell death [38]. Cyclin D1 is a protein required for progression through the G1 phase of the cell cycle, and overexpression of it has been shown to correlate with early cancer onset and tumor progression and it can lead to oncogenesis by increasing angiogenesis via VEGF production [39,40]. Therefore, inhibition of gene expression of VEGF, BclxL and Cyclin D1 could prevent angiogenesis and promote apoptosis so that to regress tumor growth.
In conclusion, we disclosed inhibitory effects of curcumin in both ectopic and orthotopic mouse xenograft model of human NSCLC through targeting angiogenesis, as well as clarified STAT3 pathway was involved in curcumin-induced inhibition of tumor growth. These data suggested curcumin could be a potential drug targeting STAT3 to treat NSCLC.
Acknowledgements
This work was supported by Zhejiang Science and Technology Project of Medicine (2013KYB046).
Disclosure of conflict of interest
None.
References
- 1.Stewart B, Wild C. World cancer report 2014. International Agency for Research on Cancer. 2014 [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu P, Chen Y, Ye X. Haze, air pollution, and health in China. Lancet. 2013;382:2067–2067. doi: 10.1016/S0140-6736(13)62693-8. [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, Simms L, Shepherd FA. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D’Amico TA, Dilling TJ. NCCN guidelines insights: non-small cell lung cancer, version 4.2016. J Natl Compr Canc Netw. 2016;14:255. doi: 10.6004/jnccn.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maione P, Sacco PC, Sgambato A, Casaluce F, Rossi A, Gridelli C. Overcoming resistance to targeted therapies in NSCLC: current approaches and clinical application. Ther Adv Med Oncol. 2015;7:263–273. doi: 10.1177/1758834015595048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao YX, Pestell RG, Albanese C, Darnell JE Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 9.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B. Mutations in the EGFR kinase domain mediate Stat3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–8294. doi: 10.1158/1078-0432.CCR-05-0827. [DOI] [PubMed] [Google Scholar]
- 11.Mukohara T, Kudoh S, Yamauchi S, Kimura T, Yoshimura N, Kanazawa H, Hirata K, Wanibuchi H, Fukushima S, Inoue K. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC) Lung Cancer. 2003;41:123–130. doi: 10.1016/s0169-5002(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 12.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci U S A. 2012;109:9623. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 18.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 19.Lev-Ari S, Starr A, Katzburg S, Berkovich L, Rimmon A, Ben-Yosef R, Vexler A, Ron I, Earon G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J Nutr Biochem. 2014;25:843. doi: 10.1016/j.jnutbio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Su CC, Yang JS, Lu CC, Johua C, Wu CL, Lin JJ, Lai KC, Techun H, Lu HF, Fan MJ. Curcumin inhibits human lung large cell carcinoma cancer tumour growth in a murine xenograft model. Phytother Res. 2010;24:189–192. doi: 10.1002/ptr.2905. [DOI] [PubMed] [Google Scholar]
- 21.Perry MC, Demeule M, Régina A, Moumdjian R, Béliveau R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res. 2010;54:1192. doi: 10.1002/mnfr.200900277. [DOI] [PubMed] [Google Scholar]
- 22.Qi J, Xia G, Huang CR, Wang JX, Zhang J. JSI-124 (cucurbitacin I) inhibits tumor angiogenesis of human breast cancer through reduction of STAT3 phosphorylation. Am J Chin Med. 2015;43:337–347. doi: 10.1142/S0192415X15500226. [DOI] [PubMed] [Google Scholar]
- 23.Bimonte S, Barbieri A, Palma G, Luciano A, Rea D, Arra C. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. Biomed Res Int. 2013;2013:810423. doi: 10.1155/2013/810423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaizpena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2009;23:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 25.Lv ZD, Liu XP, Zhao WJ, Dong Q, Li FN, Wang HB, Kong B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int J Clin Exp Pathol. 2014;7:2818–2824. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang BL, Shen YM, Zhang QW, Li YL, Luo M, Liu Z, Li Y, Qian ZY, Gao X, Shi HS. Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int J Nanomedicine. 2012;2013:3521. doi: 10.2147/IJN.S45250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Zhang L, Peng H, Li Y, Xiong J, Xu Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater Sci Eng C Mater Biol Appl. 2013;33:4802–4808. doi: 10.1016/j.msec.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 28.Rocks N, Bekaert S, Coia I, Paulissen G, Gueders M, Evrard B, Heugen JV, Chiap P, Foidart JM, Noel A. Curcumin-cyclodextrin complexes potentiate gemcitabine effects in an orthotopic mouse model of lung cancer. Br J Cancer. 2012;107:1083. doi: 10.1038/bjc.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yen FL, Tsai MH, Yang CM, Liang CJ, Lin CC, Chiang YC, Lee HC, Ko HH, Lee CW. Curcumin nanoparticles ameliorate ICAM-1 expression in TNF-a-treated lung epithelial cells through p47 phox and MAPKs/AP-1 pathways. PLoS One. 2013;8:e63845–e63845. doi: 10.1371/journal.pone.0063845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tung YT, Chen HL, Lai CW, Shen CJ, Lai YW, Chen CM. Curcumin reduces pulmonary tumorigenesis in vascular endothelial growth factor (VEGF)-overexpressing transgenic mice. Mol Nutr Food Res. 2011;55:1036–1043. doi: 10.1002/mnfr.201000654. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 33.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 34.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 35.Huh JE, Lee SY. IL-6 is produced by adipose-derived stromal cells and promotes osteogenesis. Biochim Biophys Acta. 2013;1833:2608–2616. doi: 10.1016/j.bbamcr.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 37.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203–7206. [PubMed] [Google Scholar]
- 38.Korsmeyer SJ. Regulators of cell death. Trends Genet. 1995;11:101–105. doi: 10.1016/S0168-9525(00)89010-1. [DOI] [PubMed] [Google Scholar]
- 39.Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1:226. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 40.Shintani M, Okazaki A, Masuda T, Kawada M, Ishizuka M, Doki Y, Weinstein IB, Imoto M. Overexpression of cyclin DI contributes to malignant properties of esophageal tumor cells by increasing VEGF production and decreasing Fas expression. Anticancer Res. 2002;22:639–647. [PubMed] [Google Scholar]