Abstract
Ischemic preconditioning (IPC) has been introduced to protect grafts against ischemic reperfusion injury (IRI) during liver transplantation (LT) in recent years. However, the underlying molecular mechanisms of IPC are not fully understood. We aimed to confirm whether the efficacy of IPC is dependent on T cell Immunoglobulin and Mucin domain-containing molecules-1 (TIM-1). Quantitative real-time reverse transcription PCR and western blotting were used to detect the expression of genes of interest. Graft function was assessed using the levels of alanine transaminase (ALT) and aspartate transaminase (AST), percentage of apoptosis cells and pathological examination. IPC treatment alleviated graft function after ischemic reperfusion. AST, ALT, CD68, CD3 positive cells and tissue myeloperoxidase activity were decreased significantly by IPC. IPC decreased the expressions of the cytokines and chemokines. Compared with the IRI group, TIM-1 expression and TIM-1 positive cells were inhibited significantly in the IPC group. TIM-1 blockage abolished the protective effect of IPC on IRI damage. IPC could not further improve graft function and decrease the sequestration of immune cells after blocking TIM-1 signaling. IPC is a convenient therapeutic strategy against IRI during LT. The benefit of IPC depends on TIM-1 signaling.
Keywords: TIM-1, ischemic preconditioning (IPC), ischemia-reperfusion injury (IRI)
Introduction
Hepatitis B virus infection is highly prevalent in China, which could lead to more than five million patients developing irreversibly decompensated liver cirrhosis and even hepatocellular carcinoma (HCC) [1]. Liver transplantation (LT) is considered the best life-saving therapy. However, a serious shortage of donor livers is the main limit for the survival rate of patients on the waiting list for an LT. To solve this issue, several transplant centers use donor livers that exceed traditional criteria, including age beyond 60, prolonged time of warm and cold storage, and macrovesicular steatosis beyond 30% [2]. These ‘marginal’ livers are particularly sensitive to ischemia reperfusion injury (IRI), which leads to frequent primary graft non-function and promotes the progression of acute or chronic rejection [3]. Furthermore, IRI also causes damage to other organs, such as acute kidney injury [4], which is a fatal complication [5].
As early as 1986, Murry et al. confirmed that the multiple brief ischemic episodes that precede myocardial infarction were beneficial to protect the myocardium from coronary occlusion [6]. Currently, accumulating evidence shows that ischemic preconditioning (IPC) increases liver resistance to IRI and longer ischemia [7-10]. However, few studies have focused on the molecular mechanism of IPC. Moreover, the wide application of IPC in clinical practice is limited because of the direct mechanical trauma to major vascular structures, the unpredictability of the ischemic episode, and for ethical reasons. Especially in China, the donor livers are harvested mainly from donations from deceased cardiac patients. Despite remote ischemic conditioning being considered as an alternative choice that could protect kidneys from IRI after LT, it presented no protective effect on graft function [11]. In this regard, further investigation of the molecular mechanism of IPC is required urgently.
Recently, several studies reported that the T cell Immunoglobulin and Mucin domain-containing molecules-1 (TIM-1) was responsible for activation of various myeloid leukocytes [12]. TIM-1 belongs to the TIM gene family and is located on human chromosome 5q33.2 in a region that is correlated significantly with allergy, asthma, and autoimmunity [13,14]. Moreover, TIM-1 is a pattern recognition receptor that recognizes phosphatidylserine (PtdSer) [15] expressed on activated CD4+ T cells, and sustains preferentially the expression of Th2, but no or little expression of Th1 and Th17 after differentiation [16]. Crosslinking of TIM-1 with its ligand or agonist monoclonal antibody provided a potent co-stimulatory signal to increase the proliferation of naïve T cells and interleukin (IL)-4 production of Th2 cells [16]. In addition, the interaction of TIM-1 with TIM-2, TIM-3, or TIM-4 is also involved in regulating Th1, and certain dendritic cells and macrophages via PtdSer exposed on exosomes [17].
TIM-1 is also called known as kidney injury molecule (KIM-1), whose expression is localized specifically on the regenerating proximal tubule epithelial cells and is apparently upregulated in the post-ischemic rat kidney [18]. Previously, in the IRI model with LT, we found that TIM-1 expression increased in the liver with IRI, and TIM-1 blockage overcame IRI by decreasing TGF-β and IFN-γ expression, and increasing the expression of IL-4, IL-10, and IL-22 [19]. Furthermore, the use of an antagonistic anti-TIM-1 antibody decreased hepatocellular apoptosis and improved liver function by inhibiting local neutrophil infiltration, and sequestration of macrophages and T lymphocytes in LT [20].
This evidence implied that TIM-1 plays a crucial role in the activation and differentiation of T cell and immunity-mediated IRI. Here, we provide evidence that suppression of TIM-1 signaling is essential for the IPC-mediated decreases in IRI damage in LT.
Material and methods
Animals
Male C57BL/6 mice (5-week-old, 18-22 g) were obtained from the Shanghai Laboratory Animal Center, Chinese Academy Sciences, and housed in a controlled 12-h light/dark cycle environment with free access to food and water. All animal experiments were performed according to the Zhejiang University guidelines for animal care and were approved by the Animal Ethics Review Committee of Zhejiang University, and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publications, No. 8023, revised 1978).
Experimental groups
36 mice were randomly divided into 3 groups (n = 12): sham group, IRI group, and IPC+IRI group. The mice in the sham-operated group underwent surgery, with the portal vein and artery were isolated without occlusion. In the IRI group, the mice were subjected to 70% liver ischemia for 90 min, followed by 6 h of reperfusion, as previously described [20]. In the IPC+IRI group, the mice were subjected to 10 min of ischemia and 10 min of reperfusion prior to sustained ischemia, as previously described [20]. Additionally, 6 mice each from the IRI and IPC+IRI groups were randomly selected to receive an infusion containing a blocking monoclonal antibody against TIM-1 (0.5 mg/mouse i.v.; Bio X Cell, West Lebanon, NH, USA) at 1 h prior to the induction of ischemia. At the end of reperfusion, blood and liver samples were collected and preserved for subsequent procedures.
Enzyme-linked immunosorbent assay
Antibodies were diluted to 1-10 µg/mL with buffer. Subsequently, 0.1 mL of antibody was incubated in 96-well plate for 1 h. The liquor was discarded and the plate was washed three times with Tris-buffered saline and Tween-20 (TBST). Graft protein (0.1 mL/100 μg) was added to the 96-well plate and incubated for 1 h at 37°C in an incubator. After washing three times, the reaction system was further incubated with 0.1 mL enzyme labeled antibody for 1 h. Finally, the concentration of graft protein was determined by measuring the OD value at 450 nm.
Immunohistochemistry
Sections (4 µm) of graft tissues were deparaffinized and rehydrated before being subjected to heat-induced epitope retrieval. Next, the endogenous peroxidase activity was quenched using 3% hydrogen peroxide, and nonspecific binding was blocked with 5% fetal bovine serum (FBS). After incubation with primary antibodies (CD3, ab16669, Abcam, USA; CD68, ab125212, Abcam, USA). Overnight at 4°C, the sections were further incubated with corresponding conjugated-HRP secondary antibodies (anti-rabbit-HPR, 7074, CST, USA) for 1 h at room temperature. The target protein expression was visualized using 3, 3’-diaminobenzidine, followed by hematoxylin counterstain. Furthermore, the negative control was established using phosphate-buffered saline (PBS) rather than the primary antibody.
Histological examination
After the experiment ended after 6 h of reperfusion, the hepatic tissue samples harvested from the mice were fixed with 4% paraformaldehyde. The hepatic tissue samples were immediately removed and post-fixed in 4% paraformaldehyde for 24 h. Paraffin-embedded sections (3 mm thick) were stained with hematoxylin and eosin (H&E) for visualization under a light microscope (magnification, ×200; Leica Microsystems, Wetzlar, Germany).
Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) assay
The cell apoptosis of the graft was analyzed using a TUNEL staining kit (Roche, Basel, Switzerland), following the manufacturer’s instructions. The TUNEL assay was performed in triplicate and repeated ten times with independent liver tissues from each treatment group. The percentage of cell apoptosis was determined by dividing the number of TUNEL positive cells by the total number of liver cells.
Quantitative real time PCR (qRT-PCR)
The extraction of total RNA from grafts was performed using a TRIzol RNA extraction kit (Invitrogen Co.), and then reverse transcription was conducted using a Rever Tra Ace-a- reverse transcription kit (Invitrogen Co.). QRT-PCR was performed using a Takara SYBR Premix Extaq kit in the Roche LightCycler system. All experimental procedures were performed according to the manufacturer’s instructions. Primers were produced by the Shanghai Sangon Biological Engineering Technology Services Co., Ltd. The nucleotide sequences of each primer were as follows: IL-1β: AAATCTCGCAGCAGCACAT (forward), CACACACCAGCAGGTTATCA (reverse); Cxcl-1: CTGGGATTCACCTCAAGAACATC (forward), CAGGGTCAAGGCAAGCCTC (reverse); Cxcl-2: CCAACCACCAGGCTACAGG (forward), GCGTCACACTCAAGCTCTG (reverse); Tim-1: ACATATCGTGGAATCACAACGAC (forward), ACAAGCAGAAGATGGGCATTG (reverse); IL-6: CCACTTCACAAGTCGGAGGCTTA (forward), CCAGTTTGGTAGCATCCATCATTTC (reverse); TNF: TATGGCCCAGACCCTCACA (forward), GGAGTAGACAAGGTACAACCCATC (reverse); IFNγ: CATCAGCAACAACATAAGTGTCATC (forward), CATTGACAGCTTTGTGCTGGA (reverse).
Western blotting
The protein from grafts was collected with lysis buffer (Cell Signaling Technology, Beverly, MA, USA) at 4°C according to the manufacturer’s instructions. The protein concentrations were detected using the Bradford assay (Bio-Rad, Hercules, CA, USA) and a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Proteins (30-50 µg/10 µL proteins were separated by electrophoresis in 10% PAGE gels and then transferred onto 0.45 µm polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After being blocked with 5% non-fat milk in TBST buffer for 1 h, the membranes were incubated with primary antibodies overnight at 4°C. After washing three times, the membranes were incubated with conjugated-HRP secondary antibodies at room temperature for 1 h. The amount target proteins were defined using the Super Signal West Pico Chemiluminescent Substrate (Pierce, Billerica, MA, USA).
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from mouse venous blood samples and incubated with anti-PE for 30 minutes on ice. The background fluorescence was adjusted using the corresponding isotype antibody. After washing three times with PBS, TIM-1 expression was analyzed using a four-color FC500 flow cytometer (Beckman-Coulter, Miami, FL, USA) for 1×105 cellular events.
Statistical analysis
All experiments were conducted three times and the data were described as means with SD or as a frequency. Fisher’s exact test and a two-tailed Student’s t test were performed to analyze the difference in target parameters between IRI and IPC groups. All statistical analyses were performed in SPSS 19.0 software (SPSS Inc., Chicago, IL, USA), and a P-value less than 0.05 was thought to be statistically significant.
Results
IPC attenuates IRI damage in LT
To determine the effect of IPC on liver protection, the serum alanine transaminase (ALT) and aspartate transaminase (AST) levels was detected at 6 h of reperfusion, the peak of IRI. Compared with the IRI group, mice conditioned with IPC presented significantly decreased levels of ALT and AST (Figure 1A, *P < 0.05, **P < 0.01, ***P < 0.001 vs IRI; #P < 0.05 vs Tim-1+IR). Furthermore, pathological examination suggested that IPC improved the sinusoidal congestion in the graft with IRI (Figure 1B). The frequency of hepatocellular apoptosis was also detected, and TUNEL-positive cells in the IRI group was higher compared with the IPC group (Figure 1B, 1D). Western blotting further showed increased Bcl-2, and Bcl-xl levels and decreased Bax and Cleaved caspase3 levels in grafts with IRI following IPC (Figure 1C). These results implied that IPC was an effective method to protect against IRI.
Figure 1.
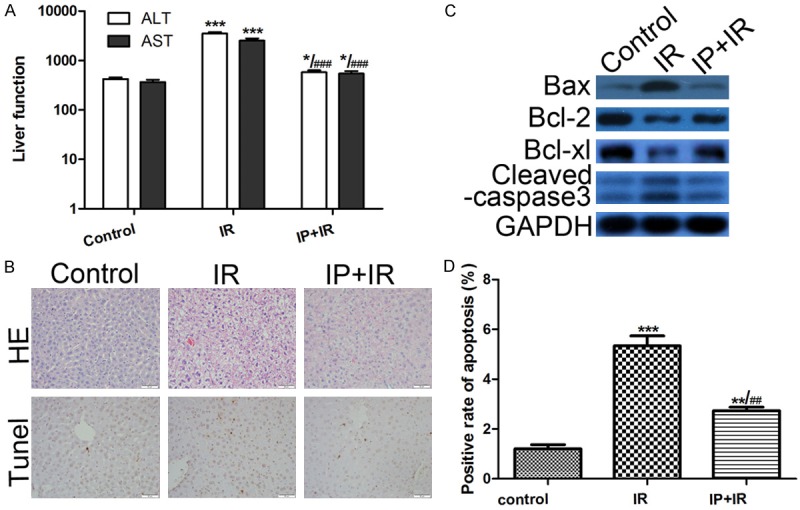
IPC attenuates IRI damage in LT. A. Ischemic preconditioning (IPC) significantly attenuated the liver damage caused by ischemic reperfusion injury (IRI) during liver transplantation (LT). *P < 0.05, **P < 0.01, ***P < 0.001 vs IRI; #P < 0.05 vs Tim-1+IR. B. Liver histology examined using hematoxylin and eosin (HE) staining, and cell apoptosis examined using TdT-mediated biotin-16-dUTP nick-end labeling (TUNEL) assay (magnification, ×400; **P < 0.01, ***P < 0.001, vs Control; ##P < 0.01 vs IR). IPC treatment improved the sinusoidal congestion in the graft with IRI. C. Western Blot was used to detect protein expression. Compared with the IRI group, the IPC group presented increased Bcl-2 and Bcl-xl expression, and decreased Bax and Cleaved caspase3 expression in the graft. D. The ratio of hepatocellular apoptosis was 12% in the IRI group and 5% in the IPC group. **P < 0.01, ***P < 0.001 vs Control; ##P < 0.01 vs IR.
IPC decreases neutrophil, T cell, and macrophage graft sequestration
Given that the accumulated neutrophils, T cells, and macrophages are the main cells that contribute to IRI progression, we performed immunohistochemical staining for CD68 and CD3 in the IRI, IPC, and control groups. After IPC treatment, the percentages of CD68 and CD3 positive cells decreased in the grafts with IRI (Figure 2A). Furthermore, a myeloperoxidase (MPO) assay was performed to further detect neutrophil infiltration into the graft. Similarly, IPC suppressed the MPO activity (U/g) significantly compared with the IRI controls (Figure 2C).
Figure 2.
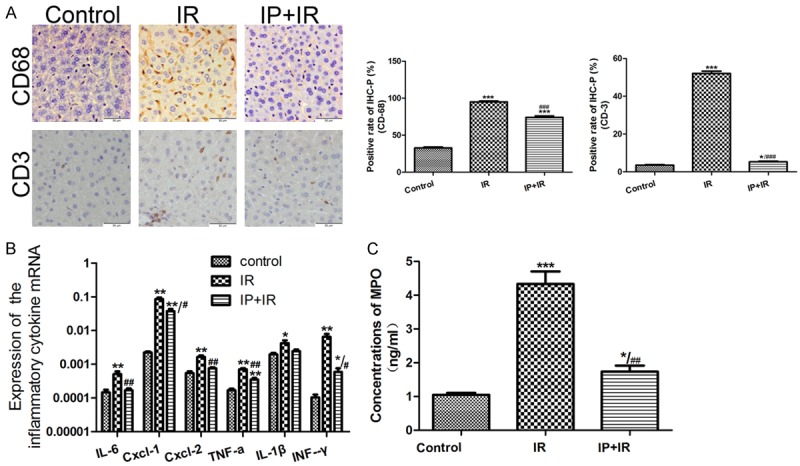
PC decreases neutrophil, T cell, and macrophage graft sequestration. A. The average percentage of CD68 and CD3 positive cells was 95, 52 in the ischemic reperfusion injury (IRI) group and 74, 5 in the ischemic preconditioning (IPC) group, respectively. *P < 0.05, ***P < 0.001 vs Control; ###P < 0.001 vs IR. B. The results of quantitative real-time reverse transcription PCR (qRT-PCR) suggested decreased expression of interleukin (IL-6), tumor necrosis factor alpha (TNF-α), interferon (IFN)-γ, IL-1β, chemokine (C-X-C motif) ligand 1 (CXCL1), and CXCL-2 after IPC treatment. *P < 0.05, **P < 0.01 vs Control; #P < 0.05, ##P < 0.01 vs IR. C. Furthermore, the myeloperoxidase (MPO) assay showed suppressed MPO activity (U/g) in IPC group compared with that in the IRI group. *P < 0.05, ***P < 0.001 vs Control; ##P < 0.01 vs IR.
In parallel, the graft expression of cytokines (IL-6, TNF-α, IFN-γ and IL-1β) and chemokines (CXCL-1 and CXCL-2) were analyzed using qRT-PCR. Compared with the IRI group, IPC mice presented significantly decreased induction of IL-6, TNF-α, IFN-γ, and IL-1β (Figure 2B). CXCL-1 and CXCL-2 predominantly drive neutrophils trafficking [21]. The reduced expression of CXCL-1 and CXCL-2 also suggest decreased neutrophil infiltration.
IPC suppressed TIM-1 expression in grafts with IRI
Given that TIM-1 is essential for immune cells sequestration in grafts with IRI, we detected TIM-1 expression by western blotting and qRT-PCR. IPC treatment apparently suppressed the TIM-1 expression induced by IRI in the graft (Figure 3A, 3B). Furthermore, the peripheral blood TIM-1 positive cells were analyzed by flow cytometry. Compared with the IRI group, the IPC group showed a lower percentage of TIM-1-positive cells in peripheral blood. There were only 3.7% TIM-1 positive cells in the control group, 16.6% in the IR group, and 9.3% in the IPC group (Figure 3C).
Figure 3.
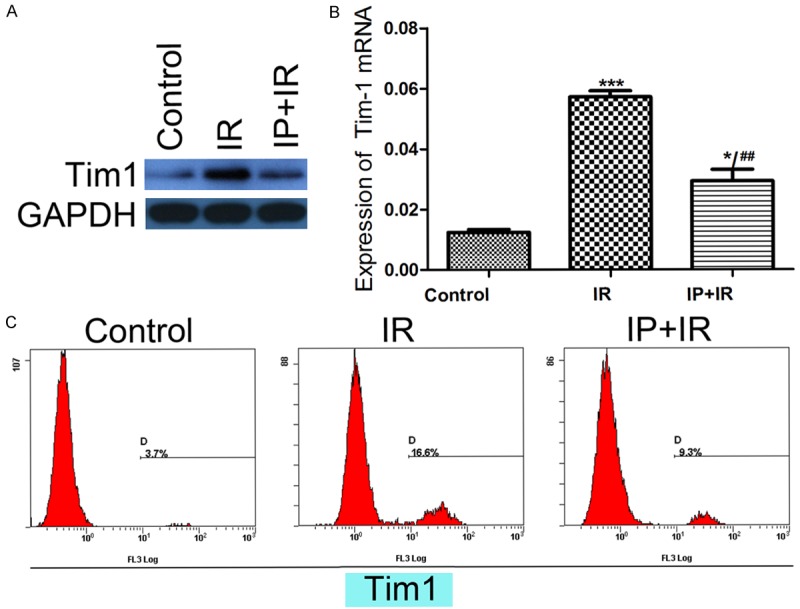
IPC suppressed TIM-1 expression in grafts with IRI. A, B. Suppressed T cell Immunoglobulin and Mucin domain-containing molecules-1 (TIM-1) expression was found in the ischemic preconditioning (IPC) group compared with the ischemic reperfusion injury (IRI) group by western blotting and quantitative real-time reverse transcription PCR (qRT-PCR). *P < 0.05, ***P < 0.001 vs Control; ##P < 0.01 vs IR. C. Furthermore, flow cytometry analysis showed 3.7% of TIM-1 positive cells in the normal group, 16.6% in the IR group, and 9.3% in the IPC group.
TIM-1 blockage abolished the protective effect of IPC on IRI damage
To further determine whether TIM-1 is essential for IPC protection, an anti-TIM-1 antibody was utilized to block TIM-1 signaling in LT. Treatment with the anti-TIM-1 antibody not only reduced the IR-induced serum ALT and AST levels, but also decreased the apoptosis rate and improved the pathological changes observed on histology. However, in the IPC group following anti-TIM-1 antibody treatment, the serum ALT and AST levels were not reduced significantly compared with the IRI graft with TIM-1 blockage (Figure 4A). Moreover, the number of apoptotic cells was not different between the IRI and IPC group after disruption of TIM-1 signaling (Figure 4B, 4D). Western blotting confirmed that TIM-1 blockage abolished the effect of IPC on increasing Bcl-2 and Bcl-xl expression, and decreasing Bax and Cleaved caspase-3 expression in grafts with IRI (Figure 4C). These results suggested that TIM-1 signaling is necessary for the effectiveness of IPC.
Figure 4.
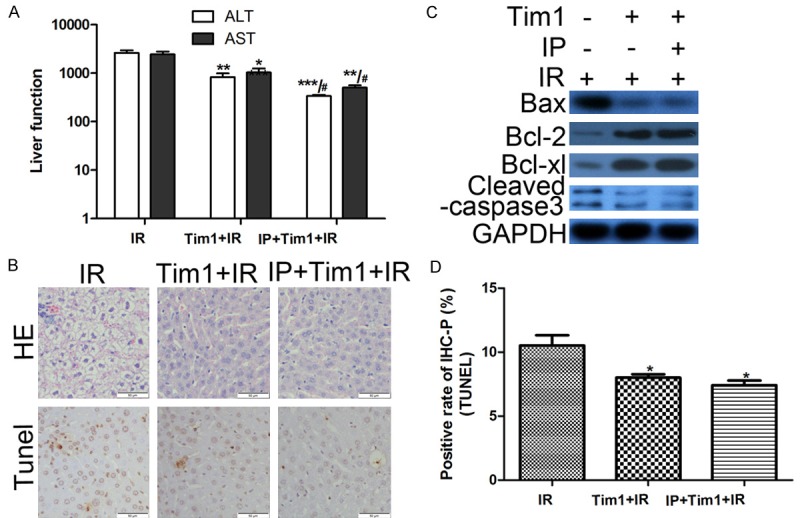
TIM-1 blockage abolished the protective effect of IPC on IRI damage. A. Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels determined using enzyme-linked immunosorbent assay. *P < 0.05, **P < 0.01, ***P < 0.001 vs IR; #P < 0.05 vs Tim-1+IR. B, D. Liver histology examined using hematoxylin and eosin (HE) staining, and cell apoptosis examined using TdT-mediated biotin-16-dUTP nick-end labeling (TUNEL) assay (magnification, ×400; *P < 0.05, **P < 0.01 vs IR). Moreover, in the IPC group following anti-TIM-1 antibody treatment, the serum ALT and AST levels were not reduced significantly compared with the IRI graft with TIM-1 blockage. C. Protein expression was detected by Western Blot. After TIM-1 blockage, there was no difference in the expression of Bcl-2, Bcl-xl, Bax, and Cleaved caspase-3 between the IRI and IPC groups. *P < 0.05 vs IR.
Disruption of TIM-1 signaling eliminated immune regulation of IPC
After receiving anti-TIM-1 antibody treatment, neutrophil, T cell, and macrophage graft sequestration were detected in the mouse models. Compared with the IR group, the expressions of CD68 and CD3 were apparently suppressed in the IRI+TIM-1 blockage group and in the PC+TIM-1 blockage group, but similar between these two groups (Figure 5A). In addition, the MPO activity, another index of neutrophil infiltration, was highest in the IR group, and moderate in both the IRI+TIM-1 blockage and IPC+TIM-1 blockage groups, which also showed had no significant difference between them (Figure 5C). Furthermore, after TIM-1 blockage, the detection of cytokines and chemokines confirmed that IPC treatment could not decrease the expressions of IL-6, TNF-α, IFN-γ, IL-1β, CXCL-1, and CXCL-2 further (Figure 5B).
Figure 5.
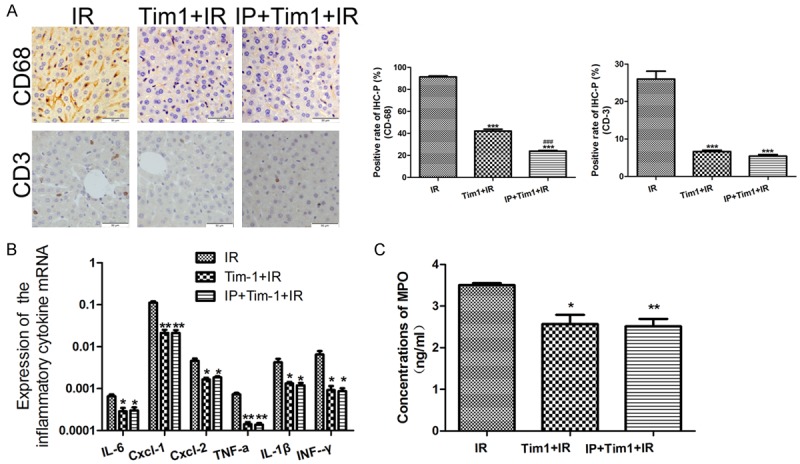
Disruption of TIM-1 signaling eliminated immune regulation of IPC. A. T cell Immunoglobulin and Mucin domain-containing molecules-1 (TIM-1) blockage simultaneously suppressed the expression of CD68 and CD3 in both the ischemic reperfusion injury (IRI) and ischemic preconditioning (IPC) groups, and no different expression was found between the two groups. ***P < 0.001 vs IR; ###P < 0.001 vs Tim-1+IR. B. After TIM-1 blockage, IPC could not decrease the expressions of interleukin (IL-6), tumor necrosis factor alpha (TNF-α), interferon (IFN)-γ, IL-1β, chemokine (C-X-C motif) ligand 1 (CXCL1), and CXCL-2 further. *P < 0.05, **P < 0.01 vs IR. C. In addition, the myeloperoxidase (MPO) activity was the highest in IR group, and moderate or no different in the IRI+TIM-1 blockage and IPC+TIM-1 blockage groups, respectively.
Discussion
IRI, an exogenous antigen-independent, inflammatory event, remains an unavoidable problem during LT. Although significant has been made to reduce the impact of IRI on grafts, little progress has occurred in IRI therapy. Among multiple strategies, IPC was proven to have consistent and apparently beneficial effects in a rodent LT model [22]. Furthermore, multiple centers conducted a serial of prospective randomized clinical trials in which IPC introduced by in local deceased donor livers [23], and demonstrated that IPC could protect graft against IRI injury in a deceased donor LT [24,25]. However, recently, the use of IPC has been controversial. Koneru et al. revealed that 5 minutes of IPC did not decrease graft injury caused by IRI [23]. Unexpectedly, in another study, livers from 50 deceased donors that received 10 minutes of IPC showed increased IRI in the graft after LT, and this ‘IPC paradox’ could not be explained by the researchers [26]. Desai et al. reviewed these paradoxical reports and concluded that the different efficacy of IPC was potentially caused differences in the recipients’ ages, the time of IPC, and the IRI severity in these studies [27].
To further investigate the efficacy and mechanism of IPC, we re-built the IRI mouse LT model and treated them with IPC. In the present study, the graft sample was collected at 6 h after reperfusion, when the liver dysfunction was the most serious. After treatment with IPC, the level of AST, ALT and the rate of apoptotic cells decreased significantly. However, compared with normal controls, the liver function was still poorer in the IPC treatment group. These results suggested that IPC was an effective therapy against IRI; however, its efficacy was limited by IRI severity. To the best our knowledge, few studies have focused on the evaluation of IRI severity. For example, Li et al. revealed that renalase was responsive sensitively to oxidative stress and could be detected in the peripheral blood, which indicated the possibility of renalase as a promising biomarker for evaluating IRI severity [28]. In addition, the breath pentane level correlated with prognosis of IRI in swine and might be a useful predictor of IRI severity [29]. However, the current biomarkers are unhelpful to standardize the application of IPC. Therefore, a precise biomarker or model to assess the severity of IRI is needed urgently.
In the process of IRI progression, the role of myeloid leukocytes has been explored and they are considered primarily responsible for IRI [16]. Recently, the essential role of TIM-1 co-stimulatory signaling in the differentiation and proliferation of native T cells, and the activation of macrophages, was disclosed. In a mouse IRI model, TIM-1 signaling was confirmed to be necessary for ischemia-reperfusion induced hepatocellular damage via increasing T cell, neutrophil, and macrophage sequestration [20]. Previously, we also revealed that targeting TIM-1 expressed on CD4 positive cells could suppress the activation of macrophages, resulting in decreased IRI [19]. In the present study, we found that the expression level of TIM-1 in peripheral blood and graft tissue correlated with the severity of IRI. These results suggested that the level of TIM-1 might be a promising biomarker of IRI severity.
We also confirmed that TIM-1 blockage decreased the expressions of IL-6, TNF-α, IFN-γ, IL-1β, CXCL-1, and CXCL-2 significantly, which implied that TIM-1 blockage suppressed mainly the activation of Th1 and macrophages, but whose expression was predominantly on Th2. This phenomenon indicated that TIM-1 co-stimulatory signaling has a potent adjuvant effect that is induced by the crosslinking between TIM-1 and its ligands, including TIM-1 itself [30], TIM-4 [31], IgAL [32], and PtdSer [33], respectively, expressed on different myeloid leukocytes. Besides, several reports have implied that different anti-TIM-1 antibodies have different effects on immunity. Degauque et al. reported that treatment with the anti-TIM-1 antibody 3B3 not only promoted the expansion and activation of IL-17 and IFN-γ-secreting cells, but also inhibited the proliferation of T regulatory cells (Tregs) [34]. In contrast, the lower affinity antibody, RMT1-10 was used to block TIM-1 signaling, leading to a Th1 to Th2-type cytokine switch and expansion of CD4+CD25+ Tregs, which prolonged the survival of grafts with mismatched major histocompatibility complexes (MHCs) [35]. These discoveries suggested that RMT1-10 is an effective therapy against IRI and rejection after LT.
Altering the expression of genes involved in cell death, cell cycle, stress, autophagy, and suppressing the immune response are the main mechanisms underlying IPC against IRI in LT [36]. Given the important effect of TIM-1 on the immune response, we investigated whether the therapeutic efficacy of IPC was dependent on TIM-1. After treatment with IPC, we found the TIM-1 expression of both the graft and peripheral blood decreased significantly. Furthermore, TIM-1 blockage abolished the protective effect of IPC on IRI damage, and also eliminated IPC’s suppression of the immune response.
IPC is a convenient therapeutic strategy against IRI during LT, and the benefit of IPC is proportional to the severity of IRI. High TIM-1 expression was observed in graft and peripheral blood during IRI development, and TIM-1 blockage suppressed the immune response and graft damage. Furthermore, the disruption of TIM-1 signaling abolished the effect of IPC on graft protection and sequestration of immune cells. Taken together, our results suggested that TIM-1 might be a precise biomarker of IRI severity and is responsible for the underlying mechanism of IPC.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No.81302547 and No.81570591) and Zhejiang Provincial Natural Science Foundation of China (LQ13H160006).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, Zimmerman M, Hong J, Collins TE, Gornbein J, Amersi F, Weaver M, Cao C, Chen T, Hiatt JR, Busuttil RW. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1000 liver transplants. Ann Surg. 2006;243:748–753. doi: 10.1097/01.sla.0000219669.84192.b3. discussion 753-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Kadkhodaee M, Mikaeili S, Zahmatkesh M, Golab F, Seifi B, Arab HA, Shams S, Mahdavi-Mazdeh M. Alteration of renal functional, oxidative stress and inflammatory indices following hepatic ischemia-reperfusion. Gen Physiol Biophys. 2012;31:195–202. doi: 10.4149/gpb_2012_024. [DOI] [PubMed] [Google Scholar]
- 5.O’Riordan A, Wong V, McQuillan R, McCormick PA, Hegarty JE, Watson AJ. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant. 2007;7:168–176. doi: 10.1111/j.1600-6143.2006.01602.x. [DOI] [PubMed] [Google Scholar]
- 6.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 7.Jin LM, Liu YX, Zhou L, Xie HY, Feng XW, Li H, Zheng SS. Ischemic preconditioning attenuates morphological and biochemical changes in hepatic ischemia/reperfusion in rats. Pathobiology. 2010;77:136–146. doi: 10.1159/000292647. [DOI] [PubMed] [Google Scholar]
- 8.Lloris-Carsi JM, Cejalvo D, Toledo-Pereyra LH, Calvo MA, Suzuki S. Preconditioning: effect upon lesion modulation in warm liver ischemia. Transplant Proc. 1993;25:3303–3304. [PubMed] [Google Scholar]
- 9.Matsumoto T, O’Malley K, Efron PA, Burger C, McAuliffe PF, Scumpia PO, Uchida T, Tschoeke SK, Fujita S, Moldawer LL, Hemming AW, Foley DP. Interleukin-6 and STAT3 protect the liver from hepatic ischemia and reperfusion injury during ischemic preconditioning. Surgery. 2006;140:793–802. doi: 10.1016/j.surg.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Ofluoglu E, Kerem M, Pasaoglu H, Turkozkan N, Seven I, Bedirli A, Utku Yilmaz T. Delayed energy protection of ischemic preconditioning on hepatic ischemia/reperfusion injury in rats. Eur Surg Res. 2006;38:114–121. doi: 10.1159/000093300. [DOI] [PubMed] [Google Scholar]
- 11.Kim WH, Lee JH, Ko JS, Min JJ, Gwak MS, Kim GS, Lee SK. Effect of remote ischemic postconditioning on patients undergoing living donor liver transplantation. Liver Transpl. 2014;20:1383–1392. doi: 10.1002/lt.23960. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsh DG, Neely JD, Breazeale DR, Ghosh B, Freidhoff LR, Ehrlich-Kautzky E, Schou C, Krishnaswamy G, Beaty TH. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994;264:1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 14.Encinas JA, Kuchroo VK. Mapping and identification of autoimmunity genes. Curr Opin Immunol. 2000;12:691–697. doi: 10.1016/s0952-7915(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 15.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 17.Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol. 2007;212:174–181. doi: 10.1002/jcp.21013. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Ji H, Shen X, Cai J, Gao F, Koenig KM, Batikian CM, Busuttil RW, Kupiec-Weglinski JW. Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia-reperfusion injury in mouse orthotopic liver transplantation. Am J Transplant. 2013;13:56–66. doi: 10.1111/j.1600-6143.2012.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida Y, Ke B, Freitas MC, Ji H, Zhao D, Benjamin ER, Najafian N, Yagita H, Akiba H, Busuttil RW, Kupiec-Weglinski JW. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 22.Liu A, Guo E, Yang J, Li R, Yang Y, Liu S, Hu J, Jiang X, Dirsch O, Dahmen U, Sun J, Ouyang M. Ischemic preconditioning attenuates ischemia/reperfusion injury in rat steatotic liver: role of heme oxygenase-1-mediated autophagy. Oncotarget. 2016;7:78372–78386. doi: 10.18632/oncotarget.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, de la Torre AN, Merchant A, Arora R, Samanta AK. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transpl. 2005;11:196–202. doi: 10.1002/lt.20315. [DOI] [PubMed] [Google Scholar]
- 24.Amador A, Grande L, Marti J, Deulofeu R, Miquel R, Sola A, Rodriguez-Laiz G, Ferrer J, Fondevila C, Charco R, Fuster J, Hotter G, Garcia-Valdecasas JC. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180–2189. doi: 10.1111/j.1600-6143.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- 25.Robertson FP, Magill LJ, Wright GP, Fuller B, Davidson BR. A systematic review and metaanalysis of donor ischaemic preconditioning in liver transplantation. Transpl Int. 2016;29:1147–1154. doi: 10.1111/tri.12849. [DOI] [PubMed] [Google Scholar]
- 26.Koneru B, Shareef A, Dikdan G, Desai K, Klein KM, Peng B, Wachsberg RH, de la Torre AN, Debroy M, Fisher A, Wilson DJ, Samanta AK. The ischemic preconditioning paradox in deceased donor liver transplantation-evidence from a prospective randomized single blind clinical trial. Am J Transplant. 2007;7:2788–2796. doi: 10.1111/j.1600-6143.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- 27.Desai KK, Dikdan GS, Shareef A, Koneru B. Ischemic preconditioning of the liver: a few perspectives from the bench to bedside translation. Liver Transpl. 2008;14:1569–1577. doi: 10.1002/lt.21630. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Guo J, Liu H, Niu Y, Wang L, Huang K, Wang J. Renalase as a novel biomarker for evaluating the severity of hepatic ischemia-reperfusion injury. Oxid Med Cell Longev. 2016;2016:3178562. doi: 10.1155/2016/3178562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Shi J, Sun B, Liu D, Li P, Gong Y, He Y, Liu S, Xu G, Li J, Luo A, Li E. Breath pentane as a potential biomarker for survival in hepatic ischemia and reperfusion injury--a pilot study. PLoS One. 2012;7:e44940. doi: 10.1371/journal.pone.0044940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santiago C, Ballesteros A, Tami C, Martinez-Munoz L, Kaplan GG, Casasnovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 32.Tami C, Silberstein E, Manangeeswaran M, Freeman GJ, Umetsu SE, DeKruyff RH, Umetsu DT, Kaplan GG. Immunoglobulin A (IgA) is a natural ligand of hepatitis A virus cellular receptor 1 (HAVCR1), and the association of IgA with HAVCR1 enhances virus-receptor interactions. J Virol. 2007;81:3437–3446. doi: 10.1128/JVI.01585-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, Alexopoulos S, Oukka M, Umetsu DT, DeKruyff RH, Kuchroo V, Zheng XX, Strom TB. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno T, Habicht A, Clarkson MR, Albin MJ, Yamaura K, Boenisch O, Popoola J, Wang Y, Yagita H, Akiba H, Ansari MJ, Yang J, Turka LA, Rothstein DM, Padera RF, Najafian N, Sayegh MH. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jassem W, Fuggle S, Thompson R, Arno M, Taylor J, Byrne J, Heaton N, Rela M. Effect of ischemic preconditioning on the genomic response to reperfusion injury in deceased donor liver transplantation. Liver Transpl. 2009;15:1750–1765. doi: 10.1002/lt.21936. [DOI] [PubMed] [Google Scholar]


