Abstract
Failure of cancer treatment caused by drug resistance and metastasis is mainly due to existence of cancer stem cells (CSCs). Therefore, targeting CSCs to overcome cancers is a challenging issue in clinic. In this report, in view of the important role of survivin in tumor growth and CSCs maintaining, we aimed to confirm that FL118, as a novel survivin inhibitor, may effectively inhibit lung cancer stem cells. We showed that lung cancer stem cells have the obviously higher expression of survivin than their parental cells. After treated with FL118, the survivin level in CSCs was suppressed. Consistently, lung cancer stem cells displayed significantly growth inhibition over time. Here, we compared the antitumor efficacy between FL118 and cisplatin. The data revealed that CSCs are more sensitive to FL118 than cisplatin. To further demonstrate the inhibitory effect of FL118 on CSCs, we found that FL118 down-regulated the expression of CSCs markers (ABCG2, ALDH1A1, Oct4) and drug resistant proteins (P-gp, ERCC1), suggesting that FL118 may change CSCs phenotype and improve drug-sensitivity of tumor cells. Moreover, FL118 effectively decreased the invasive ability of CSCs. These findings expand the uniqueness of FL118 as an attractive therapeutic option for cancers with drug-resistant or metastatic potential.
Keywords: FL118, cancer stem cells, drug resistance, metastasis, antitumor activity, survivin
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of total lung cancer, which is a leading cause of cancer-related deaths worldwide, and its survival rate still remains low [1]. The poor prognosis of NSCLC patients is mainly due to the traits of tumor cells including directed spreading, metastasis, recurrence and chemoresistance. The emerging evidence suggests that cancer stem cells (CSCs) play important role in the above biological behavior of tumor [2]. The CSCs are cells within a tumor that possess the capacity to self-renew and differentiate into the heterogeneous line ages of cancer cells that comprise the whole tumor [3]. Accordingly, it has been suggested that targeting CSCs could provide a new therapeutic strategy in cancer [4]. Thus, A better understanding of the signaling pathways that regulate lung CSCs maintenance and proliferation could contribute to the design of improved approaches to lung cancer treatment.
Accumulated studies have revealed that antiapoptotic protein, survivin, a unique member in the inhibitor of apoptosis (IAP) family, may act as a functional molecule in CSCs biology [5-7]. It is all known that cancer cells with stem cell-like properties possess three characteristics: increased IAP expression, activated mitotic checkpoint proteins, and elevate expression of cell cycle control proteins [8]. Similarly, survivin possesses all three characteristics [9,10]. Moreover, it’s found that only a small subset of cancer cells express survivin, and its expression overlapped with several universal CSCs markers [8]. Therefore, development of novel survivin inhibitors may overcome the challenging issues of CSCs.
Dr. Fengzhi Li and his team (Roswell Park Cancer Institute-RPCI, Buffalo, New York) has reported on a novel camptothecin derivative, designated FL118 [7,11]. More recent studies have further showed that FL118 shows strong anticancer activity in human colon and head-and-neck tumors in vitro and in vivo [12-14]. Although FL118 is not a better Top1 inhibitor than clinically used camptothecin analogues (such as irinotecan or topotecan), FL118 is able to selectively inhibit the expression of several members of the inhibitor of apoptosis family (survivin, XIAP, and cIAP2) and the Bcl-2 family (Mcl-1) [7,15]. Moreover, FL118 is effective for human tumors that acquire irinotecan and topotecan resistance due to its ability to bypass the drug resistance induced by multiple ABC transporter efflux protein [13,14]. Based on the previous studies and the properties of FL118 using survivin as a target, we hypothesize that FL118 could effectively inhibit wider ranges of malignant tumors through targeting CSCs.
In the present study, we analyzed the suppressive effect of FL118 on human NSCLC derived CSCs. On the one side, we found that FL118 can not only change the status of CSCs phenotype, but also effectively lower the growth rates of CSCs in comparison with cisplatin. On the other side, FL118 dramatically downregulated expression of resistance relative proteins, and inhibited invasive abilities of CSCs. Taken together, these observations expanded our knowledgement of the antitumor efficacy of FL118 as an attractive therapeutic option for NSCLC patients.
Materials and methods
Cell lines and cell culture
The human lung cancer cell line, A549 and H460 cells were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (HyClone SV30010) at 37°C under humidified atmosphere with 5% CO2.
Mammosphere formation assay
Stem/progenitor cells are enriched in mammospheres of cancer cells. Mammosphere formation assay is based on the unique ability of stem/progenitor cells to grow and form spheres in serum-free medium. Mammosphere culture was doneinaserum-free DMEM/F12 (Invitrogen) supplemented with B27 (Invitrogen), 20 ng/ml EGF (Invitrogen), 20 ng/ml basic fibroblast growth factor (bFGF) (Invitrogen), 5 g/ml insulin and 1% penicillin-streptomycin. Single cells prepared from mechanical and enzymatic dissociation were plated in 12-well ultra low attachment plates (Corning) at a density of 1000 cells/ml in culture. Single cell status was confirmed under microscope. Fresh mammosphere culture was added every 3-4 days. After 7 days of culture, the number of spheres (>20 μm) was counted using phase-contrast microscopy. Experiments were performed in triplicate.
Cell growth/viability assay
The CCK-8 assay was used to measure cell growth/viability. A549 and H460 cells and their stem cells (5 × 103/well) were seeded into 96-well plates, cultured for 24 h, and treated with FL118 at final concentrations of 0, 1, 10, 100, and 300 nM and 2 μg/ml cisplatin. After cultured for 24 h, 48 h and 72 h, CCK-8 was added to each well and cells were incubated at 37°C for 4 h. Then absorbance of each well was measured at 450 nm. The results were represented with the average of three parallel samples. The percentage of cell survival was calculated using the following formula: Cell survival rate (%) = (the absorbance of the drug group - absorbance of the control group)/(the absorbance of the control group-absorbance of the blank control group) × 100%.
Transwell-matrigel invasion assay
The invasiveness of cells was evaluated by a Boyden chamber assay. The polycarbonate filters (8 μm poresize, Corning) were precoated with Matrigel Matrix (Corning Incorporated, New York, USA). The A549 and H460 stem cells were prepared from mechanical and enzymatic dissociation and single cells were plated at a density of 1 × 105 cells/well in the upper chamber and treated with FL118 (10 nM) in 200 μl mammosphere culture medium, while 650 μl DMEM/F12 medium with 15% fetal bovine serum was added to the lower chamber. After incubation for 48 h, the bottom of the inserts were fixed in methanol for 20 min, stained with 0.1% crystal violet and counted under phase contrast microscope (five fields perchamber were collected). Experiments were performed in triplicate.
Western blot analysis
The cells were washed twice in ice-cold PBS and lysed in 200 μl Radio Immuno precipitation Assaylysis buffer (RIPA) with protease inhibitors Phenyl methane sulfonyl fluoride (PMSF). A BCA Protein Kit (Beyotime Biotechnology, Shanghai, China) were used to quantify the protein concentrations. Equal amounts of protein sample was loaded per well and separated by 10-12% SDS-PAGE gels, and then electrophoretically transferred onto polyvinyl diflouride (PVDF) membranes (Millipore, Billerica, MA, USA). Following blocking with 5% skim milk in TBST for 2 h at room temperature, membranes were incubated with anti-survivin (1:500) (Santa Cruz Biotechnology, USA), anti-ABCG2 (1:1000), anti-ALDH1 (1:2000), anti-ERCC1 (1:1000), anti-P-gp (1:1000) (ABCAM, Cambridge, MA, USA) antibodies at 4°C overnight, washed three times with TBST and incubated with the appropriate HRP-conjugated secondary antibodies for 2 h at room temperature. The protein bands were detected by eECL western blot kit (CWBIO, China) and visualized by autoradiography on X-Ray films (CWBIO, China). And protein levels were normalized to GAPDH (1:2000, Santa Cruz Biotechnology, USA).
Quantitative real time-PCR
Total RNA was extracted from cells with Trizol (CWBIO, China). RNA concentration and purity were determined by A260/A280 ratios. Then total RNA (1 μg) was reverse-transcribed into cDNA using FASTQuant RT Kit (TIANscript, Beijing, China) following the manufacturer’s instructions.
For quantitative assessment, real-time PCR was performed using a CFX96 Touch™ Deep Well Real-Time PCR Detection System (BIO-RAD, California, USA). The primers used in each reaction were as follows: survivin F: 5’-ATACCAGCACTTTGGGAGG-3’, R: 5’-AGAAAGGAAAGCGCAACC-3’; ERCC1F: 5’-TGGCGACGTAATTCCCGACTA-3’, R: 5’-ACAAGCAGGACCCGCAAGG-3’; MDR1F: 5’-TTGTTTGCCACCACGATA-3’, R: 5’-TGCTTCTGCCCACCACTC-3’; ABCG2F: 5’-GTTGTGATGGGCACTCTG-3’, R: 5’-CCTGTTAATCCGTTCGTT-3’; ALDH1F: 5’-GGCAGCCATTTCTTCTCA -3’, R: 5’-TGTCCAAGTCGGCATCAG-3’; β-actin F: 5’-AAGAGAGGCATCCTGACCCT-3’, R: 5’-TACATGGCTGGGGTGTTGAA-3’. Following normalization to β-actin gene, expression levels for target genes were calculated using the comparative threshold cycle (CT) method. The ΔCT values were calculated according to the formula ΔCT = CT (gene of interest) - CT (GAPDH) in correlation analysis, and the 2-ΔΔCT was calculated according to the formula ΔΔCT = ΔCT (experimental group) - ΔCT (control group) for determination of relative. Data was presented as the mean ± standard deviation (S.D.) from three independent experiments.
Annexin V/propidium Iodide (PI) double staining and analysis of cell apoptosis by flow cytometry (FCM)
The mammospheres of cancer cells were resuspended into single cell suspension and plated uniformly at a density of 2 × 105 cells/ml into 25-cm2 cell cultured flasks (5 ml/flask) with mammosphere culture medium. After cultured under the normal conditions for 24 h, the stem cells were treatment with 0 nM and 10 nM FL118. Following incubation for 24 and 48 h, cells were digested with 0.25% trypsin, dispersed into suspension by gentle pipetting, and centrifuged for 5 min at 400 g to collect the cells. Then the cells were washed twice with PBS, and resuspended in 400 μl 1 × Annexin-binding buffer up to 1 × 105 cells/ml. Subsequently, 5 μl Annexin V-FITC was added to each tube. After 15 min incubation in the dark at room temperature, 10 μl PI was mixed and incubated in the dark at 4°C for another 5 min. The resultant samples were immediately analyzed by flow cytometry. In all cases, fluorescence parameters were gated using unstained control cells and 10,000 cells were counted for each sample. Changes in the cell apoptosis were examined by FCM (BD FACSCalibur, Becton Dickinson UK Ltd., Oxford, UK). Each assay was repeated in three independent experiments.
Statistical analysis
Assay results were the average from at least 3 replicates in each of three independent experiments. All experimental data were shown as the mean ± SD. Differences between samples were analyzed using the Student’s t test. A P-value of <0.05 was considered statistically significant.
Results
FL118 significantly decreases the phenotypic expression of cancer stem cells
To investigate whether cells with mammosphere culture could exhibit cancer stem cell traits, we firstly tested mammosphere forming ability of A549 and H460 cells. As shown in Figure 1A, we found that A549 and H460 cells both possess the ability to form mammospheres. Interestingly, the number of mammospheres of H460 cells increased about 3-fold compared to A549 cells, implying that H460 might be prone to exhibit features suggestive to CSCs. Next, we examined expression of CSC phenotypic markers. As determined by real time RT-PCR analysis, ALDH1A1, Oct-4 and ABCG2 mRNA level in two kinds of mammosphere cells were increased compared with their parental cells (P<0.05, Figure 1B). Furthermore, western blotting analysis also showed the same results that the expression levels of CSC markers were obviously higher in mammosphere cells (Figure 1C).
Figure 1.
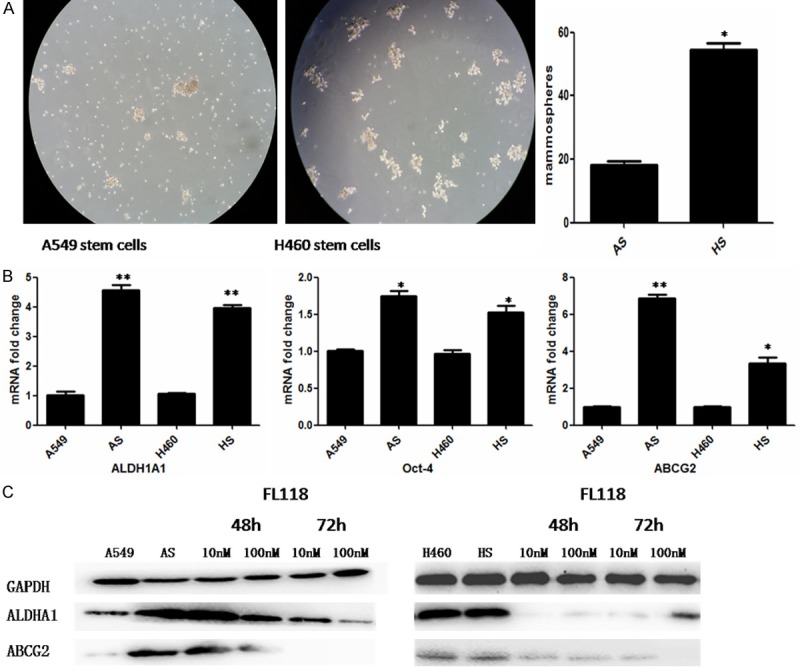
FL118 changes the phenotypic expression of CSCs derived from A549 and H460. A: Mammosphere forming ability of A549 and H460 cells. *P<0.05. B: Quantitative RT-PCR was used to quantify ALDH1A1, ABCG2 and Oct-4 mRNA expression in A549 and H460 derived stem cells. *P<0.05, **P<0.01. C: The expression of ALDH1A1 and ABCG2 of A549 and H460 derived stem cells was examined by western blotting. GAPDH servers as the control. After treated with FL118 at 10 nM and 100 nM, the level of ALDH1A1 and ABCG2 was downregulated over time. AS: A549 derived stem cells. HS: H460 derived stem cells.
To assess the inhibitory effect of FL118 on CSCs, we performed western blotting to examine the changes of CSC phenotypic markers. After treating with FL118, the expression of ABCG2 and ALDH1A1 were both significantly decreased, demonstrating that FL118 may effectively change the status of cancer stem cell-like properties (Figure 1C).
FL118 dramatically suppresses expression of resistance-associated proteins in CSCs
Emerging evidences has indicated the correlation between chemoresistance and CSCs. The mechanisms causing drug resistance in CSCs might be partly explained through the overexpression of drug transporters [2]. Accordingly, to confirm whether FL118 may overcome chemoresistant tumor cells, we detected the expression of resistance-associated proteins (ERCC1, ABCG2 and P-gp) in the A549 and H460 derived stem cells. As expected, FL118 resulted in a significant decrease of ERCC1, ABCG2, P-gpprotein (Figures 1C, 2A). However, compared with their corresponding parental cells, the expression of ERCC1 and P-gp mRNA in CSCs was not obviously altered following FL118 treatment (data not shown), except that ABCG2 mRNA was significantly decreased (Figure 2B). These data suggested that FL118 tend to eliminate the chemoresistant tumor cells through downregulating resistance-associated proteins, however, FL118 downstream targets might play distinct mechanistic role in FL118-mediated chemoresistance inhibition.
Figure 2.
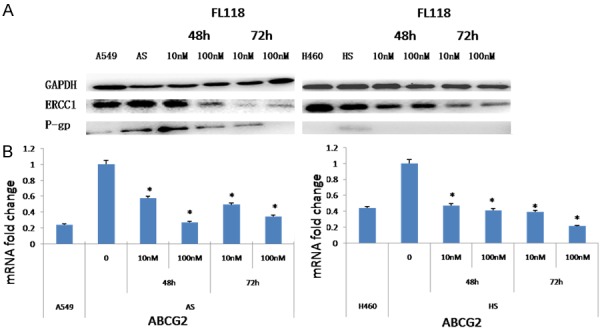
FL118 down-regulates the expression of drug-resistant proteins in the CSCs derived from A549 and H460. A: The expression of ERCC1and P-gp of A549 and H460 derived stem cells was examined by western blotting. GAPDH servers as the control. After treated with FL118 at 10 nM and 100 nM, the level of ERCC1and P-gp was downregulated over time. B: Quantitative RT-PCR was used to quantify ABCG2 mRNA expression in A549 and H460 derived stem cells after treating with FL118 for 48 h. *P<0.05. AS: A549 derived stem cells. HS: H460 derived stem cells.
FL118 is a more potent anticancer agent due to inhibition of CSCs
Recent studies have determine that cancer cells with high survivin expression are significantly more sensitive to FL118 treatment than cells which have low or undetectable survivin expression [7]. In our study, western blot and real time RT-PCR displayed that A549 and H460 derived stem cells have aberrant expression of survivin. Especially for H460 derived stem cells, there is a higher expression of survivin relative to A549 derived stem cells (Figure 3A). Consistent with the previous observations, after treating with FL118 for 48 h, survivin mRNA and protein were both down-regulated in the CSCs (Figure 3A and 3B). Meanwhile, the cells viability was significantly inhibited in a time and concentration dependent manner (Figure 3C). Satisfyingly, FL118, even at a 1 nM level, showed significantly inhibition effect on H460 derived stem cells and its parent cells. While for the A549 stem cells, FL118 significantly inhibits cell viability at the concentrations of 100-300 nM, suggesting that H460 stem cells with higher survivin expression are more sensitive to FL118 than A549 derived stem cells. On the other hand, we found that FL118 showed an superior inhibitory effect on NSCLC stem cells at concentrations of 100-300 nM compared with cisplatin. These results indicate that CSCs are more sensitive to FL118 than cisplatin, and the higher the survivin in CSCs expresses, the better FL118-mediated cell growth inhibition shows.
Figure 3.
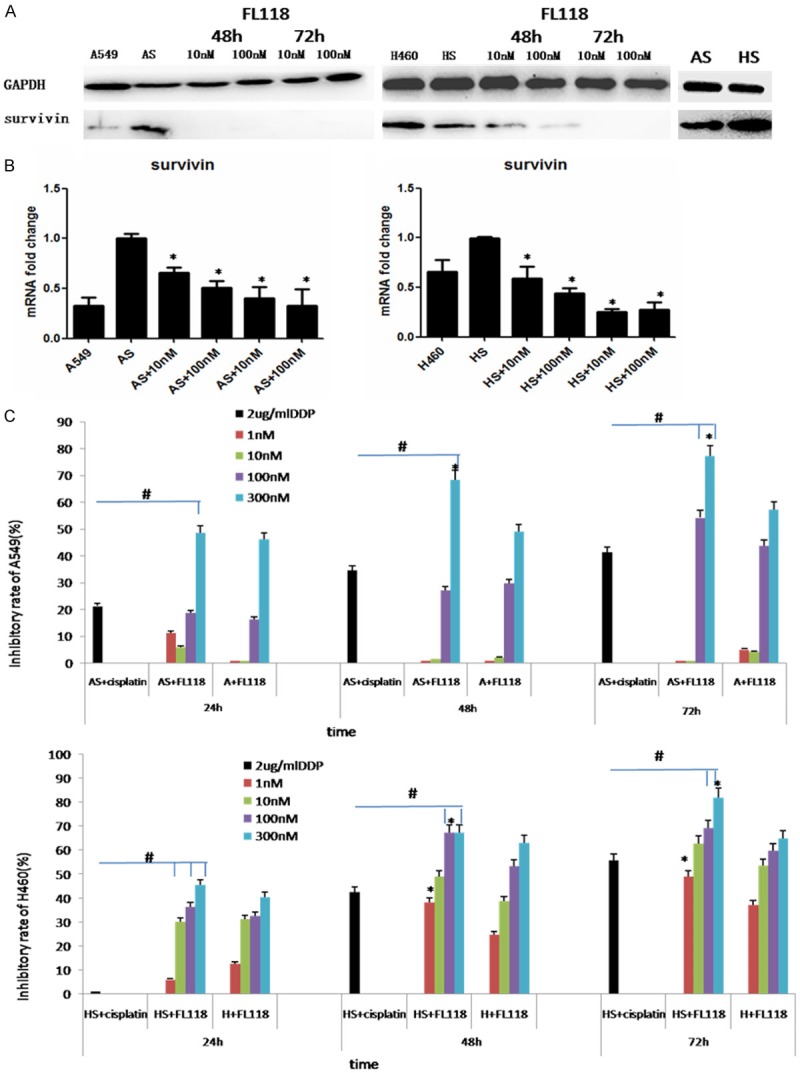
FL118 inhibits the growth of CSCs derived from A549 and H460. A: The expression of survivin of A549 and H460 derived stem cells was examined by western blotting. GAPDH servers as the control. After treated with FL118 at 10 nM and 100 nM, the level of survivin was downregulated over time. B: Quantitative RT-PCR was used to quantify survivin mRNA expression in A549 and H460 derived stem cells after treated with FL118 for 48 h. *P<0.05. C: The cell viability was detected by CCK-8 assay. In comparison with cisplatin, FL118 shows greater inhibitory effect on A549 and H460 derived stem cells, #P<0.05. The A549 and H460 derived stem cells were more sensitive to FL118 than their parental cells, *P<0.05. AS: A549 derived stem cells. HS: H460 derived stem cells.
FL118 induces apoptosis of cancer stem cells
Since survivin plays an important role in inducing apoptosis and FL118 selectively inhibits the expression of survivin, we further evaluated a definitive role for FL118 sensitivity in CSCs using Annexin V/PI staining and flow cytometry experiments. When the CSCs were treated with FL118 for 24 h and 48 h at 10 nM, a representative result was shown in Figure 4A, and the statistical analysis of these data was shown in Figure 4B. Our data demonstrated that the apoptotic rates of A549 and H460 derived stem cells decreased significantly following FL118 treatment. As the duration of the FL118 treatment increased, the apoptotic rates of cancer cells reduced significantly (P<0.05). This inhibitory effect induced by enhancement of apoptosis is in accordance with FL118-mediated cell growth inhibition detected by CCK-8 assay.
Figure 4.
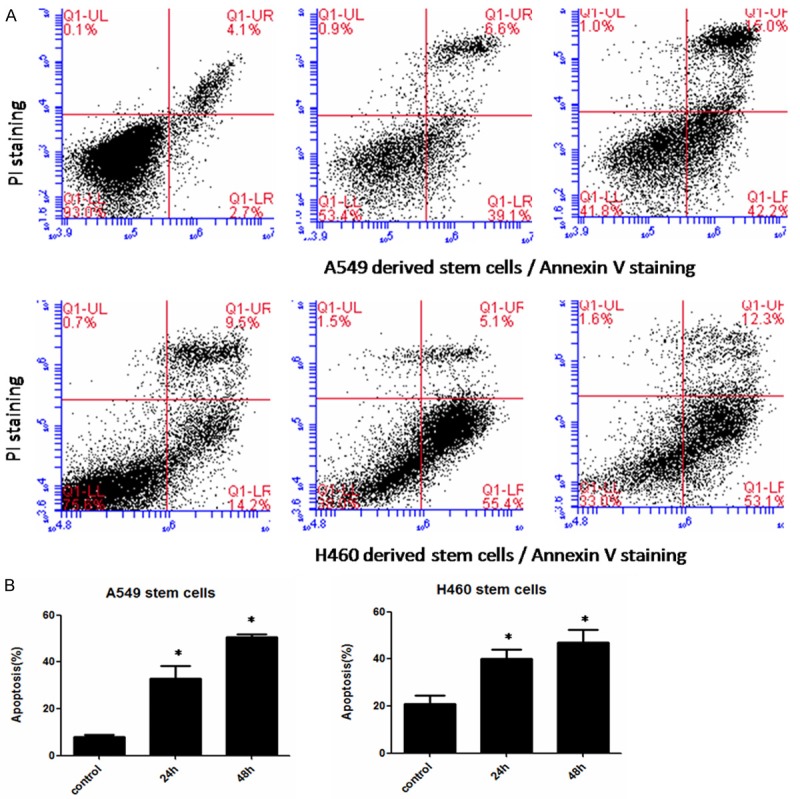
FL118 effectively increases the apoptotic rate of CSCs derived from A549 and H460. A: A representative flow cytometry result gated with PI (Y axis) and Annexin V (X axis). B: Quantitative data from A from three independent measures in parallel. After treating with FL118 for 24 h and 48 h, FL118 enhanced the CSCs apoptosis, *P<0.05.
FL118 reduces the invasive capability of cancer stem cells
In view of the potential role of CSC in metastasis of tumor, we sought to determine whether FL118 may regulate migratory and invasive capability. As shown in Figure 5A, transwell invasion assay revealed A549 and H460 stem cells had an obviously enhanced migratory and invasive capacity. However, after treating with FL118 at 10 nM for 48 h, there was a significant decrease in the number of invasive cells (Figure 5B, P<0.05). The above discoveries demonstrated FL118 effectively suppressed the migratory and invasive capabilities of NSCLC stem cells.
Figure 5.
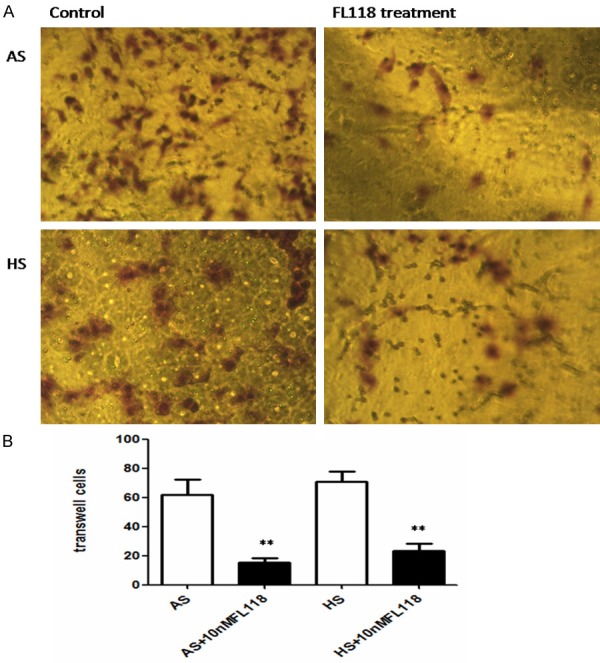
FL118 obviously decreases the invasive potential of CSCs derived from A549 and H460. A: A549 and H460 derived stem cells that had spread through the matrixgel and adhered onto the lower chamber of the filter were fixed and stained. B: The number of invasive cells. Data represent the average of three independent experiments. Following FL118 treatment, the number of invasive cells decrease significantly, **P<0.01. AS: A549 derived stem cells. HS: H460 derived stem cells.
Discussion
CSCs are a rare set of undifferentiated cells that play critical roles in the cancer initiation, development and recurrence as well as cancer therapeutic failures [15-18]. CSCs have now been identified in many human cancers, including breast, brain, lung cancers and so on. Moreover, they are confirmed as cells that form cell spheres in vitro [19-21]. It has been reported that the tumor aggressiveness and poor prognosis is mainly caused by intrinsic resistance to radiation and chemotherapy of CSCs [22]. Furthermore, the discovery of a CSC population that undergoes an epithelial-tomesenchymaltransition (EMT) demonstrates the role of CSCs in the driver of metastasis [23,24]. It’s recognized in clinic that the current treatment strategies may affect the bulk of the tumor cells, leaving therapy-resistant CSCs which contribute to disease recurrence and metastasis. Accordingly, the development of a CSC-targeted therapy may result in the complete elimination of tumors.
In this study, we found that FL118 could effectively suppress the proliferation, chemoresistance and invasiveness of NSCLC derived CSCs, suggesting that FL118 has superior antitumor efficacy. The chemical name of FL118 is 10,11-methylenedioxy-20(S)-camptothecin, a novel camptothecin derivative. However, unlike the clinically used camptothecin analogues regarded as the better Top1 inhibitors, FL118 has been showed outstanding antitumor effect because it is able to selectively inhibit the expression of survivin, XIAP, cIAP2 and Mcl-1 [7,15]. Especially for tumor cells with the higher expression of survivin, FL118 shows stronger selective inhibition in a various of tumors. It has been reported that survivin is a critical inherent and induced drug/radiation resistance factor for various cancer types during treatment [8,25,26] and a role for survivin in CSCs is independently revealed by computer analysis of the death-from-cancer signature genes. Thus, in view of the correlation between the expression of suvivin and CSCs characteristic, FL118, as a novel survivin inhibitors, may win the battle against drug resistant and metastatic cancers through targeting CSCs.
Consistent with FL118’s initial discovery, our data demonstrated a role for FL118 in suppress the growth of NSCLC derived CSCs along with higher expression of survivin (shown by Figure 3). This conclusion was further confirmed by using Annexin V/PI staining and flow cytometry analysis, showing that FL118 significantly increased the number of apoptotic CSCs through inhibiting survivin (shown by Figure 4). Additionally, we found that CSCs displayed more sensitivity to FL118 than cisplatin, one kind of traditional chemotherapeutics. Interestingly, similar with this results, our another study has confirmed that FL118 has greater effect on cisplatin-resistant NSCLC cells than its parental cell [27]. All these results implied that FL118 shows improved efficacy in treating NSCLC in comparison with cisplatin.
To further confirm FL118’s antitumor effect on CSCs, we detected the expression of pluripotent stem cell markers and resistance-associated proteins, as well as invasive capability of CSCs. As expected, after 48 h with FL118, expression of ABCG2 gene and protein in A549 and H460 derived stem cells, which is an important member of the ATP-bingding cassett (ABC) transporter family and considered as a major cancer stem cell marker, functional molecule and drug resistant factor [28], was significantly down-regulated with time (shown by Figure 1). Moreover, aldehyde dehydrogenase 1 family, member A1, also known as ALDH1A1, which has been identified as lung cancer stem cells, was also suppressed. These results indicate that FL118 can convert CSCs to nonstem cancer cells, meaning the loss of their stem cell characteristics, which further underscore the potential benefit of FL118 to increase therapeutic effectiveness in NSCLC.
It’s all known that the mechanisms causing drug resistance in CSCs might be partly explained through the overexpression of drug transporters. For example, the drug efflux pump proteins (including ABCG2, P-gp/MDR1/ABCB1, ABCD1, etc.) always expressed highly in CSCs. It has reported that breast CSCs show enhanced efficacy of chemotherapy by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense [29]. ABCB1 transporter expressing subpopulation of differentiated cells protected the CSCs against irinotecan. The present findings also displayed that ABCG2, P-gp and ERCC1 (cisplatin-resistent marker) in A549 and H460 derived CSCs were all expressed higher than their parental cells. After treating with FL118, the dramatic result occurs. The previous study confirmed that FL118 is not a substrate of the efflux pump ABCG2 and P-gp [13,14]. While our study showed that ABCG2, P-gp and ERCC1 were all clearly suppressed by FL118 (shown by Figures 1 and 2), further explaining the uniqueness of FL118 in mechanism of action to overcome drug resistance.
Equally important, we also found decreased invasion capacity of A549 and H460 derived CSCs after treated with FL118 for 48 h (shown by Figure 5), suggesting FL118 may has more potential protein targets to exert inhibitory effect on movement ability. It has been cognized that migration and invasion were association with features consistent with EMT, furthermore, EMT and stemness have a close causal relationship [23,30]. Our other studies showed FL118 decrease the expression of β-catenin and TGF-β1 (major regulatory factors of EMT) in liver cancer cells and lung cells (data not shown), and also displayed FL118 may reverse the immunophenotype of EMT in A549/DDP cells [31]. Taken together, these findings indicate FL118 may effectively overcome metastatic tumor.
In conclusion, the present study demonstrated that FL118 not only effectively inhibits the growth of NSCLC derived CSCs through down regulation of suvivin, but also significantly shows decreased invasion capacity and recover of drug sensitivity through suppressing resistance-associated proteins and immunophenotype of CSCs. Hence, this study warrants FL118 further development toward clinical application when treating NSCLC patients.
Acknowledgements
This work was sponsored by Project funded by China Postdoctoral Science Foundation. We acknowledge Dr. Fengzhi Li and his team (Roswell Park Cancer Institute-RPCI, Buffalo, New York) who found and provided FL118 for us.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH. The role of cancer stem cells in recurrent and drug-resistant lung cancer. Adv Exp Med Biol. 2016;890:57–74. doi: 10.1007/978-3-319-24932-2_4. [DOI] [PubMed] [Google Scholar]
- 3.Kong D, Li Y, Wang Z, Sarkar FH. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel) 2011;3:716–729. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuda Y, Naito Z, Kawahara K, Nakazawa N, Korc M, Ishiwata T. Nestin is a novel target for suppressing pancreatic cancer cell migration, invasion and metastasis. Cancer Biol Ther. 2011;11:512–523. doi: 10.4161/cbt.11.5.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandi S, Ulasov IV, Tyler MA, Sugihara AQ, Molinero L, Han Y, Zhu ZB, Lesniak MS. Lowdose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda S, Abe M, Onishi C, Taketani T, Purevsuren J, Yamaguchi S, Conway EM, Pelus LM. Survivin selectively modulates genes deregulated in human leukemia stem cells. J Oncol. 2011;2011:946936. doi: 10.1155/2011/946936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling X, Cao S, Cheng Q, Keefe JT, Rustum YM, Li F. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS One. 2012;7:e45571. doi: 10.1371/journal.pone.0045571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glinsky GV. Genomic models of metastatic cancer: functional analysis of death-from-cancer signature genes reveals aneuploid, anoikis-resistant, metastasis-enabling phenotype with altered cell cycle control and activated Polycomb Group (PcG) protein chromatin silencing pathway. Cell Cycle. 2006;5:1208–1216. doi: 10.4161/cc.5.11.2796. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Ling X. Survivin study: an update of “what is the next wave”? J Cell Physiol. 2006;208:476–486. doi: 10.1002/jcp.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol. 2005;247:35–88. doi: 10.1016/S0074-7696(05)47002-3. [DOI] [PubMed] [Google Scholar]
- 11.Li F. Anticancer drug FL118 is more than a survivin inhibitor: where is the Achilles’ heel of cancer? Am J Cancer Res. 2014;4:304–311. [PMC free article] [PubMed] [Google Scholar]
- 12.Ling X, Li F. An intravenous (i.v.) route-compatible formulation of FL118, a survivin, Mcl-1, XIAP, and cIAP2 selective inhibitor, improves FL118 antitumor efficacy and therapeutic index (TI) Am J Transl Res. 2013;5:139–154. [PMC free article] [PubMed] [Google Scholar]
- 13.Ling X, Liu X, Zhong K, Smith N, Prey J, Li F. FL118, a novel camptothecin analogue, overcomes irinotecan and topotecan resistance in human tumor xenograft models. Am J Transl Res. 2015;7:1765–1781. [PMC free article] [PubMed] [Google Scholar]
- 14.Westover D, Ling X, Lam H, Welch J, Jin C, Gongora C, Del Rio M, Wani M, Li F. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol Cancer. 2015;14:92. doi: 10.1186/s12943-015-0362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Ling X, Cao S, Liu X, Wan S, Jiang T, Li F. Antitumor activity of FL118, a survivin, Mcl-1, XIAP, and cIAP2 selective inhibitor, is highly dependent on its primary structure and steric configuration. Mol Pharm. 2014;11:457–467. doi: 10.1021/mp4004282. [DOI] [PubMed] [Google Scholar]
- 16.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res. 2010;156:188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Dong Z, Li Y, Yang Y, Yuan Z, Qu X, Kong B. The upregulation of signal transducer and activator of transcription 5-dependent microRNA-182 and microRNA-96 promotes ovarian cancer cell proliferation by targeting forkhead box O3 upon leptin stimulation. Int J Biochem Cell Biol. 2013;45:536–545. doi: 10.1016/j.biocel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Korkaya H, Wicha MS. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs. 2007;21:299–310. doi: 10.2165/00063030-200721050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda Y, Yoshimura H, Ueda J, Naito Z, Korc M, Ishiwata T. Nestin delineates pancreatic cancer stem cells in metastatic foci of NOD/Shi-scid IL2Rgamma(null) (NOG) mice. Am J Pathol. 2014;184:674–685. doi: 10.1016/j.ajpath.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perona R, Lopez-Ayllon BD, de Castro Carpeno J, Belda-Iniesta C. A role for cancer stem cells in drug resistance and metastasis in nonsmall-cell lung cancer. Clin Transl Oncol. 2011;13:289–293. doi: 10.1007/s12094-011-0656-3. [DOI] [PubMed] [Google Scholar]
- 22.Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat Rev. 2012;38:589–598. doi: 10.1016/j.ctrv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Charpentier M, Martin S. Interplay of stem cell characteristics, EMT, and microtentacles in circulating breast tumor cells. Cancers (Basel) 2013;5:1545–1565. doi: 10.3390/cancers5041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Zhang G, Zhang H, Zhang F, Zhou B, Ning F, Wang HS, Cai SH, Du J. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/beta-catenin/Snail signaling pathway. Eur J Pharmacol. 2014;723:156–166. doi: 10.1016/j.ejphar.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Park E, Gang EJ, Hsieh YT, Schaefer P, Chae S, Klemm L, Huantes S, Loh M, Conway EM, Kang ES, Hoe Koo H, Hofmann WK, Heisterkamp N, Pelus L, Keerthivasan G, Crispino J, Kahn M, Muschen M, Kim YM. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood. 2011;118:2191–2199. doi: 10.1182/blood-2011-04-351239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trabulo S, Cardoso AM, Santos-Ferreira T, Cardoso AL, Simoes S, Pedroso de Lima MC. Survivin silencing as a promising strategy to enhance the sensitivity of cancer cells to chemotherapeutic agents. Mol Pharm. 2011;8:1120–1131. doi: 10.1021/mp100426e. [DOI] [PubMed] [Google Scholar]
- 27.Zhang DD, Wang J, Yang ZH, Liu J, Liu ZT, Ji LX, Liu RR, Lin Q, Jiang GH. A novel camptothecin analogue FL118 reduces cisplatin resistance of non-small cell lung cancer cells. Int J Clin Exp Med. 2016;9:13501–13513. [Google Scholar]
- 28.Sun M, Yang C, Zheng J, Wang M, Chen M, Le DQ, Kjems J, Bunger CE. Enhanced efficacy of chemotherapy for breast cancer stem cells by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Acta Biomater. 2015;28:171–182. doi: 10.1016/j.actbio.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Emmink BL, Van Houdt WJ, Vries RG, Hoogwater FJ, Govaert KM, Verheem A, Nijkamp MW, Steller EJ, Jimenez CR, Clevers H, Borel Rinkes IH, Kranenburg O. Differentiated human colorectal cancer cells protect tumor-initiating cells from irinotecan. Gastroenterology. 2011;141:269–278. doi: 10.1053/j.gastro.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Garofalo M, Croce CM. Role of microRNAs in maintaining cancer stem cells. Adv Drug Deliv Rev. 2015;81:53–61. doi: 10.1016/j.addr.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stacy AE, Jansson PJ, Richardson DR. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol Pharmacol. 2013;84:655–669. doi: 10.1124/mol.113.088609. [DOI] [PubMed] [Google Scholar]


