Abstract
Neural stem cells are able to self-renew and generate glial and neuronal lineages. Neural stem cell may serve as therapeutic method for neurological disorders including spinal cord injuries, Parkinson’s disease, Huntington’s disease and Alzheimer’s disease. Long noncoding RNAs (lncRNAs) are longer than 200 nucleotides with limited protein-coding capacity. Recent studies have demonstreated that lncRNAs play an important role in several cellular processes including cell differentiation, cell development, proliferation, apoptosis, invasion and migration. However, the role of lncRNA human urothelial carcinoma associated 1 (UCA1) in the development of neural stem cells remains unknown. In this study, we showed that the expression of UCA1 was upregulated in the neural stem cell in a time-dependent manner. Knockdown of UCA1 suppressed the neural stem cell proliferation. Inhibition of UCA1 decreased the expression of nestin and the formation of neurosphere. Moreover, knockdown of UCA1 suppressed the neural stem cell differentiation to astrocyte and promoted the neural stem cell differentiation to neuron. Furthermore, we demonstrated that knockdown of UCA1 increased the expression of miR-1 in the neural stem cell and suppressed the expresion of Hes1, which is one target gene of miR-1. In addition, ectopic expression of Hes1 could impair siUCA1-induced neural stem cells proliferation. Overexpression of Hes1 suppressed siUCA1-induced β-tubulin expression and promoted siUCA1-inhibited GFAP expression in the neural stem cell. These results suggested that UCA1 regulated the neural stem cell proliferation and differentiation through regulating Hes1 expression.
Keywords: Neural stem cells, LncRNAs, UCA1, Hes1, miR-1
Introduction
Neural stem cells (NSCs) are able to self-renew and generate three types of neural cell: astrocytes, neurons and oligodendrocytes in the nervous system [1-4]. Neural stem cells exist in the developing and adult central nervous system [5-8]. Increasing data have suggested that neural stem cells may serve as potential therapeutic methods for various neurological disorders including Parkinson’s disease, Huntington’s disease, Alzheimer’s disease and spinal cord injuries [9-11]. However, there is a long way before clinical application of neural stem cells.
Long noncoding RNAs (lncRNAs) are longer than 200 nucleotides with limited protein-coding capacity [12-15]. Increasing studies have showed that lncRNAs play an important role in several cellular processes including stem cell differentiation, cell development, proliferation, apoptosis, invasion and migration [16-20]. Deregulated expression of lncRNAs is found in a lot of diseases such as gastric cancer, breast cancer, hepatocellular carcinoma, bladder cancer and lung cancer [19,21-24]. Recent data also demonstrated that lncRNAs acted a critical role in stem cell self-renewal and fate determination through regulating the expression of stem cell regulators [25-27].
In this study, we showed that knockdown of human urothelial carcinoma associated 1 (UCA1) suppressed neural stem cell proliferation and neurosphere formation. Moreover, knockdown of UCA1 promote β-tubulin-III expression and suppressed GFAP expression in the neural stem cell. Furthermore, we demonstrated that knockdown of UCA1 increased miR-1 expression in the neural stem cell and suppressed expression.
Materials and methods
Cell culture and transfection
Primary NSCs are isolated by a modified method of a published protocol. NSCs were isolated from embryos of rats and kept in the growth medium with bFGF, N2 and EGF (Gibco) supplement. Primary neurosphere was digested by 0.25% trypsin. UCA1 and control was obtained from GenePharma (Shanghai, China) and was transfected to cell using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s information. Our study was approved by the ethical board of the institute of the first hospital of Harbin Medical University and complied with Declaration of Helsinki.
Immunocytochemistry
NSCs and differentiation cells were fixed using 4% paraformaldehyde and blocked with TritonTM X-100, FBS, and donkey serum for 1 hour. Then, cells were incubated with incubated antibody (Nestin, GFAP and β-tubulin-III, Sigma) at 4°C for overnight. After three washes, cells were stained with the fluorescencelabeled secondary antibodies. Nuclei were stained with DAPI (Sigma).
Cell growth
Cells were cultured in the 96-well plate. Cell proliferation was measured by using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) following to the manufacturer’s instruction. The absorbance was assessed at 490 nm using the enzyme-labeled analyzer.
qRT-PCR
Total RNA was isolated from cell using Trizol (Invitrogen, Carlsbad, USA) according to the manufacturer’s instruction. The expression of miR-1 and UCA1 was determined by Real-time quantitative PCR (qRT-PCR) on the iQ5 real-time PCR assay system (Bio-Rad, Hercules, USA). The primers were shown in the Table 1. PCR cycle was used as follows: 94°C for 4 mins, followed by 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. U6 and GAPDH were used as the internal control for miR-1 and mRNA expression respectively.
Table 1.
Primer sequence
| Name | Sequence (5’-3’) |
|---|---|
| Real-time PCR primer sequence | |
| β-tubulin III | AGCAAGGTGCGTGAGGAGTA |
| AAGCCGGGCATGAAGAAGT | |
| Hes1 | TGAAGGATTCCAAAAATAAAATTCTCTGGG |
| CGCCTCTTCTCCATGATAGGCTTTGATGAC | |
| GAPDH | AATGGGCAGCCGTTAGGAAA |
| TGAAGGGGTCATTGATGGCA | |
| GFAP | CAACGTTAAGCTAGCCCTGGACAT |
| CTCACCATCCCGCATCTCCACAGT | |
| Nestin | GATCTAAACAGGAAGGAAATCCAGG |
| TCTAGTGTCTCATGGCTCTGGTTTT |
Western blot
Proteins were extracted from cell in the RIPA buffer (Byotime, China) and protein concentration was determined using the BCA Kit (Byotime, China). Total protein was separated using 12% SDS PAGE and transferred to the PVDF membrane (Amersham, UK). After blocking with nonfat milk in PBS, membrane was incubated with primary antibodies (Nestin, GFAP and β-tubulin-III, Sigma) for overnight. After wash three times, the membrane was immunoblotted with HRP-linked secondary antibodies. The signal was measured by ECL.
Statistical analysis
The data was expressed as the standard deviation (SD). The difference between two groups was deteceted using the Student’s t test and P<0.05 was shown as statistically significant.
Results
Neural stem cell can proliferate and differentiate into astrocyte and neuron
Isolated neural stem cells can proliferate and differentiate into neurospheres (Figure 1A). The neurosphere expressed nestin, which was the NSC-specific marker (Figure 1B). The neurosphere could differentiate into neurons and astrocytes after withdraw of bFGF (Figure 1C).
Figure 1.
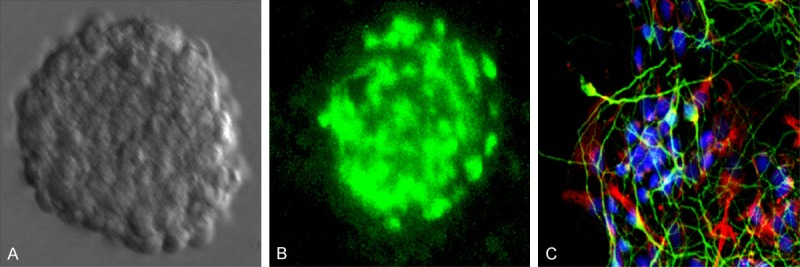
Neural stem cell can proliferate and differentiate into astrocyte and neuron. A. Representative photomicrograph of neurospheres in culture. B. Immunocytochemical staining of purified neural stem cell with Nestin. C. Immunocytochemical staining of purified neurons with β-tubulin-III (Green); astrocyte with GFAP (Red); Nucleus with DAPI (Blue).
Inhibition of UCA1 suppressed the neural stem cell proliferation
We measured the UCA1 expression in the neural stem cell. We found that the expression of UCA1 was increased in the neural stem cell in a time-dependent manner, with the maximal response on the fifth days (Figure 2A). Our data comfirmed that knockdown of UCA1 inhibited the UCA1 expression in the neural stem cell (Figure 2B). Inhibition of UCA1 suppressed the neural stem cell proliferation (Figure 2C). Moreover, knockdown of UCA1 inhibited the expression of ki-67 (Figure 2D) and cyclin D1 (Figure 2E) in the neural stem cell. Furthermore, inhibition of UCA1 decreased the nestin expression (Figure 2F) and neurosphere formation (Figure 2G).
Figure 2.
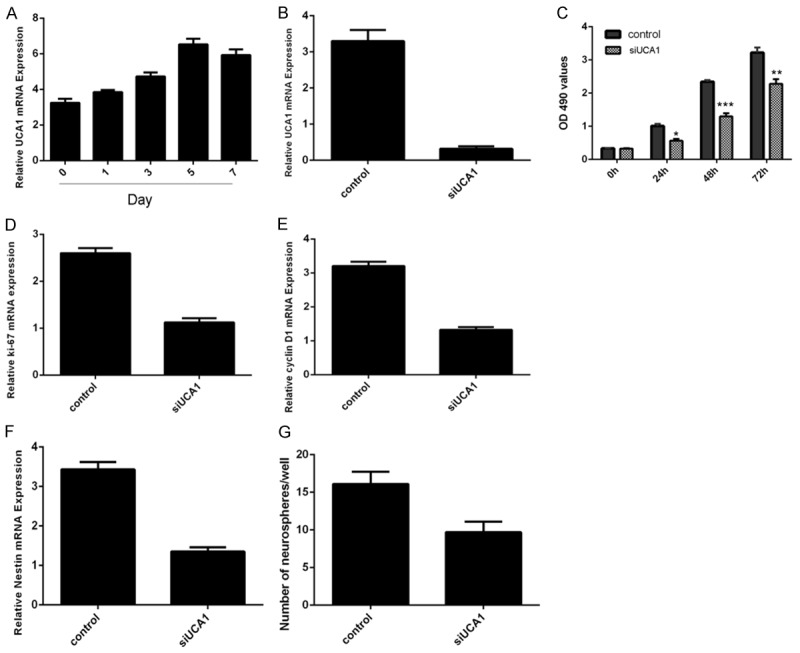
Inhibition of UCA1 suppressed the neural stem cell proliferation. A. The expression of UCA1 was detected by qRT-PCR in the neural stem cell. B. The expression of UCA1 was decreased in the neural stem cell after treated with siUCA1. C. Knockdown of UCA1 inhibited neural stem cell proliferation. D. Knockdown of UCA1 inhibited the ki-67 expression. E. Inhibition of UCA1 suppressed the cyclin D1 expression. F. Knockdown of UCA1 decreased the nestin expression. G. Inhibition expression of UCA1 decreased neurosphere formated. *P<0.05, **P<0.01 and ***P<0.001.
Inhibition of UCA1 promoted the neural stem cell differentiation into neuron
Knockdown of UCA1 could promote β-tubulin-III expression in the neural stem cell (Figure 3A and 3B). Knockdown of UCA1 suppressed the GFAP expression (Figure 3C and 3D). This effect was also confirmed by the immunofluorescence analysis (Figure 3E and 3F).
Figure 3.
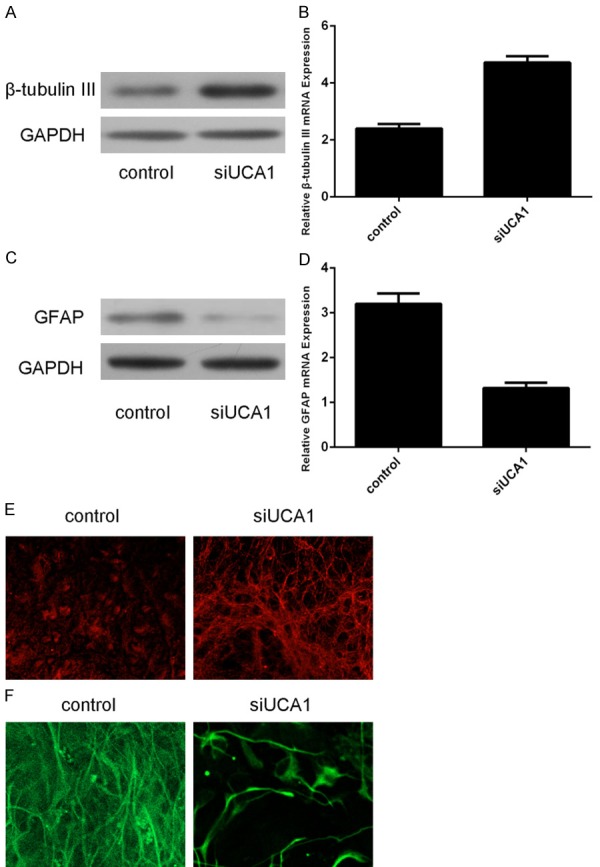
Inhibition of UCA1 promoted the neural stem cell differentiation into neuron. A. Knockdown of UCA1 could promote β-tubulin-III protein expression in the neural stem cell. B. Knockdown of UCA1 could promote β-tubulin-III mRNA expression in the neural stem cell. C. Inhibition expression of UCA1 inhibited the GFAP protein expression. D. The mRNA expression of UCA1 was measured by qR-PCR. E. Immunocytochemical staining of purified neurons with β-tubulin-III (Red). F. Immunocytochemical staining of purified astrocyte with GFAP (Green).
Knockdown of UCA1 promoted miR-1 expression and inhibited Hes1 expression
Knockdown of UCA1 increased miR-1 expression in the neural stem cell (Figure 4A). We comfirmed that the expression of miR-1 was upregulated in the neural stem cell which was treated with miR-1 mimics (Figure 4B). Overexpression of miR-1 suppressed Hes1 expression in the neural stem cell (Figure 4C and 4E). Knockdown of UCA1 also suppressed Hes1 expression (Figure 4D and 4F).
Figure 4.
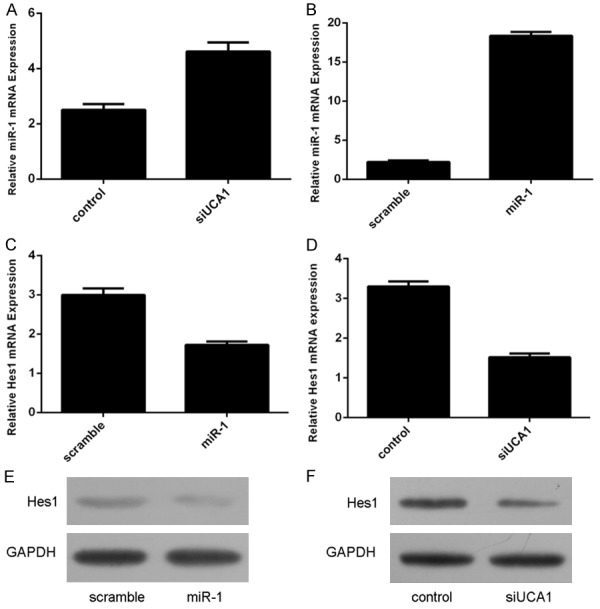
Knockdown of UCA1 promoted miR-1 expression and inhibited Hes1 expression. A. Knockdown of UCA1 increased miR-1 expression in the neural stem cell. B. The expression of miR-1 was upregulated in the neural stem cell which treated with miR-1 mimics. C. Overexpression of miR-1 suppressed Hes1 expression in the neural stem cell. D. Knockdown of UCA1 also suppressed Hes1 expression. E. The protein expression of Hes1 was measured by Western blot. F. The protein expression of Hes1 was measured by Western blot.
Knockdown of UCA1 suppressed the neural stem cell proliferation and inhibited the neural stem cell differentiation into neuron through targeting Hes1
The expression of UCA1 was upregulated in the neural stem cell after treated with pcDNA-Hes1 (Figure 5A and 5B). Ectopic expression of Hes1 could impair siUCA1-induced neural stem cells proliferation (Figure 5C). Overexpression of Hes1 suppressed siUCA1-induced β-tubulin III mRNA (Figure 5D) and protein (Figure 5E) expression in neural stem cell. Ectopic expression of Hes1 promoted the siUCA1-inhibited GFAP mRNA (Figure 5F) and protein (Figure 5G) expression in neural stem cell.
Figure 5.
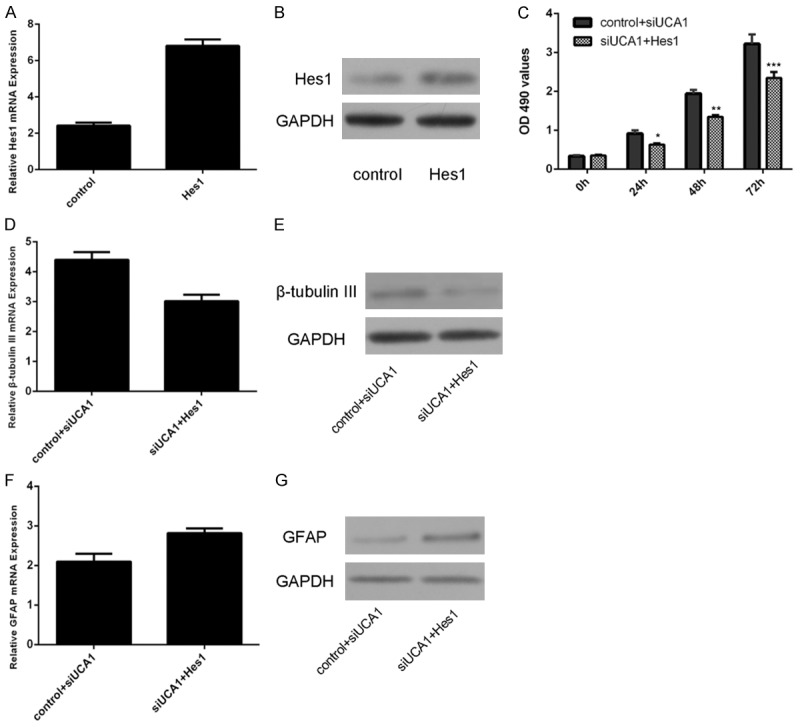
Knockdown of UCA1 suppressed the neural stem cell proliferation and inhibited the neural stem cell differentiation into neuron through targeting Hes1. A. The expression of UCA1 was upregulated in the neural stem cell after treated with pcDNA-Hes1. B. The protein expression of Hes1 was measured by Western blot. C. Ectopic expression of Hes1 could impair siUCA1-induced neural stem cells proliferation. D. Overexpression of Hes1 suppressed siUCA1-induced β-tubulin III mRNA expression. E. Overexpression of Hes1 suppressed siUCA1-induced β-tubulin III protein expression. F. Overexpression of Hes1 suppressed siUCA1-induced GFAP mRNA expression. G. Overexpression of Hes1 suppressed siUCA1-induced GFAP protein expression. *P<0.05, **P<0.01 and ***P<0.001.
Discussion
In this study, we showed that the expression of UCA1 was increased in the neural stem cell in a time-dependent manner. Knockdown of UCA1 suppressed the neural stem cell proliferation. Inhibition of UCA1 decreased the nestin expression and neurosphere formation. Moreover, knockdown of UCA1 promoted the β-tubulin-III protein and mRNA expression and suppressed the GFAP protein and mRNA expression in the neural stem cell. This effect also was also confirmed by immunofluorescence analysis. Furthermore, we demonstrated that knockdown of UCA1 increased miR-1 expression in the neural stem cell and suppressed the expression of Hes1, which was one target gene of miR-1. In addition, ectopic expression of Hes1 could impair siUCA1-induced neural stem cell proliferation. Overexpression of Hes1 suppressed siUCA1-induced β-tubulin expression and promoted the siUCA1-inhibited GFAP expression in the neural stem cell. These results suggested that UCA1 played an important role in neural stem cell proliferation and differentiation.
LncRNAs are longer than 200 nucleotides in length that are a new kind of ncRNA (non-coding RNA) with limited protein-coding capacity [28-30]. Recent studies have showed that lncRNAs act crucial roles in different biological processes such as cell development, cell differentiation, tumorigenesis, cell proliferation and migration [31-34]. UCA1 is an lncRNA first identified from bladder carcinoma and was dysregulated in various tumors such as breast cancer, melanoma, esophageal squamous cell carcinoma, colorectal cancer and gastri cancer [35-41]. UCA1 functioned as an oncogene and could promote the tumor cell proliferation, migration, invasion and inhibited cell apoptosis [42-45]. However, the role of UCA1 in the neural stem cell was unknown. In this study, we demonstrated the expression of UCA1 was increased in the neural stem cell in a time-dependent manner. Knockdown of UCA1 suppressed the neural stem cell proliferation. Inhibition of UCA1 decreased the nestin expression and neurosphere formation. Moreover, knockdown of UCA1 promoted the β-tubulin-III expression and suppressed the GFAP expression in the neural stem cell. These data suggested that UCA1 played important roles in the proliferation and differentiation of the neural stem cell.
Previous study showed that knockdown of UCA1 promoted miR-1 expression in the bladder cancer cell [46]. In this study, we aslo showed that knockdown of UCA1 enhanced the miR-1 expression in the neural stem cell. Increasing studies have showed that miR-1 play a crucial role in stem cell development and differentiation. For example, Huang et al demonstrated that miR-1 could promote the mesenchymal stem cells (MSCs) more effectively for cardiac repair and enhanced cardiac myocyte differentiation and cell survival [47]. Moreover, Huang et al [48]. showed that overexpression of miR-1 promoted the MSCs differentiation into cardiac lineage through targeting Hes1. Increasing studies have demonstrated that Hes1 acts a crucial role in the central nervous system development [49]. Ectopic expression of Hes1 inhibited the neural stem cell proliferation and differentiation into neurons [50]. In our study, we demonstrated that knockdown of UCA1 promoted Hes1 expression in the neural stem cell, which was one target gene of miR-1. Ectopic expression of Hes1 could impair siUCA1-induced neural stem cell proliferation. Overexpression of Hes1 suppressed siUCA1-induced β-tubulin expression and promoted the siUCA1-inhibited GFAP expression in the neural stem cell. These data suggested that UCA1 played a critical role in the neural stem cell proliferation and differentiation partly through enhancing Hes1 expression.
In conclusion, our data showed that knockdown of UCA1 suppressed the neural stem cell proliferation and differentiation into astrocyte and promoted the neural stem cell differentiation into neuron. Our study also showed that UCA1 regulated the neural stem cell proliferation and differentiation through regulating Hes1 expression.
Disclosure of conflict of interest
None.
References
- 1.Garg N, Po A, Miele E, Campese AF, Begalli F, Silvano M, Infante P, Capalbo C, De Smaele E, Canettieri G, Di Marcotullio L, Screpanti I, Ferretti E, Gulino A. microRNA-17-92 cluster is a direct Nanog target and controls neural stem cell through Trp53inp1. EMBO J. 2013;32:2819–2832. doi: 10.1038/emboj.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui Y, Xiao Z, Chen T, Wei J, Chen L, Liu L, Chen B, Wang X, Li X, Dai J. The miR-7 identified from collagen biomaterial-based three-dimensional cultured cells regulates neural stem cell differentiation. Stem Cells Dev. 2014;23:393–405. doi: 10.1089/scd.2013.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gioia U, Di Carlo V, Caramanica P, Toselli C, Cinquino A, Marchioni M, Laneve P, Biagioni S, Bozzoni I, Cacci E, Caffarelli E. Mir-23a and mir-125b regulate neural stem/progenitor cell proliferation by targeting Musashi1. RNA Biol. 2014;11:1105–1112. doi: 10.4161/rna.35508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C, Sun G, Li S, Lang MF, Yang S, Li W, Shi Y. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci U S A. 2010;107:1876–1881. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lattanzi A, Gentner B, Corno D, Di Tomaso T, Mestdagh P, Speleman F, Naldini L, Gritti A. Dynamic activity of miR-125b and miR-93 during murine neural stem cell differentiation and in the subventricular zone neurogenic niche. PLoS One. 2013;8:e67411. doi: 10.1371/journal.pone.0067411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aranha MM, Santos DM, Sola S, Steer CJ, Rodrigues CM. miR-34a regulates mouse neural stem cell differentiation. PLoS One. 2011;6:e21396. doi: 10.1371/journal.pone.0021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson T, Rahman S, Sansom SN, Alsio JM, Kaneda M, Smith J, O’Carroll D, Tarakhovsky A, Livesey FJ. Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs. PLoS One. 2010;5:e13453. doi: 10.1371/journal.pone.0013453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palm T, Hemmer K, Winter J, Fricke IB, Tarbashevich K, Sadeghi Shakib F, Rudolph IM, Hillje AL, De Luca P, Bahnassawy L, Madel R, Viel T, De Siervi A, Jacobs AH, Diederichs S, Schwamborn JC. A systemic transcriptome analysis reveals the regulation of neural stem cell maintenance by an E2F1-miRNA feedback loop. Nucleic Acids Res. 2013;41:3699–3712. doi: 10.1093/nar/gkt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Crit Rev Oncol Hematol. 2008;65:43–53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Y, Xiao Z, Han J, Sun J, Ding W, Zhao Y, Chen B, Li X, Dai J. MiR-125b orchestrates cell proliferation, differentiation and migration in neural stem/progenitor cells by targeting Nestin. BMC Neurosci. 2012;13:116. doi: 10.1186/1471-2202-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris L, Zalucki O, Piper M, Heng JI. Insights into the biology and therapeutic applications of neural stem cells. Stem Cells Int. 2016;2016:9745315. doi: 10.1155/2016/9745315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, Quan ZW. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583. doi: 10.1038/cddis.2014.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015;34:18. doi: 10.1186/s13046-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Li Z. Long non-coding RNA HOTAIR: a novel oncogene (review) Mol Med Rep. 2015;12:5611–5618. doi: 10.3892/mmr.2015.4161. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Li Z. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology. Oncol Lett. 2015;10:1953–1958. doi: 10.3892/ol.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–1082. doi: 10.7314/apjcp.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng Y, Tao L, Qiu J. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One. 2013;8:e73991. doi: 10.1371/journal.pone.0073991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Li X, Song Y, Zhang P, Xiao Y, Xing Y. Long non-coding RNA ANRIL is up-regulated in bladder cancer and regulates bladder cancer cell proliferation and apoptosis through the intrinsic pathway. Biochem Biophys Res Commun. 2015;467:223–228. doi: 10.1016/j.bbrc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Naemura M, Murasaki C, Inoue Y, Okamoto H, Kotake Y. Long noncoding RNA ANRIL regulates proliferation of non-small cell lung cancer and cervical cancer cells. Anticancer Res. 2015;35:5377–5382. [PubMed] [Google Scholar]
- 21.Lin L, Gu ZT, Chen WH, Cao KJ. Increased expression of the long non-coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol. 2015;10:14. doi: 10.1186/s13000-015-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu H, Zhou X, Chang H, Li H, Liu F, Ma C, Lu J. CCAT1 promotes hepatocellular carcinoma cell proliferation and invasion. Int J Clin Exp Pathol. 2015;8:5427–5434. [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139:437–445. doi: 10.1007/s00432-012-1324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XF, Liu T, Li Y, Li S. Overexpression of long non-coding RNA CCAT1 is a novel biomarker of poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2015;8:9440–9445. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Dai SQ, Zhu CS, Han HY, Han LH, Zhao B, Gao RR, Zhang J. AK048794 maintain the mouse embryonic stem cell pluripotency by functioning as miRNA sponges for miR-592. Biochem J. 2016;473:3639–3654. doi: 10.1042/BCJ20160540. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Wu F, Song Y, Li X, Wu Q, Duan Y, Jin Z. Long noncoding RNA related to periodontitis interacts with miR-182 to upregulate osteogenic differentiation in periodontal mesenchymal stem cells of periodontitis patients. Cell Death Dis. 2016;7:e2327. doi: 10.1038/cddis.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Hao Y, Jiao Y, Li Z, Yue H, Xu Z, Wang S, Cao Y, Zhao J. Differential long noncoding RNA and mRNA expression in differentiated human glioblastoma stem cells. Mol Med Rep. 2016;14:2067–2076. doi: 10.3892/mmr.2016.5505. [DOI] [PubMed] [Google Scholar]
- 28.Riva P, Ratti A, Venturin M. The long noncoding RNAs in neurodegenerative diseases: novel mechanisms of pathogenesis. Curr Alzheimer Res. 2016;13:1219–1231. doi: 10.2174/1567205013666160622112234. [DOI] [PubMed] [Google Scholar]
- 29.Soudyab M, Iranpour M, Ghafouri-Fard S. The role of long non-coding RNAs in breast cancer. Arch Iran Med. 2016;19:508–517. [PubMed] [Google Scholar]
- 30.Cai H, Xue Y, Wang P, Wang Z, Li Z, Hu Y, Shang X, Liu Y. The long noncoding RNA TUG1 regulates blood-tumor barrier permeability by targeting miR-144. Oncotarget. 2015;6:19759–19779. doi: 10.18632/oncotarget.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, Dimmeler S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 32.Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, Han L, Xia R, Wang KM, Yang JS, De W, Shu YQ, Wang ZX. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243. doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, Yin R. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36:1643–1651. doi: 10.1007/s13277-014-2763-6. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Su L, Chen X, Li P, Cai Q, Yu B, Liu B, Wu W, Zhu Z. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed Pharmacother. 2014;68:557–564. doi: 10.1016/j.biopha.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Wang XS, Zhang Z, Wang HC, Cai JL, Xu QW, Li MQ, Chen YC, Qian XP, Lu TJ, Yu LZ, Zhang Y, Xin DQ, Na YQ, Chen WF. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12:4851–4858. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- 36.Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–1927. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Xiao C, Wu CH, Hu HZ. LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20:2819–2824. [PubMed] [Google Scholar]
- 38.Wei Y, Sun Q, Zhao L, Wu J, Chen X, Wang Y, Zang W, Zhao G. LncRNA UCA1-miR-507-FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med Oncol. 2016;33:88. doi: 10.1007/s12032-016-0804-2. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Gao Z, Liao J, Shang M, Li X, Yin L, Pu Y, Liu R. lncRNA UCA1 inhibits esophageal squamous-cell carcinoma growth by regulating the Wnt signaling pathway. J Toxicol Environ Health A. 2016;79:407–418. doi: 10.1080/15287394.2016.1176617. [DOI] [PubMed] [Google Scholar]
- 40.Shang C, Guo Y, Zhang J, Huang B. Silence of long noncoding RNA UCA1 inhibits malignant proliferation and chemotherapy resistance to adriamycin in gastric cancer. Cancer Chemother Pharmacol. 2016;77:1061–1067. doi: 10.1007/s00280-016-3029-3. [DOI] [PubMed] [Google Scholar]
- 41.Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu Y, Feng Y, Liu H, Fei B, Mao Y, Zhou L, Qi X, Huang S, Hua D, Xing C, Huang Z. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep. 2016;6:23892. doi: 10.1038/srep23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Jiang Y, Wan Y, Zhang L, Qiu J, Zhou S, Cheng W. UCA1 functions as a competing endogenous RNA to suppress epithelial ovarian cancer metastasis. Tumour Biol. 2016;37:10633–41. doi: 10.1007/s13277-016-4917-1. [DOI] [PubMed] [Google Scholar]
- 43.Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao X, Chen WS, Li B. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Na XY, Liu ZY, Ren PP, Yu R, Shang XS. Long non-coding RNA UCA1 contributes to the progression of prostate cancer and regulates proliferation through KLF4-KRT6/13 signaling pathway. Int J Clin Exp Med. 2015;8:12609–12616. [PMC free article] [PubMed] [Google Scholar]
- 45.Tuo YL, Li XM, Luo J. Long noncoding RNA UCA1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci. 2015;19:3403–3411. [PubMed] [Google Scholar]
- 46.Wang T, Yuan J, Feng N, Li Y, Lin Z, Jiang Z, Gui Y. Hsa-miR-1 downregulates long non-coding RNA urothelial cancer associated 1 in bladder cancer. Tumour Biol. 2014;35:10075–10084. doi: 10.1007/s13277-014-2321-2. [DOI] [PubMed] [Google Scholar]
- 47.Huang F, Li ML, Fang ZF, Hu XQ, Liu QM, Liu ZJ, Tang L, Zhao YS, Zhou SH. Overexpression of MicroRNA-1 improves the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Cardiology. 2013;125:18–30. doi: 10.1159/000347081. [DOI] [PubMed] [Google Scholar]
- 48.Huang F, Tang L, Fang ZF, Hu XQ, Pan JY, Zhou SH. miR-1-mediated induction of cardiogenesis in mesenchymal stem cells via downregulation of Hes-1. Biomed Res Int. 2013;2013:216286. doi: 10.1155/2013/216286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeuty B. A computational model for the coordination of neural progenitor self-renewal and differentiation through Hes1 dynamics. Development. 2015;142:477–485. doi: 10.1242/dev.112649. [DOI] [PubMed] [Google Scholar]
- 50.Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: role of Hes1. Mol Cell Neurosci. 2010;43:127–135. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]


