Abstract
MicroRNAs (miRNAs) play critical roles in the tumorigenesis of prostate cancer, while the biological function of miR-4735-3p is unknown. Mitogen-activated protein kinase kinase kinase 1 (MEKK1) has been shown to induce androgen receptor (AR)-dependent apoptosis in prostate cancer cells, but the regulation of MEKK1 in prostate cancer cells remains poorly defined. Here, we showed that miR-4735-3p was a MEKK1-targeting miRNA, and was highly expressed in AR+ prostate cancer specimens. Moreover, the levels of miR-4735-3p and MEKK1 inversely correlated. MiR-4735-3p-low subjects had a better overall survival, compared to miR-4735-3p-high subjects. MiR-4735-3p targeted the 3’-UTR of MEKK1 mRNA to inhibit its protein translation. Overexpression of miR-4735-3p inhibited MEKK1-mediated cell apoptosis upon docetaxel treatment, while depletion of miR-4735-3p enhanced it. Together, our data suggest that miR-4735-3p may suppress MEKK1-mediated prostate cancer cell apoptosis during chemotherapy. Inhibition of miR-4735-3p may improve the outcome of chemotherapy for some prostate cancers.
Keywords: Prostate cancer, MEKK1, miR-4735-3p, chemotherapy
Introduction
Prostate cancer is a malignant tumor that commonly occurs in older men. Although advances in modern medicine have greatly improved the prognosis of prostate cancer, satisfactory therapeutic outcome does not occur in every instance [1-4]. The current standard of care for the treatment of metastatic prostate cancer is docetaxel chemotherapy. However, prostate cancer cells may activate autophagy machinery to resist apoptotic cell death, with undetermined molecular mechanisms [5-7]. The resistance of a specific cancer to a specific chemotherapy may result from enhanced anti-apoptotic potentials of the cancer cells [8-10]. Cellular apoptosis is regulated by apoptosis activating proteins and apoptosis suppressors [11-17]. Hence, great efforts have been made to understand the molecular carcinogenesis of prostate cancer, as well as to search an effective molecular therapy to assist the surgery and chemotherapy [18].
Mitogen activated protein (MAP) kinase pathway regulates androgen receptor (AR)-mediated gene regulation and the prostate cancer cell phenotype. Activation of MAP kinase kinase kinase 1 (MEKK1) signaling in prostate cancer cells has been shown to result in the downstream activation of MKK4 (SEK1) and subsequently JNK [19], which is associated with diverse outcomes including cell apoptosis in response to growth factor deprivation or withdrawal of extracellular matrix [20]. Constitutively active alleles of MEKK1 induce apoptosis in diverse cell types, including AR+ prostate cancer cells [21]. However, the regulation of MEKK1 in prostate cancer cells is not fully understood.
Growing evidence has suggested that aberrant expression of microRNAs (miRNAs) is involved in prostate cancer initiation, progression, outgrowth, metastases and resistance to chemotherapy [22-24]. MiRNAs are non-coding small RNAs that regulate the gene expression at protein level, through their base-pairing with the 3’-untranslated region (3’-UTR) of the mRNA of the target gene [25,26]. The levels of a specific protein could be controlled by gene expression, protein translation and protein degradation. Thus, miRNAs play a critical role in regulating intracellular protein levels in many biological events including tumorigenesis. However, miR-4735-3p is a rarely studied miRNA, and its involvement in the carcinogenesis is ill-defined.
Here, we examined the MEKK1-targeting miRNAs in the regulation of MEKK1 by miR-4735-3p in AR+ prostate cancer specimens and cells.
Materials and methods
Patient tissue specimens
Thirty-eight resected prostate cancer specimens [paired prostate cancer (PC) and the adjacent non-tumor tissue (NT)] in this study were histologically and clinically diagnosed at Tongji Hospital from 2007 to 2009. All these specimens are AR+. For the use of these clinical materials for research purposes, prior patient’s consents and approval from the Institutional Research Ethics Committee were obtained.
Cell line and reagents
A human AR+ prostate cancer cell line LNCap was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). LNCap Cells were maintained in 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, St. Louis, MO, USA) and Ham’s F12 medium (Invitrogen) supplemented with L-glutamine and 10% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA) in a humidified chamber with 5% CO2 at 37°C. Docetaxel (Sigma-Aldrich) was prepared in a stock of 1 mmol/l and applied to the cultured prostate cancer cells at 5 µmol/l.
Plasmid transfection
MiR-4735-3p-expressing and antisense plasmids were all prepared using a backbone plasmid containing a GFP reporter under CMV promoter (pcDNA3.1-CMV-GFP, Clontech, Mountain View, CA, USA). The miR-4735-3p mimic, antisense, and control null plasmids were all purchased from Sigma-Aldrich, and digested with Xhol and BamHI and subcloned with a 2A into a pcDNA3.1-CMV-GFP backbone [27]. Sequencing was performed to confirm the correct orientation of the new plasmid. Transfection was performed with Lipofectamine 2000 reagent (Invitrogen) using 1 µg plasmids, according to the instructions of the manufacturer. One day after transfection, the transfected cells were purified by flow cytometry based on their expression of GFP.
Western blot
The protein was extracted from the prostate cancer or NT specimens, or from cultured cells, in RIPA lysis buffer (1% NP40, 0.1% SDS, 100 μg/ml phenylmethylsulfonyl fluoride, 0.5% sodium deoxycholate, in PBS) on ice. The supernatants were collected after centrifugation at 12000× g at 4°C for 20 min. Protein concentration was determined using a BCA protein assay kit (Bio-rad, China), and whole lysates were mixed with 4× SDS loading buffer (125 mmol/l Tris-HCl, 4% SDS, 20% glycerol, 100 mmol/l DTT, and 0.2% bromophenol blue) at a ratio of 1:3. Samples were heated at 100°C for 5 min and were separated on SDS-polyacrylamide gels. The separated proteins were then transferred to a PVDF membrane. The membrane blots were first probed with a primary antibody. After incubation with horseradish peroxidase-conjugated second antibody, autoradiograms were prepared using the enhanced chemiluminescent system to visualize the protein antigen. The signals were recorded using X-ray film. Primary antibodies were rabbit anti-MEKK1 and anti-α-tubulin (Cell Signaling, San Jose, CA, USA). Secondary antibody is HRP-conjugated anti-rabbit (Jackson ImmunoResearch Labs, West Grove, PA, USA). Blotting images were representative from 5 repeats. α-tubulin was used as a protein loading control. The protein levels were first normalized to α-tubulin, and then normalized to the experimental controls. Densitometry of Western blots was quantified with NIH ImageJ software (Bethesda, MD, USA).
Quantitative RT-PCR
Total RNA was extracted from tissue specimens or from cultured cells with miRNeasy mini kit (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was randomly primed from 2 μg of total RNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). RT-qPCR was subsequently performed in triplicate with QuantiTect SYBR Green PCR Kit (Qiagen). All primers were purchased from Qiagen. Data were collected and analyzed using 2-ΔΔCt method for quantification of the relative mRNA expression levels. Values of genes were first normalized against α-tubulin, and then compared to the experimental controls.
MiRNA target prediction and 3’-UTR luciferase-reporter assay
MiRNAs targets were predicted as has been described before, using the algorithms TargetSan (https://www.targetscan.org) [28]. Luciferase-reporters were successfully constructed using molecular cloning technology. Plasmids for MEKK1 miRNA 3’-UTR clone and MEKK1 miRNA 3’-UTR with a site mutation at the miR-4735-3p binding site were purchased from Creative Biogene (Shirley, NY, USA). MiR-4735-3p-modified prostate cancer cells were seeded in 24-well plates for 24 hours, after which they were transfected with 1 μg of Luciferase-reporter plasmids per well. Luciferase activities were measured using the dual-luciferase reporter gene assay kit (Promega, Beijing, China), according to the manufacturer’s instructions.
Cell viability by cell counting kit-8 (CCK-8) assay
The CCK-8 detection kit (Sigma-Aldrich) was used to measure cell viability according to the manufacturer’s instructions. Briefly, cells were seeded in a 96-well microplate at a density of 5×104/ml. After 24 h, cells were treated with resveratrol. Subsequently, CCK-8 solution (20 ml/well) was added and the plate was incubated at 37°C for 2 hours. The viable cells were counted by absorbance measurements with a monochromator microplate reader at a wavelength of 450 nm. The optical density value was reported as the percentage of cell viability in relation to the control group (set as 100%).
Apoptosis assay by flow cytometry
The cultured cells were re-suspended at a density of 106 cells/ml in PBS. After double staining with FITC-Annexin V and propidium iodide (PI) from a FITC Annexin V Apoptosis Detection Kit I (Becton-Dickinson Biosciences, San Jose, CA, USA), cells were analyzed using FACScan flow cytometer (Becton-Dickinson Biosciences) equipped with Cell Quest software (Becton-Dickinson Biosciences) for determination of Annexin V+ PI- apoptotic cells.
Statistical analysis
All data were statistically analyzed using one-way ANOVA with a Bonferroni correction, followed by Fisher’s Exact Test for comparison of two groups (GraphPad Prism, GraphPad Software, Inc. La Jolla, CA, USA). Bivariate correlations were calculated by Spearman’s Rank Correlation Coefficients. Kaplan-Meier curves were used to analyze the patient survival by miR-4735-3p levels. All values are depicted as mean ± standard deviation and are considered significant if P<0.05.
Results
The levels of miR-4735-3p and MEKK1 inversely correlate in AR+ prostate cancers
We detected significantly higher levels of miR-4735-3p (Figure 1A), and significantly lower levels of MEKK1 in AR+ prostate cancer specimens (Figure 1B), compared to the paired adjacent non-tumor tissues (NT) in 38 prostate cancer specimens. To examine the relationship between miR-4735-3p and MEKK1, we examined the levels of miR-4735-3p and MEKK1 in these AR+ prostate cancer specimens. A strong inverse correlation was detected between miR-4735-3p and MEKK1 (Figure 1C, γ=-0.63, P<0.0001). These data suggest presence of a causal link between miR-4735-3p and MEKK1 in AR+ prostate cancer cells. Next, we investigated whether the levels of miR-4735-3p may correlate with overall survival of AR+ prostate cancer patients. The survival of the 38 patients were analyzed. The median value of all 38 cases was chosen as the cutoff point for separating miR-4735-3p-high cases (n=19) from miR-4735-3p-low cases (n=19). Kaplan-Meier curves were performed, showing that miR-4735-3p-low AR+ prostate cancer patients had a significantly better overall survival, compared to miR-4735-3p-high AR+ prostate cancer patients (Figure 1D).
Figure 1.
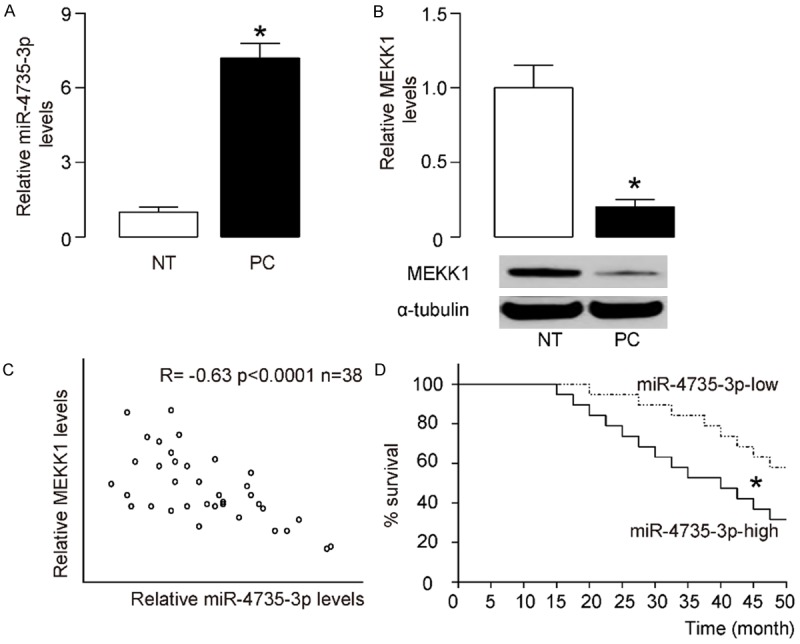
The levels of miR-4735-3p and MEKK1 inversely correlate in AR+ prostate cancer specimens. RT-qPCR on miR-4735-3p and Western blot for MEKK1 were performed on paired AR+ prostate cancer (PC) and the adjacent non-tumor tissues (NT) from 38 patients. A. miR-4735-3p levels. B. MEKK1 levels. C. A Correlation test between MEKK1 and miR-4735-3p. D. The 38 patients were followed-up for survival. The median value of all cases was chosen as the cutoff point for separating miR-4735-3p-high cases (n=19) from miR-4735-3p-low cases (n=19). Kaplan-Meier curves were performed to evaluate the overall survival of the AR+ prostate cancer patients, based on miR-4735-3p levels. *P<0.05. N=38.
MiR-4735-3p targets 3’-UTR of MEKK1 mRNA to inhibit its translation in AR+ prostate cancer cells
Since our data suggest a relationship between miR-4735-3p and MEKK1 in prostate cancer cells, we checked whether miR-4735-3p may target MEKK1 mRNA to suppress its translation. Bioinformatics analyses predicted a miR-4735-3p binding site at the 3’-UTR of MEKK1 mRNA ranged from 2270th to 2277th base site (Figure 2A). In order to figure out whether the binding of miR-4735-3p to MEKK1 mRNA in AR+ prostate cancer cells is functional, we either overexpressed miR-4735-3p, or inhibited miR-4735-3p in a human AR+ prostate cancer cell line, LNCap cells, by transfecting the cells with plasmids carrying either a miR-4735-3p-mimic (LNCap-miR-4735-3p), or a miR-4735-3p antisense (LNCap-as-miR-4735-3p). The LNCap cells were also transfected with a plasmid carrying null sequence as a control (LNCap-null). The overexpression or inhibition of miR-4735-3p in LNCap cells was confirmed by RT-qPCR (Figure 2B). MiR-4735-3p-modified LNCap cells were then transfected with 1 μg of MEKK1 3’-UTR luciferase-reporter plasmid or with 1 μg of MEKK1 3’UTR luciferase-reporter plasmid with a site mutation between 2270th to 2277th base site. The luciferase activities were quantified in these cells, suggesting that the binding of miR-4735-3p to 3’-UTR of MEKK1 mRNA results in suppression of MEKK1 protein translation (Figure 2C).
Figure 2.
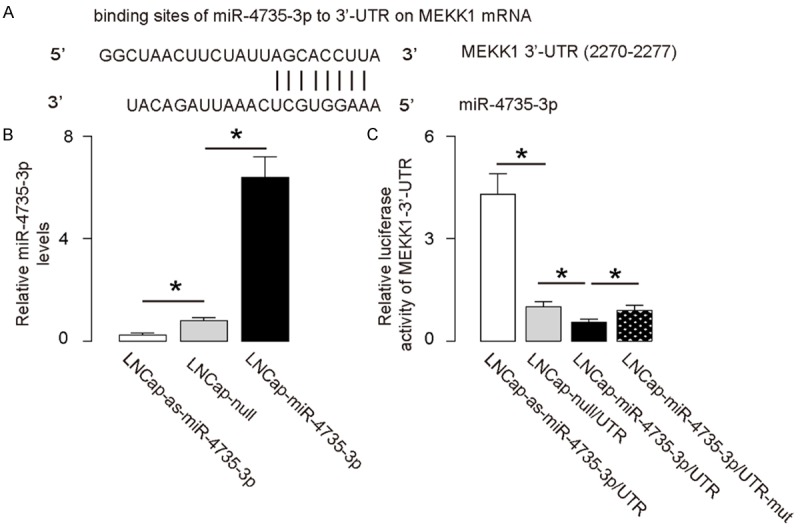
MiR-4735-3p targets 3’-UTR of MEKK1 mRNA to inhibit its expression. A. Bioinformatics analyses showing binding of miR-4735-3p to the 3’-UTR of MEKK1 mRNA. B. We either overexpressed miR-4735-3p, or inhibited miR-4735-3p in a human AR+ prostate cancer cell line, LNCap, by transfection of the cells with a miR-4735-3p-expressing plasmid (LNCap-miR-4735-3p), or with a plasmid carrying miR-4735-3p antisense (LNCap-as-miR-4735-3p). The LNCap cells were also transfected with a plasmid carrying a null sequence as a control (LNCap-null). The modification of miR-4735-3p levels in LNCap cells was confirmed by RT-qPCR. C. MiR-4735-3p-modified LNCap cells were transfected with 1 μg of MEKK1-3’UTR luciferase-reporter plasmid. The luciferase activities were examined. *P<0.05. N=5.
MiR-4735-3p reduces MEKK1 protein, but not mRNA, in AR+ prostate cancer cells
We found that modification of miR-4735-3p levels in LNCap cells did not change mRNA levels of MEKK1 (Figure 3A). However, overexpression of miR-4735-3p significantly decreased MEKK1 protein levels, while depletion of miR-4735-3p significantly increased MEKK1 protein levels in LNCap cells, by Western blot (Figure 3B). These data confirm that miR-4735-3p inhibits translation of the MEKK1 mRNA in AR+ prostate cancer cells.
Figure 3.
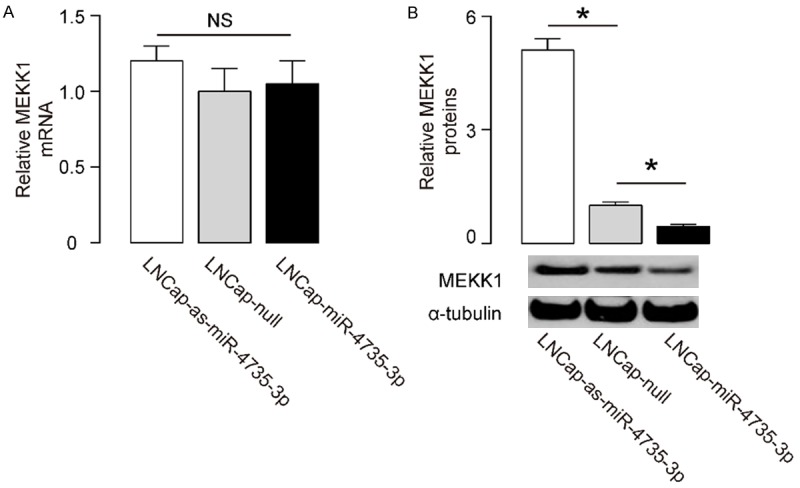
MiR-4735-3p decreases MEKK1 protein, but not mRNA, in AR+ prostate cancer cells. (A, B) The MEKK1 mRNA levels (A) and protein levels (B) in miR-4735-3p-modified LNCap cells. *P<0.05. NS: non-significant. N=5.
MiR-4735-3p decreases Docetaxel-induced AR+ prostate cancer cell apoptosis
Next, we examined the effects of miR-4735-3p modification on prostate cancer cell viability at presence of Docetaxel in a CCK-8 assay. We found that overexpression of miR-4735-3p resulted in increases in cell viability of LNCap cells, and depletion of miR-4735-3p resulted in decreases in cell viability of LNCap cells (Figure 4A). Then we did fluorescence-based apoptosis assay, and found that overexpression of miR-4735-3p resulted in decreases in LNCap cell apoptosis, and depletion of miR-4735-3p resulted in increases in LNCap cell apoptosis, shown by quantification (Figure 4B), and by representative flow charts (Figure 4C). Hence, miR-4735-3p inhibits Docetaxel-induced prostate cancer cell apoptosis. Together, our data suggest that miR-4735-3p expression in AR+ prostate cancer promotes AR+ prostate cancer cell survival against through suppressing MEKK1. Inhibition of miR-4735-3p levels in AR+ prostate cancer cells may improve the outcome of chemotherapy and the survival of the patients (Figure 5).
Figure 4.
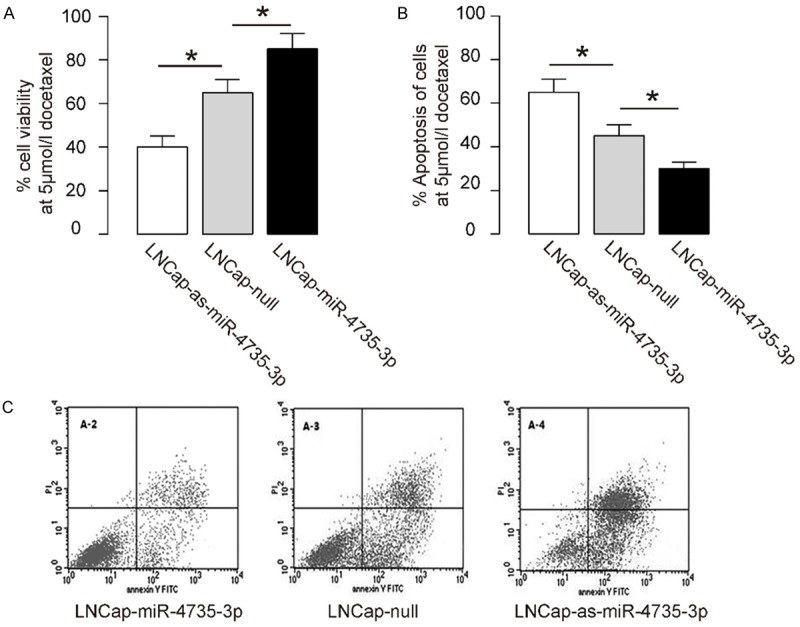
MiR-4735-3p increases Docetaxel-induced prostate cancer cell apoptosis. (A) The effects of miR-4735-3p modification on prostate cancer cell viability at presence of 5 µmol/l Docetaxel in a CCK-8 assay. (B, C) Fluorescence-based apoptosis assay on miR-4735-3p-modified LNCap cells, shown by quantification (B), and by representative flow charts (C). *P<0.05. N=5.
Figure 5.
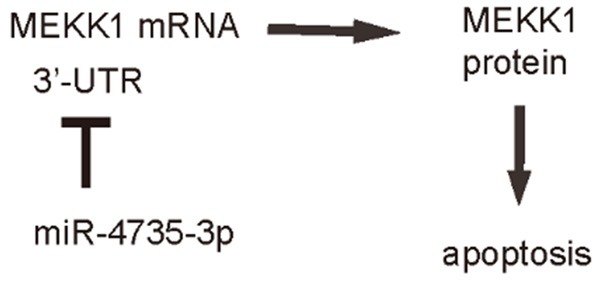
Schematic of the model. MiR-4735-3p in AR+ prostate cancer promotes cancer cell survival against chemotherapy-induced cell death. Inhibition of miR-4735-3p levels in AR+ prostate cancer cells may enhance therapeutic outcome of chemotherapy and the survival of the patients.
Discussion
MiRNAs have been shown to play a pivotal role in the processes including initiation, progression, invasion, metastases and resistance of chemotherapy of prostate cancer, which may either function by the miRNAs alone, or in a cooperative manner with other key regulators [29-31]. Hence, understanding of the regulation of aberrantly expression of miRNAs in prostate cancer carcinogenesis may help to clarify the mechanisms underlying prostate cancer carcinogenesis to improve the outcome of the therapy.
The resistance of prostate cancer cells to chemotherapy largely results from the activation of anti-apoptotic machinery and suppression of apoptotic machinery inside the prostate cancer cells [29-31]. From previous studies, we found that MEKK1 activation might be a key responsive factor upon Docetaxel treatment in prostate cancer cells. Docetaxel induced activation of apoptosis-association proteins to upregulate Cytochrome C and caspases to initiate the apoptotic process [32]. MEKK1 efficiently increases activation of apoptotic proteins, but the regulation of MEKK1 in prostate cancer cells is unclear.
Here, by sequence matching using bioinformatics analyses, we detected the candidate miRNAs that target MEKK1. Among all these miRNAs, we specifically detected a significant increase in miR-4735-3p in AR+ prostate cancer specimens, compared to the paired non-tumor tissue. Hence, we hypothesized that miR-4735-3p may target and regulate MEKK1 in AR+ prostate cancer cells. Correlation test between miR-4735-3p and MEKK1 further supported this hypothesis, and luciferase reporter assay confirmed that miR-4735-3p may bind MEKK1 mRNA to impair its translation. Then, we modified miR-4735-3p levels in prostate cancer cells, and found that it did not affect MEKK1 mRNA, but altered the protein. These data were further strengthened the proposed mechanisms.
Moreover, miR-4735-3p-mediated changes in MEKK1 seemed to result in changes in cell viability at presence of Docetaxel. We have examined several other AR+ prostate cancer cell lines, and obtained essentially similar results. Thus, a possibility of the results to be cell-line-dependence could be excluded. The effects of miR-4735-3p on cell invasion were not analyzed in the current study, but may be an attractive question to be answered in future.
To summarize, here we propose a model to explain the mechanisms underlying resistance of AR+ prostate cancer cells to chemotherapy. Prostate cancer cells upregulate miR-4735-3p, resulting in suppression of MEKK1 protein translation, which subsequently allows prostate cancer cells to survive chemotherapy. On the other hand, inhibition of miR-4735-3p levels in AR+ prostate cancer cells may enhance therapeutic outcome of chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Alshaker H, Sacco K, Alfraidi A, Muhammad A, Winkler M, Pchejetski D. Leptin signalling, obesity and prostate cancer: molecular and clinical perspective on the old dilemma. Oncotarget. 2015;6:35556–35563. doi: 10.18632/oncotarget.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perner S, Cronauer MV, Schrader AJ, Klocker H, Culig Z, Baniahmad A. Adaptive responses of androgen receptor signaling in castration-resistant prostate cancer. Oncotarget. 2015;6:35542–35555. doi: 10.18632/oncotarget.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rybak AP, Bristow RG, Kapoor A. Prostate cancer stem cells: deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget. 2015;6:1900–1919. doi: 10.18632/oncotarget.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz J, Wheler JJ, Kurzrock R. Androgen receptors beyond prostate cancer: an old marker as a new target. Oncotarget. 2015;6:592–603. doi: 10.18632/oncotarget.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Wu JB, Chung LW, Huang WC. Anticancer efficacy of SREBP inhibitor, alone or in combination with docetaxel, in prostate cancer harboring p53 mutations. Oncotarget. 2015;6:41018–41032. doi: 10.18632/oncotarget.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharaziha P, Chioureas D, Rutishauser D, Baltatzis G, Lennartsson L, Fonseca P, Azimi A, Hultenby K, Zubarev R, Ullen A, Yachnin J, Nilsson S, Panaretakis T. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6:21740–21754. doi: 10.18632/oncotarget.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mimeault M, Rachagani S, Muniyan S, Seshacharyulu P, Johansson SL, Datta K, Lin MF, Batra SK. Inhibition of hedgehog signaling improves the anti-carcinogenic effects of docetaxel in prostate cancer. Oncotarget. 2015;6:3887–3903. doi: 10.18632/oncotarget.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li DH, Pan ZK, Ye F, An HX, Wu JX. S-1-based versus 5-FU-based chemotherapy as first-line treatment in advanced gastric cancer: a meta-analysis of randomized controlled trials. Tumour Biol. 2014;35:8201–8208. doi: 10.1007/s13277-014-2099-2. [DOI] [PubMed] [Google Scholar]
- 9.Lu ZM, Luo TH, Nie MM, Fang GE, Ma LY, Xue XC, Wei G, Ke CW, Bi JW. Influence of ERCC1 and ERCC4 polymorphisms on response to prognosis in gastric cancer treated with FOLFOX-based chemotherapy. Tumour Biol. 2014;35:2941–2948. doi: 10.1007/s13277-013-1378-7. [DOI] [PubMed] [Google Scholar]
- 10.Wu XJ, Yuan P, Li ZY, Bu ZD, Zhang LH, Wu AW, Zong XL, Li SX, Shan F, Ji X, Ren H, Ji JF. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol. 2013;34:463–469. doi: 10.1007/s13277-012-0571-4. [DOI] [PubMed] [Google Scholar]
- 11.Shin D, Kwon HY, Sohn EJ, Nam MS, Kim JH, Lee JC, Ryu SY, Park B, Kim SH. Upregulation of death receptor 5 and production of reactive oxygen species mediate sensitization of PC-3 prostate cancer cells to TRAIL induced apoptosis by vitisin A. Cell Physiol Biochem. 2015;36:1151–1162. doi: 10.1159/000430286. [DOI] [PubMed] [Google Scholar]
- 12.Saleh AM, Aljada A, El-Abadelah MM, Sabri SS, Zahra JA, Nasr A, Aziz MA. The pyridoneannelated isoindigo (5’-Cl) induces apoptosis, dysregulation of mitochondria and formation of ROS in leukemic HL-60 cells. Cell Physiol Biochem. 2015;35:1958–1974. doi: 10.1159/000374004. [DOI] [PubMed] [Google Scholar]
- 13.Yun M, Lee D, Park MN, Kim EO, Sohn EJ, Kwon BM, Kim SH. Cinnamaldehyde derivative (CB-PIC) sensitizes chemo-resistant cancer cells to drug-induced apoptosis via suppression of MDR1 and its upstream STAT3 and AKT signalling. Cell Physiol Biochem. 2015;35:1821–1830. doi: 10.1159/000373993. [DOI] [PubMed] [Google Scholar]
- 14.Xiong Y, Ye T, Wang M, Xia Y, Wang N, Song X, Wang F, Liu L, Zhu Y, Yang F, Wei Y, Yu L. A novel cinnamide YLT26 induces breast cancer cells apoptosis via ROS-mitochondrial apoptotic pathway in vitro and inhibits lung metastasis in vivo. Cell Physiol Biochem. 2014;34:1863–1876. doi: 10.1159/000366385. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Huo X, Zhang C, Ma X, Han F, Wang G. Role of continuous high thoracic epidural anesthesia in hippocampal apoptosis after global cerebral ischemia in rats. Cell Physiol Biochem. 2014;34:1227–1240. doi: 10.1159/000366334. [DOI] [PubMed] [Google Scholar]
- 16.Xie M, Yi X, Wang R, Wang L, He G, Zhu M, Qi C, Liu Y, Ye Y, Tan S, Tang A. 14-Thienyl methylene matrine (YYJ18), the derivative from matrine, induces apoptosis of human nasopharyngeal carcinoma cells by targeting MAPK and PI3K/Akt pathways in vitro. Cell Physiol Biochem. 2014;33:1475–1483. doi: 10.1159/000358712. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Xia Y, Ye T, Shi X, Song X, Liu L, Zeng J, Wang N, Luo Y, Han Y, Yu L. A novel smallmolecule YLT205 induces apoptosis in human colorectal cells via mitochondrial apoptosis pathway in vitro and inhibits tumor growth in vivo. Cell Physiol Biochem. 2014;33:933–944. doi: 10.1159/000358665. [DOI] [PubMed] [Google Scholar]
- 18.Cui Y, Chen J, He Z, Xiao Y. SUZ12 depletion suppresses the proliferation of gastric cancer cells. Cell Physiol Biochem. 2013;31:778–784. doi: 10.1159/000350095. [DOI] [PubMed] [Google Scholar]
- 19.McConkey DJ, Greene G, Pettaway CA. Apoptosis resistance increases with metastatic potential in cells of the human LNCaP prostate carcinoma line. Cancer Res. 1996;56:5594–5599. [PubMed] [Google Scholar]
- 20.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 21.Abreu-Martin MT, Chari A, Palladino AA, Craft NA, Sawyers CL. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei Q, Li F, Quan H, Liu Y, Xu H. Busulfan inhibits growth of human osteosarcoma through miR-200 family microRNAs in vitro and in vivo. Cancer Sci. 2014;105:755–762. doi: 10.1111/cas.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Xiao W, Sun J, Han D, Zhu Y. MiRNA-181c inhibits EGFR-signaling-dependent MMP9 activation via suppressing Akt phosphorylation in glioblastoma. Tumour Biol. 2014;35:8653–8658. doi: 10.1007/s13277-014-2131-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Jiang C, Li D, Wang R, Wang W. MiRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in gastric cancer. Tumour Biol. 2014;35:9801–9806. doi: 10.1007/s13277-014-2273-6. [DOI] [PubMed] [Google Scholar]
- 25.Di Leva G, Croce CM. miRNA profiling of cancer. Curr Opin Genet Dev. 2013;23:3–11. doi: 10.1016/j.gde.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 2013;18:282–289. doi: 10.1016/j.drudis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Szymczak-Workman AL, Vignali KM, Vignali DA. Design and construction of 2A peptidelinked multicistronic vectors. Cold Spring Harb Protoc. 2012;2012:199–204. doi: 10.1101/pdb.ip067876. [DOI] [PubMed] [Google Scholar]
- 28.Coronnello C, Benos PV. ComiR: combinatorial microRNA target prediction tool. Nucleic Acids Res. 2013;41:W159–164. doi: 10.1093/nar/gkt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopczynska E. Role of microRNAs in the resistance of prostate cancer to docetaxel and paclitaxel. Contemp Oncol (Pozn) 2015;19:423–427. doi: 10.5114/wo.2015.56648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabris L, Ceder Y, Chinnaiyan AM, Jenster GW, Sorensen KD, Tomlins S, Visakorpi T, Calin GA. The potential of MicroRNAs as prostate cancer biomarkers. Eur Urol. 2016;70:312–322. doi: 10.1016/j.eururo.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennati M, Folini M, Gandellini P, Zaffaroni N. MicroRNAs and the response of prostate cancer to anti-cancer drugs. Curr Drug Targets. 2016;17:257–265. doi: 10.2174/1389450116666150316223341. [DOI] [PubMed] [Google Scholar]
- 32.Tamaki H, Harashima N, Hiraki M, Arichi N, Nishimura N, Shiina H, Naora K, Harada M. Bcl-2 family inhibition sensitizes human prostate cancer cells to docetaxel and promotes unexpected apoptosis under caspase-9 inhibition. Oncotarget. 2014;5:11399–11412. doi: 10.18632/oncotarget.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]


